Biosynthesis Strategies and Application Progress of Mandelic Acid Based on Biomechanical Properties
Abstract
1. Introduction
2. Methodology
2.1. Literature Search and Screening Strategy
2.1.1. Database and Search Scope
2.1.2. Keyword Combination
2.1.3. Screening Criteria
2.2. Literature Analysis and Integration Framework
2.2.1. Subject Classification and Coding
2.2.2. Quality Assessment and Data Extraction
2.2.3. Review Structure Design
2.3. Limitations
3. The Application of MA
3.1. Pharmaceuticals Field
| Domain | Category | Core Function | Typical Applications | References |
|---|---|---|---|---|
| Pharmaceuticals | Antiviral Drugs | Block viral transmission and membrane fusion | SAMMA (inhibits HIV dendritic cell transmission, prevents HSV infection) | [16,17,18] |
| Dermatological Agents | Regulate pigmentation and epidermal repair | Creams for photoaging/acne treatment; post-laser repair gels | [21,22,23,24] | |
| Cardiovascular Drugs | Inhibit platelet aggregation | (R)-O-Chloromandelic acid Clopidogrel (P2Y12 receptor antagonist); Trimethylcyclohexyl mandelate (microcirculation enhancer) | [2,25,26] | |
| Urological/Anti-inflammatory Drugs | Modulate bladder smooth muscle and inflammatory mediators | (S)-Oxybutynin (overactive bladder treatment); Methenamine mandelate (urinary antiseptic); Celecoxib and Deracoxib (COX-2 inhibitors) | [3,27,28,29] | |
| Antibiotic Synthesis | Disrupt bacterial cell wall synthesis | Cephalosporin antibiotic sidechain construction | [30,31,32] |
3.2. Chemical Industry
| Domain | Category | Core Function | Typical Applications | References |
|---|---|---|---|---|
| Chemical Industry | Chiral Separation Materials | Construct supramolecular recognition systems | Chiral stationary phases for chromatography; molecularly imprinted resolution materials | [5,6,33,34] |
| Analytical Reagents | Specific metal chelation and condensation reactions | Zirconium ion detection reagents; ketone spectrophotometric probe | [35] | |
| Advanced Dye Synthesis | Enhance fiber dyeing performance | Benzodifuranone-based disperse dyes | [36] | |
| Eco-friendly Materials | Synthesize biodegradable plastics | Poly mandelic acid (PMA) as a biodegradable polymer | [37] | |
| Fine Chemical Intermediates | Multifunctional group conversion platform | α-Aminonitriles, phenylglyoxylic acid, phenylglycine derivatives | [38] |
3.3. Agricultural Field
| Domain | Category | Core Function | Typical Applications | References |
|---|---|---|---|---|
| Agriculture | Fungicides | Disrupt pathogen membrane structure and metabolism | Mandipropamid (phosphatidylinositol synthase inhibitor); downy mildew control agents; ascomycota pathogen control | [43,44,46] |
| Insecticides | Block insect neural signaling | Cypermethrin analogs (sodium channel modulators) | [47] | |
| Herbicides | Inhibit photosynthetic systems | Metamitron (photosynthesis inhibitor) | [48] |
4. Synthetic Methods of MA
4.1. Chemical Synthesis Method
- (1)
- Phase-transfer catalysis employs quaternary ammonium salt catalysts to enable efficient synthesis under ambient conditions by enhancing mass transfer in biphasic systems, while avoiding the use of strong oxidizing or reducing agents [52]. This method benefits from mild reaction conditions and operational simplicity. However, it also faces several technical drawbacks, such as catalyst leaching, poor catalyst recyclability, and extended reaction times, which limit its industrial feasibility.
- (2)
- Asymmetric synthesis utilizes chiral ligands to directly produce enantiomerically pure (R)-MA or (S)-MA with high optical purity [53]. This approach provides excellent enantiomeric excess (ee > 98%), making it attractive for high-value applications. Nevertheless, its practical use is hindered by the high cost of chiral catalysts and the need for inert atmosphere equipment, which substantially raises production expenses and operational complexity.
- (3)
- Optical resolution methods combine chemical synthesis with chiral separation techniques to isolate optically pure products [54]. Although these methods are capable of obtaining enantiopure compounds, they generally exhibit low separation efficiency, difficulties in recovering resolving agents, and low overall yield in industrial settings. As a result, optical resolution remains challenging for large-scale or economically viable application.
4.2. Biosynthesis Method
4.2.1. Rational Design of Nitrilase Catalytic Systems
4.2.2. Equilibrium Control in Lipase-Mediated Dynamic Resolution
4.2.3. Synergistic Mechanism of Dehydrogenase-Laccase Cascade Systems
4.2.4. Microenvironment Reconfiguration in Microbial Cell Factories
| Category | Method/ Mechanism | Key Process | Advantages | Limitations | References |
