Abstract
Verticillium wilt, caused by Verticillium dahliae, poses a significant threat to olive trees (Olea europaea L.). The isolation of endophytic Streptomyces strains from olive roots has led to the discovery of several strains showing strong antifungal activity against V. dahliae, as demonstrated through in vitro and small-scale soil experiments. Molecular analyses confirmed that strain OE54 belongs to Streptomyces iranensis. The main antifungal compound identified in this strain was rapamycin. Rapamycin displayed potent antifungal effects, notably inhibiting conidiospore germination (IC50 = 87.36 μg/mL) and the hyphal growth of V. dahliae, with a minimum inhibitory concentration (MIC50) of 3.91 ng/mL. Additionally, a second rapamycin-producing strain, OE57T, was isolated. Phenotypic and genotypic analyses indicated that OE57T represents a new species, which is proposed to be named Streptomyces lacaronensis sp. nov., with OE57T designated as the type strain (=DSM 118741T; CECT 31164T). The discovery of two endophytic rapamycin-producing Streptomyces strains residing within olive roots is especially notable, given the rarity of rapamycin production among microorganisms. These findings highlight the potential of rapamycin-producing Streptomyces strains in developing biofertilizers to manage V. dahliae and reduce the impact of Verticillium wilt on olive trees and other crops.
1. Introduction
Verticillium is a genus of ascomycete fungi responsible for vascular wilt diseases, collectively termed Verticillium wilt, which affect a diverse range of plant hosts [1]. Verticillium wilt is widely regarded as one of the most devastating fungal diseases globally, posing a significant threat to numerous economically important crops, including alfalfa, almond, pistachio, peach [2,3], cotton, lettuce, ornamental plants, potato, strawberry [1], hops [4], and olive [3,5], among others. While different Verticillium species contribute to the disease, Verticillium dahliae is the most prevalent and virulent, largely due to its broad host range and extensive geographic distribution.
Olea europaea L. (olive) is one of the earliest domesticated and cultivated tree species, possessing substantial economic, historical, and social significance across many Mediterranean Basin countries. It serves primarily as the principal source of olive oil, a fundamental component of the Mediterranean diet [5]. However, in recent years, Verticillium wilt caused by V. dahliae has emerged as a severe threat to olive cultivation, with no effective control treatments currently available. This challenge is exacerbated by the pathogen’s ability to establish itself within plant tissues, rendering curative treatments ineffective. Given that V. dahliae is a soil-borne fungal pathogen that invades plants through their root systems, considerable attention has been directed toward developing biocontrol agents (BCAs) capable of colonizing the rhizosphere, or root tissues, as endophytic strains, thereby forming a protective barrier against infection [6]. Numerous studies have demonstrated the potential of various fungal BCAs in reducing the soil inoculum density and mitigating the disease severity, including Fusarium oxysporum [7,8], Mucor sp., Rhizopus sp., and Phoma sp. [9], as well as Trichoderma asperellum [10]. Additionally, entomopathogenic fungi such as Beauveria bassiana and Metarhizium brunneum have been investigated for their potential biocontrol efficacy [11]. Research has also explored the effectiveness of bacterial BCAs in managing V. dahliae infection, including various Pseudomonas strains [12,13] and members of the Bacillales order, such as Paenibacillus alvei K165 [14] and Bacillus velezensis [15].
Recent research underscores the potential efficacy of Streptomyces species as new and promising BCAs against Verticillium wilt in olive trees [16,17]. Members of the Streptomyces genus exhibit a range of distinctive properties that make them strong candidates for the development of BCAs targeting soil-borne fungal pathogens [6]. These characteristics include the following: (1) Streptomyces species are predominantly soil saprophytes and are ubiquitously distributed in diverse environments worldwide, including soils and sediments, where they are found in considerable abundance [18]. Some studies indicate that Streptomyces comprises approximately 0.06% of the bacterial abundance in soil, as determined from the 16S rRNA gene libraries [19]. (2) These species can effectively colonize the root environments of diverse plants, with a significant presence in both the rhizosphere [20] and the endosphere [21,22,23]. This colonization contributes to plant health and promotes growth, reinforcing their role in biological control strategies. (3) Streptomyces is the foremost genus in terms of bioactive secondary metabolite production, including numerous antibiotics and antifungal (AF) compounds [24]. Indeed, more than 50% of all clinically relevant antibiotics are derived from Streptomyces species [25]. (4) The antimicrobial properties of Streptomyces species extend beyond antibiotic production and are supported by additional mechanisms, such as the secretion of extracellular hydrolytic enzymes [26], the emission of volatile organic compounds [27], and the release of proteinaceous umbrella toxins [28]. (5) With over 755 validly named species (https://lpsn.dsmz.de/genus/streptomyces, accessed on 1 June 2025), Streptomyces constitutes one of the most taxonomically diverse bacterial genera. Ongoing discoveries of new species continue to expand their antimicrobial and AF capabilities, further reinforcing their importance in biological control applications.
