New Methodologies as Opportunities in the Study of Bacterial Biofilms, Including Food-Related Applications
Abstract
1. Introduction
2. Microbial Biofilm
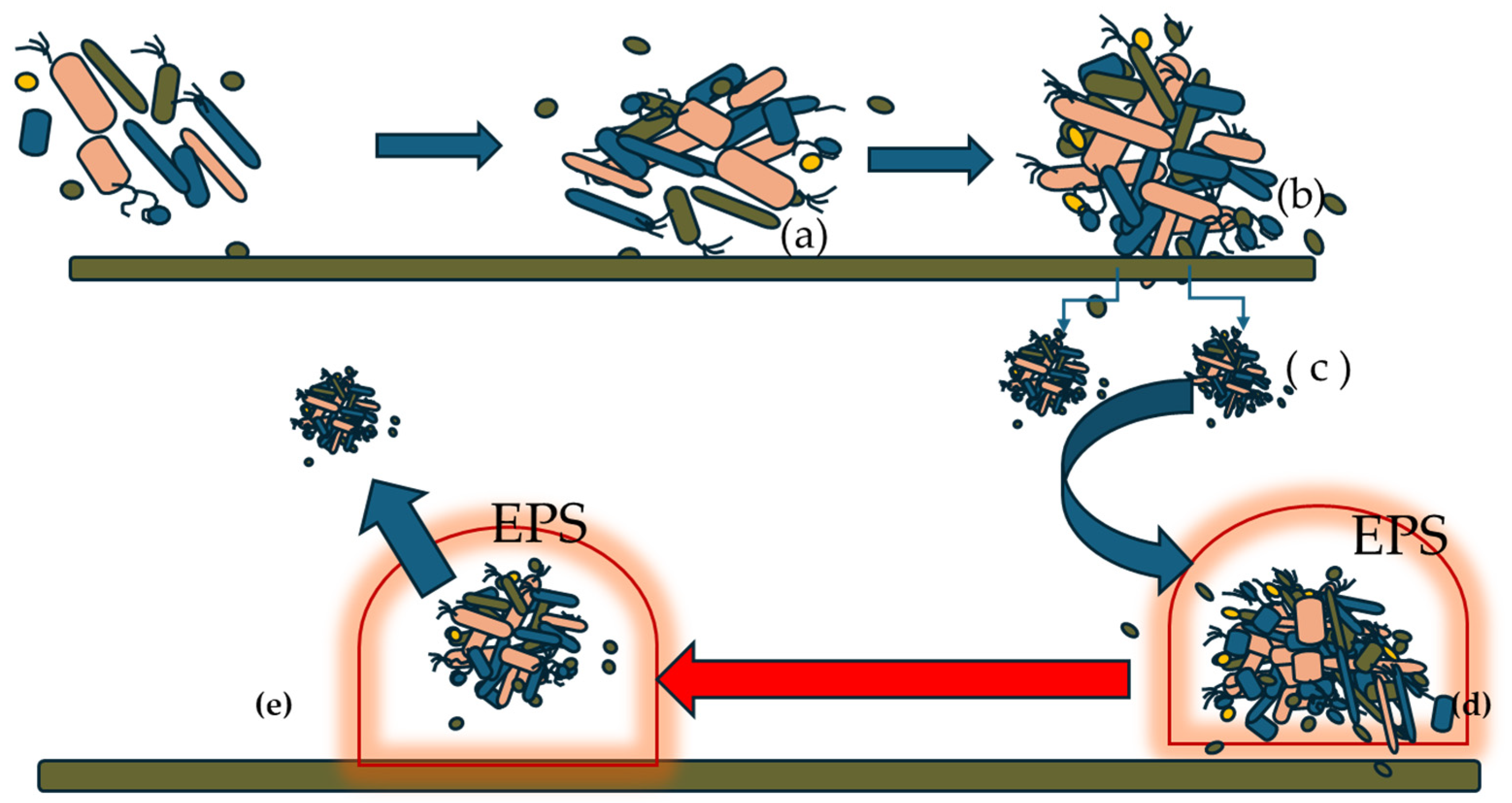
2.1. Biofilm Formation Process
- Initial Attachment: When the bacterial mass level exceeds a certain threshold, the still planktonic bacterial cells aggregate and, in the form of microcolonies, detach and colonize a specific surface. From a biophysical perspective, adhesion involves van der Waals forces and hydrophobic interactions [3].
- The transformation from planktonic to sessile cells, driven by changes in the cellular metabolic pathway, leads the bacterial cells to produce exopolysaccharides, proteins, and nucleic material. These components form a gelatinous and viscous substance that surrounds and protects the sessile cells, resembling a dome-like structure. Exopolysaccharides strengthen their adhesion and promote colonization, then proliferate and form microcolonies. Within this environment, they start to communicate via the mechanism of the so-called quorum sensing [4]. Quorum sensing (QS) plays a central role in orchestrating transcriptional regulation and phenotypic heterogeneity within maturing biofilm consortia. Through the production and detection of small signaling molecules (autoinducers), QS enables microbial populations to sense cell density and coordinate gene expression collectively. This regulation governs key biofilm-associated processes such as EPS production, motility, virulence factor expression, and stress responses. Importantly, QS also contributes to the spatial and functional diversification of cells within the biofilm, leading to phenotypic heterogeneity—an essential feature for biofilm resilience and adaptability. This heterogeneity results in distinct subpopulations with specialized roles (e.g., matrix producers, metabolically dormant cells, persisters), which complicates the effectiveness of antimicrobial interventions. Moreover, QS-mediated communication facilitates metabolic synchrony across the community, optimizing resource utilization and enhancing survival under fluctuating environmental conditions. Therefore, disrupting QS pathways represents a promising strategy to interfere with this synchrony, attenuate biofilm robustness, and sensitize microbial populations to antimicrobial agents or environmental stressors.
- Maturation of biofilm and dispersion of sessile cells.
2.2. Biofilm Resistance Mechanisms
3. Foodborne Pathogens Associated with Biofilms
Microbial Biofilm and Food Safety
4. Biofilm of Lactic Acid Bacteria
5. Biofilm of Fungi and Yeasts
6. Detection Methods to Investigate Biofilms in Food Environments
6.1. Optical Methods
6.1.1. Laser Confocal Scanning Microscopy
6.1.2. Atomic Force Microscopy
6.1.3. Scanning Electron Microscopy
6.2. Microfluidics
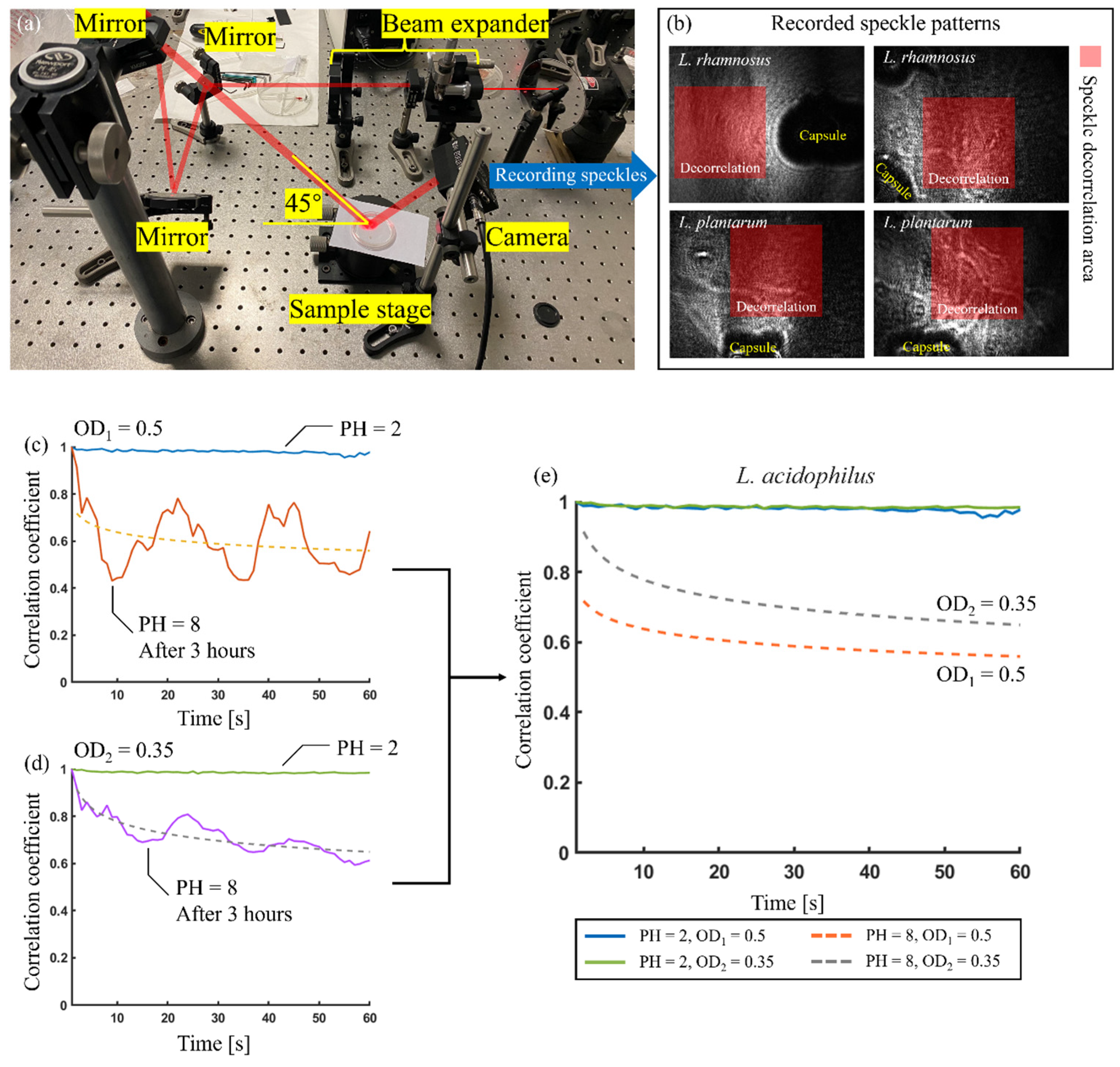
6.3. Biospeckle Laser Technology [BLT] in Microbial Studies
6.4. DNA-Based Methods [qPCR, NGS]
6.4.1. QPCR
6.4.2. NGS Technology
- Library Preparation: This step involves randomly breaking the DNA or cDNA sample into smaller fragments, after which adapter sequences are attached to both ends (5′ and 3′) through a ligation process. An optimized method called tagmentation streamlines this process by combining fragmentation and adapter ligation into a single step, significantly enhancing overall efficiency. The adapter-tagged fragments are then amplified using PCR and cleaned up—often by gel-based methods.
- Cluster Generation: The prepared library is loaded onto a flow cell containing surface-bound oligonucleotides complementary to the adapter sequences. Each DNA fragment binds to the surface and undergoes bridge amplification, a method that produces dense, clonal clusters of identical DNA strands. Once cluster formation is complete, these DNA templates are ready for sequencing.
