Modulating the Gut–Muscle Axis: Increasing SCFA-Producing Gut Microbiota Commensals and Decreasing Endotoxin Production to Mitigate Cancer Cachexia
Abstract
1. Introduction
2. Gut Microbiota Targeted Intervention May Mitigate Cancer Cachexia Linked Gut Dysbiosis, Muscle Wasting, and Systemic Inflammation
2.1. Cancer Cachexia Is Linked to Gut Dysbiosis Marked by a Loss of SCFA-Producing Gut Microbiota Commensals and Enrichment of Pro-Inflammatory Pathobionts
2.2. Cancer Cachexia-Linked Dysbiosis Depletes SCFAs, BCAAs, and Alters the Bile Acids Profile
2.3. Cancer Cachexia-Linked Dysbiosis May Lead to Pro-Inflammatory Cytokine-Mediated Skeletal Muscle and Adipose Tissue Wasting
2.4. Gut Microbiota-Targeted Therapies May Restore Eubiotic State, Thereby Mitigating Inflammation and Muscle Wasting
2.4.1. Probiotics
2.4.2. Prebiotics and Synbiotics
2.4.3. Fecal Microbiota Transplantation (FMT)
2.4.4. Postbiotics
2.4.5. Antibiotics
2.4.6. Dietary Strategies
3. Discussion
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Argilés, J.M.; Busquets, S.; Stemmler, B.; López-Soriano, F.J. Cancer cachexia: Understanding the molecular basis. Nat. Rev. Cancer 2014, 14, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Rhee, C.; Sim, J.J.; Stenvinkel, P.; Anker, S.D.; Kovesdy, C.P. Why cachexia kills: Examining the causality of poor outcomes in wasting conditions. J. Cachexia Sarcopenia Muscle 2013, 4, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.H.; Fearon, K.C. Cachexia: Prevalence and impact in medicine. Curr. Opin. Intern. Med. 2008, 7, 441–448. [Google Scholar] [CrossRef]
- Ross, P.J.; Ashley, S.; Norton, A.; Priest, K.; Waters, J.S.; Eisen, T.; Smith, I.E.; O’Brien, M.E.R. Do patients with weight loss have a worse outcome when undergoing chemotherapy for lung cancers? Br. J. Cancer 2004, 90, 1905–1911. [Google Scholar] [CrossRef]
- Wallengren, O.; Lundholm, K.; Bosaeus, I. Diagnostic criteria of cancer cachexia: Relation to quality of life, exercise capacity and survival in unselected palliative care patients. Support. Care Cancer 2013, 21, 1569–1577. [Google Scholar] [CrossRef]
- Zaorsky, N.G.; Churilla, T.M.; Egleston, B.L.; Fisher, S.G.; Ridge, J.A.; Horwitz, E.M.; Meyer, J.E. Causes of death among cancer patients. Ann. Oncol. 2017, 28, 400–407. [Google Scholar] [CrossRef]
- Argilés, J.M.; López-Soriano, F.J.; Stemmler, B.; Busquets, S. Cancer-associated cachexia—Understanding the tumour macroenvironment and microenvironment to improve management. Nat. Rev. Clin. Oncol. 2023, 20, 250–264. [Google Scholar] [CrossRef]
- Porporato, P.E. Understanding cachexia as a cancer metabolism syndrome. Oncogenesis 2016, 5, e200. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Nan, D.; Yao, W.; Huang, L.; Liu, R.; Chen, X.; Xia, W.; Sheng, H.; Zhang, H.; Liang, X.; Lu, Y. Glutamine and cancer: Metabolism, immune microenvironment, and therapeutic targets. Cell Commun. Signal 2025, 23, 45. [Google Scholar] [CrossRef] [PubMed]
- Argilés, J.M.; López-Soriano, F.J.; Toledo, M.; Betancourt, A.; Serpe, R.; Busquets, S. The cachexia score (CASCO): A new tool for staging cachectic cancer patients. J. Cachexia Sarcopenia Muscle 2011, 2, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Busquets, S.; Betancourt, A.; Seelaender, M.; Miglior, I.; Guàrdia-Olmos, J.; Peró-Cebollero, M.; López-Soriano, F.J.; Maddedu, C.; Serpe, R.; Argilés, J.M. Abstracts of the 7th Cachexia Conference, Kobe/Osaka, Japan, December 9–11, 2013. J. Cachexia Sarcopenia Muscle 2013, 4, 295–343. [Google Scholar] [CrossRef]
- Deans, D.A.C.; Tan, B.H.; Wigmore, S.J.; Ross, J.A.; De Beaux, A.C.; Paterson-Brown, S.; Fearon, K.C.H. The influence of systemic inflammation, dietary intake and stage of disease on rate of weight loss in patients with gastro-oesophageal cancer. Br. J. Cancer 2009, 100, 63–69. [Google Scholar] [CrossRef]
- Riechelmann, R.P.; Burman, D.; Tannock, I.F.; Rodin, G.; Zimmermann, C. Phase II Trial of Mirtazapine for Cancer-Related Cachexia and Anorexia. Am. J. Hosp. Palliat. Med. 2010, 27, 106–110. [Google Scholar] [CrossRef]
- Femia, R.A.; Goyette, R.E. The Science of Megestrol Acetate Delivery: Potential to Improve Outcomes in Cachexia. BioDrugs 2005, 19, 179–187. [Google Scholar] [CrossRef]
- Lynch, G.S.; Schertzer, J.D.; Ryall, J.G. Therapeutic approaches for muscle wasting disorders. Pharmacol. Ther. 2007, 113, 461–487. [Google Scholar] [CrossRef]
- Ovesen, L.; Allingstrup, L.; Hannibal, J.; Mortensen, E.L.; Hansen, O.P. Effect of dietary counseling on food intake, body weight, response rate, survival, and quality of life in cancer patients undergoing chemotherapy: A prospective, randomized study. J. Clin. Oncol. 1993, 11, 2043–2049. [Google Scholar] [CrossRef]
- Walsh, D.; Nelson, K.A.; Mahmoud, F. Established and potential therapeutic applications of cannabinoids in oncology. Support. Care Cancer 2003, 11, 137–143. [Google Scholar] [CrossRef]
- Loprinzi, C.L.; Kugler, J.W.; Sloan, J.A.; Mailliard, J.A.; Krook, J.E.; Wilwerding, M.B.; Rowland, K.M.; Camoriano, J.K.; Novotny, P.J.; Christensen, B.J. Randomized Comparison of Megestrol Acetate Versus Dexamethasone Versus Fluoxymesterone for the Treatment of Cancer Anorexia/Cachexia. J. Clin. Oncol. 1999, 17, 3299–3306. [Google Scholar] [CrossRef]
- Ruiz Garcia, V.; López-Briz, E.; Carbonell Sanchis, R.; Gonzalvez Perales, J.L.; Bort-Martí, S. Megestrol acetate for treatment of anorexia-cachexia syndrome. Cochrane Database Syst. Rev. 2013, 2019, 3299–3306. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.; Arends, J.; Baracos, V. Understanding the mechanisms and treatment options in cancer cachexia. Nat. Rev. Clin. Oncol. 2013, 10, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Bindels, L.B.; Thissen, J.-P. Nutrition in cancer patients with cachexia: A role for the gut microbiota? Clin. Nutr. Exp. 2016, 6, 74–82. [Google Scholar] [CrossRef]
- Herremans, K.M.; Riner, A.N.; Cameron, M.E.; Trevino, J.G. The Microbiota and Cancer Cachexia. Int. J. Mol. Sci. 2019, 20, 6267. [Google Scholar] [CrossRef]
- Ziemons, J.; Smidt, M.L.; Damink, S.O.; Rensen, S.S. Gut microbiota and metabolic aspects of cancer cachexia. Best. Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101508. [Google Scholar] [CrossRef]
