Foodborne Botulism Caused by Clostridium botulinum Subtype A5(b3) by Self-Packaged Vacuum Spicy Rabbit Heads
Abstract
1. Introduction
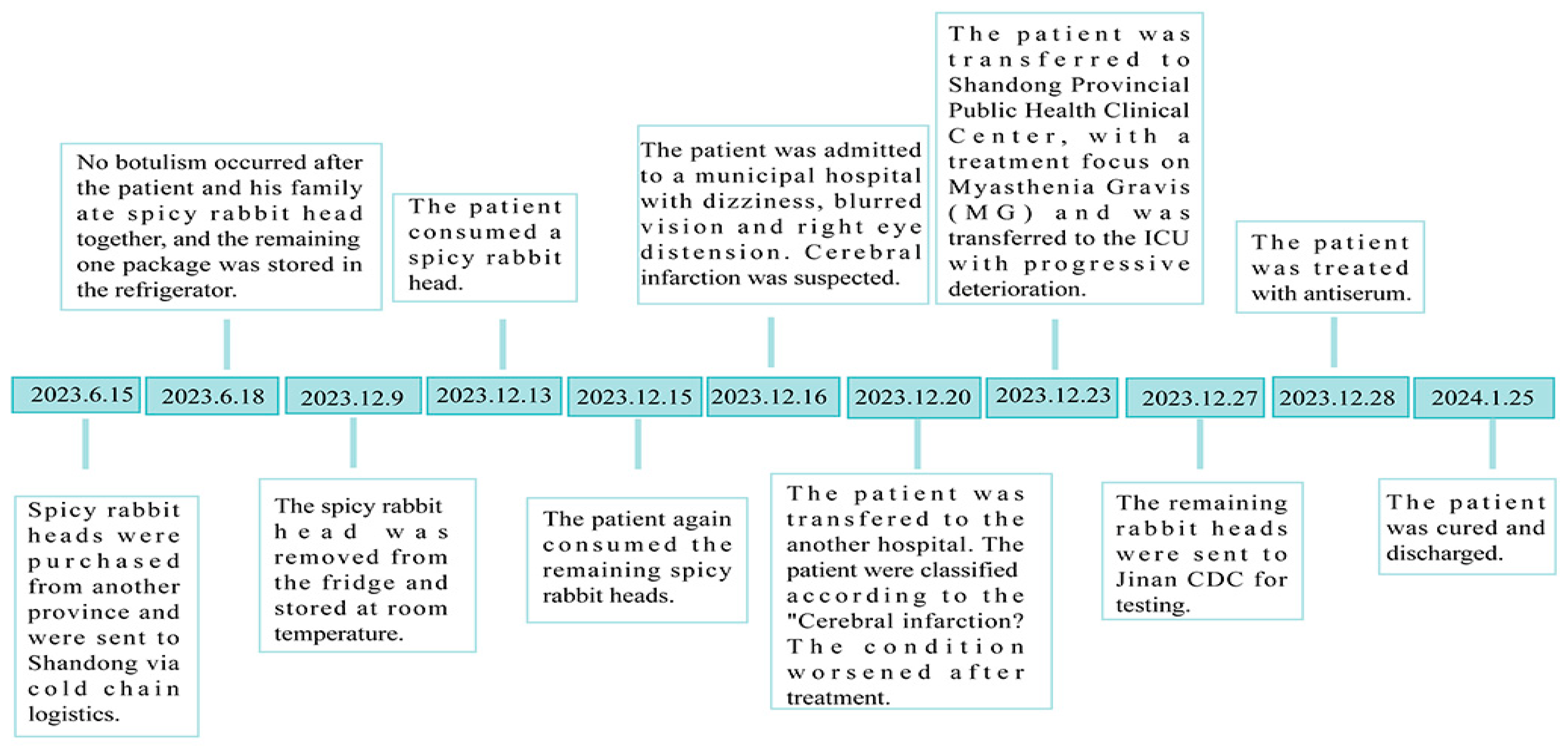
2. Epidemiological Investigation
3. Materials and Methods
3.1. Isolation of Clostridium botulinum
3.2. DNA Extraction and Real-Time Quantitative PCR Typing
3.3. Toxin Detection of Patient Samples
3.4. Assessment of BoNT Toxin by Mouse Bioassay Experiments
3.5. Analysis of Whole-Genome Sequencing (WGS) and Genomic Characteristic Collinearity of C. botulinum Strains
4. Results
4.1. Detection of BoNT and BoNT Genes
4.2. Isolation and Identification of C. botulinum
4.3. Botulinum Neurotoxin Types and MLST Typing
4.4. Gene Cluster of the Neurotoxins, Antibiotic Resistance Genes, and Virulence Factors
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rasetti-Escargueil, C.; Lemichez, E.; Popoff, M.R. Public health risk associated with botulism as foodborne zoonoses. Toxins 2019, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Centurioni, D.A.; Egan, C.T.; Perry, M.J. Current developments in diagnostic assays for laboratory confirmation and investigation of botulism. J. Clin. Microbiol. 2022, 60, e0013920. [Google Scholar] [CrossRef] [PubMed]
- Erbguth, F.J. Historical notes on botulism, Clostridium botulinum, botulinum toxin, and the idea of the therapeutic use of the toxin. Mov. Disord. 2004, 19 (Suppl. S8), S2–S6. [Google Scholar] [CrossRef]
- Rawson, A.M.; Dempster, A.W.; Humphreys, C.M.; Minton, N.P. Pathogenicity and virulence of Clostridium botulinum. Virulence 2023, 14, 2205251. [Google Scholar] [CrossRef]
- Bowe, B.K.; Wentz, T.G.; Gregg, B.M.; Tepp, W.H.; Schill, K.M.; Sharma, S.; Pellett, S. Genomic Diversity, Competition, and toxin production by group I and II Clostridium botulinum strains used in food challenge studies. Microorganisms 2022, 10, 1895. [Google Scholar] [CrossRef]
- Carter, A.T.; Peck, M.W. Genomes, neurotoxins and biology of Clostridium botulinum Group I and Group II. Res. Microbiol. 2015, 166, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Hill, K.K.; Smith, T.J. Genetic diversity within Clostridium botulinum serotypes, botulinum neurotoxin gene clusters and toxin subtypes. Curr. Top. Microbiol. Immunol. 2013, 364, 1–20. [Google Scholar]