- Sequencing: Illumina’s sequencing-by-synthesis (SBS) technology utilizes a unique approach involving reversible terminator-bound nucleotides. All four fluorescently labeled dNTPs are added in each sequencing cycle. This concurrent incorporation helps minimize bias and dramatically lowers raw error rates compared to other sequencing platforms, thanks to the natural competition among bases. The approach delivers highly accurate, base-by-base reads and effectively mitigates sequence-specific errors—especially those found in repetitive regions or homopolymer stretches.
- Data Analysis: After sequencing, the resulting reads are mapped against a reference genome. This alignment enables various types of downstream analyses, such as identifying insertions and deletions [indels], detecting single-nucleotide polymorphisms (SNPs), quantifying gene expression in RNA-seq experiments, and performing metagenomic or phylogenetic studies.
6.5. CRISPR-Cas Systems
- (1)
- EPS Synthesis Genes: Genes such as pel, pga, and bcs are essential to produce the extracellular polymeric substances that form the biofilm matrix in P. aeruginosa and E. coli. Disrupting these genes prevents bacteria from developing a protective biofilm structure.
- (2)
- Adhesion and Fimbriae Genes: Genes encoding cell surface proteins [csgA, fimH, and fnbA], which can facilitate bacterial attachment and biofilm initiation in S. aureus and E. coli, can be selectively silenced using CRISPR to reduce biofilm formation at its earliest stages.
- (3)
- Quorum Sensing Regulators: Some genes, such as luxS, lasR, and rhlR in P. aeruginosa, are responsible for bacterial cell-to-cell communication. The disruption of such pathways impedes bacteria from coordinating the formation of biofilms, thereby inhibiting maturation and persistence [232].
6.6. Organoids
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, G.; Kaplan, H.B.; Kolter, R. Biofilm formation as microbial development. Annu. Rev. Microbiol. 2000, 54, 49–79. [Google Scholar] [CrossRef]
- Parsek, M.R.; Greenberg, E.P. Sociomicrobiology: The connections between quorum sensing and biofilms. Trends Microbiol. 2005, 13, 27–33. [Google Scholar] [CrossRef]
- Fazeli-Nasab, B.; Sayyed, R.Z.; Mojahed, L.S.; Rahmani, A.F.; Ghafari, M.; Antonius, S. Biofilm production: A strategic mechanism for survival of microbes under stress conditions. Biocatal. Agric. Biotechnol. 2022, 42, 102337. [Google Scholar] [CrossRef]
- Kaplan, J.Á. Biofilm dispersal: Mechanisms clinical implications potential therapeutic uses. J. Dent. Res. 2010, 89, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xue, J.; Zhu, H.; Chen, S.; Wang, Y.; Xiao, Z.; Luo, Y. Advances in biofilm characterization: Utilizing rheology and atomic force microscopy in foods and related fields. Adv. Compos. Hybrid Mater. 2024, 7, 143. [Google Scholar] [CrossRef]
- Branda, S.S.; Vik, Å.; Friedman, L.; Kolter, R. Biofilms: The matrix revisited. Trends Microbiol. 2005, 13, 20–26. [Google Scholar] [CrossRef]
- Das, T.; Sehar, S.; Manefield, M. The roles of extracellular DNA in the structural integrity of extracellular polymeric substance and bacterial biofilm development. Environ. Microbiol. Rep. 2013, 5, 778–786. [Google Scholar] [CrossRef]
- Billings, N.; Birjiniuk, A.; Samad, T.S.; Doyle, P.S.; Ribbeck, K. Material properties of biofilms—A review of methods for understanding permeability and mechanics. Rep. Prog. Phys. 2015, 78, 036601. [Google Scholar] [CrossRef]
- Abraham, W.R. Controlling biofilms of gram-positive pathogenic bacteria. Curr. Med. Chem. 2006, 13, 1509–1524. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.Y.; Prentice, E.L.; Webber, M.A. Mechanisms of antimicrobial resistance in biofilms. npj Antimicrob. Resist. 2024, 2, 27. [Google Scholar] [CrossRef] [PubMed]
- Mah, T.F.C.; O’Toole, G.A. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001, 9, 34–39. [Google Scholar] [CrossRef]
- Lewis, K. Persister cells, dormancy and infectious disease. Nat. Rev. Microbiol. 2007, 5, 48–56. [Google Scholar] [CrossRef]
- Poole, K. Multidrug efflux pumps and antimicrobial resistance in Pseudomonas aeruginosa and related organisms. J. Mol. Microbiol. Biotechnol. 2001, 3, 255–264. [Google Scholar] [PubMed]
- Madsen, J.S.; Burmølle, M.; Hansen, L.H.; Sørensen, S.J. The interconnection between biofilm formation and horizontal gene transfer. FEMS Immunol. Med. Microbiol. 2012, 65, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Billón, M.; Llambías-Cabot, A.E.; Jordana-Lluch, E.; Oliver, A.; Macià, M.D. Mechanisms of antibiotic resistance in Pseudomonas aeruginosa biofilms. Biofilm 2023, 5, 100129. [Google Scholar] [CrossRef] [PubMed]
- Coppola, F.; Ferluga, G.; Iacumin, L.; Bernardi, C.; Pellegrini, M.; Comi, G. Hygienic Quality of Air-Packed and Refrigerated or Frozen Stored Döner Kebab and Evaluation of the Growth of Intentionally Inoculated Listeria monocytogenes. Microorganisms 2025, 13, 701. [Google Scholar] [CrossRef]
- Francolino, R.; Martino, M.; Nazzaro, F.; Sirignano, C.; Fratianni, F.; Coppola, F.; De Feo, V. Chemical Profile and Bioactivities of Three Species of Mentha Growing in the Campania Region, Southern Italy. Plants 2025, 14, 360. [Google Scholar] [CrossRef]
- Dula, S.; Ajayeoba, T.A.; Ijabadeniyi, O.A. Bacterial biofilm formation on stainless steel in the food processing environment and its health implications. Folia Microbiol. 2021, 66, 293–302. [Google Scholar] [CrossRef]
- Møretrø, T.; Langsrud, S. Listeria monocytogenes: Biofilm formation and persis-tence in food-processing environments. Biofilms 2004, 1, 107–121. [Google Scholar] [CrossRef]
- Steenackers, H.; Hermans, K.; Vanderleyden, J.; De Keersmaecker, S.C. Salmonella biofilms: An overview on occurrence, structure, regulation and eradication. Food Res. Int. 2012, 45, 502–531. [Google Scholar] [CrossRef]
- Meng, F.; Lyu, F.; Bie, X.; Lu, Y.; Lu, Z. Advances in transcriptomic analysis of Salmonella biofilms and their correlation with food safety. Curr. Opin. Food Sci. 2024, 55, 101110. [Google Scholar] [CrossRef]
- Smith, J.L.; Fratamico, P.M. Escherichia coli as a Pathogen. In Foodborne Diseases; Academic Press: Cambridge, MA, USA, 2017; pp. 189–208. [Google Scholar] [CrossRef]
- Rahal, E.A.; Kazzi, N.; Nassar, F.J.; Matar, G.M. Escherichia coli O157: H7—Clinical aspects and novel treatment approaches. Front. Cell. Infect. Microbiol. 2012, 2, 138. [Google Scholar] [CrossRef]
- Hokmabad, B.V.; Martinez-Calvo, A.; Gonzalez La Corte, S.; Datta, S.S. Spatial self-organization of confined bacterial suspensions. bioRxiv 2016. [Google Scholar] [CrossRef]
- Singh, S.; Singh, S.K.; Chowdhury, I.; Singh, R. Understanding the mechanism of bacterial biofilms resistance to antimicrobial agents. Open Microbiol. J. 2017, 11, 53. [Google Scholar] [CrossRef]
- Yang, Y.; Yan, X.; Redding, M.; Gu, G.; Luo, Y.; Patel, J.; Nou, X. Biofilm formation of Escherichia coli O157:H7 strains associated with recent reoccurring lettuce outbreaks. Food Microbiol. 2025, 128, 104728. [Google Scholar] [CrossRef]
- Nasu, T.; Maeda, S. Escherichia coli persisters in biofilm can perform horizontal gene transfer by transformation. Biochem. Biophys. Res. Commun. 2024, 738, 150549. [Google Scholar] [CrossRef]
- Bai, X.; Nakatsu, C.H.; Bhunia, A.K. Bacterial Biofilms and Their Implications in Pathogenesis and Food Safety. Foods 2021, 10, 2117. [Google Scholar] [CrossRef]
- Coppola, F.; Nazzaro, F.; Fratianni, F.; Lombardi, S.J.; Grazia, L.; Coppola, R.; Tremonte, P. Pumpkin Oil and Its Effect on the Quality of Naples-Style Salami Produced from Buffalo Meat. Foods 2025, 14, 1077. [Google Scholar] [CrossRef]
- Bhosale, S.; Brahmane, P.; Kubade, A.; Desale, R. Biofilm in the dairy industry: Detection and common process for control biofilms. Pharma Innov. J. 2021, 10, 809–817. [Google Scholar]
- Elafify, M.; Liao, X.; Feng, J.; Ahn, J.; Ding, T. Biofilm formation in food industries: Challenges and control strategies for food safety. Food Res. Int. 2024, 190, 114650. [Google Scholar] [CrossRef]
- Idrees, M.; Sawant, S.; Karodia, N.; Rahman, A. Staphylococcus aureus biofilm: Morphology, genetics, pathogenesis and treatment strategies. Int. J. Environ. Res. Public Health 2021, 18, 7602. [Google Scholar] [CrossRef]
- Nazzaro, F.; Ombra, M.N.; Coppola, F.; De Giulio, B.; d’Acierno, A.; Coppola, R.; Fratianni, F. Antibacterial Activity and Prebiotic Properties of Six Types of Lamiaceae Honey. Antibiotics 2024, 13, 868. [Google Scholar] [CrossRef]
- Coppola, F.; Abdalrazeq, M.; Fratianni, F.; Ombra, M.N.; Testa, B.; Zengin, G.; Zavala, J.F.A.; Nazzaro, F. Rosaceae Honey: Antimicrobial Activity and Prebiotic Properties. Antibiotics 2025, 14, 298. [Google Scholar] [CrossRef]
