Essential Oils as an Antifungal Alternative to Control Several Species of Fungi Isolated from Musa paradisiaca: Part III
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Purification of Microorganisms
2.2. Morphological Identification
2.3. Molecular Identification Through DNA Sequencing
2.4. Ex Vivo Fungal Activity
2.5. In Vitro Antifungal Activity with Essential Oils
3. Results
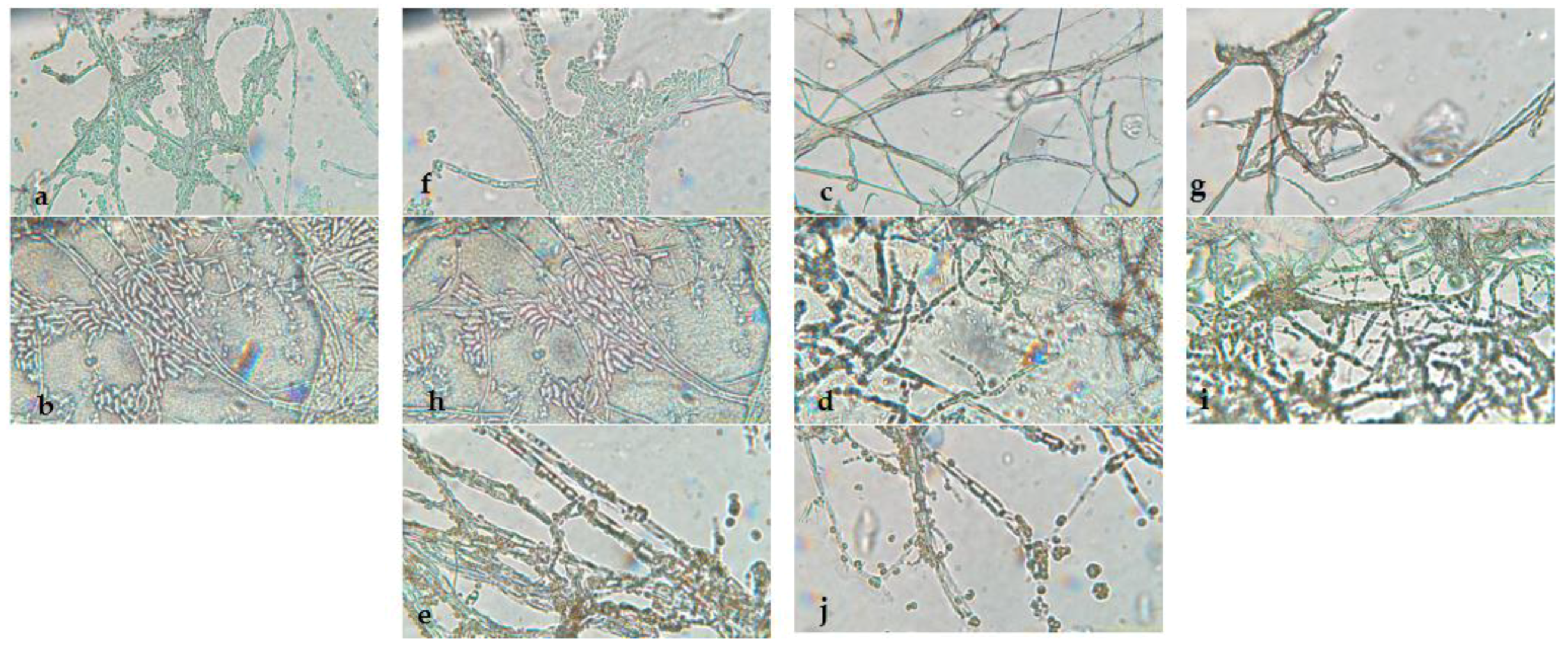
3.1. Morphological Identification
3.2. Molecular Identification Through DNA Sequencing
3.3. Fungal Activity Ex Vivo
3.4. In Vitro Antifungal Activity with Essential Oils
4. Discussion
4.1. Morphological Identification
4.2. Molecular Identification Through DNA Sequencing
4.3. Ex Vivo Fungal Activity
4.4. In Vitro Antifungal Activity with Essential Oils
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tang, Q.; Liu, D.; Zhang, J. Post-harvest control of fungal diseases in bananas using essential oils. J. Postharvest Biol. 2019, 45, 42–49. [Google Scholar] [CrossRef]
- Mata Anchundia, D.; Suatunce Cunuhay, P.; Poveda Morán, R. Análisis económico del banano orgánico y convencional en la provincia Los Ríos, Ecuador. Avances 2021, 23, 419–430. [Google Scholar]
- Borges, C.V.; Amorim, E.P.; Leonel, M.; Gomez Gomez, H.A.; Santos, T.P.R.d.; Ledo, C.A.d.S.; Belin, M.A.F.; Almeida, S.L.d.; Minatel, I.O.; Lima, G.P.P. Post-harvest physicochemical profile and bioactive compounds of 19 bananas and plantains genotypes. Bragantia 2018, 78, 284–296. [Google Scholar] [CrossRef]
- Vallejo-Rojas, V.; Rivera-Ferre, M.G.; Ravera, F. The agri-food system (re)configuration: The case study of an agroecological network in the Ecuadorian Andes. Agric. Hum. Values 2022, 39, 1301–1327. [Google Scholar] [CrossRef]
- Alvindia, D.G.; Natsuaki, K.T. Biocontrol of crown rot-causing Colletotrichum musae by Burkholderia sp. Crop Prot. 2008, 27, 953–957. [Google Scholar] [CrossRef]
- Ruiz Medina, M.D.; Ruales, J. Postharvest Alternatives in Banana Cultivation. Agronomy 2024, 14, 2109. [Google Scholar] [CrossRef]
- Aguilar-Anccota, R.; Arévalo-Quinde, C.G.; Morales-Pizarro, A.; Galecio-Julca, M. Hongos asociados a la necrosis de haces vasculares en el cultivo de banano orgánico: Síntomas, aislamiento e identificación, y alternativas de manejo integrado. Sci. Agropecu. 2021, 12, 249–256. [Google Scholar] [CrossRef]
- Capa Benítez, L.B.; Alaña Castillo, T.P.; Benítez Narváez, R.M. Importancia de la producción de banano orgánico. Caso: Provincia de El Oro, Ecuador. Rev. Univ. Y Soc. 2016, 8, 64–71. [Google Scholar]
- Agronomía, C. Tecnología Poscosecha. Agron. Costarric. 2005, 29, 207–209. [Google Scholar]
- Mari, M.; Torres, R.; Vanneste, J.L. Biological control of postharvest diseases: Opportunities and challenges. Front. Microbiol. 2014, 5, 1–17. [Google Scholar] [CrossRef]