This study describes the isolation and characterization of endophytic Streptomyces strains from the internal root tissues of olive plants, followed by their selection based on the potent AF activity against Verticillium dahliae. The two most effective isolates were identified as rapamycin producers, with one representing a new species within the Streptomyces genus. Ultimately, this research seeks to improve the health of vegetatively propagated olive plants in nursery settings by promoting root colonization by these beneficial microorganisms or developing biofertilizers to control this devastating disease.
2. Materials and Methods
2.1. Isolation of Culturable Endophytic Streptomycetes from Olive Roots
Root samples from three adult olive trees were collected in a plot belonging to the company Río Lacarón S.L. (La Garrovilla, Spain), at 215 m above sea level (38°55′08.1” N 6°31′27.7” W). Root samples were placed in sterile plastic bags and maintained at 4 °C until processing. Endophytic streptomycetes were isolated from the interior of root tissue samples. Roots were washed in sterile phosphate buffer (PBS) and sonicated (160 W; 2 min) to dislodge soil and organic matter from the sample surface. After drying at room temperature (RT), roots were cut into 2.0 cm-long fragments. Root fragments were surface-sterilized by immersing in 20 mL of Tween 20 (0.1%) for 30 s, followed by sodium hypochlorite (1%) for 6 min, and then Na2S2O3 (2.5%) for 10 min to remove the residual chlorine. Next, samples were washed three times with sterile water, submerged in 70% (v/v) ethanol for 6 min, followed by being subjected to three washes with sterile water and air-dried in a laminar flow hood (Telstar AV-100; Telstar, Terrassa, Spain). To confirm the effectiveness of the surface disinfection process, 0.2 mL of liquid from the final washing step was spread onto International Streptomyces Project 2 (ISP 2) [29] agar media and incubated at 28 °C. The isolation of endophytic streptomycetes was carried out from 5 g of previously disinfected root material. This material was cut into small fragments (using a scalpel under sterile conditions) that were later frozen in liquid nitrogen and ground into a fine powder using a mortar and pestle. Then, 20 mL of a solution containing yeast extract (6%, w/v) and sodium dodecyl sulfate (0.5%; w/v) were added to the samples and incubated at 40 °C for 15–30 min. Ten-fold serial dilutions of the samples were plated on starch-casein agar (SCA) [30]) and ISP 2 agar media, containing pimaricin (100 μg/mL) and nalidixic acid (50 μg/mL), to avoid fungi and Gram-negative bacterial growth, respectively. Plates were incubated at 28 °C for 3 to 7 days. Different isolates were selected based on their morphological and cultural characteristics, such as colony properties, presence/absence of aerial mycelia, spore mass color, distinctive reverse colony color, and production of diffusible pigments. The isolates were routinely cultivated and maintained the isolates on MEY (Maltose Yeast Extract) medium [31] at 4 °C. Spore-producing isolates were preserved as spore suspensions at −20 °C in glycerol (40%; w/v).
2.2. In Vitro Selection of Isolates Based on Antifungal Activity in Plate Assays
The AF activity of all isolated Streptomyces strains was checked using an in vitro plate assay [22]. Briefly, isolates were inoculated onto potato dextrose agar (PDA) plates (Sigma-Aldrich, Madrid, Spain) within a 1.0 cm2 area, with four isolates per plate, positioned 1 cm from the plate’s edge. A 0.5 cm agar plug containing the V. dahliae V937I strain [32] was placed at the center of each plate. The plates were incubated at 25 °C for up to 12 days, after which the growth inhibition zones were measured. To quantify the AF activity of the most effective isolates, they were further tested individually on MEY agar plates, where bacterial colonies were arranged in a circular pattern 1 cm from the plate’s edge. A V. dahliae-containing agar plug was placed at the center, and plates were incubated at 25 °C for up to 10 days. The inhibition index (I) was calculated using the formula: I index (%) = [(Rc − R)/Rc] × 100, where R represents the radius of the fungal colony in the presence of the bacterial isolate, and Rc is the maximum radius of the fungal colony in the control condition. Each assay was performed in triplicate for all tested strains.