- Bindels, L.B.; Neyrinck, A.M.; Loumaye, A.; Catry, E.; Walgrave, H.; Cherbuy, C.; Leclercq, S.; Van Hul, M.; Plovier, H.; Pachikian, B.; et al. Increased gut permeability in cancer cachexia: Mechanisms and clinical relevance. Oncotarget 2018, 9, 18224–18238. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Tremaroli, V.; Bäckhed, F. Functional interactions between the gut microbiota and host metabolism. Nature 2012, 489, 242–249. [Google Scholar] [CrossRef]
- Genton, L.; Mareschal, J.; Charretier, Y.; Lazarevic, V.; Bindels, L.B.; Schrenzel, J. Targeting the Gut Microbiota to Treat Cachexia. Front. Cell. Infect. Microbiol. 2019, 9, 305. [Google Scholar] [CrossRef]
- Przewłócka, K.; Folwarski, M.; Kaźmierczak-Siedlecka, K.; Skonieczna-Żydecka, K.; Kaczor, J.J. Gut-Muscle Axis Exists and May Affect Skeletal Muscle Adaptation to Training. Nutrients 2020, 12, 1451. [Google Scholar] [CrossRef]
- Lin, R.; Liu, W.; Piao, M.; Zhu, H. A review of the relationship between the gut microbiota and amino acid metabolism. Amino Acids 2017, 49, 2083–2090. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, S.; Kim, H.; Garcia-Perez, I.; Reza, M.M.; Martin, K.A.; Kundu, P.; Cox, L.M.; Selkrig, J.; Posma, J.M.; Zhang, H.; et al. The gut microbiota influences skeletal muscle mass and function in mice. Sci. Transl. Med. 2019, 11, eaan5662. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Yu, J.; Li, Y.; Yang, F.; Yu, H.; Xue, M.; Zhang, F.; Jiang, X.; Ji, X.; Bao, Z. Depletion of gut microbiota induces skeletal muscle atrophy by FXR-FGF15/19 signalling. Ann. Med. 2021, 53, 508–522. [Google Scholar] [CrossRef] [PubMed]
- Salvi, P.S.; Cowles, R.A. Butyrate and the Intestinal Epithelium: Modulation of Proliferation and Inflammation in Homeostasis and Disease. Cells 2021, 10, 1775. [Google Scholar] [CrossRef]
- Oliphant, K.; Allen-Vercoe, E. Macronutrient metabolism by the human gut microbiome: Major fermentation by-products and their impact on host health. Microbiome 2019, 7, 91. [Google Scholar] [CrossRef]
- Van Der Beek, C.M.; Dejong, C.H.C.; Troost, F.J.; Masclee, A.A.M.; Lenaerts, K. Role of short-chain fatty acids in colonic inflammation, carcinogenesis, and mucosal protection and healing. Nutr. Rev. 2017, 75, 286–305. [Google Scholar] [CrossRef]
- Sakakida, T.; Ishikawa, T.; Doi, T.; Morita, R.; Endo, Y.; Matsumura, S.; Ota, T.; Yoshida, J.; Hirai, Y.; Mizushima, K.; et al. Water-soluble dietary fiber alleviates cancer-induced muscle wasting through changes in gut microenvironment in mice. Cancer Sci. 2022, 113, 1789–1800. [Google Scholar] [CrossRef]
- Pötgens, S.A.; Thibaut, M.M.; Joudiou, N.; Sboarina, M.; Neyrinck, A.M.; Cani, P.D.; Claus, S.P.; Delzenne, N.M.; Bindels, L.B. Multi-compartment metabolomics and metagenomics reveal major hepatic and intestinal disturbances in cancer cachectic mice. J. Cachexia Sarcopenia Muscle 2021, 12, 456–475. [Google Scholar] [CrossRef]
- Ubachs, J.; Ziemons, J.; Soons, Z.; Aarnoutse, R.; Van Dijk, D.P.J.; Penders, J.; Van Helvoort, A.; Smidt, M.L.; Kruitwagen, R.F.P.M.; Baade-Corpelijn, L.; et al. Gut microbiota and short-chain fatty acid alterations in cachectic cancer patients. J. Cachexia Sarcopenia Muscle 2021, 12, 2007–2021. [Google Scholar] [CrossRef]
- Pötgens, S.A.; Brossel, H.; Sboarina, M.; Catry, E.; Cani, P.D.; Neyrinck, A.M.; Delzenne, N.M.; Bindels, L.B. Klebsiella oxytoca expands in cancer cachexia and acts as a gut pathobiont contributing to intestinal dysfunction. Sci. Rep. 2018, 8, 12321. [Google Scholar] [CrossRef]
- Puppa, M.J.; White, J.P.; Sato, S.; Cairns, M.; Baynes, J.W.; Carson, J.A. Gut barrier dysfunction in the ApcMin/+ mouse model of colon cancer cachexia. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2011, 1812, 1601–1606. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Erbay, E. Nutrient sensing and inflammation in metabolic diseases. Nat. Rev. Immunol. 2008, 8, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Guo, C.; Zhang, D.; Zhang, J.; Wang, X.; Geng, C. The Altered Tight Junctions: An Important Gateway of Bacterial Translocation in Cachexia Patients with Advanced Gastric Cancer. J. Interferon Cytokine Res. 2014, 34, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Panebianco, C.; Villani, A.; Potenza, A.; Favaro, E.; Finocchiaro, C.; Perri, F.; Pazienza, V. Targeting Gut Microbiota in Cancer Cachexia: Towards New Treatment Options. Int. J. Mol. Sci. 2023, 24, 1849. [Google Scholar] [CrossRef]
- Bindels, L.B.; Beck, R.; Schakman, O.; Martin, J.C.; De Backer, F.; Sohet, F.M.; Dewulf, E.M.; Pachikian, B.D.; Neyrinck, A.M.; Thissen, J.-P.; et al. Restoring Specific Lactobacilli Levels Decreases Inflammation and Muscle Atrophy Markers in an Acute Leukemia Mouse Model. PLoS ONE 2012, 7, e37971. [Google Scholar] [CrossRef]
- Sciorati, C.; Touvier, T.; Buono, R.; Pessina, P.; François, S.; Perrotta, C.; Meneveri, R.; Clementi, E.; Brunelli, S. Necdin is expressed in cachectic skeletal muscle to protect fibers from tumor-induced wasting. J. Cell Sci. 2009, 122, 1119–1125. [Google Scholar] [CrossRef][Green Version]
- Talbert, E.E.; Metzger, G.A.; He, W.A.; Guttridge, D.C. Modeling human cancer cachexia in colon 26 tumor-bearing adult mice. J. Cachexia Sarcopenia Muscle 2014, 5, 321–328. [Google Scholar] [CrossRef]
- Bindels, L.B.; Neyrinck, A.M.; Claus, S.P.; Le Roy, C.I.; Grangette, C.; Pot, B.; Martinez, I.; Walter, J.; Cani, P.D.; Delzenne, N.M. Synbiotic approach restores intestinal homeostasis and prolongs survival in leukaemic mice with cachexia. ISME J. 2016, 10, 1456–1470. [Google Scholar] [CrossRef]
- De Maria, Y.N.L.F.; Aciole Barbosa, D.; Menegidio, F.B.; Santos, K.B.N.H.; Humberto, A.C.; Alencar, V.C.; Silva, J.F.S.; Costa De Oliveira, R.; Batista, M.L., Jr.; Nunes, L.R.; et al. Analysis of mouse faecal dysbiosis, during the development of cachexia, induced by transplantation with Lewis lung carcinoma cells. Microbiology 2021, 167, 001088. [Google Scholar] [CrossRef]