- Villa-Martínez, A.; Pérez-Leal, R.; Morales-Morales, H.A.; Basurto-Sotelo, M.; Soto-Parra, J.M.; Martínez-Escudero, E. Situación actual en el control de Fusarium spp. y evaluación de la actividad antifúngica de extractos vegetales. Acta Agronómica 2015, 64, 194–205. [Google Scholar] [CrossRef]
- Goicoechea, N. To what extent are soil amendments useful to control Verticillium wilt? Pest Manag. Sci. 2009, 65, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Pabon Montoya, B.; Córdova Chávez, M.; Alban Alcivar, J.; Jaramillo Robles, A. Efectos antifúngicos de extractos botánicos sobre el crecimiento micelial de Colletotrichum sp. a nivel in vitro, causante de antracnosis en la fruta de aguacate. 593 Digit. Publ. CEIT 2024, 9, 869–879. [Google Scholar] [CrossRef]
- Alzate, D.; Mier, G.; Afanador, L.; Durango, D.; García, C. Evaluación de la fitotoxicidad y la actividad antifúngica contra Colletotrichum acutatum de los aceites esenciales de tomillo (Thymus vulgaris), limoncillo (Cymbopogon citratus), y sus componentes mayoritarios. Vitae 2009, 16, 116–125. [Google Scholar]
- Bandoni, A.L.; Retta, D.; Lira, P.M.D.L.; van Baren, C.M. ¿Son realmente útiles los aceites esenciales? Boletín Latinoam. Y Caribe Plantas Med. Y Aromáticas 2009, 8, 317–322. [Google Scholar]
- Oliva, M.D.L.M.; Lorello, I.M.; Baglio, C.; Posadaz, A.; Carezzano, M.E.; Paletti Rovey, M.F.; Huallpa, C.L.; Juliani, H.R. Chemical composition, antioxidant, and antimicrobial activities of rosemary (Salvia rosmarinus Spenn.) essential oils from Argentina. J. Med. Act. Plants 2023, 12, 38–52. [Google Scholar]
- Pilozo, G.; Villavicencio-Vásquez, M.; Chóez-Guaranda, I.; Murillo, D.V.; Pasaguay, C.D.; Reyes, C.T.; Maldonado-Estupinan, M.; Ruiz-Barzola, O.; Leon-Tamariz, F.; Manzano, P. Chemical, antioxidant, and antifungal analysis of oregano and thyme essential oils from Ecuador: Effect of thyme against Lasiodiplodia theobromae and its application in banana rot. Heliyon 2024, 10, e31443. [Google Scholar] [CrossRef] [PubMed]
- Flores-Villa, E.; Sáenz-Galindo, A.; Castañeda-Facio, A.O.; Narro-Céspedes, R.I. Romero (Rosmarinus officinalis L.): Su origen, importancia y generalidades de sus metabolitos secundarios. TIP Rev. Espec. En Cienc. Químico-Biológicas 2020, 7. [Google Scholar] [CrossRef]
- Farias, A.P.P.; Monteiro Odos, S.; da Silva, J.K.R.; Figueiredo, P.L.B.; Rodrigues, A.A.C.; Monteiro, I.N.; Maia, J.G.S. Chemical composition and biological activities of two chemotype-oils from Cinnamomum verum J. Presl growing in North Brazil. J. Food Sci. Technol. 2020, 57, 3176–3183. [Google Scholar] [CrossRef] [PubMed]
- Pinto, E.; Silva, C.; Costa, L. Eugenol as an antifungal agent: Mechanisms and applications. J. Appl. Microbiol. 2018, 124, 1089–1099. [Google Scholar] [CrossRef]