|---|---|---|---|---|---|
| Biosynthesis | Nitrilase Catalysis | Active-site engineering to enhance stress fields | High enantioselectivity (ee > 98%), improved catalytic efficiency | Product racemization during release, low productivity | [56,61,65] |
| Lipase-mediated Resolution | Dynamic kinetic resolution with immobilized lipases | Reusable enzymes, high ee (>98%) | Trade-off between substrate conversion and optical purity | [66,67] | |
| Dehydrogenase-Laccase Cascade | Reductive amination and laccase-mediated cofactor regeneration | Theoretical 100% yield, resolves electron transfer bottlenecks | Complex multienzyme coordination, copper cluster strain modulation challenges | [59,68,69,70] | |
| Microbial Cell Factories | Membrane tension engineering and metabolic flux control | High titer/optical purity, one-pot biosynthesis | Requires advanced genetic/metabolic engineering | [71,72,73,74,77] |
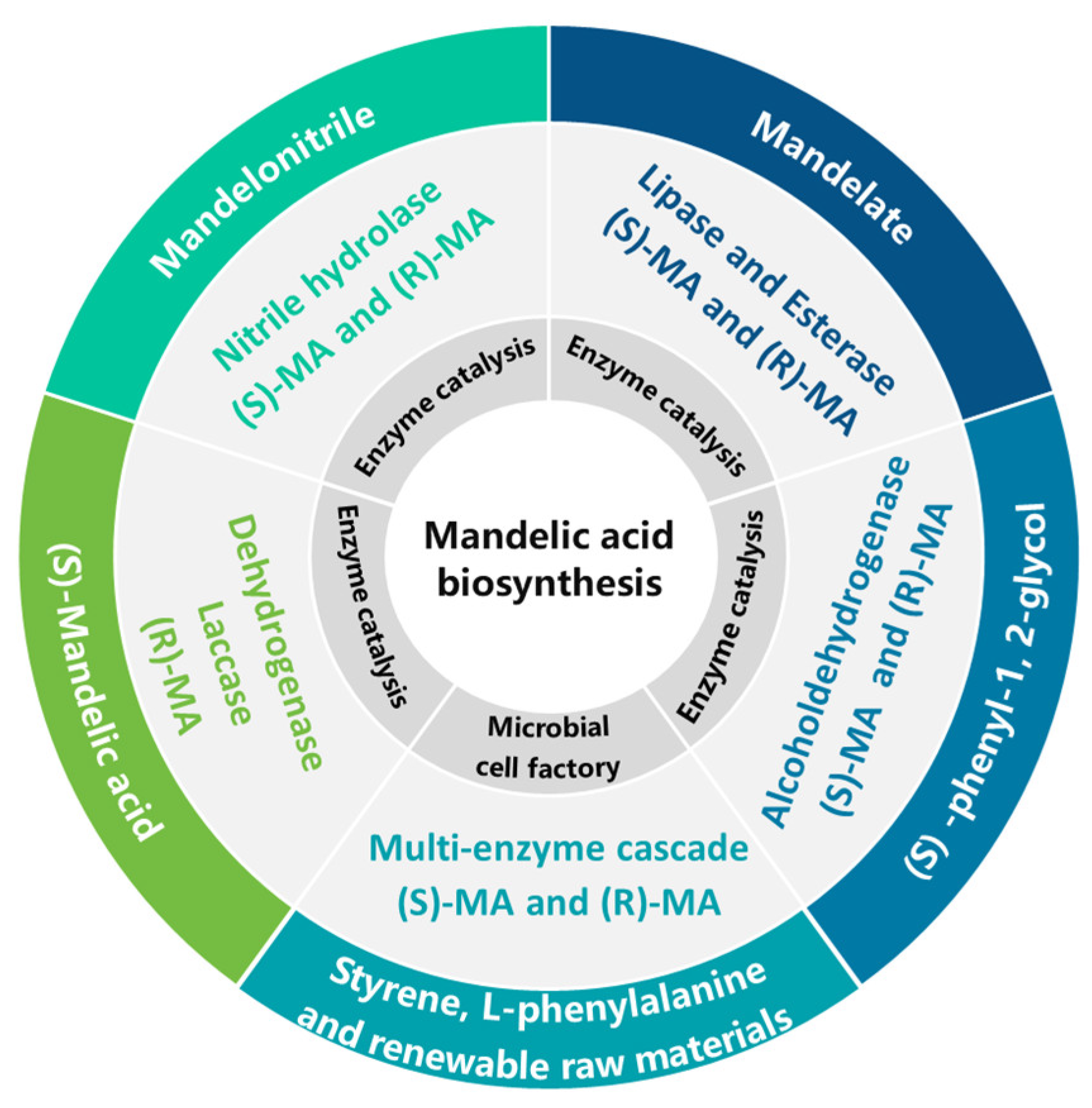
5. Advances in the Biosynthesis of MA
5.1. Synthesis of (S)-MA and (R)-MA from Mandelonitrile
5.2. Synthesis of (S)-MA and (R)-MA from MA Esters
5.3. Synthesis of (S)-MA and (R)-MA from (S)-Phenyl-1,2-glycol
5.4. Synthesis of (S)-MA and (R)-MA from Styrene, Bio-Based L-Phenylalanine, and Renewable Feedstocks
5.5. Synthesis of (R)-MA from (S)-MA
6. Prospect
6.1. From Enzyme Engineering to System Integration: Technological Evolution
6.2. From Laboratory to Industrialization: Potential Challenges
6.3. The Future Directions of Green Biosynthesis
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Choińska, R.; Dąbrowska, K.; Świsłocka, R.; Lewandowski, W.; Świergiel, A.H. Antimicrobial Properties of Mandelic Acid, Gallic Acid and their Derivatives. Mini Rev. Med. Chem. 2021, 21, 2544–2550. [Google Scholar] [CrossRef]
- Martinkova, L.; Kren, V. Biocatalytic production of mandelic acid and analogues: A review and comparison with chemical processes. Appl. Microbiol. Biotechnol. 2018, 102, 3893–3900. [Google Scholar] [CrossRef]
- Dehghani, Z.; Akhond, M.; Jangi, S.R.H.; Absalan, G. Highly sensitive enantioselective spectrofluorimetric determination of R-/S-mandelic acid using l-tryptophan-modified amino-functional silica-coated N-doped carbon dots as novel high-throughput chiral nanoprobes. Talanta 2024, 266, 124977. [Google Scholar] [CrossRef]
- Norvaiša, K.; O’bRien, J.E.; Osadchuk, I.; Twamley, B.; Borovkov, V.; Senge, M.O. Importance of molecular symmetry for enantiomeric excess recognition by NMR. Chem. Commun. 2022, 58, 5423–5426. [Google Scholar] [CrossRef]
- Han, Y.; Kou, M.; Zhang, H.; Qiu, H.; Shi, Y.P. Chiral fluorescent carbon dots for tyrosine enantiomers: Discrimination, mechanism and cell imaging. Sens. Actuators B Chem. 2025, 422, 136677. [Google Scholar] [CrossRef]
- Sakai, R.; Mato, Y.; Ishimaru, H.; Ogata, K.; Kurose, S.; Ozawa, S.; Umeda, S.; Tsuda, K.; Satoh, T.; Kakuchi, T. Colorimetric Sensing of Chirality Based on Synergistic Effect of Multiple Chiral Amide Receptors Consecutively Organized along Poly (phenylacetylene) Backbone. Macromolecules 2024, 57, 11450–11460. [Google Scholar] [CrossRef]
- Świsłocka, R.; Świderski, G.; Nasiłowska, J.; Sokołowska, B.; Wojtczak, A.; Lewandowski, W. Research on the Electron Structure and Antimicrobial Properties of Mandelic Acid and Its Alkali Metal Salts. Int. J. Mol. Sci. 2023, 24, 3078. [Google Scholar] [CrossRef]
- Matejczyk, M.; Ofman, P.; Świsłocka, R.; Parcheta, M.; Lewandowski, W. The study of biological activity of mandelic acid and its alkali metal salts in wastewaters. Environ. Res. 2022, 205, 112429. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Shi, H.; Siddique, F.; Xian, J.; Song, A.; Wang, B.; Wu, Z.; Cui, Z.N. Design and Synthesis of Mandelic Acid Derivatives for Suppression of Virulence via T3SS against Citrus Canker. J. Agric. Food Chem. 2024, 72, 9611–9620. [Google Scholar] [CrossRef]
- Kolano, M.; Ohnmacht, B.; Lemmer, A.; Kraume, M. How Fluid Pseudoplasticity and Elasticity Affect Propeller Flows in Biogas Fermenters. Chem. Ing. Tech. 2024, 96, 1570–1584. [Google Scholar] [CrossRef]
- Wang, Z.K.; Gong, J.S.; Feng, D.T.; Su, C.; Li, H.; Rao, Z.M.; Lu, Z.M.; Shi, J.S.; Xu, Z.H. Geometric Remodeling of Nitrilase Active Pocket Based on ALF-Scanning Strategy To Enhance Aromatic Nitrile Substrate Preference and Catalytic Efficiency. Appl. Environ. Microbiol. 2023, 89, e0022023. [Google Scholar] [CrossRef]
- Zeng, X.H.; Du, H.; Zhao, H.M.; Xiang, L.; Feng, N.X.; Li, H.; Li, Y.W.; Cai, Q.Y.; Mo, C.H.; Wong, M.H.; et al. Insights into the binding interaction of substrate with catechol 2, 3-dioxygenase from biophysics point of view. J. Hazard. Mater. 2020, 391, 122211. [Google Scholar] [CrossRef] [PubMed]