- Jeong, S.M.; Jin, E.-J.; Wei, S.; Bae, J.-H.; Ji, Y.; Jo, Y.; Jeong, J.-H.; Im, S.J.; Ryu, D. The impact of cancer cachexia on gut microbiota composition and short-chain fatty acid metabolism in a murine model. BMB Rep. 2023, 56, 404–409. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, R.; Yin, Z.; Chen, X.; Mao, R.; Zheng, X.; Yuan, M.; Li, H.; Lu, Y.; Liu, S.; et al. Gut microbiota-driven metabolic alterations reveal the distinct pathogenicity of chemotherapy-induced cachexia in gastric cancer. Pharmacol. Res. 2024, 209, 107476. [Google Scholar] [CrossRef] [PubMed]
- Byndloss, M.X.; Olsan, E.E.; Rivera-Chávez, F.; Tiffany, C.R.; Cevallos, S.A.; Lokken, K.L.; Torres, T.P.; Byndloss, A.J.; Faber, F.; Gao, Y.; et al. Microbiota-activated PPAR-γ signaling inhibits dysbiotic Enterobacteriaceae expansion. Science 2017, 357, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Zhang, W.; Shen, Q.; Miao, C.; Chen, L.; Li, Y.; Gu, X.; Fan, M.; Ma, Y.; Wang, H.; et al. Bile acid metabolism dysregulation associates with cancer cachexia: Roles of liver and gut microbiome. J. Cachexia Sarcopenia Muscle 2021, 12, 1553–1569. [Google Scholar] [CrossRef]
- Bennani-Baiti, N.; Walsh, D. Animal models of the cancer anorexia–cachexia syndrome. Support. Care Cancer 2011, 19, 1451–1463. [Google Scholar] [CrossRef]
- DeBoer, M.D. Animal models of anorexia and cachexia. Expert Opin. Drug Discov. 2009, 4, 1145–1155. [Google Scholar] [CrossRef]
- Jabes, D.L.; De Maria, Y.N.L.F.; Aciole Barbosa, D.; Santos, K.B.N.H.; Carvalho, L.M.; Humberto, A.C.; Alencar, V.C.; Costa De Oliveira, R.; Batista, M.L.; Menegidio, F.B.; et al. Fungal Dysbiosis Correlates with the Development of Tumor-Induced Cachexia in Mice. J. Fungi 2020, 6, 364. [Google Scholar] [CrossRef]
- Ni, Y.; Lohinai, Z.; Heshiki, Y.; Dome, B.; Moldvay, J.; Dulka, E.; Galffy, G.; Berta, J.; Weiss, G.J.; Sommer, M.O.A.; et al. Distinct composition and metabolic functions of human gut microbiota are associated with cachexia in lung cancer patients. ISME J. 2021, 15, 3207–3220. [Google Scholar] [CrossRef]
- Varian, B.J.; Goureshetti, S.; Poutahidis, T.; Lakritz, J.R.; Levkovich, T.; Kwok, C.; Teliousis, K.; Ibrahim, Y.M.; Mirabal, S.; Erdman, S.E. Beneficial bacteria inhibit cachexia. Oncotarget 2016, 7, 11803–11816. [Google Scholar] [CrossRef]
- De Clercq, N.C.; Van Den Ende, T.; Prodan, A.; Hemke, R.; Davids, M.; Pedersen, H.K.; Nielsen, H.B.; Groen, A.K.; De Vos, W.M.; Van Laarhoven, H.W.M.; et al. Fecal Microbiota Transplantation from Overweight or Obese Donors in Cachectic Patients with Advanced Gastroesophageal Cancer: A Randomized, Double-blind, Placebo-Controlled, Phase II Study. Clin. Cancer Res. 2021, 27, 3784–3792. [Google Scholar] [CrossRef]
- Bindels, L.B.; Neyrinck, A.M.; Salazar, N.; Taminiau, B.; Druart, C.; Muccioli, G.G.; François, E.; Blecker, C.; Richel, A.; Daube, G.; et al. Non digestible oligosaccharides modulate the gut microbiota to control the development of leukemia and associated cachexia in mice. PLoS ONE 2015, 10, e0131009. [Google Scholar] [CrossRef]
- Castellani, C.; Singer, G.; Kaiser, M.; Kaiser, T.; Huang, J.; Sperl, D.; Kashofer, K.; Fauler, G.; Guertl-Lackner, B.; Höfler, G.; et al. Neuroblastoma causes alterations of the intestinal microbiome, gut hormones, inflammatory cytokines, and bile acid composition. Pediatr. Blood Cancer 2017, 64. [Google Scholar] [CrossRef] [PubMed]
- Hakozaki, T.; Nolin-Lapalme, A.; Kogawa, M.; Okuma, Y.; Nakamura, S.; Moreau-Amaru, D.; Tamura, T.; Hosomi, Y.; Takeyama, H.; Richard, C.; et al. Cancer Cachexia among Patients with Advanced Non-Small-Cell Lung Cancer on Immunotherapy: An Observational Study with Exploratory Gut Microbiota Analysis. Cancers 2022, 14, 5405. [Google Scholar] [CrossRef] [PubMed]
- Frost, G.; Sleeth, M.L.; Sahuri-Arisoylu, M.; Lizarbe, B.; Cerdan, S.; Brody, L.; Anastasovska, J.; Ghourab, S.; Hankir, M.; Zhang, S.; et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat. Commun. 2014, 5, 3611. [Google Scholar] [CrossRef]
- Goffredo, M.; Mass, K.; Parks, E.J.; Wagner, D.A.; McClure, E.A.; Graf, J.; Savoye, M.; Pierpont, B.; Cline, G.; Santoro, N. Role of Gut Microbiota and Short Chain Fatty Acids in Modulating Energy Harvest and Fat Partitioning in Youth. J. Clin. Endocrinol. Metab. 2016, 101, 4367–4376. [Google Scholar] [CrossRef]
- González Hernández, M.A.; Canfora, E.E.; Jocken, J.W.E.; Blaak, E.E. The Short-Chain Fatty Acid Acetate in Body Weight Control and Insulin Sensitivity. Nutrients 2019, 11, 1943. [Google Scholar] [CrossRef]
- Burri, E.; Beglinger, C. The use of fecal calprotectin as a biomarker in gastrointestinal disease. Expert Rev. Gastroenterol. Hepatol. 2014, 8, 197–210. [Google Scholar] [CrossRef]
- Thibaut, M.M.; Gillard, J.; Dolly, A.; Roumain, M.; Leclercq, I.A.; Delzenne, N.M.; Muccioli, G.G.; Bindels, L.B. Bile Acid Dysregulation Is Intrinsically Related to Cachexia in Tumor-Bearing Mice. Cancers 2021, 13, 6389. [Google Scholar] [CrossRef]
- Sharma, M.; Kambadur, R.; Sriram, S.; Lokireddy, S.; McFarlane, C.D. Molecular targets of cancer cachexia: Opportunities for pharmanutritional approaches. PharmaNutrition 2014, 2, 126–128. [Google Scholar] [CrossRef]
- Tisdale, M.J. Mechanisms of Cancer Cachexia. Physiol. Rev. 2009, 89, 381–410. [Google Scholar] [CrossRef]
- Argilés, J.M.; García-Martínez, C.; Llovera, M.; López, F.J. The Role of Tumor Necrosis Factor-αα in Muscle Wasting Disorders. Basic Appl. Myol. 1998, 8, 371–380. [Google Scholar]
- Patel, H.J.; Patel, B.M. TNF-α and cancer cachexia: Molecular insights and clinical implications. Life Sci. 2017, 170, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.-Y.; Choi, Y.S.; Yeom, C.H.; Kwak, S.M.; Yoon, H.M.; Kim, D.G.; Koh, S.-J.; Park, J.; Lee, M.A.; Lee, Y.J.; et al. Interleukin-6 but not tumour necrosis factor-alpha predicts survival in patients with advanced cancer. Support. Care Cancer 2013, 21, 3071–3077. [Google Scholar] [CrossRef] [PubMed]
- Klein, G.L.; Petschow, B.W.; Shaw, A.L.; Weaver, E. Gut barrier dysfunction and microbial translocation in cancer cachexia: A new therapeutic target. Curr. Opin. Support. Palliat. Care 2013, 7, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Tazuke, Y.; Drongowski, R.A.; Teitelbaum, D.H.; Coran, A.G. Interleukin-6 changes tight junction permeability and intracellular phospholipid content in a human enterocyte cell culture model. Pediatr. Surg. Int. 2003, 19, 321–325. [Google Scholar] [CrossRef]