- Ruiz Medina, M.D.; Quimbita Yupangui, Y.; Ruales, J. Effect of a Protein–Polysaccharide Coating on the Physicochemical Properties of Banana (Musa paradisiaca) During Storage. Coatings 2025, 15, 812. [Google Scholar] [CrossRef]
- Abadias, M.; Teixidó, N.; Usall, J.; Viñas, I. Evaluation of alternative strategies to control postharvest blue mould of apple caused by Penicillium expansum. Int. J. Food Microbiol. 2008, 122, 25–31. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.; Saban, K. Review of essential oils as anti-fungal agents for plant fungal diseases. Ziraat Fakültesi Dergisi. 2019, 14, 294–301. Available online: https://dergipark.org.tr/en/pub/sduzfd/issue/50576/542012 (accessed on 8 May 2025).
- Zhang, W.; Li, B.; Lv, Y.; Wei, S.; Zhang, S.; Hu, Y. Synergistic effects of combined cinnamaldehyde and nonanal vapors against Aspergillus flavus. Int. J. Food Microbiol. 2023, 402, 110277. [Google Scholar] [CrossRef] [PubMed]
- Haro-González, J.N.; Castillo-Herrera, G.A.; Martínez-Velázquez, M.; Espinosa-Andrews, H. Clove Essential Oil (Syzygium aromaticum L. Myrtaceae): Extraction, Chemical Composition, Food Applications, and Essential Bioactivity for Human Health. Molecules 2021, 26, 6387. [Google Scholar] [CrossRef] [PubMed]
- Kintzios, S.E. Oregano. In Handbook of Herbs and Spices, 2nd ed.; Peter, K.V., Ed.; Woodhead Publishing: Cambridge, UK, 2012; pp. 417–436. [Google Scholar] [CrossRef]
- Nazzarro, F.; Fratianni, F.; Coppola, R.; De Feo, V. Essential Oils and Antifungal Activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef] [PubMed]
- Arcila-Lozano, C.C.; Loarca-Piña, G.; Lecona-Uribe, S.; González de Mejía, E. El orégano: Propiedades, composición y actividad biológica de sus componentes. Arch. Latinoam. Nutr. 2004, 54, 100–111. [Google Scholar] [PubMed]
- Prashar, A.; Locke, I.C.; Evans, C.S. Cytotoxicity of clove (Syzygium aromaticum) oil and its major components to human skin cells. Cell Prolif. 2006, 39, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Selles, S.M.A.; Kouidri, M.; Belhamiti, B.T.; Ait Amrane, A. Chemical composition, in-vitro antibacterial and antioxidant activities of Syzygium aromaticum essential oil. Food Meas. 2020, 14, 2352–2358. [Google Scholar] [CrossRef]
- Ruiz Medina, M.; Ávila, J.; Ruales, J. Diseño de un recubrimiento comestible bioactivo para aplicarlo en la frutilla (Fragaria vesca) como proceso de postcosecha. Rev. Iberoam. Tecnol. Postcosecha 2016, 17, 276–287. [Google Scholar]
- Aquino-Martínez, J.G.; Vázquez-García, L.M.; Reyes-Reyes, B.G. Biocontrol in vitro e in vivo de Fusarium oxysporum Schlecht. f. sp. dianthi (Prill. y Delacr.) Snyder y Hans. con Hongos Antagonistas Nativos de la Zona Florícola de Villa Guerrero, Estado de México. Rev. Mex. Fitopatol. 2008, 26, 127–137. [Google Scholar]