- Rossetto, O.; Pirazzini, M.; Montecucco, C. Botulinum neurotoxins: Genetic, structural and mechanistic insights. Nat. Rev. Microbiol. 2014, 12, 535–549. [Google Scholar] [CrossRef]
- Rummel, A. The long journey of botulinum neurotoxins into the synapse. Toxicon Off. J. Int. Soc. Toxinol. 2015, 107, 9–24. [Google Scholar] [CrossRef]
- Peck, M.W.; Smith, T.J.; Anniballi, F.; Austin, J.W.; Bano, L.; Bradshaw, M.; Cuervo, P.; Cheng, L.W.; Derman, Y.; Dorner, B.G.; et al. Historical perspectives and guidelines for botulinum neurotoxin subtype nomenclature. Toxins 2017, 9, 38. [Google Scholar] [CrossRef]
- Matsumura, T.; Fujinaga, Y. Functional analysis of botulinum hemagglutinin (HA). Methods Mol. Biol. 2020, 2132, 191–200. [Google Scholar] [PubMed]
- Gonzalez-Escalona, N.; Timme, R.; Raphael, B.H.; Zink, D.; Sharma, S.K. Whole-genome single-nucleotide-polymorphism analysis for discrimination of Clostridium botulinum group I strains. Appl. Environ. Microbiol. 2014, 80, 2125–2132. [Google Scholar] [CrossRef] [PubMed]
- Sobel, J. Botulism. Clin. Infect. Dis. 2005, 41, 1167–1173. [Google Scholar] [CrossRef]
- Peck, M.W. Biology and genomic analysis of Clostridium botulinum. Adv. Microb. Physiol. 2009, 55, 183–265. [Google Scholar]
- Le Bouquin, S.; Lucas, C.; Souillard, R.; Le Maréchal, C.; Petit, K.; Kooh, P.; Jourdan-Da Silva, N.; Meurens, F.; Guillier, L.; Mazuet, C. Human and animal botulism surveillance in France from 2008 to 2019. Front. Public Health 2022, 24, 1003917. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.A.; Tchao, C.; Prystajecky, N.; Weedmark, K.; Tcholakov, Y.; Lefebvre, M.; Austin, J.W. Foodborne Botulism, Canada, 2006–2021. Emerg. Infect. Dis. 2023, 29, 1730–1737. [Google Scholar]
- Li, H.Q.; Guo, Y.C.; Tian, T.; Guo, W.H.; Liu, C.Q.; Liang, X.C.; Liu, J.; Li, W.; Fu, P. Epidemiological analysis of foodborne botulism outbreaks-China, 2004–2020. China CDC Wkly. 2022, 4, 788–792. [Google Scholar]
- Ma, X.; Li, K.; Li, F.; Su, J.; Meng, W.; Sun, Y.; Sun, H.; Sun, J.; Yuan, Y.; Lin, Y.; et al. Tracing Foodborne botulism events caused by Clostridium botulinum in Xinjiang province, China, using a core genome sequence typing scheme. Microbiol. Spectr. 2022, 10, e0116422. [Google Scholar] [CrossRef]
- GB 4789.12-2016; Microbiological Examination of Food Hygiene—Examination of Clostridium Botulinum and Botulinus Toxin. Standards Press of China: Beijing, China, 2017.