- Otto, M. Staphylococcal Biofilms. In Bacterial Biofilms; Romeo, T., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 207–228. [Google Scholar] [CrossRef]
- Di Ciccio, P.; Vergara, A.; Festino, A.R.; Paludi, D.; Zanardi, E.; Ghidini, S.; Ianieri, A. Biofilm formation by Staphylococcus aureus on food contact surfaces: Relationship with temperature and cell surface hydrophobicity. Food Control 2015, 50, 930–936. [Google Scholar] [CrossRef]
- Gutiérrez, D.; Delgado, S.; Vázquez-Sánchez, D.; Martínez, B.; Cabo, M.L.; Rodríguez, A.; Herrera, J.J.; García, P. Incidence of Staphylococcus aureus and analysis of associated bacterial communities on food industry surfaces. Appl. Environ. Microbiol. 2012, 78, 8547–8554. [Google Scholar] [CrossRef]
- Nilofar; Zengin, G.; Uba, A.I.; Abul, N.; Gulcin, I.; Koyuncu, I.; Yuksekdag, O.; Ponnaiya, S.K.M.; Tessappan, S.; Nazzaro, F.; et al. A multifunctional natural treasure based on a “one stone, many birds” strategy for designing health-promoting applications: Tordylium apulum. Food Biosci. 2024, 62, 105088. [Google Scholar] [CrossRef]
- Lombardi, S.J.; Pannella, G.; Coppola, F.; Vergalito, F.; Maiuro, L.; Succi, M.; Sorrentino, E.; Tremonte, P.; Coppola, R. Plant-Based Ingredients Utilized as Fat Replacers and Natural Antimicrobial Agents in Beef Burgers. Foods 2024, 13, 3229. [Google Scholar] [CrossRef]
- Rossi, E.; La Rosa, R.; Bartell, J.A.; Marvig, R.L.; Haagensen, J.A.; Sommer, L.M.; Molin, S.; Johansen, H.K. Pseudomonas aeruginosa adaptation and evolution in patients with cystic fibrosis. Nature Rev. Microbiol. 2021, 19, 331–342. [Google Scholar] [CrossRef]
- Khammassi, M.; Polito, F.; Caputo, L.; Abidi, A.; Mabrouk, Y.; Nazzaro, F.; Fratianni, F.; Anouar, E.H.; Snoussi, M.; Noumi, E.; et al. Antibacterial, antibiofilm, and chemical profiles of Ammi visnaga L. and Foeniculum vulgare mill. Essential oils, and ADMET, molecular docking investigation of essential oils major components. Fitoterapia 2024, 177, 106047. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gu, N.; Huang, T.Y.; Zhong, F.; Peng, G. Pseudomonas aeruginosa: A Typical Biofilm Forming Pathogen and an Emerging but Underestimated Pathogen in Food Processing. Front. Microbiol. 2023, 13, 1114199. [Google Scholar] [CrossRef]
- Stewart, P.S.; Franklin, M.J. Physiological heterogeneity in biofilms. Nat. Rev. Microbiol. 2008, 6, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Zhang, J.; Li, J.; Feng, L.; Wang, Y.; Zhu, J. Biofilm formation of Pseudomonas fluorescens induced by a novel diguanylate cyclase modulated c-di-GMP promotes spoilage of large yellow croaker (Larimichthys crocea). Food Res. Int. 2025, 208, 116231. [Google Scholar] [CrossRef]
- Comi, G.; Iacumin, L. Spoilage of meat and fish. In The Microbiological Quality of Food, 2nd ed.; Woodhead Publishing: Cambridge, UK, 2024; pp. 221–248. [Google Scholar] [CrossRef]
- Hamid, Z.; Meyrick, B.K.; Macleod, J.; Heath, E.A.; Blaxland, J. The application of ozone within the food industry, mode of action, current and future applications, and regulatory compliance. Lett. Appl. Microbiol. 2024, 77, ovae101. [Google Scholar] [CrossRef]
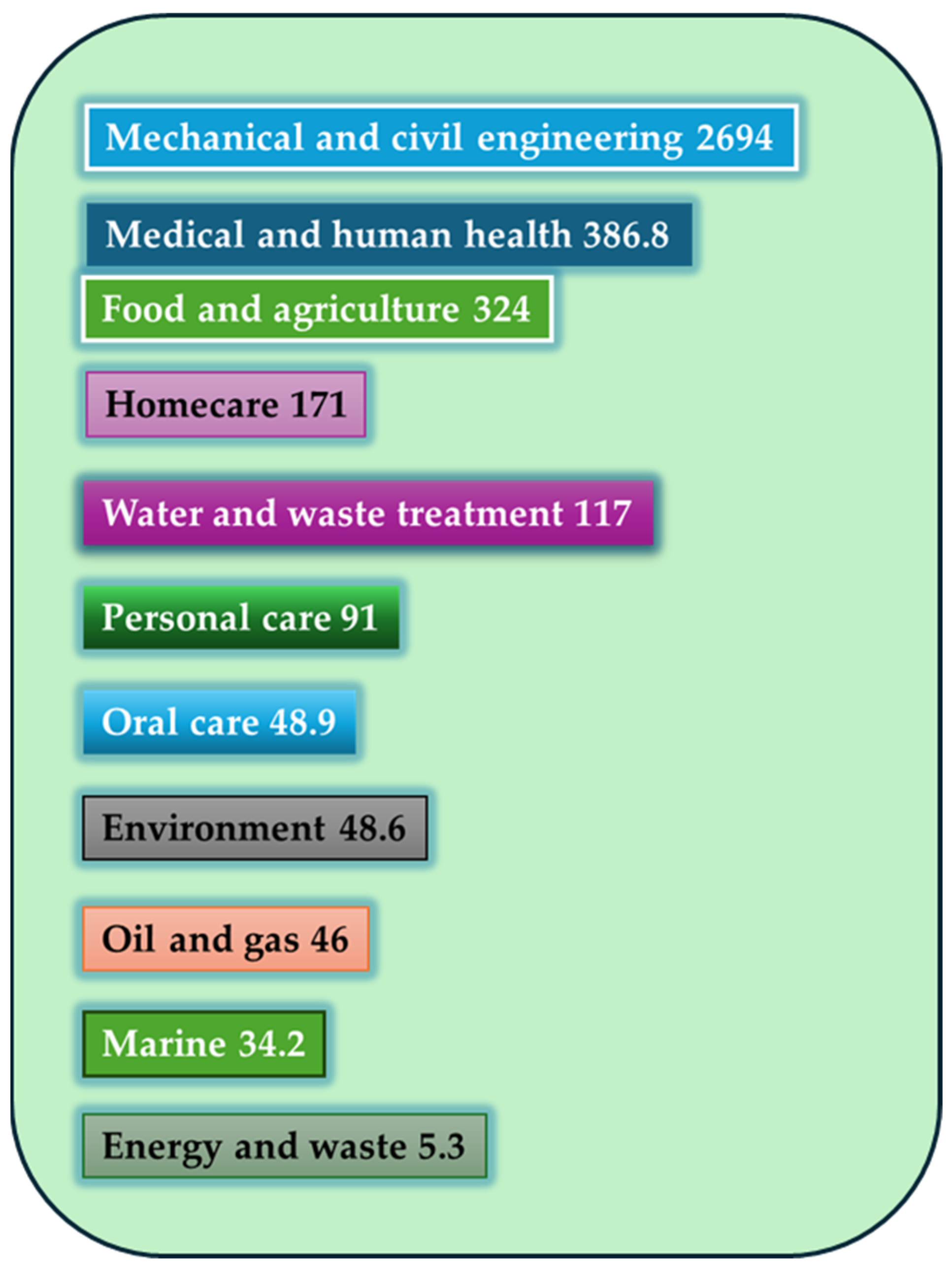
- Cámara, M.; Green, W.; MacPhee, C.E.; Rakowska, P.D.; Raval, R.; Richardson, M.C.; Slater-Jefferies, J.; Steventon, K.; Webb, J.S. Economic significance of biofilms: A multidisciplinary and cross-sectoral challenge. NPJ Biofilms Microbiomes 2022, 8, 42. [Google Scholar] [CrossRef]
- Mishra, A.; Tabassum, N.; Aggarwal, A.; Kim, Y.; Khan, F. Artificial Intelligence-Driven Analysis of Antimicrobial-Resistant and Biofilm-Forming Pathogens on Biotic and Abiotic Surfaces. Antibiotics 2024, 13, 788. [Google Scholar] [CrossRef]
- Fleming, D.; Rumbaugh, K. The Consequences of Biofilm Dispersal on the Host. Sci. Rep. 2018, 8, 10738. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wuertz, S. Bacteria and archaea on Earth and their abundance in biofilms. Nat. Rev. Microbiol. 2019, 17, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.; Benoliel, C.; Drider, D.; Dhulster, P.; Chihib, N.E. Biofilm formation and persistence on abiotic surfaces in the context of food and medical environments. Arch. Microbiol. 2014, 196, 453–472. [Google Scholar] [CrossRef]
- Snoussi, M.; Noumi, E.; Hajlaoui, H.; Bouslama, L.; Hamdi, A.; Saeed, M.; Alreshidi, M.; Adnan, M.; Aouadi, K.; Ghannay, S.; et al. Phytochemical Profiling of Allium subhirsutum L. Aqueous Extract with Antioxidant, Antimicrobial, Antibiofilm, and Anti-Quorum Sensing Properties: In Vitro and In Silico Studies. Plants 2021, 11, 495. [Google Scholar] [CrossRef] [PubMed]
- Joo, H.S.; Otto, M. Molecular basis of in vivo biofilm formation by bacterial pathogens. Chem. Biol. 2012, 19, 1503–1513. [Google Scholar] [CrossRef]
- Yin, W.; Wang, Y.; Liu, L.; He, J. Biofilms: The microbial protective clothing in extreme environments. Int. J. Mol. Sci. 2019, 20, 3423. [Google Scholar] [CrossRef]
- Sharma, S.; Mohler, J.; Mahajan, S.D.; Schwartz, S.A.; Bruggemann, L.; Aalinkeel, R. Microbial biofilm: A review on formation, infection, antibiotic resistance, control measures, and innovative treatment. Microorganisms 2023, 11, 1614. [Google Scholar] [CrossRef]
- Carrascosa, C.; Raheem, D.; Ramos, F.; Saraiva, A.; Raposo, A. Microbial biofilms in the food industry: A comprehensive review. Int. J. Environ. Res. Public Health 2021, 18, 2014. [Google Scholar] [CrossRef]
- Galié, S.; García-Gutiérrez, C.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Biofilms in the food industry: Health aspects and control methods. Front. Microbiol. 2018, 9, 898. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.S.; Flint, S.H.; Schmid, J.; Brooks, J.D. The role of surface charge and hydrophobicity in the attachment of Anoxybacillus flavithermus isolated from milk powder. J. Ind. Microbiol. Biotechnol. 2010, 37, 1111–1119. [Google Scholar] [CrossRef]
- Marchand, S.; Vandriesche, G.; Coorevits, A.; Coudijzer, K.; De Jonghe, V.; Dewettinck, K.; De Vos, P.; Devreese, B.; Heyndrickx, M.; De Block, J. Heterogeneity of heat-resistant proteases from milk Pseudomonas species. Int. J. Food Microbiol. 2009, 133, 68–77. [Google Scholar] [CrossRef]
- Liu, X.; Yao, H.; Zhao, X.; Ge, C. Biofilm formation and control of foodborne pathogenic bacteria. Molecules 2023, 28, 2432. [Google Scholar] [CrossRef]
- García-Cayuela, T.; Korany, A.M.; Bustos, I.; de Cadiñanos, L.P.G.; Requena, T.; Peláez, C.; Martínez-Cuesta, M.C. Adhesion abilities of dairy Lactobacillus plantarum strains showing an aggregation phenotype. Food Res. Int. 2014, 57, 44–50. [Google Scholar] [CrossRef]
- Aleksanyan, T.; Hakobyan, L.L.; Dimov, S.; Haertlé, T.; Bazukyan, I. Important properties of lactic acid bacteria and their role in industry. In Microbial Essentialism; Academic Press: Cambridge, MA, USA, 2024; pp. 1–46. [Google Scholar] [CrossRef]
- Comi, G.; Colautti, A.; Bernardi, C.E.M.; Stella, S.; Orecchia, E.; Coppola, F.; Iacumin, L. Leuconostoc gelidum is the major species responsible for the spoilage of cooked sausage packaged in a modified atmosphere, and hop extract is the best inhibitor tested. Microorganisms 2024, 12, 1175. [Google Scholar] [CrossRef] [PubMed]
- Colautti, A.; Camprini, L.; Ginaldi, F.; Comi, G.; Reale, A.; Coppola, F.; Iacumin, L. Safety traits, genetic and technological characterization of Lacticaseibacillus rhamnosus strains. LWT 2024, 207, 116578. [Google Scholar] [CrossRef]
- Yao, P.; Mohd Esah, E.; Zhao, C. Regulatory Mechanisms and Applications of Lactobacillus Biofilms in the Food Industry. Front. Microbiol. 2025, 15, 1465373. [Google Scholar] [CrossRef]
- Pannella, G.; Lombardi, S.J.; Coppola, F.; Vergalito, F.; Iorizzo, M.; Succi, M.; Coppola, R. Effect of biofilm formation by Lactobacillus plantarum on the malolactic fermentation in model wine. Foods 2020, 9, 797. [Google Scholar] [CrossRef]
- Mgomi, F.C.; Yang, Y.R.; Cheng, G.; Yang, Z.Q. Lactic acid bacteria biofilms and their antimicrobial potential against pathogenic microorganisms. Biofilm 2023, 5, 100118. [Google Scholar] [CrossRef]
- Aponte, M.; Blaiotta, G.; La Croce, F.; Mazzaglia, A.; Farina, V.; Settanni, L.; Moschetti, G. Use of Selected Autochthonous Lactic Acid Bacteria for Spanish-Style Table Olive Fermentation. Food Microbiol. 2012, 30, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Arena, M.P.; Capozzi, V.; Spano, G.; Fiocco, D. The potential of lactic acid bacteria to colonize biotic and abiotic surfaces and the investigation of their interactions and mechanisms. Appl. Microbiol. Biotechnol. 2017, 101, 2641–2657. [Google Scholar] [CrossRef]
- Kukhtyn, M.; Berhilevych, O.; Kravcheniuk, K.; Shynkaruk, O.; Horyuk, Y. Formation of biofilms on dairy equipment and the influence of disinfectants on them. East. Eur. J. Enterp. Technol. 2017, 5, 26–33. [Google Scholar] [CrossRef][Green Version]