- Li, W.; Li, G.; Zhang, H. Postharvest disease of banana caused by Fusarium musae: A public health concern. J. Appl. Microbiol. 2021, 131, 828–837. [Google Scholar] [CrossRef]
- Barrera Necha, L.L.; García Barrera, L.J. Actividad antifúngica de aceites esenciales y sus compuestos sobre el crecimiento de Fusarium sp. aislado de papaya (Carica papaya). Rev. Científica UDO Agrícola 2008, 8, 33–41. [Google Scholar]
- Ultee, A.; Bennik, M.H.J.; Moezelaar, R. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 2002, 68, 1561–1568. [Google Scholar] [CrossRef] [PubMed]
- Pina-Vaz, C.; Gonçalves Rodrigues, A.; Pinto, E.; Costa-de-Oliveira, S.; Tavares, C.; Salgueiro, L.; Cavaleiro, C.; Gonçalves, M.J.; Martinez-de-Oliveira, J. Antifungal activity of Thymus oils and their major compounds. J. Eur. Acad. Dermatol. Venereol. 2004, 18, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Samarakoon, K.W.; Thong, P.H.; Jeewanthi, R.K.C. Evaluation of antifungal activity of cassia and holy basil essential oils against postharvest banana pathogens. Chem. Pap. 2020, 74, 3113–3121. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. Chemical components and pharmacological benefits of Basil (Ocimum basilicum): A review. Int. J. Food Prop. 2020, 23, 1961–1970. [Google Scholar] [CrossRef]
- Salazar, E.; Hernández, R.; Tapia, A.; Gómez-Alpízar, L. Identificación molecular del hongo Colletotrichum spp., aislado de banano (Musa spp.) de la altura en la zona de Turrialba y determinación de su sensibilidad a fungicidas poscosecha. Agron. Costarric. 2012, 36, 53–68. [Google Scholar] [CrossRef]
- Tortora, G.J.; Funke, B.R.; Case, C.L. Introducción a la Microbiología; Editorial Médica Panamericana: Madrid, Spain, 2007. [Google Scholar]
- Agu, K.; Awah, N.; Nnadozie, A. Isolation, identification, and pathogenicity of fungi associated with cocoyam (Colocasia esculenta) spoilage. Food Sci. Technol. 2016, 4, 103–106. [Google Scholar] [CrossRef]
- Suárez, L.; Rangel, A. Aislamiento de microorganismos para control biológico de Moniliophthora roreri. Acta Agronómica 2013, 62, 370–378. [Google Scholar]
- Orwa, P.; Mugambi, G.; Wekesa, V.; Mwirichia, R. Isolation of haloalkaliphilic fungi from Lake Magadi in Kenya. Heliyon 2020, 6, e02823. [Google Scholar] [CrossRef] [PubMed]
- Vilaplana, R.; Pazmiño, L.; Valencia-Chamorro, S. Control of anthracnose, caused by Colletotrichum musae, on postharvest organic banana by thyme oil. Postharvest Biol. Technol. 2018, 138, 56–63. [Google Scholar] [CrossRef]
- Morales, R.; Henríquez, G. Aislamiento e identificación del moho causante de antracnosis en musa paradisiaca l. (plátano) en cooperativa san carlos, el salvador y aislamiento de mohos y levaduras con capacidad antagonista. Crea Cienc. Rev. Científica 2021, 13, 84–94. [Google Scholar] [CrossRef]