- Narsale, A.A.; Carson, J.A. Role of interleukin-6 in cachexia: Therapeutic implications. Curr. Opin. Support. Palliat. Care 2014, 8, 321–327. [Google Scholar] [CrossRef]
- Prado, B.L.; Qian, Y. Anti-cytokines in the treatment of cancer cachexia. Ann. Palliat. Med. 2019, 8, 67–79. [Google Scholar] [CrossRef]
- Kent, L.W.; Rahemtulla, F.; Hockett, R.D.; Gilleland, R.C.; Michalek, S.M. Effect of Lipopolysaccharide and Inflammatory Cytokines on Interleukin-6 Production by Healthy Human Gingival Fibroblasts. Infect. Immun. 1998, 66, 608–614. [Google Scholar] [CrossRef]
- Dehoux, M.J.M.; Van Beneden, R.P.; Fernández-Celemín, L.; Lause, P.L.; Thissen, J.-P.M. Induction of MafBx and Murf ubiquitin ligase mRNAs in rat skeletal muscle after LPS injection. FEBS Lett. 2003, 544, 214–217. [Google Scholar] [CrossRef]
- Fang, W.; Tseng, Y.; Lee, T.; Fu, Y.; Chang, W.; Lo, W.; Lin, C.; Lo, Y. Triptolide prevents LPS-induced skeletal muscle atrophy via inhibiting NF-κB/TNF-α and regulating protein synthesis/degradation pathway. Br. J. Pharmacol. 2021, 178, 2998–3016. [Google Scholar] [CrossRef]
- Li, Y.-P.; Chen, Y.; John, J.; Moylan, J.; Jin, B.; Mann, D.L.; Reid, M.B. TNF-α acts via p38 MAPK to stimulate expression of the ubiquitin ligase atrogin1/MAFbx in skeletal muscle. FASEB J. 2005, 19, 362–370. [Google Scholar] [CrossRef]
- Lang, C.H.; Frost, R.A.; Vary, T.C. Regulation of muscle protein synthesis during sepsis and inflammation. Am. J. Physiol.-Endocrinol. Metab. 2007, 293, E453–E459. [Google Scholar] [CrossRef] [PubMed]
- Kodani, H.; Aoi, W.; Hirata, M.; Takami, M.; Kobayashi, Y.; Kuwahata, M. Skeletal muscle metabolic dysfunction with circulating carboxymethyl-lysine in dietary food additive-induced leaky gut. FASEB J. 2024, 38, e23715. [Google Scholar] [CrossRef]
- Kitamura, H.; Kimura, S.; Shimamoto, Y.; Okabe, J.; Ito, M.; Miyamoto, T.; Naoe, Y.; Kikuguchi, C.; Meek, B.; Toda, C.; et al. Ubiquitin-specific protease 2-69 in macrophages potentially modulates metainflammation. FASEB J. 2013, 27, 4940–4953. [Google Scholar] [CrossRef]
- Carvalho, B.M.; Guadagnini, D.; Tsukumo, D.M.L.; Schenka, A.A.; Latuf-Filho, P.; Vassallo, J.; Dias, J.C.; Kubota, L.T.; Carvalheira, J.B.C.; Saad, M.J.A. Modulation of gut microbiota by antibiotics improves insulin signalling in high-fat fed mice. Diabetologia 2012, 55, 2823–2834. [Google Scholar] [CrossRef]
- Stoeva, M.K.; Garcia-So, J.; Justice, N.; Myers, J.; Tyagi, S.; Nemchek, M.; McMurdie, P.J.; Kolterman, O.; Eid, J. Butyrate-producing human gut symbiont, Clostridium butyricum, and its role in health and disease. Gut Microbes 2021, 13, 1907272. [Google Scholar] [CrossRef]
- Culp, E.J.; Goodman, A.L. Cross-feeding in the gut microbiome: Ecology and mechanisms. Cell Host Microbe 2023, 31, 485–499. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- LeBlanc, J.G.; Laiño, J.E.; Del Valle, M.J.; Vannini, V.; Van Sinderen, D.; Taranto, M.P.; De Valdez, G.F.; De Giori, G.S.; Sesma, F. B-Group vitamin production by lactic acid bacteria—Current knowledge and potential applications: Vitamin production by LAB. J. Appl. Microbiol. 2011, 111, 1297–1309. [Google Scholar] [CrossRef]
- Rudzki, L.; Stone, T.W.; Maes, M.; Misiak, B.; Samochowiec, J.; Szulc, A. Gut microbiota-derived vitamins–underrated powers of a multipotent ally in psychiatric health and disease. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 107, 110240. [Google Scholar] [CrossRef]
- Duncan, S.H.; Holtrop, G.; Lobley, G.E.; Calder, A.G.; Stewart, C.S.; Flint, H.J. Contribution of acetate to butyrate formation by human faecal bacteria. Br. J. Nutr. 2004, 91, 915–923. [Google Scholar] [CrossRef]
- Scheppach, W. Effects of short chain fatty acids on gut morphology and function. Gut 1994, 35, S35–S38. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Wu, W.; Liu, Z.; Cong, Y. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J. Gastroenterol. 2017, 52, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fellows, R.; Denizot, J.; Stellato, C.; Cuomo, A.; Jain, P.; Stoyanova, E.; Balázsi, S.; Hajnády, Z.; Liebert, A.; Kazakevych, J.; et al. Microbiota derived short chain fatty acids promote histone crotonylation in the colon through histone deacetylases. Nat. Commun. 2018, 9, 105. [Google Scholar] [CrossRef] [PubMed]
- Ho, R.H.; Chan, J.C.Y.; Fan, H.; Kioh, D.Y.Q.; Lee, B.W.; Chan, E.C.Y. In Silico and in Vitro Interactions Between Short Chain Fatty Acids and Human Histone Deacetylases. Biochemistry 2017, 56, 4871–4878. [Google Scholar] [CrossRef]
- Carlsson, A.H.; Yakymenko, O.; Olivier, I.; Håkansson, F.; Postma, E.; Keita, Å.V.; Söderholm, J.D. Faecalibacterium prausnitzii supernatant improves intestinal barrier function in mice DSS colitis. Scand. J. Gastroenterol. 2013, 48, 1136–1144. [Google Scholar] [CrossRef]
- Martín, R.; Miquel, S.; Chain, F.; Natividad, J.M.; Jury, J.; Lu, J.; Sokol, H.; Theodorou, V.; Bercik, P.; Verdu, E.F.; et al. Faecalibacterium prausnitzii prevents physiological damages in a chronic low-grade inflammation murine model. BMC Microbiol. 2015, 15, 67. [Google Scholar] [CrossRef]
- Miquel, S.; Martín, R.; Lashermes, A.; Gillet, M.; Meleine, M.; Gelot, A.; Eschalier, A.; Ardid, D.; Bermúdez-Humarán, L.G.; Sokol, H.; et al. Anti-nociceptive effect of Faecalibacterium prausnitzii in non-inflammatory IBS-like models. Sci. Rep. 2016, 6, 19399. [Google Scholar] [CrossRef]
- Morelli, L.; Capurso, L. FAO/WHO Guidelines on Probiotics: 10 Years Later. J. Clin. Gastroenterol. 2012, 46, S1–S2. [Google Scholar] [CrossRef]
- Roobab, U.; Batool, Z.; Manzoor, M.F.; Shabbir, M.A.; Khan, M.R.; Aadil, R.M. Sources, formulations, advanced delivery and health benefits of probiotics. Curr. Opin. Food Sci. 2020, 32, 17–28. [Google Scholar] [CrossRef]
- Silva, D.R.; Sardi, J.D.C.O.; Pitangui, N.D.S.; Roque, S.M.; Silva, A.C.B.D.; Rosalen, P.L. Probiotics as an alternative antimicrobial therapy: Current reality and future directions. J. Funct. Foods 2020, 73, 104080. [Google Scholar] [CrossRef]