- Dertli, E.; Çon, A.H. Microbial diversity of traditional sourdough and kefir fermentation. Curr. Opin. Food Sci. 2017, 13, 26–31. [Google Scholar] [CrossRef]
- Fanning, S.; Mitchell, A.P. Fungal Biofilms. PLoS Pathog. 2012, 8, e1002585. [Google Scholar] [CrossRef]
- Karaulli, J.; Xhaferaj, N.; Coppola, F.; Testa, B.; Letizia, F.; Kyçyk, O.; Iorizzo, M. Bioprospecting of Metschnikowia pulcherrima Strains, Isolated from a Vineyard Ecosystem, as Novel Starter Cultures for Craft Beer Production. Fermentation 2024, 10, 513. [Google Scholar] [CrossRef]
- Köhler, J.R.; Hube, B.; Puccia, R.; Casadevall, A.; Perfect, J.R. Fungi that infect humans. Microbiol. Spectr. 2017, 5, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Verstrepen, K.J.; Klis, F.M. Flocculation, adhesion and biofilm formation in yeasts. Mol. Microbiol. 2006, 60, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Perpetuini, G.; Rossetti, A.P.; Rapagnetta, A.; Arfelli, G.; Prete, R.; Tofalo, R. Wine barrel biofilm as a source of yeasts with non-conventional properties. Microorganisms 2024, 12, 880. [Google Scholar] [CrossRef]
- Zara, G.; Budroni, M.; Mannazzu, I.; Fancello, F.; Zara, S. Yeast biofilm in food realms: Occurrence and control. World J. Microbiol. Biotechnol. 2020, 36, 1–10. [Google Scholar] [CrossRef]
- Willaert, R.G. Adhesins of yeasts: Protein structure and interactions. J. Fungi 2018, 4, 119. [Google Scholar] [CrossRef]
- Lipke, P.N.; Klotz, S.A.; Dufrêne, Y.F.; Jackson, D.N.; Garcia-Sherman, M.C. Amyloid-like β-aggregates as force-sensitive switches in fungal biofilms and infections. Microbiol. Mol. Biol. Rev. 2018, 82, 10–112. [Google Scholar] [CrossRef]
- Moreno-García, J.; Mauricio, J.C.; Moreno, J.; García-Martínez, T. Stress responsive proteins of a flor yeast strain during the early stages of biofilm formation. Process Biochem. 2016, 51, 578–588. [Google Scholar] [CrossRef]
- Vopálenská, I.; Šťovíček, V.; Janderová, B.; Váchová, L.; Palková, Z. Role of distinct dimorphic transitions in territory colonizing and formation of yeast colony architecture. Environ. Microbiol. 2010, 12, 264–277. [Google Scholar] [CrossRef]
- Faria-Oliveira, F.; Carvalho, J.; Ferreira, C.; Hernáez, M.L.; Gil, C.; Lucas, C. Quantitative differential proteomics of yeast extracellular matrix: There is more to it than meets the eye. BMC Microbiol. 2015, 15, 271. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Testa, B.; Coppola, F.; Iorizzo, M.; Di Renzo, M.; Coppola, R.; Succi, M. Preliminary characterisation of Metschnikowia pulcherrima to be used as a starter culture in red winemaking. Beverages 2024, 10, 88. [Google Scholar] [CrossRef]
- Testa, B.; Coppola, F.; Letizia, F.; Albanese, G.; Karaulli, J.; Ruci, M.; Iorizzo, M. Versatility of Saccharomyces cerevisiae 41CM in the brewery sector: Use as a starter for “Ale” and “Lager” craft beer production. Processes 2022, 10, 2495. [Google Scholar] [CrossRef]
- Fleet, G.H.; Mian, M.A. The occurrence and growth of yeasts in dairy products. Int. J. Food Microbiol. 1987, 4, 145–155. [Google Scholar] [CrossRef]
- Tournas, V.H.; Katsoudas, E. Mould and yeast flora in fresh berries, grapes and citrus fruits. Int. J. Food Microbiol. 2005, 105, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Salo, S.; Wirtanen, G. Disinfectant efficacy on foodborne spoilage yeast strains. Food Bioprod. Process. 2005, 83, 288–296. [Google Scholar] [CrossRef]
- Colautti, A.; Orecchia, E.; Coppola, F.; Iacumin, L.; Comi, G. Cyberlindnera fabianii, an uncommon yeast responsible for gluten bread spoilage. Foods 2024, 13, 2381. [Google Scholar] [CrossRef]
- Domínguez-Manzano, J.; Olmo-Ruiz, C.; Bautista-Gallego, J.; Arroyo-López, F.N.; Garrido-Fernández, A.; Jiménez-Díaz, R. Biofilm formation on abiotic and biotic surfaces during Spanish style green table olive fermentation. Int. J. Food Microbiol. 2012, 157, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Vitzilaiou, E.; Stoica, I.M.; Knøchel, S. Microbial biofilm communities on reverse osmosis membranes in whey water processing before and after cleaning. J. Membr. Sci. 2019, 587, 117174. [Google Scholar] [CrossRef]
- Buonanno, A.; Imparato, M.; Maione, A.; Carraturo, F.; Galdiero, E.; Guida, M.; de Alteriis, E. The biotherapeutic potential of a novel probiotic Kluyveromyces marxianus isolated from a sourdough starter against vaginal Candida albicans strains. J. Fungi 2025, 11, 147. [Google Scholar] [CrossRef]
- Lawrence, J.R.; Neu, T.R. Confocal laser scanning microscopy for analysis of microbial biofilms. Methods Enzymol. 1999, 310, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Holah, J.; Gibson, H. Food industry biofilms. In Biofilms: Recent Advances in Their Study and Control; CRC Press: Boca Raton, FL, USA, 2000; pp. 211–235. ISBN 0-203-35307-2. [Google Scholar]
- Chatterjee, S.; Biswas, N.; Datta, A.; Dey, R.; Maiti, P. Atomic force microscopy in biofilm study. Microscopy 2014, 63, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Miquet, E.E.; Cabrera, H.; Grassi, H.C.; Andrades, E.J.; Otero, I.; Rodríguez, D.; Darias, J.G. Digital imaging information technology for biospeckle activity assessment relative to bacteria and parasites. Lasers Med. Sci. 2017, 32, 1375–1386. [Google Scholar] [CrossRef]
- Bianco, V.; Nazzaro, F.; Wang, Z.; Behal, J.; Grilli, S.; Ferraro, P. Motility-based screening of probiotic bacteria candidates by label-free coherent light tester. In Optical Methods for Inspection, Characterization, and Imaging of Biomaterials VI; SPIE: Bellingham, WA, USA, 2023; Volume 12622, pp. 248–251. [Google Scholar] [CrossRef]
- Elliott, A.D. Confocal microscopy: Principles and modern practices. Curr. Protoc. Cytom. 2020, 92, e68. [Google Scholar] [CrossRef]
- Sánchez-Martín, J.; Serrano-Heredia, S.M.; Carrasco Jiménez, E.; Valero, A.; López-Cabo, M. Biopreservation strategies to control cross-contamination of Listeria monocytogenes biofilm cells on cold-smoked rainbow trout. SSRN Electron. J. 2024, 5170601. [Google Scholar] [CrossRef]
- Olszewska, M.A.; Dev Kumar, G.; Hur, M.; Diez-Gonzalez, F. Inactivation of dried cells and biofilms of Listeria monocytogenes by exposure to blue light at different wavelengths and the influence of surface materials. Appl. Environ. Microbiol. 2023, 89, e01147-23. [Google Scholar] [CrossRef]
- Sun, J.; Wang, D.; Sun, Z.; Liu, F.; Du, L.; Wang, D. The combination of ultrasound and chlorogenic acid to inactivate Staphylococcus aureus under planktonic, biofilm, and food systems. Ultrason. Sonochem. 2021, 80, 105801. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Peng, C.; Chi, F.; Yu, C.; Yang, Q.; Li, Z. Antibacterial and antibiofilm activities of chlorogenic acid against Yersinia enterocolitica. Front. Microbiol. 2022, 13, 885092. [Google Scholar] [CrossRef]
- Rieu, A.; Briandet, R.; Habimana, O.; Garmyn, D.; Guzzo, J.; Piveteau, P. Listeria monocytogenes EGD-e biofilms: No mushrooms but a network of knitted chains. Appl. Environ. Microbiol. 2008, 74, 4491–4497. [Google Scholar] [CrossRef]
- Ladewig, L.; Tufail, M.A.; Kinfu, B.M.; Fokt, H.; Baines, J.F.; Schmitz, R.A. Exploring new Bacteroidota strains: Functional diversity and probiotic characteristics. bioRxiv 2025. [Google Scholar] [CrossRef]
- Liu, H.; Ma, J.; Yang, P.; Geng, F.; Li, X.; Lü, J.; Wang, Y. Comparative analysis of biofilm characterization of probiotic Escherichia coli. Front. Microbiol. 2024, 15, 1365562. [Google Scholar] [CrossRef] [PubMed]
- Chandla, S.; Harjai, K.; Shukla, G. Synergistic effect of biogenics derived from potential probiotics together with zingerone against biofilm formation by Pseudomonas aeruginosa PAO1. Probiotics Antimicrob. Proteins 2021, 13, 1481–1497. [Google Scholar] [CrossRef]
- Wilson, R.M.; Walker, J.M.; Beld, J.; Yin, K. Lactobacillus acidophilus (strain Scav) postbiotic metabolites reduce infection and modulate inflammation in an in vivo model of Pseudomonas aeruginosa wound infection. J. Appl. Microbiol. 2025, 136, lxaf061. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, H. Application of atomic force microscopy in food microorganisms. Trends Food Sci. Technol. 2019, 87, 73–83. [Google Scholar] [CrossRef]
- Ahmed, T. Atomic Force Microscopy: A New Look at Microbes; Springer Nature: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Olivares, A.; Barraza, V.; Aguayo, S. Micro-and nano-scale adhesion of oral bacteria to biomaterials using atomic force microscopy: A systematic review. Jpn. Dental Sci. Rev. 2025, 61, 41–54. [Google Scholar] [CrossRef]
- Deza, M.; Araujo, M.; Garrido, M. Inactivation of Escherichia coli, Listeria monocytogenes, Pseudomonas aeruginosa and Staphylococcus aureus on stainless steel and glass surfaces by neutral electrolysed water. Lett. Appl. Microbiol. 2005, 40, 341–346. [Google Scholar] [CrossRef]