- Funnell-Harris, D.; Prom, L. Isolation and characterization of grain mold fungi Cochliobolus and Alternaria spp. from sorghum using semiselective media and DNA sequence analyses. Can. J. Microbiol. 2013, 59, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Fernández, J.P.; Wang-Wong, A.; Muñoz-Fonseca, M. Microorganismos asociados a la enfermedad conocida como pudrición suave del fruto de banano (Musa sp.) y alternativas de control microbiológicas y químicas a nivel in vitro *. Agron. Costarric. 2022, 46, 61–76. [Google Scholar] [CrossRef]
- Ruiz Medina, M.D.; Ruales, J. Aceites esenciales como alternativa antifúngica para el control de diversas especies de hongos aislados de Musa paradisiaca: Parte I. Preprints 2025, 2025051213. [Google Scholar] [CrossRef]
- Johanna, S.V.; Natalia, C.G.; Ximena Carolina, P.M. Manual de Microbiología General: Principios Básicos de Laboratorio; Editorial Tadeo Lozano: Bogotá, Colombia, 2014. [Google Scholar]
- Espinel-Ingroff, A.; Montero, D.; Martin-Mazuelos, E. Long-term preservation of fungal isolates in commercially prepared cryogenic microbank vials. J. Clin. Microbiol. 2004, 42, 1257–1259. [Google Scholar] [CrossRef] [PubMed]
- Siller-Ruiz, M.; Hernández-Egido, S.; Sánchez-Juanes, F.; González-Buitrago, J.M.; Muñoz-Bellido, J.L. Métodos rápidos de identificación de bacterias y hongos. Enfermedades Infecc. Microbiol. Clínica 2017, 35, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Acurio Vásconez, R.D.; España Imbaquingo, C.K.; Acurio Vásconez, R.D.; España Imbaquingo, C.K. Aislamiento, caracterización y evaluación de Trichoderma spp. como promotor de crecimiento vegetal en pasturas de Raygrass (Lolium perenne) y trébol blanco (Trifolium repens). LA GRANJA Rev. Cienc. De La Vida 2017, 25, 53–61. [Google Scholar] [CrossRef]
- Carpio-Coba, C.F.; Noboa-Silva, V.F.; Salazar-Castañeda, E.P.; Lema-Saigua, E.R. Caracterización macroscópica y microscópica de cuatro especies forestales de la amazonia del sur de Ecuador. Polo Del Conoc. 2021, 6, 886–904. Available online: https://polodelconocimiento.com/ojs/index.php/es/article/view/2986 (accessed on 8 May 2025).[Green Version]
- Bellemain, E.; Carlsen, T.; Brochmann, C.; Coissac, E.; Taberlet, P.; Kauserud, H. ITS as an environmental DNA barcode for fungi: An in silico approach reveals potential PCR biases. BMC Microbiol. 2010, 10, 189. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, C.; Tarqui, M.; Huiza, P.; Quispe, A. Determinación de Secuencias de ADN en Bioedit. ResearchGate n.d. Available online: https://www.researchgate.net/publication/370895529_Determinacion_de_secuencias_de_ADN_en_Bioedit (accessed on 25 February 2025).