- Kell, D.B. Control of Metabolite Efflux in Microbial Cell Factories: Current Advances and Future Prospects in Fermentation Microbiology and Biotechnology, 4th ed.; CRC Press: Boca Raton, FL, USA, 2018; pp. 117–138. [Google Scholar]
- Christianson, J.C.; Jarosch, E.; Sommer, T. Mechanisms of substrate processing during ER-associated protein degradation. Nat. Rev. Mol. Cell Biol. 2023, 24, 777–796. [Google Scholar] [CrossRef] [PubMed]
- Ghoula, M.; Janel, N.; Camproux, A.; Moroy, G. Exploring the Structural Rearrangements of the Human Insulin-Degrading Enzyme through Molecular Dynamics Simulations. Int. J. Mol. Sci. 2022, 23, 1746. [Google Scholar] [CrossRef]
- Young, I.C.; Benhabbour, S.R. Multipurpose prevention technologies: Oral, parenteral, and vaginal dosage forms for prevention of HIV/STIs and unplanned pregnancy. Polymers 2021, 13, 2450. [Google Scholar] [CrossRef]
- Tian, Y. Check for updates Chapter 22 Polyvinylamine and Its Derivative as Effective Carrier for Targeted Delivery of Small RNAs. RNA Amplif. Anal. Methods Protoc. 2024, 2822, 353. [Google Scholar]
- Wang, Y.; Jia, Z.; Jiang, J.; Mao, X.; Pan, X.; Wu, J. Highly regioselective ring-opening polymerization of cyclic diester for alternating sequence-controlled copolymer synthesis of mandelic acid and glycolic acid. Macromolecules 2019, 52, 7564–7571. [Google Scholar] [CrossRef]
- Molotkovsky, R.J.; Alexandrova, V.V.; Galimzyanov, T.R.; Jiménez-Munguía, I.; Pavlov, K.V.; Batishchev, O.V.; Akimov, S.A. Lateral membrane heterogeneity regulates viral-induced membrane fusion during HIV entry. Int. J. Mol. Sci. 2018, 19, 1483. [Google Scholar] [CrossRef]
- Chang, Y.; Xu, H.; Motaleb, M.A.; Liu, J. Characterization of the flagellar collar reveals structural plasticity essential for spirochete motility. mBio 2021, 12, e02494-21. [Google Scholar] [CrossRef]
- Ghunawat, S.; Sarkar, R.; Garg, V.K. Comparative Study of 35% Glycolic Acid, 20% Salicylic–10% Mandelic Acid, and Phytic Acid Combination Peels in the Treatment of Active Acne and Postacne Pigmentation. J. Cutan. Aesthetic Surg. 2019, 12, 158. [Google Scholar] [CrossRef]
- Edison, B.L.; Smith, H.A.; Li, W.H.; Parsa, R.; Green, B.A.; Konish, P.; Dufort, M.; Tierney, N.K. 18295 Mandelic Acid, a Lipophilic Alpha Hydroxy Acid, Reduces Lipid Production, Enhances Exfoliation and Provides Clinical and Patient Perceivable Benefits to Oily and Photodamaged Skin. J. Am. Acad. Dermatol. 2020, 83, AB97. [Google Scholar] [CrossRef]
- Müller, E.; Sosedov, O.; Gröning, J.A.D.; Stolz, A. Synthesis of (R)-mandelic acid and (R)-mandelic acid amide by recombinant E. coli strains expressing a (R)-specific oxynitrilase and an arylacetonitrilase. Biotechnol. Lett. 2021, 43, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, S.W.; Culbertson, E.J. Effects of Topical Mandelic Acid Treatment on Facial Skin Viscoelasticity. Facial Plast. Surg. 2018, 34, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Saeed, A.; Shahzad, D.; Faisal, M.; Larik, F.A.; El-Seedi, H.R.; Channar, P.A. Developments in the synthesis of the antiplatelet and antithrombotic drug (S)-clopidogrel. Chirality 2017, 29, 684–707. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, T.C.; Thakur, N.; Kumar, V. Arylacetonitrilases: Potential Biocatalysts for Green Chemistry. Appl. Biochem. Biotechnol. 2024, 196, 1769–1785. [Google Scholar] [CrossRef]
- Masumoto, S.; Suzuki, M.; Kanai, M.; Shibasaki, M. A practical synthesis of (S)-oxybutynin. Tetrahedron Lett. 2002, 43, 8647–8651. [Google Scholar] [CrossRef]
- Dash, R.; Biswal, J.; Yadav, M.; Sharma, T.; Mohapatra, S.; Prusty, S.K. Novel atorvastatin-curcumin conjugate nanogel, a selective COX2 inhibitor with enhanced biopharmaceutical profile: Design, synthesis, in silico, in vitro, and in vivo investigation. J. Drug Deliv. Sci. Technol. 2023, 81, 104211. [Google Scholar] [CrossRef]
- Lin, D.; Xu, X.; Chen, L.; Chen, L.; Deng, M.; Chen, J.; Ren, Z.; Lei, L.; Wang, J.; Deng, J.; et al. Supramolecular nanofiber of indomethacin derivative confers highly cyclooxygenase-2 (COX-2) selectivity and boosts anti-inflammatory efficacy. J. Control. Release 2023, 364, 272–282. [Google Scholar] [CrossRef]
- Cao, S.; Zhao, B.; Zhang, X.; Hou, X. Advances in the application of photocatalysis, electrocatalysis, and biocatalysis in the synthesis of mandelic acid. Shandong Chem. Ind. 2024, 53, 95–97. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, C.; Lei, J.; Chen, L.; Hu, H.; Zeng, S.; Yu, L. Studies on the L-2-hydroxy-acid oxidase 2 catalyzed metabolism of S-mandelic acid and its analogues. Drug Metab. Pharmacokinet. 2019, 34, 187–193. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, J.; Tao, Z.; Luo, L.; Huang, C.; Liu, B.; Shi, H.; Tang, L.; Ou, Z. Highly efficient synthesis of the chiral ACE inhibitor intermediate (R)-2-hydroxy-4-phenylbutyrate ethyl ester via engineered bi-enzyme coupled systems. Bioresour. Bioprocess. 2024, 11, 99. [Google Scholar] [CrossRef]