- Wang, H.; Li, J.P.; Li, X.B.; Kou, J.T.; Zhang, J. Rapid quantitative detection of botulinum toxin type A by using lanthanide fluorescence technology. Chin. Front. Health Quar. 2020, 43, 235–237. [Google Scholar]
- Sharma, S.K.; Eblen, B.S.; Bull, R.L.; Burr, D.H.; Whiting, R.C. Evaluation of lateral-flow Clostridium botulinum neurotoxin detection kits for food analysis. Appl. Environ. Microbiol. 2005, 71, 3935–3941. [Google Scholar] [CrossRef]
- Chen, S.F.; Zhou, Y.Q.; Chen, Y.R.; Gu, J. Fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Min, M.; Bai, L.; Peng, X.; Guo, L.; Wan, K.; Qiu, Z. An outbreak of botulinum types A, B, and E associated with vacuum-packaged salted fish and ham. J. Emerg. Med. 2021, 60, 760–763. [Google Scholar] [CrossRef]
- Pernu, N.; Keto-Timonen, R.; Lindström, M.; Korkeala, H. High prevalence of Clostridium botulinum in vegetarian sausages. Food Microbiol. 2020, 91, 103512. [Google Scholar] [CrossRef]
- Otofuji, T.; Tokiwa, H.; Takahashi, K. A food-poisoning incident caused by Clostridium botulinum toxin A in Japan. Epidemiol. Infect. 1987, 99, 167–172. [Google Scholar] [CrossRef]
- Lonati, D.; Schicchi, A.; Crevani, M.; Buscaglia, E.; Scaravaggi, G.; Maida, F.; Cirronis, M.; Petrolini, V.M.; Locatelli, C.A. Foodborne botulism: Clinical diagnosis and medical treatment. Toxins 2020, 12, 509. [Google Scholar] [CrossRef] [PubMed]
- Sebaihia, M.; Peck, M.W.; Minton, N.P.; Thomson, N.R.; Holden, M.T.; Mitchell, W.J.; Carter, A.T.; Bentley, S.D.; Mason, D.R.; Crossman, L.; et al. Genome sequence of a proteolytic (Group I) Clostridium botulinum strain Hall A and comparative analysis of the clostridial genomes. Genome Res. 2007, 17, 1082–1092. [Google Scholar] [CrossRef] [PubMed]
- Brunt, J.; van Vliet, A.H.; Carter, A.T.; Stringer, S.C.; Amar, C.; Grant, K.A.; Godbole, G.; Peck, M.W. Diversity of the genomes and neurotoxins of strains of Clostridium botulinum group I and Clostridium sporogenes associated with foodborne, infant and wound botulism. Toxins 2020, 12, 586. [Google Scholar] [CrossRef]
- Hutson, R.A.; Zhou, Y.; Collins, M.D.; Johnson, E.A.; Hatheway, C.L.; Sugiyama, H. Genetic characterization of Clostridium botulinum type A containing silent type B neurotoxin gene sequences. J. Biol. Chem. 1996, 271, 10786–10792. [Google Scholar] [CrossRef]
- Dover, N.; Barash, J.R.; Hill, K.K.; Davenport, K.W.; Teshima, H.; Xie, G.; Arnon, S.S. Clostridium botulinum strain Af84 contains three neurotoxin gene clusters: Bont/A2, bont/F4 and bont/F5. PLoS ONE 2013, 8, e61205. [Google Scholar] [CrossRef]
- Nawrocki, E.M.; Bradshaw, M.; Johnson, E.A. Botulinum neurotoxin-encoding plasmids can be conjugatively transferred to diverse clostridial strains. Sci. Rep. 2018, 8, 3100. [Google Scholar] [CrossRef]
- Benoit, R.M. Botulinum neurotoxin diversity from a gene-centered view. Toxins 2018, 10, 310. [Google Scholar] [CrossRef]
- Dover, N.; Barash, J.R.; Hill, K.K.; Detter, J.C.; Arnon, S.S. Novel structural elements within the nonproteolytic Clostridium botulinum type F toxin gene cluster. Appl. Environ. Microbiol. 2011, 77, 1904–1906. [Google Scholar] [CrossRef] [PubMed]
- Skarin, H.; Segerman, B. Horizontal gene transfer of toxin genes in Clostridium botulinum: Involvement of mobile elements and plasmids. Mob. Genet. Elements. 2011, 1, 213–215. [Google Scholar] [CrossRef]