- Hutkins, R.W.; Krumbeck, J.A.; Bindels, L.B.; Cani, P.D.; Fahey, G., Jr.; Goh, Y.J.; Hamaker, B.; Martens, E.C.; Mills, D.A.; Vaughan, E.; et al. Prebiotics: Why definitions matter. Curr. Opin. Biotechnol. 2016, 37, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Scott, K.P.; Rastall, R.A.; Tuohy, K.M.; Hotchkiss, A.; Dubert-Ferrandon, A.; Gareau, M.; Murphy, E.F.; Saulnier, D.; Loh, G.; et al. Dietary prebiotics: Current status and new definition. Food Sci. Technol. Bull. Funct. Foods 2010, 7, 1–19. [Google Scholar] [CrossRef]
- Kolida, S.; Gibson, G.R. Synbiotics in Health and Disease. Annu. Rev. Food Sci. Technol. 2011, 2, 373–393. [Google Scholar] [CrossRef]
- Faber, J.; Vos, P.; Kegler, D.; Van Norren, K.; Argilés, J.M.; Laviano, A.; Garssen, J.; Van Helvoort, A. Beneficial immune modulatory effects of a specific nutritional combination in a murine model for cancer cachexia. Br. J. Cancer 2008, 99, 2029–2036. [Google Scholar] [CrossRef]
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Yu, D.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D.; et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009, 461, 1282–1286. [Google Scholar] [CrossRef]
- Cox, M.A.; Jackson, J.; Stanton, M.; Rojas-Triana, A.; Bober, L.; Laverty, M.; Yang, X.; Zhu, F.; Liu, J.; Wang, S.; et al. Short-chain fatty acids act as antiinflammatory mediatorsby regulating prostaglandin E2 and cytokines. World J. Gastroenterol. 2009, 15, 5549. [Google Scholar] [CrossRef]
- Wang, J.-L.; Chen, Y.-S.; Huang, K.-C.; Yeh, C.-H.; Chen, M.C.-M.; Wu, L.S.-H.; Chiu, Y.-H. Resistant Starch-Encapsulated Probiotics Attenuate Colorectal Cancer Cachexia and 5-Fluorouracil-Induced Microbial Dysbiosis. Biomedicines 2024, 12, 1450. [Google Scholar] [CrossRef]
- Malta, F.A.P.S.; Gonçalves, D.C. A triple-masked, two-center, randomized parallel clinical trial to assess the superiority of eight weeks of grape seed flour supplementation against placebo for weight loss attenuation during perioperative period in patients with cachexia associated with colorectal cancer: A study protocol. Front. Endocrinol. 2024, 14, 1146479. [Google Scholar] [CrossRef]
- Migliori, M.; Panichi, V.; De La Torre, R.; Fitó, M.; Covas, M.; Bertelli, A.; Muñoz-Aguayo, D.; Scatena, A.; Paoletti, S.; Ronco, C. Anti-Inflammatory Effect of White Wine in CKD Patients and Healthy Volunteers. Blood Purif. 2015, 39, 218–223. [Google Scholar] [CrossRef]
- Barroso, E.; Muñoz-González, I.; Jiménez, E.; Bartolomé, B.; Moreno-Arribas, M.V.; Peláez, C.; Del Carmen Martínez-Cuesta, M.; Requena, T. Phylogenetic profile of gut microbiota in healthy adults after moderate intake of red wine. Mol. Nutr. Food Res. 2017, 61, 1600620. [Google Scholar] [CrossRef]
- Muñoz-González, I.; Jiménez-Girón, A.; Martín-Álvarez, P.J.; Bartolomé, B.; Moreno-Arribas, M.V. Profiling of Microbial-Derived Phenolic Metabolites in Human Feces after Moderate Red Wine Intake. J. Agric. Food Chem. 2013, 61, 9470–9479. [Google Scholar] [CrossRef] [PubMed]
- Queipo-Ortuño, M.I.; Boto-Ordóñez, M.; Murri, M.; Gomez-Zumaquero, J.M.; Clemente-Postigo, M.; Estruch, R.; Cardona Diaz, F.; Andrés-Lacueva, C.; Tinahones, F.J. Influence of red wine polyphenols and ethanol on the gut microbiota ecology and biochemical biomarkers. Am. J. Clin. Nutr. 2012, 95, 1323–1334. [Google Scholar] [CrossRef] [PubMed]
- Stephen, A.M.; Cummings, J.H. The microbial contribution to human faecal mass. J. Med. Microbiol. 1980, 13, 45–56. [Google Scholar] [CrossRef]
- Kim, J.Y.; Whon, T.W.; Lim, M.Y.; Kim, Y.B.; Kim, N.; Kwon, M.-S.; Kim, J.; Lee, S.H.; Choi, H.-J.; Nam, I.-H.; et al. The human gut archaeome: Identification of diverse haloarchaea in Korean subjects. Microbiome 2020, 8, 114. [Google Scholar] [CrossRef]
- Ben-Amor, K.; Heilig, H.; Smidt, H.; Vaughan, E.E.; Abee, T.; De Vos, W.M. Genetic Diversity of Viable, Injured, and Dead Fecal Bacteria Assessed by Fluorescence-Activated Cell Sorting and 16S rRNA Gene Analysis. Appl. Environ. Microbiol. 2005, 71, 4679–4689. [Google Scholar] [CrossRef]
- Hanssen, N.M.J.; De Vos, W.M.; Nieuwdorp, M. Fecal microbiota transplantation in human metabolic diseases: From a murky past to a bright future? Cell Metab. 2021, 33, 1098–1110. [Google Scholar] [CrossRef]
- Porcari, S.; Baunwall, S.M.D.; Occhionero, A.S.; Ingrosso, M.R.; Ford, A.C.; Hvas, C.L.; Gasbarrini, A.; Cammarota, G.; Ianiro, G. Fecal microbiota transplantation for recurrent C. difficile infection in patients with inflammatory bowel disease: A systematic review and meta-analysis. J. Autoimmun. 2023, 141, 103036. [Google Scholar] [CrossRef]
- Van Der Vossen, E.W.J.; Davids, M.; Voermans, B.; Wortelboer, K.; Hartstra, A.V.; Koopen, A.M.; De Groot, P.; Levin, E.; Nieuwdorp, M. Disentangle beneficial effects of strain engraftment after fecal microbiota transplantation in subjects with MetSyn. Gut Microbes 2024, 16, 2388295. [Google Scholar] [CrossRef]
- Camarillo-Guerrero, L.F.; Almeida, A.; Rangel-Pineros, G.; Finn, R.D.; Lawley, T.D. Massive expansion of human gut bacteriophage diversity. Cell 2021, 184, 1098–1109.e9. [Google Scholar] [CrossRef]
- Li, J.J.; Zhu, M.; Kashyap, P.C.; Chia, N.; Tran, N.H.; McWilliams, R.R.; Bekaii-Saab, T.S.; Ma, W.W. The role of microbiome in pancreatic cancer. Cancer Metastasis Rev. 2021, 40, 777–789. [Google Scholar] [CrossRef]
- Kim, K.O.; Gluck, M. Fecal Microbiota Transplantation: An Update on Clinical Practice. Clin. Endosc. 2019, 52, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Davar, D.; Dzutsev, A.K.; McCulloch, J.A.; Rodrigues, R.R.; Chauvin, J.-M.; Morrison, R.M.; Deblasio, R.N.; Menna, C.; Ding, Q.; Pagliano, O.; et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science 2021, 371, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Ott, S.J.; Waetzig, G.H.; Rehman, A.; Moltzau-Anderson, J.; Bharti, R.; Grasis, J.A.; Cassidy, L.; Tholey, A.; Fickenscher, H.; Seegert, D.; et al. Efficacy of Sterile Fecal Filtrate Transfer for Treating Patients With Clostridium difficile Infection. Gastroenterology 2017, 152, 799–811.e7. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, T.S.; Koefoed, A.K.; Jakobsen, R.R.; Deng, L.; Castro-Mejía, J.L.; Brunse, A.; Neve, H.; Vogensen, F.K.; Nielsen, D.S. Bacteriophage-mediated manipulation of the gut microbiome—Promises and presents limitations. FEMS Microbiol. Rev. 2020, 44, 507–521. [Google Scholar] [CrossRef]