- Lu, Y.; Swisher, J.H.; Meyer, T.Y.; Coates, G.W. Chirality-directed regioselectivity: An approach for the synthesis of alternating poly (lactic-co-glycolic acid). J. Am. Chem. Soc. 2021, 143, 4119–4124. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, P.D.S., Jr.; Tenorio, J.C.; Alves, D.C. Diastereomeric Double Salts of Carvedilol with Mandelic Acids. A Structural Analysis Reveals the Failure in Resolution. SSRN 2024. [Google Scholar] [CrossRef]
- Belcher, R.; Sykes, A.; Tatlow, J.C. Mandelic acid and halogen-substituted mandelic acids as reagents for the determination of zirconium. Anal. Chim. Acta 1954, 10, 34–47. [Google Scholar] [CrossRef]
- Zambare, A.A.; Bagal, M.S.; Sharma, S.J.; Sekar, M. NLOphoric unsymmetrically substituted D-π-A benzodifuranone dyes: Density functional theory, time dependent-density functional theory, and non-linear optical studies. Comput. Theor. Chem. 2024, 1241, 114919. [Google Scholar] [CrossRef]
- Jeswani, H.K.; Perry, M.R.; Shaver, M.P.; Azapagic, A. Biodegradable and conventional plastic packaging: Comparison of life cycle environmental impacts of poly (mandelic acid) and polystyrene. Sci. Total Environ. 2023, 903, 166311. [Google Scholar] [CrossRef] [PubMed]
- Pavlačková, J.; Egner, P.; Mokrejš, P.; Janalíková, M. Formulating Sustainable Emulsions: Mandelic Acid and Essential Oils as Natural Preservatives. Molecules 2024, 29, 4510. [Google Scholar] [CrossRef]
- Qu, T.; Shao, Y.; Csinos, A.S.; Ji, P. Sensitivity of Phytophthora nicotianae From Tobacco to Fluopicolide, Mandipropamid, and Oxathiapiprolin. Plant Disaster 2016, 100, 2119–2125. [Google Scholar] [CrossRef]
- Li, S.; Li, D.; Xiao, T.; Zhang, S.; Song, Z.; Ma, H. Design, Synthesis, Fungicidal Activity, and Unexpected Docking Model of the First Chiral Boscalid Analogues Containing Oxazolines. J. Agric. Food Chem. 2016, 64, 8927–8934. [Google Scholar] [CrossRef]
- Hou, S.; Xie, D.; Yang, J.; Niu, X.; Hu, D.; Wu, Z. Design, synthesis and antifungal evaluation of novel mandelic acid derivatives containing a 1,3,4-oxadiazothioether moiety. Chem. Biol. Drug Des. 2021, 98, 166–174. [Google Scholar] [CrossRef]
- Hao, K.; Lin, B.; Nian, F.; Gao, X.; Wei, Z.; Luo, G.; Lu, Y.; Lan, M.; Yang, J.; Wu, G. RNA-seq analysis of the response of plant-pathogenic oomycete Phytophthora parasitica to the fungicide dimethomorph. Rev. Argent. Microbiol. 2019, 51, 268–277. [Google Scholar] [CrossRef]
- Wang, J.; Shi, H.; Lu, A. Design, Synthesis, and Antifungal/Anti-Oomycete Activities of Novel 1, 2, 4-Triazole Derivatives Containing Carboxamide Fragments. J. Fungi 2024, 10, 160. [Google Scholar] [CrossRef]
- Zheng, Y.; Shi, D.; Song, D.; Chen, K.; Wen, F.; Zhang, J.; Xue, W.; Wu, Z. Novel mandelic acid derivatives containing piperazinyls as potential candidate fungicides against Monilinia fructicola: Design, synthesis and mechanism study. Bioorganic Chem. 2024, 151, 107647. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Chen, B. Role of cell membrane homeostasis in the pathogenicity of pathogenic filamentous fungi. Virulence 2024, 15, 2299183. [Google Scholar] [CrossRef] [PubMed]
- Guan, X. Functional Analysis of Cytoskeletal Signalling in the Defence Response of Grapevine. Ph.D. Thesis, Karlsruher Institut für Technologie (KIT), Karlsruhe, Germany, 2013. [Google Scholar]
- Liu, C.; Wu, M.; Qu, J.; Huang, X.; Zeng, Q.; Ha, M. JNK and Jag1/Notch2 co-regulate CXCL16 to facilitate cypermethrin-induced kidney damage. Ecotoxicol. Environ. Saf. 2022, 238, 113582. [Google Scholar] [CrossRef] [PubMed]
- Bera, P.; Biswas, S. Nanoscale Zr(IV) MOF-Embedded Chitosan on Paper Composite for Aqueous Phase Detection of the Herbicide Metamitron and the Food Colorant Tartrazine. ACS Appl. Nano Mater. 2024, 7, 25369–25379. [Google Scholar] [CrossRef]
- Huang, Q.; Tao, Y.; Li, H.; Guo, L.; Wang, L.; Ban, C.; Shen, G. (R,S)-Mandelic acid in pure and binary solvents solubility measurement and its correlation with thermodynamic models. J. Mol. Liq. 2021, 338, 116768. [Google Scholar] [CrossRef]
- Nandanwar, S.U.; Rathod, S.; Bansal, V.; Bokade, V.V. A Review on Selective Production of Acetophenone from Oxidation of Ethylbenzene over Heterogeneous Catalysts in a Decade. Catal. Lett. 2021, 151, 2116–2131. [Google Scholar] [CrossRef]
- Henry, M.C.; Minty, L.; Kwok, A.C.W.; Elwood, J.M.L.; Foulis, A.J.; Pettinger, J.; Jamieson, C. One-Pot Oxidative Amidation of Aldehydes via the Generation of Nitrile Imine Intermediates. J. Org. Chem. 2024, 89, 7913–7926. [Google Scholar] [CrossRef]