- Jonas, K.; Tomenius, H.; Kader, A.; Normark, S.; Römling, U.; Belova, L.M.; Melefors, Ö. Roles of curli, cellulose and BapA in Salmonella biofilm morphology studied by atomic force microscopy. BMC Microbiol. 2007, 7, 70. [Google Scholar] [CrossRef]
- Park, B.J.; Haines, T.; Abu-Lail, N.I. A correlation between the virulence and the adhesion of Listeria monocytogenes to silicon nitride: An atomic force microscopy study. Colloids Surf. B Biointerfaces 2009, 73, 237–243. [Google Scholar] [CrossRef]
- Pesce, G.; Rusciano, G.; Sasso, A.; Isticato, R.; Sirec, T.; Ricca, E. Surface charge and hydrodynamic coefficient measurements of Bacillus subtilis spore by optical tweezers. Colloids Surf. B Biointerfaces 2014, 116, 568–575. [Google Scholar] [CrossRef]
- Zeng, G.; Vad, B.S.; Dueholm, M.S.; Christiansen, G.; Nilsson, M.; Tolker-Nielsen, T.; Otzen, D.E. Functional bacterial amyloid increases Pseudomonas biofilm hydrophobicity and stiffness. Front. Microbiol. 2015, 6, 1099. [Google Scholar] [CrossRef]
- Formosa-Dague, C.; Feuillie, C.; Beaussart, A.; Derclaye, S.; Kucharíková, S.; Lasa, I.; Van Dijick Dufrêne, Y.F. Sticky matrix: Adhesion mechanism of the staphylococcal polysaccharide intercellular adhesin. ACS Nano 2016, 10, 3443–3452. [Google Scholar] [CrossRef]
- Guerin, J.; Burgain, J.; Francius, G.; El-Kirat-Chatel, S.; Beaussart, A.; Scher, J.; Gaiani, C. Adhesion of Lactobacillus rhamnosus GG surface biomolecules to milk proteins. Food Hydrocoll. 2018, 82, 296–303. [Google Scholar] [CrossRef]
- Nahle, S.; El Khoury, A.; Assaf, J.C.; Louka, N.; Chokr, A.; Atoui, A. A promising innovative technique for mycotoxin detoxification from beverages using biofilms of lactic acid bacteria. Innov. Food Sci. Emerg. Technol. 2022, 82, 103165. [Google Scholar] [CrossRef]
- Assaf, J.C.; Khoury, A.E.; Chokr, A.; Louka, N.; Atoui, A. A novel method for elimination of aflatoxin M1 in milk using Lactobacillus rhamnosus GG biofilm. Int. J. Dairy Technol. 2019, 72, 248–256. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Q.; Zhao, J.; Zhang, H.; Chen, W. Lactobacillus-derived components for inhibiting biofilm formation in the food industry. World J. Microbiol. Biotechnol. 2024, 40, 117. [Google Scholar] [CrossRef] [PubMed]
- Zdarta, A.; Kaczorek, E. Nanomechanical changes in probiotic bacteria under antibiotics exposure: Implications on Lactobacillus biofilm formation. Biochim. Biophys. Acta Mol. Cell Res. 2023, 1870, 119533. [Google Scholar] [CrossRef]
- Giordani, B.; Parolin, C.; Vitali, B. Lactobacilli as anti-biofilm strategy in oral infectious diseases: A mini-review. Front. Med. Technol. 2021, 3, 769172. [Google Scholar] [CrossRef]
- Relucenti, M.; Familiari, G.; Donfrancesco, O.; Taurino, M.; Li, X.; Chen, R.; Selan, L. Microscopy methods for biofilm imaging: Focus on SEM and VP-SEM pros and cons. Biology 2021, 10, 51. [Google Scholar] [CrossRef]
- Goldstein, J.; Newbury, D.; Michael, J.R.; Ritchie, N.W.M.; Scott, J.H.J. Scanning Electron Microscopy and X-Ray Microanalysis; Springer Science+Business Media: New York, NY, USA, 2018. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef]
- Høiby, N.; Ciofu, O.; Johansen, H.K.; Song, Z.-J.; Moser, C.; Jensen, P.Ø.; Molin, S.; Givskov, M.; Tolker-Nielsen, T.; Bjarnsholt, T. The clinical impact of bacterial biofilms. Int. J. Oral Sci. 2010, 3, 55–65. [Google Scholar] [CrossRef]
- Percival, S.L.; Suleman, L.; Vuotto, C.; Donelli, G. Healthcare-associated infections, medical devices and biofilms: Risk, tolerance and control. J. Med. Microbiol. 2015, 64, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Dunne, W.M. Bacterial adhesion: Seen any good biofilms lately? Clin. Microbiol. Rev. 2002, 15, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Filipić, B.; Ušjak, D.; Rambaher, M.H.; Oljacic, S.; Milenković, M.T. Evaluation of novel compounds as anti-bacterial or anti-virulence agents. Front. Cell. Infect. Microbiol. 2024, 14, 1370062. [Google Scholar] [CrossRef]
- Khan, S.T.; Ahamed, M.; Al-Khedhairy, A.; Musarrat, J. Biocidal effect of copper and zinc oxide nanoparticles on human oral microbiome and biofilm formation. Mater. Lett. 2013, 97, 67–70. [Google Scholar] [CrossRef]
- Weerarathne, P.; Payne, J.; Saha, J.; Kountoupis, T.; Jadeja, R.; Jaroni, D. Evaluating the efficacy of sodium acid sulfate to reduce Escherichia coli O157:H7 and its biofilms on food-contact surfaces. LWT 2021, 139, 110501. [Google Scholar] [CrossRef]
- Dhowlaghar, N.; Bansal, M.; Schilling, M.W.; Nannapaneni, R. Scanning electron microscopy of Salmonella biofilms on various food-contact surfaces in catfish mucus. Food Microbiol. 2018, 74, 143–150. [Google Scholar] [CrossRef]
- Neu, T.R.; Lawrence, J.R. Innovative techniques, sensors, and approaches for imaging biofilms at different scales. Trends Microbiol. 2015, 23, 233–242. [Google Scholar] [CrossRef]
- Heydorn, A.; Nielsen, A.T.; Hentzer, M.; Sternberg, C.; Givskov, M.; Ersbøll, B.K.; Molin, S. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology 2000, 146, 2395–2407. [Google Scholar] [CrossRef]
- Yuan, L.; Straub, H.; Shishaeva, L.; Ren, Q. Microfluidics for biofilm studies. Annu. Rev. Anal. Chem. 2023, 16, 139–159. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, S.; García-Aznar, J.M.; Gonzalo-Asensio, J. Microfluidic devices for studying bacterial taxis, drug testing and biofilm formation. Microb. Biotechnol. 2022, 15, 395–414. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, Y.D.; Vogeleer, P.; Jacques, M.; Harel, J. High-throughput microfluidic method to study biofilm formation and host-pathogen interactions in pathogenic Escherichia coli. Appl. Environ. Microbiol. 2015, 81, 2827–2840. [Google Scholar] [CrossRef] [PubMed]
- Al Ghamdi, H.; Zabermawi, N.; Aly, M.M. Biofilm formation of foodborne pathogens and strategies of its prevention and biocontrol: A review. Appl. Food Biotechnol. 2024, 12, 1–8. [Google Scholar] [CrossRef]
- Ranjbaran, M.; Verma, M.S. Microfluidics at the interface of bacteria and fresh produce. Trends Food Sci. Technol. 2022, 128, 102–117. [Google Scholar] [CrossRef]
- Xiang, X.; Wang, X.; Shang, Y.; Ding, Y. Microfluidic intestine-on-a-chip: Current progress and further perspectives of probiotic-foodborne pathogen interactions. Trends Food Sci. Technol. 2023, 134, 207–221. [Google Scholar] [CrossRef]
- Meroni, G.; Panelli, S.; Zuccotti, G.; Bandi, C.; Drago, L.; Pistone, D. Probiotics as therapeutic tools against pathogenic biofilms: Have we found the perfect weapon? Microbiol. Res. 2021, 12, 916–937. [Google Scholar] [CrossRef]
- Dreier, M.; Berthoud, H.; Shani, N.; Wechsler, D.; Junier, P. Development of a high-throughput microfluidic qPCR system for the quantitative determination of quality-relevant bacteria in cheese. Front. Microbiol. 2021, 11, 619166. [Google Scholar] [CrossRef]
- Jin, R.; Song, J.; Liu, C.; Lin, R.; Liang, D.; Aweya, J.J.; Yang, S. Synthetic microbial communities: Novel strategies to enhance the quality of traditional fermented foods. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13388. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Hansen, M.F.; Røder, H.L.; Wang, N.; Burmølle, M.; He, G. Mixed-species biofilms in the food industry: Current knowledge and novel control strategies. Crit. Rev. Food Sci. Nutr. 2020, 60, 2277–2293. [Google Scholar] [CrossRef]
- Rashtchi, P. Exploring Lactiplantibacillus Plantarum Biofilm Formation and the Impact of Biotic, Environmental and Physical Factors. Ph.D. Thesis, Wageningen University and Research, Wageningen, The Netherlands, 2024. [Google Scholar]
- Bazzaz, S.; Abbasi, A.; Ghotbabad, A.G.; Pourjafar, H.; Hosseini, H. Novel encapsulation approaches in the functional food industry: With a focus on probiotic cells and bioactive compounds. Probiotics Antimicro. Prot. 2024, 1–39. [Google Scholar] [CrossRef]
- Ghosh, S.; Ahmad, R.; Zeyaullah, M.; Khare, S.K. Microbial nano-factories: Synthesis and biomedical applications. Front. Chem. 2021, 9, 626834. [Google Scholar] [CrossRef] [PubMed]
- Gomes, L.C.; Mergulhao, F.J.M. A selection of platforms to evaluate surface adhesion and biofilm formation in controlled hydrodynamic conditions. Microorganisms 2021, 9, 1993. [Google Scholar] [CrossRef]
- Mirghani, M.E.S.; Liyana, Y.; Parveen, J. Bioactivity analysis of lemongrass (Cymbopogon citratus) essential oil. Int. Food Res. J. 2012, 19, 569. [Google Scholar]
- Oliveira, N.M.; Martinez-Garcia, E.; Xavier, J.; Durham, W.M.; Kolter, R.; Kim, W.; Foster, K.R. Biofilm formation as a response to ecological competition. PLoS Biol. 2015, 13, e1002191. [Google Scholar] [CrossRef]