2.3. Analysis of Antifungal Activity in Small-Scale Soil Experiments
The AF activity in soil of selected isolates was evaluated using small-scale soil tests, following the methodology described by Calvo-Peña et al. (2023) [16]. Soil samples were collected from a depth of 20–30 cm after removing the uppermost 5 cm of surface soil. Five subsamples were taken, combined, and homogenized for analysis. Physicochemical properties of the soil were determined at the Laboratory of Instrumental Techniques (University of León, Spain). Parameters analyzed included soil texture and mechanical composition, organic carbon content, pH, electrical conductivity, nitrogen levels, available phosphorus, exchangeable potassium, micronutrients (Fe, Cu, Mn, and Zn), magnesium, available boron, and total and active carbonates. These analyses were conducted following standard procedures as described by Calvo-Peña et al. (2023) [16].
2.4. In Vitro Confrontation of Endophytic Streptomyces Strains
To check potential antagonistic interactions between endophytic Streptomyces strains, pairwise confrontation assays were conducted following the methodology initially described by Schrey et al. (2012) [33], with minor modifications as detailed by Calvo-Peña et al. (2023) [16].
2.5. Molecular Identification of Strains and Genome Sequencing
Strains exhibiting the highest AF activity were identified at the genus level through partial sequencing of the 16S rRNA gene. Genomic DNA was extracted following the protocol described by Hopwood et al. (1985) [34]. The 16S rRNA genes were amplified using the oligonucleotide primers 27F and 1492R [35], and the resulting sequences were compared to those of type strains available in the EzTaxon-e database [36]. Sequence alignments were performed using MEGA v11.0 (http://www.megasoftware.net/), and evolutionary distances were calculated using the Kimura two-parameter (K2P) model for nucleotide sequences [37].
For putative species-level identification, a multilocus sequence analysis (MLSA) was conducted using five housekeeping genes: atpD (ATP synthase F1, β-subunit), gyrB (DNA gyrase B subunit), recA (recombinase A), rpoB (RNA polymerase, β-subunit), and trpB (tryptophan synthase, β-subunit) [38]. Amplification of these genes was performed using primers and conditions described by Guo et al. (2008) [39] and Rong et al. (2009) [40]. The GenBank accession numbers for the housekeeping gene sequences are listed in Table 1.

Table 1.
GenBank accession numbers of DNA sequences corresponding to partially sequenced genes used in the MLSA analysis of the Streptomyces sp. OE54 and OE57T strains.
A phylogenetic tree was constructed based on the concatenation of the five housekeeping genes. Sequences were manually trimmed to uniform positions before alignment using MEGA v11.0, incorporating sequences from type strains obtained from the ARS Microbial Genomic Sequence Database (https://blogs.cornell.edu/buckley/streptomyces-sequence-database/, accessed on 20 January 2025). Phylogenetic analysis was performed using the maximum likelihood method with the Kimura two-parameter model (Kimura, 1980 [37]). MLSA evolutionary distances were calculated using MEGA v11.0 based on K2P distance. Strains exhibiting an MLSA evolutionary distance of ≤0.007 were considered conspecific, following the empirical guidelines established by Rong and Huang (2012) [38].
The genomes of strains OE54 and OE57T were sequenced using next-generation sequencing (NGS) technologies, employing both the PacBio Sequel II system and the Illumina platform, as provided by Macrogen Inc. (Seoul, Republic of Korea). De novo genome assembly was performed using Canu v2.2 [41], and assembly quality was assessed with QUAST v5.3.0 [42]. Draft genome annotation was conducted using the RAST-SEED webserver (https://rast.nmpdr.org/) [43]. The genome sequences of strains OE54 and OE57T have been deposited in the GenBank database under the accession numbers JBLHDJ000000000 and JBLHDK000000000, respectively. The associated BioProject accession number is PRJNA1185486.