- Yadav, G.D.; Sowbna, P.R. Process intensification and waste minimization in liquid–liquid–liquid phase transfer catalyzed selective synthesis of mandelic acid. Chem. Eng. Res. Des. 2012, 90, 1281–1291. [Google Scholar] [CrossRef]
- Battaglia, V.; Meninno, S.; Lattanzi, A. Asymmetric Organocatalysed Synthesis of (R)-Mandelic Acid Esters and α-Alkoxy Derivatives from Commercial Sources. Chem. Eur. J. 2025, 31, e202403769. [Google Scholar] [CrossRef]
- Mao, Z.; Xia, K.; Zhang, K.; Chen, H.; Li, M.; Abdukader, A.; Jin, W. Visible light-induced oxidative esterification of mandelic acid with alcohols: A new synthesis of α-ketoesters. Green Chem. 2024, 26, 6046–6050. [Google Scholar] [CrossRef]
- Xia, Y.; Zhao, J.; Saeed, M.; Hussain, N.; Chen, X.; Guo, Z.; Yong, Y.; Chen, H. Molecular modification strategies of nitrilase for its potential application in agriculture. J. Agric. Food Chem. 2024, 72, 15106–15121. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.D.; Cai, X.; Liu, Z.Q.; Zheng, Y.G. Nitrilase: A promising biocatalyst in industrial applications for green chemistry. Crit. Rev. Biotechnol. 2021, 41, 72–93. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.T.; Su, W.C.; Chen, Q.X.; Shen, D.Y.; Zhuang, J.X. The inhibitory kinetics and mechanism of glycolic acid on lipase. J. Biomol. Struct. Dyn. 2020, 38, 2021–2028. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Nakajima-Kambe, T. Isolation and characterization of a novel bacterium that promotes the degradation of poly (glycolic acid) by its extracellular esterase under thermophilic conditions. Polym. Degrad. Stab. 2024, 230, 111007. [Google Scholar] [CrossRef]
- Sulej, J.; Piątek-Gołda, W.; Grąz, M.; Szałapata, K.; Waśko, P.; Janik-Zabrotowicz, E.; Osińska-Jaroszuk, M. Immobilisation of cellobiose dehydrogenase and laccase on chitosan particles as a multi-enzymatic system for the synthesis of lactobionic acid. J. Funct. Biomater. 2023, 14, 383. [Google Scholar] [CrossRef]
- Wang, P.; Li, D.; Yang, J.; Jiang, L.; Feng, J.; Yang, C.; Shi, R. Immobilization of (S)-mandelate dehydrogenase and its catalytic performance on stereoselective transformation of mandelic acid. J. Taiwan Inst. Chem. Eng. 2014, 45, 744–748. [Google Scholar] [CrossRef]
- Stolz, A.; Eppinger, E.; Sosedov, O.; Kiziak, C. Comparative Analysis of the Conversion of Mandelonitrile and 2-Phenylpropionitrile by a Large Set of Variants Generated from a Nitrilase Originating from Pseudomonas fluorescens EBC191. Molecules 2019, 24, 4232. [Google Scholar] [CrossRef]
- Sun, H.; Wang, H.; Gao, W.; Chen, L.; Wu, K.; Wei, D. Directed evolution of nitrilase PpL19 from Pseudomonas psychrotolerans L19 and identification of enantio-complementary mutants toward mandelonitrile. Biochem. Biophys. Res. Commun. 2015, 468, 820–825. [Google Scholar] [CrossRef]
- Bian, S.Q.; Wang, Z.K.; Gong, J.S.; Su, C.; Li, H.; Xu, Z.H.; Shi, J.S. Protein Engineering of Substrate Specificity toward Nitrilases: Strategies and Challenges. J. Agric. Food Chem. 2025, 73, 1775–1789. [Google Scholar] [CrossRef] [PubMed]
- Baum, S.; Williamson Dael, S.; Sewell, T.; Stolz, A. Conversion of Sterically Demanding α,α-Disubstituted Phenylacetonitriles by the Arylacetonitrilase from Pseudomonas fluorescens EBC191. Appl. Environ. Microbiol. 2012, 78, 48–57. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, X.H.; Liu, Z.Q.; Xue, Y.P.; Wang, Y.S.; Yang, B.; Zheng, Y.G. Production of R-Mandelic Acid Using Nitrilase from Recombinant E. coli Cells Immobilized with Tris (Hydroxymethyl) Phosphine. Appl. Biochem. Biotechnol. 2018, 184, 1024–1035. [Google Scholar] [CrossRef] [PubMed]
- Arana-Peña, S.; Rios, N.S.; Carballares, D.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. Immobilization of lipases via interfacial activation on hydrophobic supports: Production of biocatalysts libraries by altering the immobilization conditions. Catal. Today 2021, 362, 130–140. [Google Scholar] [CrossRef]
- Lima, R.N.; Anjos, C.S.; Porto, A.L. Biocatalytic synthesis of lipophilic amides by the lipase of Candida antarctica type B. Mol. Catal. 2022, 530, 112635. [Google Scholar] [CrossRef]
- Chen, X.; Yang, C.; Wang, P.; Zhang, X.; Bao, B.; Li, D.; Shi, R. Stereoselective biotransformation of racemic mandelic acid using immobilized laccase and (S)-mandelate dehydrogenase. Bioresour. Bioprocess. 2017, 4, 2. [Google Scholar] [CrossRef][Green Version]
- Arregui, L.; Ayala, M.; Gómez-Gil, X.; Gutiérrez-Soto, G.; Hernández-Luna, C.E.; Santos, M.H.; Levin, L.; Rojo-Domínguez, A.; Romero-Martínez, D.; Saparrat, M.C.N.; et al. Laccases: Structure, function, and potential application in water bioremediation. Microb. Cell Factories 2019, 18, 200. [Google Scholar] [CrossRef]