- Alvand, Z.M.; Parseghian, L.; Aliahmadi, A.; Rahimi, M.; Rafati, H. Nanoencapsulated Thymus daenensis and Mentha piperita essential oil for bacterial and biofilm eradication using microfluidic technology. Int. J. Pharm. 2024, 651, 123751. [Google Scholar] [CrossRef]
- Rahmati, F.; Hosseini, S.S.; Mahuti Safai, S.; Asgari Lajayer, B.; Hatami, M. New insights into the role of nanotechnology in microbial food safety. 3 Biotech 2020, 10, 1–15. [Google Scholar] [CrossRef]
- Heuberger, L.; Messmer, D.; Scherrer, D.; Lörtscher, E.; Schoenenberger, A.; Palivan, C.G. Microfluidic giant polymer vesicles equipped with biopores for high-throughput screening of bacteria. Adv. Sci. 2024, 11, 2307103. [Google Scholar] [CrossRef]
- Anjum, M.; Laitila, A.; Ouwehand, A.C.; Forssten, S.D. Current perspectives on gastrointestinal models to assess probiotic-pathogen interactions. Front. Microbiol. 2022, 13, 831455. [Google Scholar] [CrossRef] [PubMed]
- Droumpali, A.; Liu, Y.; Ferrer-Florensa, X.; Sternberg, C.; Dimaki, M.; Andersen, A.J.; Taboryski, R. Biosynthesis enhancement of tropodithietic acid (TDA) antibacterial compound through biofilm formation by marine bacteria Phaeobacter inhibens on micro-structured polymer surfaces. RSC Adv. 2023, 13, 33159–33166. [Google Scholar] [CrossRef]
- Pandiselvam, R.; Mayookha, V.P.; Kothakota, A.; Ramesh, S.V.; Thirumdas, R.; Juvvi, P. Biospeckle laser technique–A novel non-destructive approach for food quality and safety detection. Trends Food Sci. Technol. 2020, 97, 1–13. [Google Scholar] [CrossRef]
- Mandracchia, B.; Palpacuer, J.; Nazzaro, F.; Bianco, V.; Rega, R.; Ferraro, P.; Grilli, S. Biospeckle decorrelation quantifies the performance of alginate-encapsulated probiotic bacteria. IEEE J. Sel. Top. Quantum Electron. 2018, 25, 1–6. [Google Scholar] [CrossRef]
- Balmages, I.; Liepins, J.; Zolins, S.; Bliznuks, D.; Lihacova, I.; Lihachev, A. Laser speckle imaging for early detection of microbial colony forming units. Biomed. Opt. Express 2021, 12, 1609–1620. [Google Scholar] [CrossRef]
- Gåsvik, K.J. Optical Metrology; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Federico, A.; Kaufmann, G.H.; Galizzi, G.E.; Rabal, H.; Trivi, M.; Arizaga, R. Simulation of dynamic speckle sequences and its application to the analysis of transient processes. Opt. Commun. 2006, 260, 493–499. [Google Scholar] [CrossRef]
- Murialdo, S.E.; Sendra, G.H.; Passoni, L.I.; Arizaga, R.; Gonzalez, J.F.; Rabal, H.; Trivi, M. Analysis of bacterial chemotactic response using dynamic laser speckle. J. Biomed. Opt. 2009, 14, 64015. [Google Scholar] [CrossRef] [PubMed]
- Murialdo, S.E.; Passoni, L.I.; Guzman, M.N.; Sendra, G.H.; Rabal, H.; Trivi, M.; Gonzalez, J.F. Discrimination of motile bacteria from filamentous fungi using dynamic speckle. J. Biomed. Opt. 2012, 17, 56011. [Google Scholar] [CrossRef]
- Yoon, J.; Lee, K.; Park, Y. A simple and rapid method for detecting living microorganisms in food using laser speckle decorrelation. arXiv 2016, arXiv:1603.07343. [Google Scholar] [CrossRef]
- Loutfi, H.; Pellen, F.; Le Jeune, B.; Lteif, R.; Kallassy, M.; Le Brun, G.; Abboud, M. Realtime monitoring of bacterial growth kinetics in suspensions using laser speckle imaging. Sci. Rep. 2020, 10, 408. [Google Scholar] [CrossRef]
- Kim, H.; Singh, A.K.; Bhunia, A.K.; Bae, E. Laser-induced speckle scatter patterns in Bacillus colonies. Front. Microbiol. 2014, 5, 537. [Google Scholar] [CrossRef] [PubMed]
- Grassi, H.C.; García, L.C.; Lobo-Sulbarán, M.L.; Velásquez, A.; Andrades-Grassi, F.A.; Cabrera, H.; Andrades-Grassi, J.E.; Andrades, E.D.J. Quantitative laser biospeckle method for the evaluation of the activity of Trypanosoma cruzi using VDRL plates and digital analysis. PLoS Negl. Trop. Dis. 2016, 10, e0005169. [Google Scholar] [CrossRef]
- Zhou, K.; Zhou, C.; Sapre, A.; Pavlock, J.H.; Weaver, A.; Muralidharan, R.; Noble, J.; Chung, T.; Kovac, J.; Liu, Z. Dynamic laser speckle imaging meets machine learning to enable rapid antibacterial susceptibility testing (DyRAST). ACS Sens. 2020, 5, 3140–3149. [Google Scholar] [CrossRef]
- Goodman, J.W. Some fundamental properties of speckle. J. Opt. Soc. Am. 1976, 66, 1145–1150. [Google Scholar] [CrossRef]
- Bianco, V.; Marchesano, V.; Finizio, A.; Paturzo, M.; Ferraro, P. Self-propelling bacteria mimic coherent light decorrelation. Opt. Express 2015, 23, 9388–9396. [Google Scholar] [CrossRef] [PubMed]
- Bianco, V.; Memmolo, P.; Paturzo, M.; Ferraro, P. On-speckle suppression in IR digital holography. Opt. Lett. 2016, 41, 5226–5229. [Google Scholar] [CrossRef]
- Kreis, T. Handbook of Holographic Interferometry: Optical and Digital Methods; Wiley VCH: Weinheim, Germany, 2006; ISBN 3-527-40546-1. [Google Scholar]
- Oleandro, E.; Grilli, S.; Rega, R.; Mugnano, M.; Bianco, V.; Valentino, M.; Mandracchia, B.; Nazzaro, F.; Coppola, R.; Ferraro, P. Biospeckle analysis and biofilm electrostatic tests, two useful methods in microbiology. Appl. Microbiol. 2021, 1, 557–572. [Google Scholar] [CrossRef]
- Ansari, M.Z.; Mujeeb, A. Assessment of microscopic repair dynamics in self-healing polymer by modeling laser speckle images. Laser Phys. 2018, 28, 126003. [Google Scholar] [CrossRef]
- van der Kooij, H.M. Let There Be Light: Quantitative Imaging of Nanoscale Dynamics in Polymer Materials. Ph.D. Thesis, Wageningen University and Research, Wageningen, The Netherlands, 2020. [Google Scholar]
- Al-Ogaidi, M.; Al-Temeemy, A.A. Bio-speckle laser technology in revealing microbial radial growth: A review. Pak. J. Life Soc. Sci. 2024, 22, 1890–1901. [Google Scholar] [CrossRef]
- Rohde, A.; Hammerl, J.A.; Boone, I.; Jansen, W.; Fohler, S.; Klein, G.; Dieckmann, R.; Al Dahouk, S. Overview of validated alternative methods for the detection of foodborne bacterial pathogens. Trends Food Sci. Technol. 2017, 62, 113–118. [Google Scholar] [CrossRef]
- Aronsson, K.; Rönner, U. Influence of pH, water activity and temperature on the inactivation of Escherichia coli and Saccharomyces cerevisiae by pulsed electric fields. Innov. Food Sci. Emerg. Technol. 2001, 2, 105–112. [Google Scholar] [CrossRef]
- Jo, Y.; Jung, J.; Kim, M.; Park, H.; Kang, S.-J.; Park, Y. Label-free identification of individual bacteria using Fourier transform light scattering. Opt. Express 2015, 23, 15792–15805. [Google Scholar] [CrossRef]
- Liu, L.; Ngadi, M. Hyperspectral imaging for food quality and safety control. Ethiop. J. Appl. Sci. Technol. 2013, 1, 51–59. [Google Scholar]
- Bianco, V.; Mandracchia, B.; Nazzaro, F.; Marchesano, V.; Gennari, O.; Paturzo, M.; Grilli, S.; Ferraro, P. Food quality inspection by speckle decorrelation properties of bacteria colonies. In Proceedings of the Optical Methods for Inspection, Characterization, and Imaging of Biomaterials III, Munich, Germany, 25–29 June 2017; Volume 10333, pp. 264–269. [Google Scholar] [CrossRef]
- Ramírez-Miquet, E.E.; Otero, I.; Rodríguez, D.; Darias, J.G.; Combarro, A.M.; Contreras, O.R. Differences in activity profile of bacterial cultures studied by dynamic speckle patterns. In Proceedings of the Imaging, Manipulation, and Analysis of Biomolecules, Cells, and Tissues XI, San Francisco, CA, USA, 2–7 February 2013; 85871P. pp. 216–223. [Google Scholar] [CrossRef]
- Wang, Z.; Giugliano, G.; Behal, J.; Schiavo, M.; Memmolo, P.; Miccio, L.; Grilli, S.; Nazzaro, F.; Ferraro, P.; Bianco, V. All-optical dual module platform for motility-based functional scrutiny of microencapsulated probiotic bacteria. Biomed. Opt. Express 2024, 15, 2202–2223. [Google Scholar] [CrossRef] [PubMed]
- Nagaraju, D.; Yanjarappa, S.M.; Purushothama, C.R.A. (Eds.) Molecular Approaches for the Detection of Fungal Phytopathogens; CRC Press: Boca Raton, FL, USA, 2025; ISBN 9781040266762. [Google Scholar]
- Loutfi, H.; Pellen, F.; Le Jeune, B.; Lteif, R.; Kallassy, M.; Le Brun, G.; Abboud, M. Interpretation of the bacterial growth process based on the analysis of the speckle field generated by calibrated scattering media. Opt. Express 2020, 28, 28648–28655. [Google Scholar] [CrossRef] [PubMed]
- Passoni, I.; Rabal, H.; Meschino, G.; Trivi, M. Probability mapping images in dynamic speckle classification. Appl. Opt. 2013, 52, 726–733. [Google Scholar] [CrossRef]
- Soni, A.; Dixit, Y.; Reis, M.M.; Brightwell, G. Hyperspectral imaging and machine learning in food microbiology: Developments and challenges in detection of bacterial, fungal, and viral contaminants. Compr. Rev. Food Sci. Food Saf. 2022, 21, 3717–3745. [Google Scholar] [CrossRef]
- Zhu, H.; Luo, J.; He, S. Detecting multiple mixed bacteria using dual-mode hyperspectral imaging and deep neural networks. Appl. Sci. 2024, 14, 1525. [Google Scholar] [CrossRef]
- da Costa Ferreira, L.; Carvalho, I.C.B.; Jorge, L.A.D.C.; Quezado-Duval, A.M.; Rossato, M. Hyperspectral imaging for the detection of plant pathogens in seeds: Recent developments and challenges. Front. Plant Sci. 2024, 15, 1387925. [Google Scholar] [CrossRef]