2.6. Cultural and Growth Properties
The cultural characteristics of the strains were assessed on various agar media, including ISP 1 (DSMZ 1764), ISP 2 (DSMZ 987), ISP 3 (DSMZ 609), ISP 4 (DSMZ 547), ISP 5 (DSMZ 993), ISP 6 (DSMZ 1269), ISP 7 (DSMZ 1619) [29], nutrient agar (DSMZ 1) (Scharlab S.L., Sentmenat, Spain), Bennett’s agar (DSMZ 548) (HiMedia Laboratories GmbH, Modautal, Germany), and trypticase soy agar (TSA) (DSMZ 535) (Scharlab S.L.). Plates were incubated at 28 °C for seven days. Growth tolerance was evaluated across a range of temperatures (4 °C to 45 °C) and pH values (5.0 to 12.0) using DSMZ 65 medium (glucose 4 g/L; yest extract, 4 g/L; malt extract, 10 g/L; CaCO3, 2 g/L; agar, 20 g/L; pH 7.2). All tests were conducted in duplicate with bacterial suspensions standardized to 5 on the McFarland scale. The coloration of aerial and substrate mycelium, as well as the production of diffusible pigments, was recorded and compared against standard color charts. The close phylogenomic neighbors, Streptomyces rapamycinicus DSM 41530T and Streptomyces iranensis DSM 41954T, were included in this study and obtained from the Leibniz Institute DSMZ—German Collection of Microorganisms and Cell Cultures.
2.7. Phenotypic and Chemotaxonomic Properties
Freeze-dried biomass from a 7-day old culture of strain OE57T and its phylogenomic neighbor, Streptomyces rapamycinicus DSM 41530T, prepared in ISP 2 medium with shaking at 120 rpm, was used for chemotaxonomic analysis. Standard chromatographic procedures were applied to determine isomeric forms of diaminopimelic acid (A2pm) [44], whole cell sugars [45], and the polar lipid profile of strain OE57T [46,47]. Cellular fatty acids and isoprenoid quinones of the strain were extracted from wet biomass prepared under the same growth conditions as mentioned above. Cellular fatty acids were analyzed after conversion into fatty acid methyl esters by gas chromatography (GC) using an Agilent 6890N equipment (Agilent Technologies, Santa Clara, CA, USA) as described by Sasser [48]. The fatty acid pattern was confirmed via gas chromatography–mass spectrometry (GC-MS) using an Agilent GC–MS 7000D instrument, as described in Vieira et al. (2021) [49]. The position of double bonds was determined by a derivatization with dimethyl sulfide and a further GC-MS measurement [50]. Menaquinone extracts were analyzed by high-performance liquid chromatography (HPLC), and identified with both a diode-array detector (DAD) and high-resolution MS [51]. The biochemical and enzymatic properties of the OE54 and OE57T strains and their phylogenomic relatives (S. iranensis DSM 41954T and S. rapamycinicus DSM 41530T) were characterized using API-ZYM and API 20NE test strips (bioMérieux, Lyon, France). For API 20NE, inoculation of the cupules was performed at 28 °C with a bacterial suspension standardized to a turbidity of 5 on the McFarland scale, and results were recorded at 24 and 48 h. API-ZYM assays were conducted at 28 °C instead of the standard 37 °C, with an incubation period of 24 h.
2.8. Phylogeny and Comparative Genomic Analyses
For preliminary taxonomic identification at the genus level, partial 16S rRNA gene sequences were analyzed as described above. Complete 16S rRNA gene sequences (average length 1485 bp), obtained from whole-genome sequencing, were used for more refined phylogenetic analyses. Pairwise 16S rRNA gene sequence similarities between the strains and their closest phylogenetic relatives were estimated using the parameters described by Meier-Kolthoff et al. (2013) [52], as implemented in the Genome-to-Genome Distance Calculator (GGDC) 2.1 (http://ggdc.dsmz.de) [53]. Reference strain sequences were retrieved from the EzBioCloud database (https://www.ezbiocloud.net/) [54]. Phylogenetic relationships were inferred using maximum-likelihood (ML) trees based on both 16S rRNA gene and whole-genome sequences. These analyses were conducted using the Type (Strain) Genome Server (TYGS) v1.0 (https://tygs.dsmz.de/), a bioinformatics tool for genome-based taxonomic classification [55]. The TYGS platform utilizes an extensive, continuously updated genome database containing taxonomic and nomenclatural information for all available type strains. This system enables full-genome phylogenetic comparisons by aligning the genome sequence of the strain of interest with those of type strains, facilitating taxonomic placement. Genomic relatedness between the strains and their closest phylogenomic relatives was assessed using digital DNA–DNA hybridization (dDDH) values calculated with GGDC 2.1 [56] and differences in genomic G + C content.