- Tamura, K.; Stecher, G.; Kumar, S. Molecular Evolutionary Genetics Analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhao, J.; Dai, X.; Li, X. Extracción y análisis de composiciones químicas de productos naturales y plantas. Separations 2023, 10, 598. [Google Scholar] [CrossRef]
- National Institute of Standards and Technology. Essential Oils: The Effects of Processing and Standards for Quality Control; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2011. [Google Scholar]
- Cavanagh, H.M.; Wilkinson, J.M. Biological activities of lavender essential oil. Phytother. Res. 2002, 16, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Baser, K.H.C.; Buchbauer, G. Handbook of Essential Oils: Science, Technology, and Applications; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Letseka, T.E.; Sepheka, N.J.; Dubery, I.A.; George, M.J. Bioprospecting of Essential Oil-Bearing Plants: Rapid Screening of Volatile Organic Compounds Using Headspace Bubble-in-Drop Single-Drop Microextraction for Gas Chromatography Analysis. Plants 2022, 11, 2749. [Google Scholar] [CrossRef] [PubMed]
- Chacón-Cascante, A.; Crespi, J.M. Historical overview of the European Union banana import policy. Agron. Costarric. 2006, 30, 111–127. [Google Scholar]
- Aguilar-Anccota, R.; Apaza-Apaza, S.; Maldonado, E.; Calle-Cheje, Y.; Rafael-Rutte, R.; Montalvo, K. Control in vitro e in vivo de Thielavipsis paradoxa y Collettrichum musae cn biofungicidas en frutos de banano orgánico. Manglar 2024, 21, 57–63. [Google Scholar] [CrossRef]
- Magri, A.; Curci, M.; Battaglia, V.; Fiorentino, A.; Petriccione, M. Essential Oils in Postharvest Treatment against Microbial Spoilage of the Rosaceae Family Fruits. AppliedChem 2023, 3, 196–216. [Google Scholar] [CrossRef]
- Hassan, O.; Jong, Y.; Taehyun, C.; Jun, S. Molecular and morphological characterization of Colletotrichum species in the Colletotrichum gleosporoides. Plant Dis. 2018, 102, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Ranasinghe, L.; Jayawardena, B.; Abeywickrama, K. Fungicidal activity of essential oils of Cinnamomum zeylanicum (L.) and Syzygium aromaticum (L.) Merr et LM Perry Against Crown Rot Anthracnose pathogens Isol. banana. Lett. Appl. Microbiol. 2002, 35, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Jabnoun-Khiareddine, H.; Mahjoub, M.; Daami-Remadi, M. Morphological Variability Within and Among Verticillium Species Collected in Tunisia. Tunis. J. Plant Prot. 2010, 5, 19–38. [Google Scholar]
- Nuangmek, W.; Kumla, J.; Khuna, S.; Lumyong, S.; Suwannarach, N. Identificación y caracterización de especies de Fusarium causantes de la pudrición de la sandía en el norte de Tailandia. Plants 2023, 12, 956. [Google Scholar] [CrossRef]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual; Blackwell Publishing: Ames, IA, USA, 2006. [Google Scholar]
- Yang, R.; Li, Y.; Zhao, H.; Sun, X.; Chen, W.; Li, P.; Li, X.; Wu, C.; Ma, M.; Gong, G. Identificación y caracterización de especies de Colletotrichum asociadas con el maíz en Sichuan, China. J. Fungi 2024, 10, 799. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, A.; Sanfuentes, E.; Duran, A.; Valenzuela, S. Cancro resinoso del pino: ¿una amenaza potencial para las plantaciones de Pinus radiata en Chile? Gayana Botánica 2016, 73, 369–380. [Google Scholar] [CrossRef]