- Elgahwash, R.G.A.; Blažić, M.; Balaž, A.M.; Prodanović, R. Lactobionic acid production via mutant cellobiose dehydrogenase/laccase continuous enzymatic regeneration of electron acceptors. Biocatal. Biotransform. 2024, 42, 316–323. [Google Scholar] [CrossRef]
- Luo, Z.; Wu, G.; Kong, M.; Chen, Z.; Zhuang, Z.; Fan, J.; Chen, T. Structured illumination-based super-resolution live-cell quantitative FRET imaging. Photonics Res. 2023, 11, 887–896. [Google Scholar] [CrossRef]
- Reifenrath, M.; Boles, E. Engineering of hydroxymandelate synthases and the aromatic amino acid pathway enables de novo biosynthesis of mandelic and 4-hydroxymandelic acid with Saccharomyces cerevisiae. Metab. Eng. 2018, 45, 246–254. [Google Scholar] [CrossRef]
- Lukito, B.R.; Wang, Z.; Balaji, S.S.; Li, Z. Production of (R)-mandelic acid from styrene, L-phenylalanine, glycerol, or glucose via cascade biotransformations. Bioresour. Bioprocess. 2021, 8, 22. [Google Scholar] [CrossRef]
- Lv, B.; Zeng, Y.; Zhang, H.; Li, Z.; Xu, Z.; Wang, Y.; Gao, Y.; Chen, Y.; Fu, X. Mechanosensitive channels mediate hypoionic shock-induced aminoglycoside potentiation against bacterial persisters by enhancing antibiotic uptake. Antimicrob. Agents Chemother. 2022, 66, e01125-21. [Google Scholar] [CrossRef] [PubMed]
- Nie, Z.; Liu, P.; Wang, Y.; Guo, X.; Tan, Z.; Shen, J.; Tang, Z.; Lin, J.; Sun, J.; Zheng, P.; et al. Directed evolution and rational design of mechanosensitive channel MscCG2 for improved glutamate excretion efficiency. J. Agric. Food Chem. 2021, 69, 15660–15669. [Google Scholar] [CrossRef] [PubMed]
- Balleza, D.; Alessandrini, A.; Beltrán García, M.J. Role of lipid composition, physicochemical interactions, and membrane mechanics in the molecular actions of microbial cyclic lipopeptides. J. Membr. Biol. 2019, 252, 131–157. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, H.; Martinac, B. Mechanosensitive channels of Corynebacterium glutamicum functioning as exporters of l-glutamate and other valuable metabolites. Curr. Opin. Chem. Biol. 2020, 59, 77–83. [Google Scholar] [CrossRef]
- Mateo, C.; Chmura, A.; Rustler, S.; Rantwijk, F.; Stolz, A.; Sheldon, R.A. Synthesis of enantiomerically pure (S)-mandelic acid using an oxynitrilase–nitrilase bienzymatic cascade: A nitrilase surprisingly shows nitrile hydratase activity. Tetrahedron Asymmetry 2006, 17, 320–323. [Google Scholar] [CrossRef]
- Rustler, S.; Motejadded, H.; Altenbuchner, J.; Stolz, A. Simultaneous expression of an arylacetonitrilase from Pseudomonas fluorescens and a (S)-oxynitrilase from Manihot esculenta in Pichia pastoris for the synthesis of (S)-mandelic acid. Appl. Microbiol. Biotechnol. 2008, 80, 87–97. [Google Scholar] [CrossRef]
- Chmura, A.; Rustler, S.; Paravidino, M.; Rantwijk, F.; Stolz, A.; Sheldon, R.A. The combi-CLEA approach: Enzymatic cascade synthesis of enantiomerically pure (S)-mandelic acid. Tetrahedron Asymmetry 2013, 24, 1225–1232. [Google Scholar] [CrossRef]
- Rucká, L.; Volkova, O.; Pavlík, A.; Kaplan, O.; Kracík, M.; Nešvera, J.; Martínková, L.; Pátek, M. Expression control of nitrile hydratase and amidase genes in Rhodococcus erythropolis and substrate specificities of the enzymes. Antonie Leeuwenhoek 2014, 105, 1179–1190. [Google Scholar] [CrossRef]
- Zhou, S.P.; Xue, Y.P.; Zheng, Y.G. Maximizing the potential of nitrilase: Unveiling their diversity, catalytic proficiency, and versatile applications. Biotechnol. Adv. 2024, 72, 108352. [Google Scholar] [CrossRef]
- Dadashipour, M.; Asano, Y. Hydroxynitrile lyases: Insights into biochemistry, discovery, and engineering. ACS Catal. 2011, 1, 1121–1149. [Google Scholar] [CrossRef]
- Sheldon, R.A.; van Pelt, S. Enzyme immobilisation in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef]
- Chen, S.; Liu, F.; Zhang, K.; Huang, H.; Wang, H.; Zhou, J.; Zhang, J.; Gong, Y.; Zhang, D.; Chen, Y.; et al. An efficient enzymatic aminolysis for kinetic resolution of aromatic α-hydroxyl acid in non-aqueous media. Tetrahedron Lett. 2016, 57, 5312–5314. [Google Scholar] [CrossRef]
- He, Y.C.; Ma, C.L.; Zhang, X.; Li, L.; Xu, J.H.; Wu, M.X. Highly enantioselective oxidation of racemic phenyl-1,2-ethanediol to optically pure (R)-(-)-mandelic acid by a newly isolated Brevibacterium lutescens CCZU12-1. Appl. Microbiol. Biotechnol. 2013, 97, 7185–7194. [Google Scholar] [CrossRef]
- Gennaro, P.D.; Bernasconi, S.; Orsini, F.; Corretto, E.; Sello, G. Multienzymatic preparation of 3-[(1R)-1-hydroxyethyl]benzoic acid and (2S)-hydroxy(phenyl)ethanoic acid. Tetrahedron Asymmetry 2010, 21, 1885–1889. [Google Scholar] [CrossRef]