2.9. In Silico Screening for Secondary Metabolites
The presence of putative biosynthetic gene clusters (BGCs) in the genome sequences of the strains and their closest phylogenomic relatives was analyzed using the antiSMASH web tool v7.0 [57]. antiSMASH predicts secondary metabolite BGCs by identifying relevant genes and grouping them into clusters based on sequence similarity and genomic proximity. The tool employs domain recognition through HMMER to detect key enzyme domains, and the identified clusters are compared against multiple databases to predict the potential metabolites they may encode [58]. The analysis was performed using the relaxed strictness setting with all additional features enabled, including ClusterBLAST (https://docs.antismash.secondarymetabolites.org/modules/clusterblast/, accessed on 15 December 2024)and MIBiG cluster comparisons (https://mibig.secondarymetabolites.org/, accesed on 15 December 2024). For clusters of particular interest, manual curation and further assessments were conducted using BLAST (v2.16.0. https://blast.ncbi.nlm.nih.gov/Blast.cgi, accessed on 15 December 2024). Genes of unknown function were analyzed with InterProScan (version 5.72-103.0; https://ebi.ac.uk/interpro/about/interproscan; accessed on 15 December 2024) to predict their potential roles within the clusters. Additionally, CAGECAT (https://cagecat.bioinformatics.nl; accessed on 15 December 2024) [59] and BiG-SCAPE v1.1.9 (https://git.wageningenur.nl/medema-group/BiG-SCAPE; accessed on 15 December 2024) [60] were used to assess similarities among the identified clusters.
2.10. Fermentation, Culture Extract Preparation, and HPLC Analysis of Antifungal Activity
To analyze the production of AF compounds in liquid media, crude extracts of strains OE54 and OE57T grown in ISP 2 medium were prepared following the method described by Das et al. (2018) [61]. Briefly, the cell-free culture supernatant was vigorously mixed with ethyl acetate in a 1:1 (v/v) ratio for 30 min, followed by separation of the organic layer. The ethyl acetate extract was evaporated under vacuum using a CentriVap concentrator (Labconco, Kansas City, MO, USA), and the residue was dissolved in 80% methanol to a final concentration of 1 mg/mL. To test AF activity, 60 μL of the crude extract were used following the AF bioassay described above. The remaining extract was filtered through Corning® Costar® Spin-X® centrifuge tube filters (0.45 μm pore size; Merck KGaA, Darmstadt, Germany) and stored at −20 °C until further analysis. HPLC analysis of the crude extracts was performed using an Agilent 1200 Series Gradient HPLC System (Agilent Technologies), following the chromatographic method described by Awla et al. (2020) [62]. The system was equipped with a quaternary pump delivery system (G1311A), a preparative autosampler (G1329A), a diode array multi-wavelength detector (G7115A), and an analytical fraction collector (G1364F) with an Autosampler Thermostat (G1330B). A 10 μL sample was injected and resolved using an analytical Lichospher RP18 column (40 × 250 mm; 5 μm) (Teknokroma, San Cugat del Vallés, Spain).