- Sendra, G.H.; Arizaga, R.; Rabal, H.; Trivi, M. Decomposition of biospeckle images in temporary spectral bands. Opt. Lett. 2005, 30, 1641–1643. [Google Scholar] [CrossRef]
- Ram, B.G.; Oduor, P.; Igathinathane, C.; Howatt, K.; Sun, X. A systematic review of hyperspectral imaging in precision agriculture: Analysis of its current state and future prospects. Comput. Electron. Agric. 2024, 222, 109037. [Google Scholar] [CrossRef]
- Zhang, Y.; Ceylan Koydemir, H.; Shimogawa, M.M.; Yalcin, S.; Guziak, A.; Liu, T.; Oguz, I.; Huang, Y.; Bai, B.; Luo, Y. Motility-based label-free detection of parasites in bodily fluids using holographic speckle analysis and deep learning. Light Sci. Appl. 2018, 7, 108. [Google Scholar] [CrossRef]
- Briers, D.; Duncan, D.D.; Hirst, E.; Kirkpatrick, S.J.; Larsson, M.; Steenbergen, W.; Stromberg, T.; Thompson, O.B. Laser speckle contrast imaging: Theoretical and practical limitations. J. Biomed. Opt. 2013, 18, 66018. [Google Scholar] [CrossRef]
- Pérez-González, O.; Rodríguez-Villarreal, R.A.; López-Arroyo, J.I.; Maldonado-Blanco, M.G.; Rodríguez-Guerra, R. Mexican strains of Hirsutella isolated from Diaphorina citri (Hemiptera: Liviidae): Morphologic and molecular characterization. Fla. Entomol. 2015, 98, 290–297. [Google Scholar] [CrossRef]
- Ansari, M.Z.; Ramírez-Miquet, E.E.; Otero, I.; Rodríguez, D.; Darias, J.G. Real time and online dynamic speckle assessment of growing bacteria using the method of motion history image. J. Biomed. Opt. 2016, 21, 66006. [Google Scholar] [CrossRef] [PubMed]
- Almatroudi, A. Investigating biofilms: Advanced methods for comprehending microbial behavior and antibiotic resistance. Front. Biosci. (Landmark Ed.) 2024, 29, 133. [Google Scholar] [CrossRef]
- Díez López, C.; Kayser, M.; Vidaki, A. Estimating the time since deposition of saliva stains with a targeted bacterial DNA approach: A proof-of-principle study. Front. Microbiol. 2021, 12, 647933. [Google Scholar] [CrossRef]
- Lazarevic, V.; Gaïa, N.; Girard, M.; Mauffrey, F.; Ruppé, E.; Schrenzel, J. Effect of bacterial DNA enrichment on detection and quantification of bacteria in an infected tissue model by metagenomic next-generation sequencing. ISME Commun. 2022, 2, 122. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, T.; Soteyome, T.; Mao, Y.; Luo, Y.; Yuan, L.; Xu, Z. The survival and enterotoxin gene expression of Staphylococcus aureus planktonic and biofilm cells in quick-frozen food. LWT 2023, 187, 115354. [Google Scholar] [CrossRef]
- Nahar, S.; Jeong, H.L.; Kim, Y.; Ha, A.J.W.; Roy, P.K.; Park, S.H.; Ha, S.D. Inhibitory effects of Flavourzyme on biofilm formation, quorum sensing, and virulence genes of foodborne pathogens Salmonella Typhimurium Escherichia coli. Food Res. Int. 2021, 147, 110461. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, L.; Hu, L.; Wang, Z.; Wang, X.; Dong, Q. Adhesion and kinetics of biofilm formation and related gene expression of Listeria monocytogenes in response to nutritional stress. Food Res. Int. 2022, 156, 111143. [Google Scholar] [CrossRef]
- Lou, X.; Wu, Y.; Huang, Z.; Zhang, W.; Xiao, X.; Wu, J.; Fang, Z. Biofilm formation and associated gene expression changes in Cronobacter from cereal related samples in China. Food Microbiol. 2024, 118, 104409. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, Z.; Feng, L.; Qu, D.; Zhu, J. Identification of microbial communities and multi-species biofilms contamination in seafood processing environments with different hygiene conditions. Food Microbiol. 2024, 122, 104553. [Google Scholar] [CrossRef]
- Kim, Y.K.; Roy, P.K.; Ashrafudoulla, M.; Nahar, S.; Toushik, S.H.; Hossain, M.I.; Ha, S.D. Antibiofilm effects of quercetin against Salmonella enterica biofilm formation and virulence, stress response, and quorum-sensing gene expression. Food Control 2022, 137, 108964. [Google Scholar] [CrossRef]
- Wen, Q.H.; Wang, R.; Zhao, S.Q.; Chen, B.R.; Zeng, X.A. Inhibition of biofilm formation of foodborne Staphylococcus aureus by the citrus flavonoid naringenin. Foods 2021, 10, 2614. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Armenta, F.J.; Hernandez-Oñate, M.A.; Martinez-Tellez, M.A.; Lopez-Zavala, A.A.; Gonzalez-Aguilar, G.A.; Gutierrez-Pacheco, M.M.; Ayala-Zavala, J.F. Quercetin repressed the stress response factor (sigB) and virulence genes (prfA, actA, inlA, and inlC), lower the adhesion, and biofilm development of Listeria monocytogenes. Food Microbiol. 2020, 87, 103377. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Hao, L.; Zhou, R.; Jin, Y.; Huang, J.; Wu, C. Multispecies biofilms in fermentation: Biofilm formation, microbial interactions, and communication. Compr. Rev. Food Sci. Food Saf. 2022, 21, 3346–3375. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; El-Omar, E.M. Application of molecular techniques in Helicobacter pylori detection: Limitations and improvements. Helicobacter 2021, 26, e12841. [Google Scholar] [CrossRef]
- Ogeh, D.N. Exploiting Public Human Genome NGS Datasets to Characterize Repetitive DNA and Recover Assembly Gaps. Doctoral Dissertation, University of Leicester, Leicester, UK, 2018. [Google Scholar]
- Caldera, J.R.; Anikst, V.; Gray, H.; Tsan, A.; Sono, R.; Yang, S. Performance Evaluation of a Commercial Automated Library Preparation System for Clinical Microbial Whole-Genome Sequencing Assays. J. Mol. Diagn. 2024, 26, 719–726. [Google Scholar] [CrossRef]
- Nanda, A.S.; Wu, K.; Irkliyenko, I.; Woo, B.; Ostrowski, M.S.; Clugston, A.S.; Ramani, V. Direct transposition of native DNA for sensitive multimodal single-molecule sequencing. Nat. Genet. 2024, 56, 1300–1309. [Google Scholar] [CrossRef]
- Syed, M.A.; Ali, S.; Hussain, T. (Eds.) Microbiology in the Era of Artificial Intelligence: Nanotechnology, Quantum, and Next Generation Sequencing; CRC Press: Boca Raton, FL, USA, 2025; ISBN 9781032712376. [Google Scholar]
- Hemmati, M.A.; Monemi, M.; Asli, S.; Mohammadi, S.; Foroozanmehr, B.; Haghmorad, D.; Eslami, M. Using new technologies to analyze gut microbiota and predict cancer risk. Cells 2024, 13, 1987. [Google Scholar] [CrossRef]
- Hemdan, B.A.; El-Liethy, M.A.; ElMahdy, M.E.I.; El-Taweel, G.E. Metagenomics analysis of bacterial structure communities within natural biofilm. Heliyon 2019, 5, e02271. [Google Scholar] [CrossRef]
- Sharma, B.; Mishra, A.; Sahni, P.K.; Sharma, U. Next-generation sequencing: Role in microbial biofilm study. In Microbial Biofilms; Yadav, M.K., Vidal, J.E., Song, J.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 237–247. [Google Scholar] [CrossRef]
- Mayer, P.; Smith, A.C.; Hurlow, J.; Morrow, B.R.; Bohn, G.A.; Bowler, P.G. Assessing biofilm at the bedside: Exploring reliable accessible biofilm detection methods. Diagnostics 2024, 14, 2116. [Google Scholar] [CrossRef]
- Mohammad, Z.H.; Arias-Rios, E.V.; Ahmad, F.; Juneja, V.K. Microbial contamination in the food processing environment. In Microbial Biotechnology in the Food Industry: Advances, Challenges, and Potential Solutions; Springer Nature: Cham, Switzerland, 2024; pp. 15–43. [Google Scholar]
- Shin, J.M.; Luo, T.; Lee, K.H.; Guerreiro, D.; Botero, T.M.; McDonald, N.J.; Rickard, A.H. Deciphering endodontic microbial communities by next-generation sequencing. J. Endod. 2018, 44, 1080–1087. [Google Scholar] [CrossRef] [PubMed]
- Moulic, A.G.; Deshmukh, P.; Gaurkar, S.S. A comprehensive review on biofilms in otorhinolaryngology: Understanding the pathogenesis, diagnosis, and treatment strategies. Cureus 2024, 16, e57634. [Google Scholar] [CrossRef]
- Dave, M.; Tattar, R. Antimicrobial resistance genes in the oral microbiome. Evid. Based Dent. 2025, 73, 001866. [Google Scholar] [CrossRef]
- Ghosh, S.; Nag, M.; Lahiri, D.; Sarkar, T.; Pati, S.; Kari, Z.A.; Nirmal, N.P.; Edinur, H.A.; Ray, R.R. Engineered biofilm: Innovative nextgen strategy for quality enhancement of fermented foods. Front. Nutr. 2022, 9, 808630. [Google Scholar] [CrossRef]
- Wang, C.; Cui, Y.; Qu, X. Mechanisms and improvement of acid resistance in lactic acid bacteria. Arch. Microbiol. 2018, 200, 195–201. [Google Scholar] [CrossRef]
- Mojica, F.J.M.; Díez-Villaseñor, C.; García-Martínez, J.; Soria, E. Intervening sequences of regularly spaced prokaryotic repeats derive from foreign genetic elements. J. Mol. Evol. 2005, 60, 174–182. [Google Scholar] [CrossRef]
- Zuberi, A.; Ahmad, N.; Ahmad, H.; Saeed, M.; Ahmad, I. Beyond antibiotics: CRISPR/Cas9 triumph over biofilm-associated antibiotic resistance infections. Front. Cell Infect. Microbiol. 2024, 14, 1408569. [Google Scholar] [CrossRef]