- Sun, Z.; Ning, Y.; Liu, L.; Liu, Y.; Sun, B.; Jiang, W.; Yang, C.; Yang, S. Metabolic engineering of the L-phenylalanine pathway in Escherichia coli for the production of S- or R-mandelic acid. Microb. Cell Factories 2011, 10, 71. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Xu, J. Preparation of (S)-mandelic acid from racemate using growing cells of Pseudomonas putida ECU1009 with (R)-mandelate degradation activity. Biochem. Eng. J. 2006, 30, 11–15. [Google Scholar] [CrossRef]
- Takakura, Y.; Ono, T.; Danjo, K.; Nozaki, H. Efficient enzymatic production of benzaldehyde from l-phenylalanine with a mutant form of 4-hydroxymandelate synthase. Biosci. Biotechnol. Biochem. 2022, 86, 1718–1725. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, J.; He, P.; Moran, G.R.; Harrison, D.H.T. Two Roads Diverged: The Structure of Hydroxymandelate Synthase from Amycolatopsis orientalis in Complex with 4-Hydroxymandelate. Biochemistry 2008, 47, 2002–2013. [Google Scholar] [CrossRef]
- Luo, Z.; Lee, S. Metabolic engineering of Escherichia coli for the production of benzoic acid from glucose. Metab. Eng. 2020, 62, 298–311. [Google Scholar] [CrossRef]
- Lukito, B.R.; Sekar, B.S.; Wu, S.; Li, Z. Whole Cell-Based Cascade Biotransformation for the Production of (S)-Mandelic Acid from Styrene, L-Phenylalanine, Glucose, or Glycerol. Adv. Synth. Catal. 2019, 361, 3560–3568. [Google Scholar] [CrossRef]
- Yao, C.; Cao, Y.; Wu, S.; Li, S.; He, B. An organic solvent and thermally stable lipase from Burkholderia ambifaria YCJ01: Purification, characteristics and application for chiral resolution of mandelic acid. J. Mol. Catal. B Enzym. 2013, 85–86, 105–110. [Google Scholar] [CrossRef]
- Cao, Y.; Wu, S.; Li, J.; Wu, B.; He, B. Highly efficient resolution of mandelic acid using lipase from Pseudomonas stutzeri LC2-8 and a molecular modeling approach to rationalize its enantioselectivity. J. Mol. Catal. B Enzym. 2014, 99, 108–113. [Google Scholar] [CrossRef]
- Guo, F.; Ye, L.; Li, A.; Yang, X.; Yang, C.; Yu, H. Insight into the role of halogen bond in the activity of d-mandelate dehydrogenase toward halogenated substrates. Tetrahedron Lett. 2016, 57, 1944–1948. [Google Scholar] [CrossRef]
- Durao, P.; Bento, I.; Fernandes, A.T.; Melo, E.P.; Lindley, P.F.; Martins, L.O. Perturbations of the T1 copper site in the CotA laccase from Bacillus subtilis: Structural, biochemical, enzymatic and stability studies. JBIC J. Biol. Inorg. Chem. 2006, 11, 514–526. [Google Scholar] [CrossRef]
- Hitaishi, V.P.; Clément, R.; Quattrocchi, L.; Parent, P.; Duché, D.; Zuily, L.; Ilbert, M.; Lojou, E.; Mazurenko, L. Interplay between orientation at electrodes and copper activation of Thermus thermophilus laccase for O2 reduction. J. Am. Chem. Soc. 2019, 142, 1394–1405. [Google Scholar] [CrossRef]
- Durao, P.; Chen, Z.; Silva, C.S.; Soares, C.M.; Pereira, M.M.; Todorovic, S.; Hildebrandt, P.; Bento, I.; Lindley, P.F.; Martins, L.O. Proximal mutations at the type 1 copper site of CotA laccase: Spectroscopic, redox, kinetic and structural characterization of I494A and L386A mutants. Biochem. J. 2008, 412, 339–346. [Google Scholar] [CrossRef]
- Janusz, G.; Pawlik, A.; Świderska-Burek, U.; Polak, J.; Sulej, J.; Jarosz-Wilkołazka, A.; Paszczyński, A. Laccase properties, physiological functions, and evolution. Int. J. Mol. Sci. 2020, 21, 966. [Google Scholar] [CrossRef]
- Dewanti, A.R.; Mitra, B.A. Transient Intermediate in the Reaction Catalyzed by (S)-Mandelate Dehydrogenase from Pseudomonas putida. Biochemistry 2003, 42, 12893–12901. [Google Scholar] [CrossRef]
- Blank, L.M.; Ebert, B.E.; Buehler, K.; Bühler, B. Redox Biocatalysis and Metabolism: Molecular Mechanisms and Metabolic Network Analysis. Antioxid. Redox Signal. 2010, 13, 349–394. [Google Scholar] [CrossRef]
- Bell, E.L.; Finnigan, W.; France, S.P.; Green, A.P.; Hayes, M.A.; Hepworth, L.J.; Lovelock, S.L.; Niikura, H.; Osuna, S.; Romero, E.; et al. Biocatalysis. Nat. Rev. Methods Primers 2021, 1, 46. [Google Scholar] [CrossRef]
- Wang, Z.; Balaji, S.; Sekar Li, Z. Recent advances in artificial enzyme cascades for the production of value-added chemicals. Bioresour. Technol. 2021, 323, 124551. [Google Scholar] [CrossRef]
- Gong, X.; Zhang, J.; Gan, Q.; Teng, Y.; Hou, J.; Lyu, Y.; Liu, Z.; Wu, Z.; Dai, R.; Zou, Y.; et al. Advancing microbial production through artificial intelligence-aided biology. Biotechnol. Adv. 2024, 74, 108399. [Google Scholar] [CrossRef]
- Sekar, B.S.; Lukito, B.R.; Li, Z. Production of Natural 2-Phenylethanol from Glucose or Glycerol with Coupled Escherichia coli Strains Expressing l-Phenylalanine Biosynthesis Pathway and Artificial Biocascades. ACS Sustain. Chem. Eng. 2019, 7, 12231–12239. [Google Scholar]