2.11. Purification of Rapamycin and Epoxinnamide by Vacuum Flash Chromatography (VFC)
Strain OE54 was cultivated in 500 mL indented Erlenmeyer flasks containing 125 mL of ISP 2 medium. Each flask was inoculated with 10 agar plugs (0.5 cm diameter) of the bacterial strain, which had been previously grown on SCA plates until sporulation was achieved. Cultures were incubated at 30 °C with agitation at 150 rpm for 72 h. The resulting fermentation broth (6.5 L) was mixed with the adsorption resin Amberlite XAD-1180 (Dupont, Mississauga, ON, Canada) and filtered using Radifil RW50 as a filtration aid (Agrovin, Alcázar de San Juan, Spain). The exhausted broth was discarded, and the resin–mycelium mixture was extracted with 3 L of EtOAc/MeOH (3:1). Following filtration and solvent evaporation under vacuum, 2.90 g of crude extract was obtained. The crude extract was analyzed by LC-MS and tested for AF activity using a plate bioassay. A 2 mg aliquot of the extract was dissolved in 400 μL of MeOH and analyzed using a 1290 Infinity II HPLC system (Agilent) coupled to an Agilent 6230 time-of-flight LC/MS (LC/TOF) mass spectrometer. Chromatographic separation was performed on an Agilent Zorbax Eclipse Plus C18 RRHD column (2.1 × 50 mm, 1.8 μm particle size) with a gradient system of MeOH/H2O (0.1% formic acid), increasing from 20% to 100% MeOH over 8 min. UV detection was performed at 220 nm with a flow rate of 0.6 mL/min. Upon confirming that the crude extract exhibited AF activity, it was subjected to VFC using silica gel (40–60 μm, 60 Å) (Thermo Fisher Scientific Inc., Waltham, MA, USA) as the stationary phase and eluted with a stepwise gradient of hexane/EtOAc/MeOH. The obtained fractions were analyzed by LC-MS, as described above, and tested for AF activity. Those active fractions were combined and further purified by C18 reversed-phase column chromatography using MeOH/H2O (9:1). Some active fractions were pooled and subjected to an additional round of VFC using a stepwise gradient of dichloromethane/MeOH. This process yielded 12 fractions (F1′–F12′), each of which was tested again for AF activity.
2.12. Structural Elucidation of Rapamycin and Epoxinnamide
The purified compound was analyzed by nuclear magnetic resonance (NMR) for structural elucidation. The molecular formula of the major compound detected in the active fractions was determined using high-resolution electrospray ionization mass spectrometry (HRESIMS) and Nuclear Magnetic Resonance (NMR) spectroscopy. Structural elucidation was conducted through extensive NMR analyses, including 1H NMR, 13C NMR, 1H-1H COSY, gHSQC, and gHMBC experiments. 1H and 13C NMR spectra were recorded on a Varian Mercury 400 spectrometer (Agilent Technologies) operating at 400 MHz and 100 MHz, respectively. gHMQC and gHMBC experiments were performed using an inverse resonance probe. Chemical shifts are reported in parts per million (ppm) relative to the solvent signals (CDCl3: δH 7.26, δC 77.0). Mass spectrometric data were acquired using an Agilent 6230 time-of-flight LC/MS (LC/TOF) mass spectrometer, providing high-resolution mass measurements for molecular formula determination.
2.13. Analysis of Rapamycin’s Inhibitory Effect on Conidiospore Germination and Hyphal Growth
The effect of rapamycin on conidiospore germination was tested using a protocol based on López-Moral et al. (2022) [63]. Conidial suspensions were prepared from 12-day-old Verticillium dahliae V937I colonies grown on PDA and adjusted to a final concentration of 1 × 106 conidia/mL. A 5 mg/mL stock solution of rapamycin (Thermo Fisher Scientific Inc.) was prepared in DMSO and diluted in 50% ethanol to generate working solutions (50 to 350 μg/mL). This approach minimized any potential inhibitory effects of DMSO and ethanol on conidiospore germination. For the germination assay, a 5 μL drop of the conidial suspension was placed in the center of a microscope coverslip and mixed with 5 μL of rapamycin solution. A negative control (0 μg/mL rapamycin) was prepared by mixing 5 μL of conidial suspension with 5 μL of 50% ethanol. Coverslips were placed inside Petri dishes containing water agar to create humid chambers and incubated at 25 °C in the dark for 10 h. Following incubation, conidial germination was halted by adding a 5 μL drop of 0.01% acid fuchsin in lactoglycerol (lactic acid:glycerol:water, 1:2:1) to each coverslip. The coverslips were then mounted onto slides for microscopic examination. The experiment was conducted in triplicate for each concentration and repeated twice. For each coverslip, 100 randomly selected conidia were examined under ×400 phase-contrast microscopy Olympus CX41 (Olympus Europe, Hamburg, Germany), and germinated conidia were counted. Conidia were considered germinated if the germ tube length was at least equal to the longitudinal axis of the conidia. The relative germination inhibition (RGI, %) was calculated as RGI = [(Gcontrol − Grap)/Gcontrol] × 100, where Gcontrol represents the percentage of germinated conidia in the control group (35% ethanol), and Grap represents the percentage of germinated conidia in the presence of rapamycin. The data were used to generate a regression line, and the 50% inhibitory concentration (IC50) was determined via Probit analysis of mortality data. The effect of rapamycin on hyphal growth and the calculation of minimum inhibitory concentration (MIC) was determined using different rapamycin concentrations on PDA plates [64], with rapamycin concentrations ranging from 0 to 20 ng/mL.