- Carter, A.T.; Mason, D.R.; Grant, K.A.; Franciosa, G.; Aureli, P.; Peck, M.W. Further characterization of proteolytic Clostridium botulinum type A5 reveals that neurotoxin formation is unaffected by loss of the cntR (botR) promoter sigma factor binding site. J. Clin. Microbiol. 2010, 48, 1012–1013. [Google Scholar] [CrossRef]
- Michael, W.P.; Sandra, C.S.; Andrew, T.C. Clostridium botulinum in the post-genomic era. Food Microbiol. 2011, 28, 183–191. [Google Scholar]
- Carter, A.T.; Pearson, B.M.; Crossman, L.C.; Drou, N.; Heavens, D.; Baker, D.; Febrer, M.; Caccamo, M.; Grant, K.A.; Peck, M.W. Complete genome sequence of the proteolytic Clostridium botulinum type A5 (B3) strain H04402065. J. Bacteriol. 2011, 193, 2351–2352. [Google Scholar] [CrossRef]
- Dover, N.; Barash, J.R.; Arnon, S.S. Novel Clostridium botulinum toxin gene arrangement with subtype A5 and partial subtype B3 botulinum neurotoxin genes. J. Clin. Microbiol. 2009, 47, 2349–2350. [Google Scholar] [CrossRef]
- Mazuet, C.; Legeay, C.; Sautereau, J.; Ma, L.; Bouchier, C.; Bouvet, P.; Popoff, M.R. Diversity of group I and II Clostridium botulinum strains from France including recently identified subtypes. Genome Biol. Evol. 2016, 8, 1643–1660. [Google Scholar] [CrossRef] [PubMed]
- Xuan, H.Y.; Cai, J.X.; Xia, L.N. Research progress of cfr gene mediated bacteria resistance. J. Agric. Sci. Technol. 2019, 21, 43–50. [Google Scholar]
- Peck, M.W.; van Vliet, A.H. Impact of Clostridium botulinum genomic diversity on food safety. Curr. Opin. Food Sci. 2016, 10, 52–59. [Google Scholar] [CrossRef]
- Rao, A.K.; Sobel, J.; Chatham-Stephens, K.; Luquez, C. Clinical guidelines for diagnosis and treatment of botulism. MMWR Recomm. Rep. 2021, 70, 1–30. [Google Scholar] [CrossRef] [PubMed]


| Sample | MBA | HSFICA | Colloidal Gold Method | Real-Time PCR | Isolated Strains |
|---|---|---|---|---|---|
| Patient fecal | A | A | A | A and B | JNPF1 (C. botulinum) |
| Spicy rabbit head 1 | A | A | A | A and B | JNSRH1 (C. botulinum) |
| Spicy rabbit head 2 | A | A | A | A and B | JNSRH2 (C. botulinum) |
| TPGY culture of JNPF1 | A | N/A | N/A | N/A | C. botulinum |
| TPGY culture of JNSRH1 | A | N/A | N/A | N/A | C. botulinum |
| TPGY culture of JNSRH2 | A | N/A | N/A | N/A | C. botulinum |
| Patient serum | N/A | A | A | N/A | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, W.; Ma, C.; Liu, M.; Li, Y.; Zhou, L.; Shi, Y.; Xu, X.; Liu, H. Foodborne Botulism Caused by Clostridium botulinum Subtype A5(b3) by Self-Packaged Vacuum Spicy Rabbit Heads. Microorganisms 2025, 13, 1662. https://doi.org/10.3390/microorganisms13071662
Cui W, Ma C, Liu M, Li Y, Zhou L, Shi Y, Xu X, Liu H. Foodborne Botulism Caused by Clostridium botulinum Subtype A5(b3) by Self-Packaged Vacuum Spicy Rabbit Heads. Microorganisms. 2025; 13(7):1662. https://doi.org/10.3390/microorganisms13071662
Chicago/Turabian StyleCui, Wen, Chuanmin Ma, Ming Liu, Yan Li, Lin Zhou, Yuwen Shi, Xuefang Xu, and Hui Liu. 2025. "Foodborne Botulism Caused by Clostridium botulinum Subtype A5(b3) by Self-Packaged Vacuum Spicy Rabbit Heads" Microorganisms 13, no. 7: 1662. https://doi.org/10.3390/microorganisms13071662
APA StyleCui, W., Ma, C., Liu, M., Li, Y., Zhou, L., Shi, Y., Xu, X., & Liu, H. (2025). Foodborne Botulism Caused by Clostridium botulinum Subtype A5(b3) by Self-Packaged Vacuum Spicy Rabbit Heads. Microorganisms, 13(7), 1662. https://doi.org/10.3390/microorganisms13071662