- Xu, Y.; Wu, Y.; Lv, X.; Sun, G.; Zhang, H.; Chen, T.; Du, G.; Li, J.; Liu, L. Design and construction of novel biocatalyst for bioprocessing: Recent advances and future outlook. Bioresour. Technol. 2021, 332, 125071. [Google Scholar] [CrossRef]
- Xue, C.; Liu, F.; Xu, M.; Tang, I.C.; Zhao, J.; Bai, F.; Yang, S.T. Butanol production in acetone-butanol-ethanol fermentation with in situ product recovery by adsorption. Bioresour. Technol. 2016, 219, 158–168. [Google Scholar] [CrossRef]
- Lukito, B.R.; Wu, S.; Saw, H.J.J.; Li, Z. One-Pot Production of Natural 2-Phenylethanol from L-Phenylalanine via Cascade Biotransformations. ChemCatChem 2019, 11, 831–840. [Google Scholar] [CrossRef]
- Zhou, Y.; Sekar, B.S.; Wu, S.; Li, Z. Benzoic acid production via cascade biotransformation and coupled fermentation-biotransformation. Biotechnol. Bioeng. 2020, 117, 2340–2350. [Google Scholar] [CrossRef]
- Zhou, L.; Tao, C.; Shen, X.; Sun, X.; Wang, J.; Yuan, Q. Unlocking the potential of enzyme engineering via rational computational design strategies. Biotechnol. Adv. 2024, 73, 108376. [Google Scholar] [CrossRef]
- Wang, H.; Liu, R.; Wang, B.; Hong, Y.; Cui, Z.; Ni, Q. Multitype Perception Method for Drug-Target Interaction Prediction. IEEE/ACM Trans. Comput. Biol. Bioinform. 2023, 20, 3489–3498. [Google Scholar] [CrossRef]
- Casilli, F.; Canyelles-Niño, M.; Roelfes, G.; Alonso-Cotchico, L. Computation-guided engineering of distal mutations in an artificial enzyme. Faraday Discuss. 2024, 252, 262–278. [Google Scholar] [CrossRef]
- Goshisht, M.K. Machine Learning and Deep Learning in Synthetic Biology: Key Architectures, Applications, and Challenges. ACS Omega 2024, 9, 9921–9945. [Google Scholar] [CrossRef]
- Volk, M.J.; Lourentzou, I.; Mishra, S.; Vo, L.T.; Zhai, C.; Zhao, H. Biosystems Design by Machine Learning. ACS Synth. Biol. 2020, 9, 1514–1533. [Google Scholar] [CrossRef] [PubMed]







| Category | Method/ Mechanism | Key Process | Advantages | Limitations | References |
|---|---|---|---|---|---|
| Chemical Synthesis | Traditional Methods | Cyanohydrin Hydrolysis; α-Haloacetophenone Hydrolysis | Mature technology, widely applicable | High toxicity (cyanides/halogens), high energy input, hazardous wastewater | [49,50,51] |
| Phase-transfer Catalysis | Biphasic reaction with quaternary ammonium salts under ambient conditions | Mild conditions, avoids strong oxidants/reductants | Catalyst leaching, poor recyclability, long reaction time | [52] | |
| Asymmetric Synthesis | Chiral ligands for single-enantiomer production | High optical purity (ee > 98%) | High cost of chiral catalysts, inert atmosphere required | [53] | |
| Optical Resolution | Racemate synthesis and chiral separation | Obtains enantiopure products | Low efficiency, resolving agent recovery challenges, low yield | [54] |
| Category | Brief Description | Examples | References |
|---|---|---|---|
| Synthesis from Mandelonitrile | Stereoselective nitrilase systems optimized via enzyme engineering and molecular dynamics | M113F/R128K mutants (S)-MA; Dual-enzyme system (cassava D-HNL) | [55,63,77] |
| Synthesis from MA Esters | Lipase/esterase-mediated dynamic resolution with mechanical asymmetry in active sites | Pseudomonas sp. esterase (R)-MA; Candida antarctica lipase ammonolysis | [57,58,85] |
| Synthesis from (S)-Phenyl-1,2-glycol | Aldehyde-ketone dehydrogenase systems with mechanical stress modulation | Brevibacterium lutescens CCZU12-1 (R)-MA; dehydrogenase (S)-MA | [23,86,87] |
| Synthesis from Styrene/Renewables | Modular cascade systems for green synthesis using engineered microbes | E. coli LZ37; AoHmaS mutant; Six-step E. coli system | [71,72,88,89,90] |
| (R)-MA from (S)-MA | Enzyme cofactor regeneration and redox potential optimization | Burkholderia cepacia lipase; Dehydrogenase-laccase dual system; Laccase T1 copper mutants | [59,60,94] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, J.; An, Y.; Gao, H. Biosynthesis Strategies and Application Progress of Mandelic Acid Based on Biomechanical Properties. Microorganisms 2025, 13, 1722. https://doi.org/10.3390/microorganisms13081722
Yin J, An Y, Gao H. Biosynthesis Strategies and Application Progress of Mandelic Acid Based on Biomechanical Properties. Microorganisms. 2025; 13(8):1722. https://doi.org/10.3390/microorganisms13081722
Chicago/Turabian StyleYin, Jingxin, Yi An, and Haijun Gao. 2025. "Biosynthesis Strategies and Application Progress of Mandelic Acid Based on Biomechanical Properties" Microorganisms 13, no. 8: 1722. https://doi.org/10.3390/microorganisms13081722
APA StyleYin, J., An, Y., & Gao, H. (2025). Biosynthesis Strategies and Application Progress of Mandelic Acid Based on Biomechanical Properties. Microorganisms, 13(8), 1722. https://doi.org/10.3390/microorganisms13081722