- Van Lier, Y.F.; Davids, M.; Haverkate, N.J.E.; De Groot, P.F.; Donker, M.L.; Meijer, E.; Heubel-Moenen, F.C.J.I.; Nur, E.; Zeerleder, S.S.; Nieuwdorp, M.; et al. Donor fecal microbiota transplantation ameliorates intestinal graft-versus-host disease in allogeneic hematopoietic cell transplant recipients. Sci. Transl. Med. 2020, 12, eaaz8926. [Google Scholar] [CrossRef]
- DeFilipp, Z.; Bloom, P.P.; Torres Soto, M.; Mansour, M.K.; Sater, M.R.A.; Huntley, M.H.; Turbett, S.; Chung, R.T.; Chen, Y.-B.; Hohmann, E.L. Drug-Resistant E. coli Bacteremia Transmitted by Fecal Microbiota Transplant. N. Engl. J. Med. 2019, 381, 2043–2050. [Google Scholar] [CrossRef]
- Cammarota, G.; Ianiro, G.; Tilg, H.; Rajilić-Stojanović, M.; Kump, P.; Satokari, R.; Sokol, H.; Arkkila, P.; Pintus, C.; Hart, A.; et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut 2017, 66, 569–580. [Google Scholar] [CrossRef]
- Żółkiewicz, J.; Marzec, A.; Ruszczyński, M.; Feleszko, W. Postbiotics—A Step Beyond Pre- and Probiotics. Nutrients 2020, 12, 2189. [Google Scholar] [CrossRef]
- Wegh, C.A.M.; Geerlings, S.Y.; Knol, J.; Roeselers, G.; Belzer, C. Postbiotics and Their Potential Applications in Early Life Nutrition and Beyond. Int. J. Mol. Sci. 2019, 20, 4673. [Google Scholar] [CrossRef]
- Malagón-Rojas, J.N.; Mantziari, A.; Salminen, S.; Szajewska, H. Postbiotics for Preventing and Treating Common Infectious Diseases in Children: A Systematic Review. Nutrients 2020, 12, 389. [Google Scholar] [CrossRef]
- Tsilingiri, K.; Rescigno, M. Postbiotics: What else? Benef. Microbes 2013, 4, 101–107. [Google Scholar] [CrossRef] [PubMed]
- De Marco, S.; Sichetti, M.; Muradyan, D.; Piccioni, M.; Traina, G.; Pagiotti, R.; Pietrella, D. Probiotic Cell-Free Supernatants Exhibited Anti-Inflammatory and Antioxidant Activity on Human Gut Epithelial Cells and Macrophages Stimulated with LPS. Evid.-Based Complement. Altern. Med. 2018, 2018, 1756308. [Google Scholar] [CrossRef] [PubMed]
- Geraldelli, D.; Ribeiro, M.C.; Medeiros, T.C.; Comiran, P.K.; Martins, K.O.; Oliveira, M.F.; Oliveira, G.A.; Dekker, R.F.H.; Barbosa-Dekker, A.M.; Alegranci, P.; et al. Tumor development in rats and cancer cachexia are reduced by treatment with botryosphaeran by increasing apoptosis and improving the metabolic profile. Life Sci. 2020, 252, 117608. [Google Scholar] [CrossRef] [PubMed]
- Van Doorn-Schepens, M.L.M.; Abis, G.S.A.; Oosterling, S.J.; Van Egmond, M.; Poort, L.; Stockmann, H.B.A.C.; Bonjer, H.J.; Savelkoul, P.H.M.; Budding, A.E. The effect of selective decontamination on the intestinal microbiota as measured with IS-pro: A taxonomic classification tool applicable for direct evaluation of intestinal microbiota in clinical routine. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 1337–1345. [Google Scholar] [CrossRef]
- Martínez-Pérez, M.; Fernández-Fernández, R.; Morón, R.; Nieto-Sánchez, M.T.; Yuste, M.E.; Díaz-Villamarín, X.; Fernández-Varón, E.; Vázquez-Blanquiño, A.; Alberola-Romano, A.; Cabeza-Barrera, J.; et al. Selective Digestive Decontamination: A Comprehensive Approach to Reducing Nosocomial Infections and Antimicrobial Resistance in the ICU. J. Clin. Med. 2024, 13, 6482. [Google Scholar] [CrossRef]
- Kenneth, M.J.; Wu, C.-C.; Fang, C.-Y.; Hsu, T.-K.; Lin, I.-C.; Huang, S.-W.; Chiu, Y.-C.; Hsu, B.-M. Exploring the Impact of Chemotherapy on the Emergence of Antibiotic Resistance in the Gut Microbiota of Colorectal Cancer Patients. Antibiotics 2025, 14, 264. [Google Scholar] [CrossRef]
- Im, E.-J.; Lee, C.-H.; Moon, P.-G.; Rangaswamy, G.G.; Lee, B.; Lee, J.M.; Lee, J.-C.; Jee, J.-G.; Bae, J.-S.; Kwon, T.-K.; et al. Sulfisoxazole inhibits the secretion of small extracellular vesicles by targeting the endothelin receptor A. Nat. Commun. 2019, 10, 1387. [Google Scholar] [CrossRef]
- Chitti, S.V.; Marzan, A.L.; Shahi, S.; Kang, T.; Fonseka, P.; Mathivanan, S. Repurposing of Antibiotic Sulfisoxazole Inhibits Lipolysis in Pre-Clinical Model of Cancer-Associated Cachexia. Biology 2021, 10, 700. [Google Scholar] [CrossRef]
- Byerley, L.O.; Lorenzen, B.; Chang, H.-M.; Hartman, W.G.; Keenan, M.J.; Page, R.; Luo, M.; Dowd, S.E.; Taylor, C.M. Gut Microbial Dysbiosis Differs in Two Distinct Cachectic Tumor-Bearing Models Consuming the Same Diet. Nutrients 2024, 16, 1076. [Google Scholar] [CrossRef]
- Davar, D.; Zarour, H.M. Facts and Hopes for Gut Microbiota Interventions in Cancer Immunotherapy. Clin. Cancer Res. 2022, 28, 4370–4384. [Google Scholar] [CrossRef]
- Bruera, E. ABC of palliative care: Anorexia, cachexia, and nutrition. BMJ 1997, 315, 1219–1222. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Isenring, E.; Yates, P. The prevalence of nutrition impact symptoms and their relationship to quality of life and clinical outcomes in medical oncology patients. Support. Care Cancer 2009, 17, 83–90. [Google Scholar] [CrossRef]
- Yoon, S.L.; Grundmann, O. Relevance of Dietary Supplement Use in Gastrointestinal-Cancer-Associated Cachexia. Nutrients 2023, 15, 3391. [Google Scholar] [CrossRef]
- Löhr, J.; Oliver, M.R.; Frulloni, L. Synopsis of recent guidelines on pancreatic exocrine insufficiency. United Eur. Gastroenterol. J. 2013, 1, 79–83. [Google Scholar] [CrossRef]
- Tang, Y.-L.; Zhu, L.; Tao, Y.; Lu, W.; Cheng, H. Role of targeting TLR4 signaling axis in liver-related diseases. Pathol.-Res. Pract. 2023, 244, 154410. [Google Scholar] [CrossRef]
- Doyle, A.; Zhang, G.; Fattah, E.A.A.; Eissa, N.T.; Li, Y. Toll-like receptor 4 mediates lipopolysaccharide-induced muscle catabolism via coordinate activation of ubiquitin-proteasome and autophagy-lysosome pathways. FASEB J. 2011, 25, 99–110. [Google Scholar] [CrossRef]
- Tamayo-Torres, E.; Garrido, A.; De Cabo, R.; Carretero, J.; Gómez-Cabrera, M.C. Molecular mechanisms of cancer cachexia. Role of exercise training. Mol. Asp. Med. 2024, 99, 101293. [Google Scholar] [CrossRef]
- Imhann, F.; Bonder, M.J.; Vich Vila, A.; Fu, J.; Mujagic, Z.; Vork, L.; Tigchelaar, E.F.; Jankipersadsing, S.A.; Cenit, M.C.; Harmsen, H.J.M.; et al. Proton pump inhibitors affect the gut microbiome. Gut 2016, 65, 740–748. [Google Scholar] [CrossRef]