- Hille, F.; Richter, H.; Wong, S.P.; Bratovič, M.; Ressel, S.; Charpentier, E. The biology of CRISPR-cas: Backward and forward. Cell 2018, 172, 1239–1259. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Zhou, W.; Zhou, J.; He, Z.; Zhou, Y.; Han, J.; Qu, D. CRISPR-Cas systems are present predominantly on chromosome and its relationship with MEGs in Vibrio species. Arch. Microbiol. 2022, 204, 76. [Google Scholar] [CrossRef]
- Mayorga-Ramos, A.; Zúñiga-Miranda, J.; Carrera-Pacheco, S.E.; Barba-Ostria, C.; Guamán, L.P. CRISPR-cas-based antimicrobials: Design, challenges, and bacterial mechanisms of resistance. ACS Infect. Dis. 2023, 9, 1283–1302. [Google Scholar] [CrossRef]
- Bikard, D.; Euler, C.W.; Jiang, W.; Nussenzweig, P.M.; Goldberg, G.W.; Duportet, X.; Fischetti, V.A.; Marraffini, L.A. Exploiting CRISPR-Cas nucleases to produce sequence-specific antimicrobials. Nat. Biotechnol. 2014, 32, 1146–1150. [Google Scholar] [CrossRef] [PubMed]
- Crawley, A.B.; Henriksen, E.D.; Stout, E.; Brandt, K.; Barrangou, R. Characterizing the activity of abundant, diverse and active CRISPR-Cas systems in lactobacilli. Sci. Rep. 2018, 8, 11544. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Zheng, P.; Sun, J.; Wang, M. Microbial base editing: A powerful emerging technology for microbial genome engineering. Trends Biotechnol. 2021, 39, 165–180. [Google Scholar] [CrossRef] [PubMed]
- Citorik, R.J.; Mimee, M.; Lu, T.K. Sequence-specific antimicrobials using efficiently delivered RNA-guided nucleases. Nat. Biotechnol. 2014, 32, 1141–1145. [Google Scholar] [CrossRef]
- Waters, E.M.; Neill, D.R.; Kaman, B.; Sahota, J.S.; Clokie, M.R.J.; Winstanley, C.; Kadioglu, A. Phage therapy is highly effective against chronic lung infections with antibiotic-resistant Pseudomonas aeruginosa. Thorax 2020, 75, 306–310. [Google Scholar] [CrossRef]
- Gelsinger, D.R.; Vo, P.L.H.; Klompe, S.E.; Ronda, C.; Wang, H.H.; Sternberg, S.H. Bacterial genome engineering using CRISPR-associated transposases. Nat. Protoc. 2024, 19, 752–790. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.C.; Tagliaferri, T.L.; de Oliveira Mendes, T.A. Potential of the endogenous and artificially inserted CRISPR-Cas system for controlling virulence and antimicrobial resistance of food pathogens. Food Chem. Adv. 2023, 2, 100229. [Google Scholar] [CrossRef]
- Crawley, A.B.; Henriksen, J.R.; Barrangou, R. CRISPR-based genome editing in lactic acid bacteria: Applications and beyond. FEMS Microbiol. Lett. 2018, 365, fny048. [Google Scholar] [CrossRef]
- Lu, Y.; Li, F.; Bai, J.; Ledesma-Amaro, R.; Liu, D.; He, Q.; Deng, R. Rapid screening of antimicrobial probiotics using CRISPR cascade. Biosens. Bioelectron. 2022, 216, 114673. [Google Scholar] [CrossRef]
- van Pijkeren, J.; Barrangou, R. Genome Editing of Food-Grade Lactobacilli to Develop Therapeutic Probiotics. Microbiol. Spectr. 2017, 5, 1–16. [Google Scholar] [CrossRef]
- Barrangou, R.; Notebaart, R. A CRISPR-Directed Microbiome Manipulation across the Food Supply Chain. Trends Microbiol. 2019, 27, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Vries, R.G.; Snippert, H.J.; van de Wetering, M.; Barker, N.; Stange, D.E.; Van Es, J.H.; Abo, A.; Kujala, P.; Peters, P.J. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009, 459, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Stange, D.E.; Ferrante, M.; Vries, R.G.; Van Es, J.H.; Van Den Brink, S.; Clevers, H. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 2011, 141, 1762–1772. [Google Scholar] [CrossRef]
- Zhang, R.R.; Koido, M.; Tadokoro, T.; Ouchi, R.; Matsuno, T.; Ueno, Y.; Taniguchi, H. Human iPSC-derived posterior gut progenitors are expandable and capable of forming gut and liver organoids. Stem Cell Rep. 2018, 10, 780–793. [Google Scholar] [CrossRef]
- Hirota, A.; AlMusawi, S.; Nateri, A.S.; Ordóñez-Morán, P.; Imajo, M. Biomaterials for intestinal organoid technology and personalized disease modeling. Acta Biomater. 2021, 132, 272–287. [Google Scholar] [CrossRef] [PubMed]
- Yu, J. Vascularized organoids: A more complete model. Int. J. Stem Cells 2021, 14, 127–137. [Google Scholar] [CrossRef]
- Seidlitz, T.; Koo, B.K.; Stange, D.E. Gastric organoids—An in vitro model system for the study of gastric development and road to personalized medicine. Cell Death Differ. 2021, 28, 68–83. [Google Scholar] [CrossRef]
- Shiota, J.; Samuelson, L.C.; Razumilava, N. Hepatobiliary organoids and their applications for studies of liver health and disease: Are we there yet? Hepatology 2021, 74, 2251–2263. [Google Scholar] [CrossRef]
- Chauhdari, T.; Zaidi, S.A.; Su, J.; Ding, Y. Organoids meet microfluidics: Recent advancements, challenges, and future of organoids-on-chip. Vitr. Models 2025, 1, 1–18. [Google Scholar] [CrossRef]
- Van den Bossche, S.; Ostyn, L.; Vandendriessche, V.; Rigauts, C.; De Keersmaecker, H.; Nickerson, C.A.; Crabbé, A. The development and characterization of in vivo-like three-dimensional models of bronchial epithelial cell lines. Eur. J. Pharm. Sci. 2023, 190, 106567. [Google Scholar] [CrossRef]
- Fritsche, E.; Haarmann-Stemmann, T.; Kapr, J.; Galanjuk, S.; Hartmann, J.; Mertens, P.R.; Koch, K. Stem cells for next level toxicity testing in the 21st century. Small 2021, 17, 2006252. [Google Scholar] [CrossRef] [PubMed]
- Bluhmki, T.; Bitzer, S.; Gindele, J.A.; Schruf, E.; Kiechle, T.; Webster, M.; Schymeinsky, J.; Ries, R.; Gantner, F.; Bischoff, D.; et al. Development of a miniaturized 96-Transwell air–liquid interface human small airway epithelial model. Sci. Rep. 2020, 10, 13022. [Google Scholar] [CrossRef] [PubMed]
- De Breij, A.; Riool, M.; Cordfunke, R.A.; Malanovic, N.; De Boer, L.; Koning, R.I.; Ravensbergen, E.; Franken, M.; Van Der Heijde, T.; Boekema, B.K. The antimicrobial peptide SAAP-148 combats drug-resistant bacteria and biofilms. Sci. Transl. Med. 2018, 10, eaan4044. [Google Scholar] [CrossRef]
- Nickerson, C.A.; Honer zu Bentrup, K.; Ott, C.M. Three-dimensional cell culture models for drug discovery and infectious disease. Bioforum Eur. 2005, 6, 34–36. [Google Scholar] [CrossRef]
- Griffith, L.G.; Swartz, M.A. Capturing complex 3D tissue physiology in vitro. Nat. Rev. Mol. Cell Biol. 2006, 7, 211–224. [Google Scholar] [CrossRef]
- Dutta, D.; Clevers, H. Organoid culture systems to study host–pathogen interactions. Curr. Opin. Immunol. 2017, 48, 15–22. [Google Scholar] [CrossRef]
- Mahieu, L.; Van Moll, L.; De Vooght, L.; Delputte, P.; Cos, P. In vitro modelling of bacterial pneumonia: A comparative analysis of widely applied complex cell culture models. FEMS Microbiol. Rev. 2024, 48, fuae007. [Google Scholar] [CrossRef] [PubMed]
- Forbester, J.L.; Goulding, D.; Vallier, L.; Hannan, N.; Hale, C.; Pickard, D.; Dougan, G. Interaction of Salmonella enterica serovar Typhimurium with intestinal organoids derived from human induced pluripotent stem cells. Infect. Immun. 2015, 83, 2926–2934. [Google Scholar] [CrossRef]
- Kim, H.J.; Huh, D.; Hamilton, G.; Ingber, D.E. Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab. Chip 2012, 12, 2165–2174. [Google Scholar] [CrossRef]
- Park, S.E.; Georgescu, A.; Huh, D. Organoids-on-a-chip. Science 2019, 364, 960–965. [Google Scholar] [CrossRef]
- Benam, K.H.; Gilchrist, S.; Kleensang, A.; Satz, A.B.; Willett, C.; Zhang, Q. Exploring new technologies in biomedical research. Drug Discov. Today 2019, 24, 1242–1247. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.N.; Ingber, D.E. Microfluidic organs-on-chips. Nat. Biotechnol. 2014, 32, 760–772. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Yuan, X.; Lu, X.; Wu, J.; Luo, C.; Zhang, T.; Xie, R. The Use of Gut Organoids: To Study the Physiology and Disease of the Gut Microbiota. J. Cell Mol. Med. 2025, 29, e70330. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coppola, F.; Fratianni, F.; Bianco, V.; Wang, Z.; Pellegrini, M.; Coppola, R.; Nazzaro, F. New Methodologies as Opportunities in the Study of Bacterial Biofilms, Including Food-Related Applications. Microorganisms 2025, 13, 1062. https://doi.org/10.3390/microorganisms13051062
Coppola F, Fratianni F, Bianco V, Wang Z, Pellegrini M, Coppola R, Nazzaro F. New Methodologies as Opportunities in the Study of Bacterial Biofilms, Including Food-Related Applications. Microorganisms. 2025; 13(5):1062. https://doi.org/10.3390/microorganisms13051062
Chicago/Turabian StyleCoppola, Francesca, Florinda Fratianni, Vittorio Bianco, Zhe Wang, Michela Pellegrini, Raffaele Coppola, and Filomena Nazzaro. 2025. "New Methodologies as Opportunities in the Study of Bacterial Biofilms, Including Food-Related Applications" Microorganisms 13, no. 5: 1062. https://doi.org/10.3390/microorganisms13051062
APA StyleCoppola, F., Fratianni, F., Bianco, V., Wang, Z., Pellegrini, M., Coppola, R., & Nazzaro, F. (2025). New Methodologies as Opportunities in the Study of Bacterial Biofilms, Including Food-Related Applications. Microorganisms, 13(5), 1062. https://doi.org/10.3390/microorganisms13051062