- Sherif, M.; El-Debaiky, S.A.; El-Samawaty, A.R.M.; Baka, Z.A.M.; Elsharkawy, M.M.; El-Bebany, A.F. The Role of Mycotoxins in Interactions between Fusarium graminearum and F. verticillioides Growing in Saprophytic Cultures and Co-Infecting Maize Plants. Toxins 2023, 15, 575. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, Y.-D.; Zhang, D.-D.; Zhang, Y.-Y.; Wang, D.; Song, J.; Zhang, J.; Li, R.; Kong, Z.-Q.; Klosterman, S.J.; et al. Características biológicas de las cepas MAT1-1 y MAT1-2 de Verticillium dahliae. Int. J. Mol. Sci. 2021, 22, 7148. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, G.; Prasannath, K. Recent Advances in Molecular Diagnostics of Fungal Plant Pathogens: A Mini Review. Front. Cell. Infect. Microbiol. 2021, 10, 600234. [Google Scholar] [CrossRef] [PubMed]
- Rojo-Báez, I.; Álvarez-Rodríguez, B.; García-Estrada, R.; León-Félix, J.; Sañudo Barajas, J.A.; Allende, R. Situación actual de Colletotrichums spp. en México: Taxonomía, caracterización, patogénesis y control. Rev. Mex. De Fitopatol. Mex. J. Phytopathol. 2017, 35, 549–570. [Google Scholar] [CrossRef]
- Ekwomadu, T.I.; Mwanza, M. Hongos patógenos de Fusarium, identificación, efectos adversos, manejo de enfermedades y seguridad alimentaria mundial: Una revisión de las últimas investigaciones. Agricultura 2023, 13, 1810. [Google Scholar] [CrossRef]
- Perrier, X. Combinando enfoques biológicos para esclarecer la evolución de los plátanos comestibles. Ethnobot. Res. Appl. 2009, 7, 199–216. Available online: https://ethnobotanyjournal.org/index.php/era/article/view/362 (accessed on 8 May 2025). [CrossRef]
- Sharma, R.R.; Singh, D.; Singh, R. Biological control of postharvest diseases of fruits and vegetables by microbial antagonists: A review. Biol. Control 2009, 50, 205–221. [Google Scholar] [CrossRef]
- Ramudingana, P.; Makhado, N.; Kamutando, C.N.; Thantsha, M.S.; Mamphogoro, T.P. Agentes de biocontrol fúngico en el manejo de pérdidas poscosecha de productos frescos: Una revisión exhaustiva. J. Fungi 2025, 11, 82. [Google Scholar] [CrossRef] [PubMed]
- RuizMedina, M.; Ruales, J. Aceites esenciales como alternativa antifúngica para el control de diversas especies de hongos aislados de Musa paradisiaca: Parte II. Preprints 2025, 2025011894. [Google Scholar] [CrossRef]









| Parameter | Details |
|---|---|
| Fungal species | Fusarium pseudocircinatum, Colletotrichum tengchongense, Fusarium napiforme, Fusarium verticilloides, Verticillium dahliae |
| Source of fungal isolates | Infected banana peel samples from Ecuadorian Musa paradisiaca |
| Inoculum preparation | 106 conidia/mL for each fungal species |
| Medium used | Potato Dextrose Agar (PDA) supplemented with 0.05% chloramphenicol to prevent bacterial growth |
| Essential oils tested | Oregano (Origanum vulgare), Rosemary (Salvia rosmarinus), Clove (Syzygium aromaticum), Thyme (Thymus vulgaris), Cinnamon (Cinnamomum verum), Basil (Ocimum basilicum) |
| Essential oil concentrations | 200, 400, 600, 800, 1000 ppm |
| Inoculation method | Wound method: 100 µL of adjusted fungal inoculum applied to the banana fruit |
| Incubation conditions | At 25 ± 2 °C and 75 ± 5% relative humidity, 24 h monitoring intervals for in vitro assays; at approximately 13 ± 1 °C and 92 ± 3% relative humidity for ex vivo assays |
| Control treatment | PDA medium without essential oils (negative control) |
| Duration of observation | 6 weeks for ex vivo assays, 20 wounds per fungal species, and 24 h for in vitro assays |
| Organism | Fragment | NCBI | % Identity |
|---|---|---|---|
| Fusarium pseudocircinatum | ITS | MG838060.1 | 99.54% |
| Fusarium napiforme | ITS | ON204349.1 | 98.71% |
| Colletotrichum tengchongense | ITS | OL842169.1 | 99.66% |
| Fusarium verticilloides | ITS | PQ416097.1 | 99.01% |
| Verticillium dahliae | ITS | NR 126124.1 | 99.38% |
| MG838060.1 | H1 | ON204349.1 | H2 | OL842169.1 | H3 | PQ416097.1 | H4 | NR_126124.1 | H5 | |