- VanderVeen, B.N.; Cardaci, T.D.; Bullard, B.M.; Madden, M.; Li, J.; Velazquez, K.T.; Kubinak, J.L.; Fan, D.; Murphy, E.A. Involvement of the gut microbiota in cancer cachexia. Am. J. Physiol.-Cell Physiol. 2024, 327, C661–C670. [Google Scholar] [CrossRef]
- Hugenholtz, F.; De Vos, W.M. Mouse models for human intestinal microbiota research: A critical evaluation. Cell. Mol. Life Sci. 2018, 75, 149–160. [Google Scholar] [CrossRef]
- Montassier, E.; Gastinne, T.; Vangay, P.; Al-Ghalith, G.A.; Bruley Des Varannes, S.; Massart, S.; Moreau, P.; Potel, G.; De La Cochetière, M.F.; Batard, E.; et al. Chemotherapy-driven dysbiosis in the intestinal microbiome. Aliment. Pharmacol. Ther. 2015, 42, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Pin, F.; Barreto, R.; Couch, M.E.; Bonetto, A.; O’Connell, T.M. Cachexia induced by cancer and chemotherapy yield distinct perturbations to energy metabolism. J. Cachexia Sarcopenia Muscle 2019, 10, 140–154. [Google Scholar] [CrossRef] [PubMed]
- Johns, N.; Stretch, C.; Tan, B.H.L.; Solheim, T.S.; Sørhaug, S.; Stephens, N.A.; Gioulbasanis, I.; Skipworth, R.J.E.; Deans, D.A.C.; Vigano, A.; et al. New genetic signatures associated with cancer cachexia as defined by low skeletal muscle index and weight loss. J. Cachexia Sarcopenia Muscle 2017, 8, 122–130. [Google Scholar] [CrossRef] [PubMed]
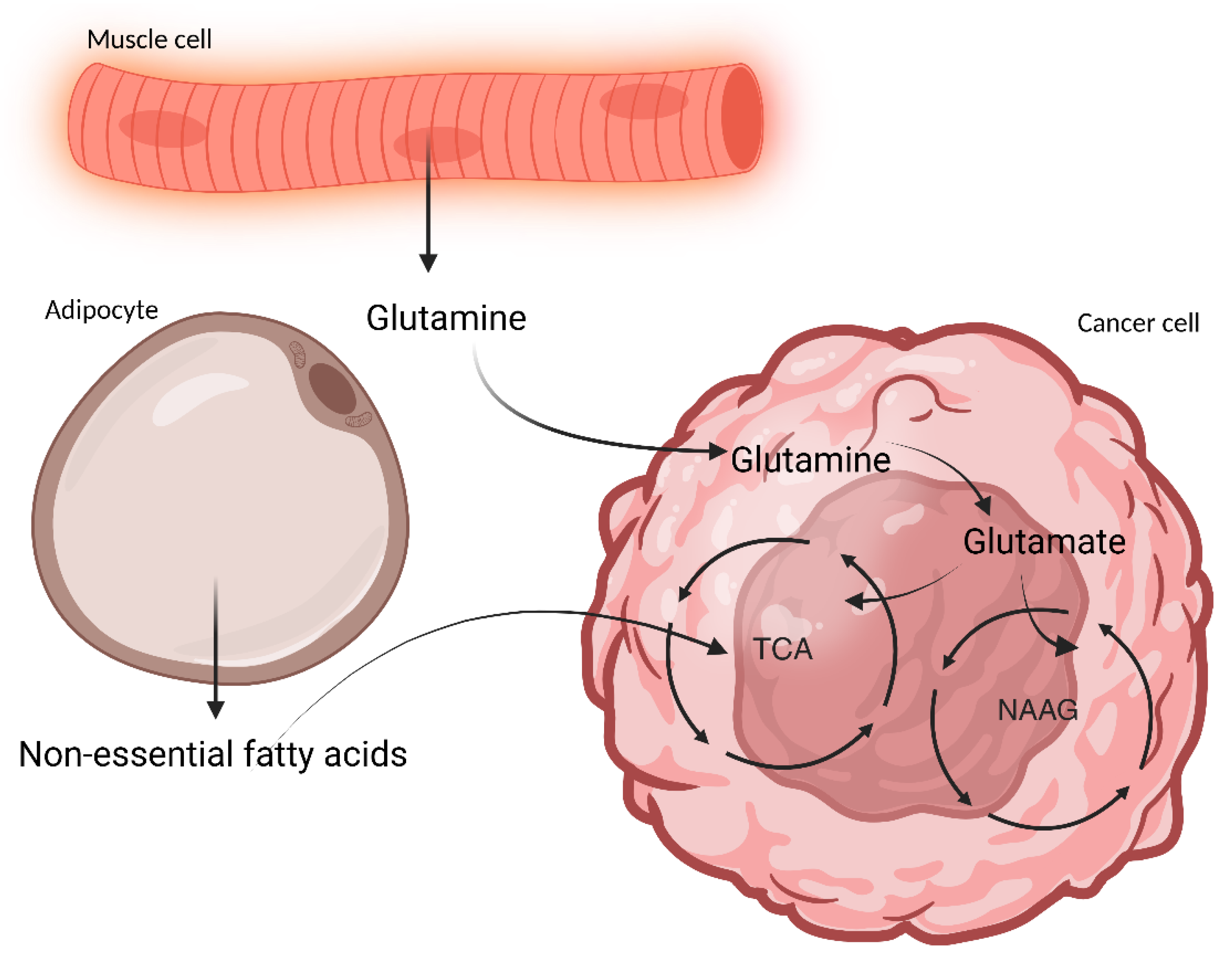
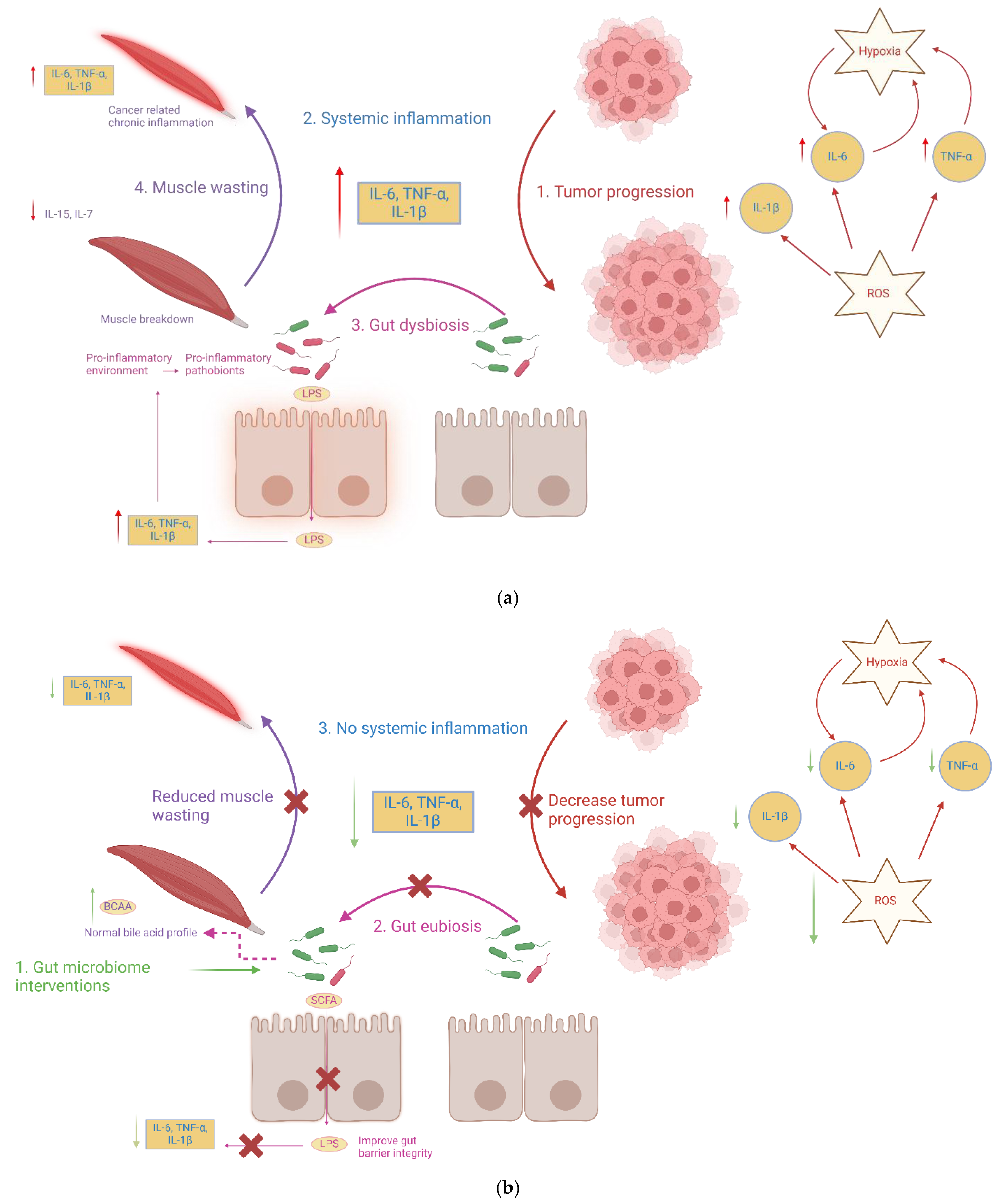
| Study (Year) | Population/Model | Gut Microbiota Changes | SCFA Changes | Inflammation and Immune Markers | Cachexia and Metabolic Outcomes | Gut Barrier | Intervention and Outcome |
|---|---|---|---|---|---|---|---|
| Bindels et al., 2018 [26] | Colon cancer cachexia (C26) + parallel human cohort (152 colorectal and lung cancer patients with or without cachexia) | (Mouse) Enterobacteriaceae expansion, loss of butyrate-producers. (Human) Similar dysbiosis in cachectic vs. non-cachectic (b.i.) | (Mouse) ↓ Cecal acetate, butyrate. (Human) ↓ acetate (significant), overall ↓ SCFAs (trend) (b.i.) | (Mouse) ↑ inflammatory cytokines, acute-phase response. (Human) ↑ IL-6, ↑ LPS-binding protein (LBP) in cachexia (b.i.). | Cachexia severity correlated with gut barrier dysfunction and systemic inflammation. | Increased (mice) (b.i.) | Anti-IL-6 antibody in mice prevented dysbiosis and muscle wasting. No microbiota-targeted intervention in humans. |
| Sakakida et al., 2022 [37] | Colon cancer cachexia (C26) with high-fiber diet | ↑ SCFA-producing taxa with fiber (e.g., Lachnospiraceae, Bifidobacterium) | Likely ↑ SCFAs (acetate, propionate, butyrate) | Fiber-fed cachectic mice: less muscle wasting, higher body/muscle mass. Suggests reduced systemic inflammation (IL-6, TNF-α not explicitly measured). | Partial rescue of cachexia without affecting tumor progression; improved metabolic/inflammatory status. | Not measured | Prebiotic fiber (partially hydrolyzed guar gum) increased muscle mass, body weight. |
| Pötgens et al., 2021 [38] | Colon cancer cachexia (C26) | ↓ Diversity (↓ Lachnospiraceae, Ruminococcaceae, ↑ Enterobacteriaceae), altered fungal composition (mycobiome) | ↓ Butyrate, ↓ acetate in cachectic mice | ↑ IL-6, TNF-α, acute-phase proteins. Multi-omics: ↑ LPS biosynthesis, ↓ amino acid biosynthesis, altered bile acids, correlating with hepatic inflammation, muscle proteolysis. | Significant host–microbe metabolic disruption (↓ plasma amino acids, ↑ purine catabolism, etc.). | Increased (“leaky gut”) | Observational; highlights need to restore SCFAs, reduce endotoxin, rebalance bile acids. |
| Ubachs et al., 2021 [39] | Mixed cancers (pancreatic, breast, lung, ovarian); n = 107 (33 cachectic) | Cachexia: ↑ Proteobacteria (esp. Enterobacteriaceae), ↑ Veillonella. ↓ Megamonas, Peptococcus vs. non-cachectic | ↓ Fecal acetate (significant), propionate/butyrate trended lower | High CRP, pro-inflammatory cytokines common in cachexia. ↑ fecal calprotectin correlated with Enterobacteriaceae, Peptococcus → colonic inflammation. | Bacterial richness similar across groups. Proteobacteria bloom linked to weight loss, systemic inflammation. | Likely increased | Observational; suggests targeting Proteobacteria overgrowth, restoring SCFAs. |
| Pötgens et al., 2018 [40] | Colon cancer cachexia (C26) | ↓ Lachnospiraceae, Ruminococcaceae, Porphyromonadaceae families. ↑ Enterobacteriace-ae (Klebsiella oxytoca). Loss of butyrate-producers (b.i.) | ↓ Butyrate, ↓ acetate (b.i.) | Cachexia: severe muscle wasting, ↑ IL-6. Klebsiella overgrowth increased inflammation. | Identified Klebsiella as a potential target; no deeper metabolic analysis here. | Increased (“leaky gut”) (b.i.) | Observational; probiotic (Faecalibacterium prausnitzii) did not improve barrier or cachexia. |
| Jiang et al., 2014 [43] | Gastric cancer: cachectic vs. non-cachectic patients | ↓ Microbiota diversity in cachectic | Not reported | Cachexia group: ↑ gut permeability (sugar test), ↑ claudin, ↓ occludin, ↑ bacterial translocation, ↑ IL-6/TNF-α, CRP > 10 mg/L, >10% weight loss. | Demonstrates link: dysbiosis → leaky gut → systemic inflammation → cachexia. | Increased | Observational (no intervention). Reinforces link between dysbiosis (and likely reduced diversity), leaky gut, and systemic inflammation in human cachexia. |
| Bindels, Beck, et al., 2012 [45] | Murine acute leukemia (Ba/F3): cachectic vs. non-cachectic | ↓ Lactobacillus spp. (L. reuteri, L. gasseri) Dysbiosis: ↓ Firmicutes, ↑ Proteobacteria (b.i.) | Not measured | Cachexia: ↑ muscle atrophy genes (Atrogin-1, MuRF1), ↑ IL-6, TNF-α, G-CSF, MCP-1, IL-4. Probiotic lowered these cytokines. | Severe muscle wasting, anorexia. Probiotic reduced IL-6 and muscle atrophy markers. | Not assessed | Probiotic (L. reuteri + L. gasseri) attenuated cachexia (↓ IL-6, ↓ muscle loss). |
| Bindels et al., 2016 [48] | Murine leukemia (Ba/F3) & colon cancer (C26) | ↓ Lactobacillus spp., ↑ Parabacteroides goldsteinii, Enterobacteriace-ae. (b.i.) Synbiotic restored beneficial taxa | ↓ C.ecal acetate, butyrate (C26 model) (b.i.) | Cachexia: ↑ IL-6. Synbiotic prolonged survival, indicating reduced inflammatory tone | Rapid muscle wasting, shortened survival. Synbiotic attenuated cachexia severity, improved lifespan. | Increased permeability | Synbiotic (inulin + L. reuteri) normalized gut microbiota, improved barrier, reduced muscle wasting. |
| De Maria et al., 2021 [49] | Lung cancer cachexia (Lewis lung carcinoma in mice) | ↑ Diversity in cachexia. ↑ Firmicutes (Staphylococcaceae, Turicibacteraceae, Lachnospiraceae, Ruminococcaceae), ↑ minor phyla (Cyanobacteria, Tenericutes, TM7), ↓ Bacteroidetes | Not measured | Cachexia: severe muscle wasting. Likely ↑ IL-6, TNF-α (not explicitly shown). Possible immune shifts (Th17/Treg). | Suggests lung cancer cachexia differs from GI cancer models. | Not measured | Observational; indicates tumor-type–specific dysbiosis, no intervention tested. |
| Jeong et al., 2023 [50] | Lung cancer cachexia (Lewis lung carcinoma in mice) | ↓ α-diversity in cachexia. ↑ Bifidobacterium, Romboutsia, ↓ Streptococcus | ↓ Fecal acetate, ↓ butyrate | Marked muscle wasting. Likely ↑ IL-6 (not detailed). Loss of Streptococcus may disrupt cross-feeding. | Reinforces SCFA depletion as common in cachexia; specific taxa shifts vary by tumor type. | Not measured | Observational; highlights need for SCFA-boosting strategies to mitigate cachexia. |
| Ni et al., 2021 [57] | NSCLC patients (n = 31; 12 cachectic, 19 non-cachectic) | Cachexia: distinct β-diversity but no α-diversity loss. ↑ Klebsiella oxytoca, ↓ Faecalibacterium prausnitzii, Prevotella copri, Lactobacillus spp. | Overall ↓ SCFAs predicted (loss of multiple SCFA producers) | Cachexia: >5% weight loss. Microbiome shift: ↑ LPS/endotoxin synthesis, ↓ beneficial metabolic pathways (drives systemic inflammation, malnutrition). Branched-chain amino acids (BCAAs) significantly depleted. | “Pro-inflammatory, catabolic” microbiome profile. | Likely increased | Observational; suggests targeting Klebsiella and restoring SCFA-producers to mitigate cachexia. Non-cachetic patients: ↑ BCAAs ↑ 3-oxocholic acid showing positive correlation with Prevotella copri and Lactobacillus gasseri, respectively. |
| Varian et al., 2016 [58] | Colorectal cancer cachexia (ApcMin/+ mice) | Cachexia-associated dysbiosis (details not fully specified) | Not reported | Cachexia: severe muscle wasting. Probiotic group: ↑ muscle mass, ↓ atrophy, ↑ thymus size, ↓ FoxN1 (lower systemic inflammation). | Probiotic inhibited cachexia progression, prolonged survival. | Not reported | Probiotic (Lactobacillus reuteri) reduced muscle wasting, extended survival. |
| de Clercq et al., 2021 [59] | Metastatic gastroesophageal cancer, n = 24 (12 allogeneic FMT, 12 placebo) | Baseline dysbiosis in advanced cancer. Allogeneic FMT (healthy obese donors) transiently ↑ microbial diversity, ↑ SCFA-producers | Donors on high-fiber diets → likely ↑ SCFAs; recipient SCFAs not measured | No significant cachexia improvement (weight/appetite) with allogeneic vs. autologous FMT. | Allogeneic FMT group had higher disease control at 12 wks, trend of longer survival (365 vs. 227 days; p = 0.057), but weight loss persisted. | Not assessed | FMT did not reverse cachexia but improved chemo tolerance, hinted at survival benefit. |
| Bindels et al., 2015 [60] | Murine acute leukemia (cachexia), 2 weeks on 5% POS vs. control | ↑ Bacteroidetes, Bacteroides dorei, Bifidobacterium spp., Roseburia spp. (a.i.) | ↑ Acetate ↓ Isovalerate and other BCAA-derived SCFAs (a.i.) | Cachexia: anorexia, fat loss. Prebiotic reduced anorexia/adipose wasting (implying lower inflammation, though cytokines not shown). | Improved food intake, preserved fat mass with prebiotic (a.i.). | Not measured | Prebiotic (pectic oligosaccharides) improved appetite and reduced fat loss. |
| Castellani et al., 2017 [61] | Neuroblastoma cachexia (mouse model) | Minimal change vs. controls (slight ↓ Firmicutes, not significant) | No difference reported | Cachexia: ↑ IL-6, TNF-α. Altered gut hormones (↑ GLP-1, ↑ PYY). Marked muscle wasting despite stable microbiota. | ↓ Secondary bile acids (e.g., lithocholic, deoxycholic), suggesting tumor-driven or inflammation-driven cachexia. | Not measured | No microbiota-targeted intervention (observational). Demonstrates cachexia can occur with minimal dysbiosis. |
| Hakozaki et al., 2022 [62] | NSCLC on immunotherapy (n = 113; 57 cachectic, 56 non-cachectic) | Cachexia: ↑ Escherichia/Shigella, Hungatella, ↓ Anaerostipes, Blautia. | Not measured, but fewer butyrate-producers → likely ↓ SCFAs | Cachexia: higher neutrophil–lymphocyte ratio, worse LIPI → severe systemic inflammation. Shorter survival under immunotherapy. | Loss of SCFA producers suggests lower anti-inflammatory metabolites (e.g., butyrate). | Not tested | Observational; Anaerostipes and E. ventriosum were associated with longer progression-free survival and overall survival. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roy, S.; Alizadeh Bahmani, A.H.; Davids, M.; Herrema, H.; Nieuwdorp, M. Modulating the Gut–Muscle Axis: Increasing SCFA-Producing Gut Microbiota Commensals and Decreasing Endotoxin Production to Mitigate Cancer Cachexia. Microorganisms 2025, 13, 1356. https://doi.org/10.3390/microorganisms13061356
Roy S, Alizadeh Bahmani AH, Davids M, Herrema H, Nieuwdorp M. Modulating the Gut–Muscle Axis: Increasing SCFA-Producing Gut Microbiota Commensals and Decreasing Endotoxin Production to Mitigate Cancer Cachexia. Microorganisms. 2025; 13(6):1356. https://doi.org/10.3390/microorganisms13061356
Chicago/Turabian StyleRoy, Sagnik, Amir Hossein Alizadeh Bahmani, Mark Davids, Hilde Herrema, and Max Nieuwdorp. 2025. "Modulating the Gut–Muscle Axis: Increasing SCFA-Producing Gut Microbiota Commensals and Decreasing Endotoxin Production to Mitigate Cancer Cachexia" Microorganisms 13, no. 6: 1356. https://doi.org/10.3390/microorganisms13061356
APA StyleRoy, S., Alizadeh Bahmani, A. H., Davids, M., Herrema, H., & Nieuwdorp, M. (2025). Modulating the Gut–Muscle Axis: Increasing SCFA-Producing Gut Microbiota Commensals and Decreasing Endotoxin Production to Mitigate Cancer Cachexia. Microorganisms, 13(6), 1356. https://doi.org/10.3390/microorganisms13061356