| MG838060.1 | ID | 0.002 | 2.439 | 2.704 | 2.949 | 2.947 | 2.492 | 2.511 | 2.293 | 2.294 |
| H1 | 0.005 | ID | 2.439 | 2.704 | 2.949 | 2.947 | 2.492 | 2.511 | 2.293 | 2.294 |
| ON204349.1 | 2.752 | 2.752 | ID | 2.251 | 2.401 | 2.366 | 2.298 | 2.308 | 2.345 | 2.333 |
| H2 | 5.106 | 5.106 | 2.524 | ID | 2.695 | 2.676 | 2.609 | 2.528 | 2.306 | 2.295 |
| OL842169.1 | 5.62 | 5.62 | 3.998 | 4.639 | ID | 0.002 | 2.124 | 1.943 | 2.468 | 2.575 |
| H3 | 5.656 | 5.656 | 4.075 | 4.671 | 0.003 | ID | 2.124 | 1.943 | 2.468 | 2.575 |
| PQ416097.1 | 4.511 | 4.511 | 3.786 | 4.445 | 2.397 | 2.397 | ID | 0.005 | 2.3 | 2.302 |
| H4 | 4.393 | 4.393 | 3.778 | 3.829 | 2.401 | 2.401 | 0.01 | ID | 2.3 | 2.302 |
| NR_126124.1 | 3.52 | 3.52 | 3.871 | 4.226 | 5.121 | 5.121 | 3.683 | 3.683 | ID | 0.004 |
| H5 | 3.499 | 3.499 | 3.902 | 4.358 | 5.193 | 5.193 | 3.66 | 3.66 | 0.006 | ID |
| Essential Oil | Fungus | Concentration [ppm] | ||||
|---|---|---|---|---|---|---|
| 200 | 400 | 600 | 800 | 1000 | ||
| Cinnamon | Fusarium pseudocircinatum | + | + | − | − | − |
| Colletotrichum tengchongense | − | − | − | − | − | |
| Fusarium verticilloides | + | − | − | − | − | |
| Fusarium napiforme | − | − | − | − | − | |
| Verticillium dahliae | − | − | − | − | − | |
| Clove | Fusarium pseudocircinatum | + | + | + | + | + |
| Colletotrichum tengchongense | + | + | − | − | − | |
| Fusarium verticilloides | + | + | − | − | − | |
| Fusarium napiforme | − | − | − | − | − | |
| Verticillium dahliae | + | − | − | − | − | |
| Basil | Fusarium pseudocircinatum | + | + | + | + | + |
| Colletotrichum tengchongense | + | + | + | + | + | |
| Fusarium verticilloides | + | + | + | + | + | |
| Fusarium napiforme | + | + | + | + | − | |
| Verticillium dahliae | + | + | + | + | + | |
| Oregano | Fusarium pseudocircinatum | + | − | − | − | − |
| Colletotrichum tengchongense | + | + | − | − | − | |
| Fusarium verticilloides | + | − | − | − | − | |
| Fusarium napiforme | − | − | − | − | − | |
| Verticillium dahliae | − | − | − | − | − | |
| Rosemary | Fusarium pseudocircinatum | + | + | + | + | + |
| Colletotrichum tengchongense | + | + | + | + | + | |
| Fusarium verticilloides | + | + | + | + | + | |
| Fusarium napiforme | + | + | + | − | − | |
| Verticillium dahliae | + | + | + | + | + | |
| Thyme | Fusarium pseudocircinatum | + | + | + | + | − |
| Colletotrichum tengchongense | + | + | − | − | − | |
| Fusarium verticilloides | + | + | − | − | − | |
| Fusarium napiforme | − | − | − | − | − | |
| Verticillium dahlia | + | − | − | − | − | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz Medina, M.D.; Ruales, J. Essential Oils as an Antifungal Alternative to Control Several Species of Fungi Isolated from Musa paradisiaca: Part III. Microorganisms 2025, 13, 1663. https://doi.org/10.3390/microorganisms13071663
Ruiz Medina MD, Ruales J. Essential Oils as an Antifungal Alternative to Control Several Species of Fungi Isolated from Musa paradisiaca: Part III. Microorganisms. 2025; 13(7):1663. https://doi.org/10.3390/microorganisms13071663
Chicago/Turabian StyleRuiz Medina, Maritza D., and Jenny Ruales. 2025. "Essential Oils as an Antifungal Alternative to Control Several Species of Fungi Isolated from Musa paradisiaca: Part III" Microorganisms 13, no. 7: 1663. https://doi.org/10.3390/microorganisms13071663
APA StyleRuiz Medina, M. D., & Ruales, J. (2025). Essential Oils as an Antifungal Alternative to Control Several Species of Fungi Isolated from Musa paradisiaca: Part III. Microorganisms, 13(7), 1663. https://doi.org/10.3390/microorganisms13071663