3. Results
3.1. Isolation and Selection of Culturable Endophytic Streptomycetes Associated with Olive Roots and Their Antifungal Activity Against V. dahliae
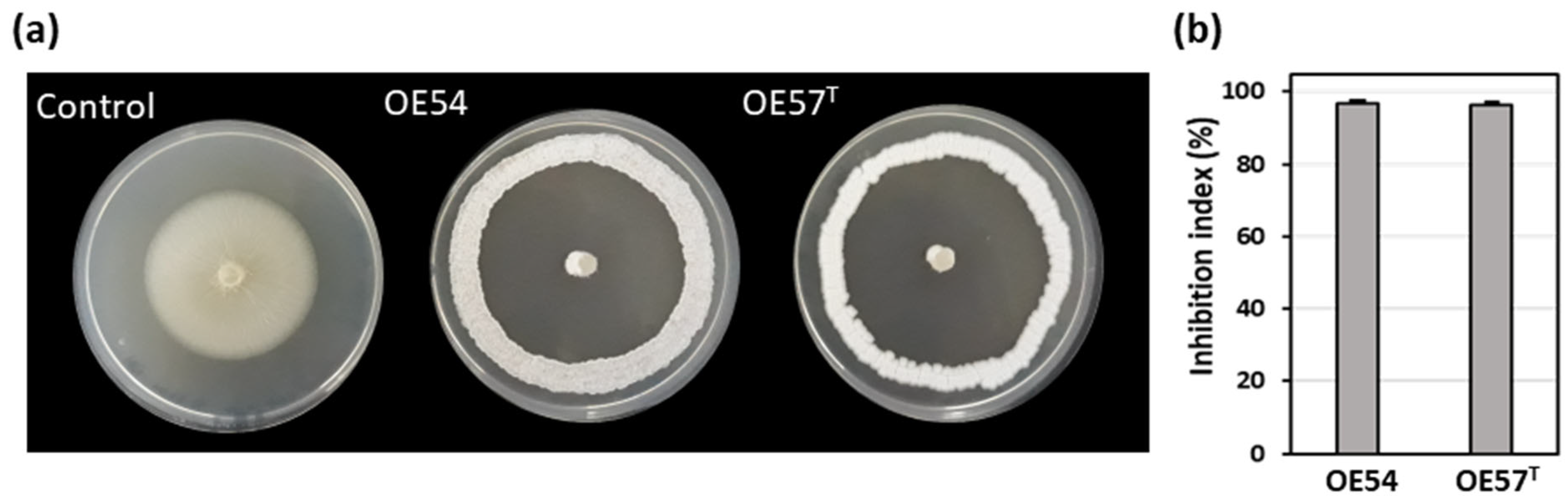
A total of 106 putative different endophytic Streptomyces strains (designated OE1 to OE106, where OE denotes Olive Endophyte) were isolated from the internal tissues of olive roots. Selection was based on distinct morphological and cultural characteristics. In an in vitro bioassay-based screening, 27.3% (29 out of 106) of the isolates exhibited AF activity against V. dahliae strain V937I. Among them, two strains demonstrated particularly strong inhibitory effects on V. dahliae mycelial growth: strain OE54, with an inhibition index of 96.86% (±0.68), and strain OE57T, with an inhibition index of 96.21% (±0.91) (Figure 1).
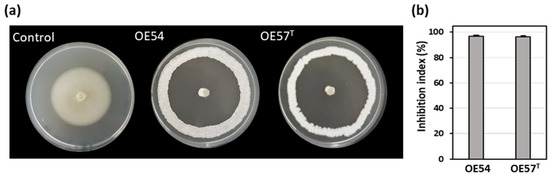
Figure 1.
(a) Antifungal activity of the endophytic isolates OE54 and OE57T against the phytopathogenic fungus Verticillium dahliae V937I, as determined by an in vitro bioassay-based screening; and (b) quantification of the antifungal activity by calculating the inhibition index. Data shown represent the mean values from three independent experiments, each conducted in duplicate.
3.2. Evaluation of Antifungal Activity in a Small-Scale Soil Assay
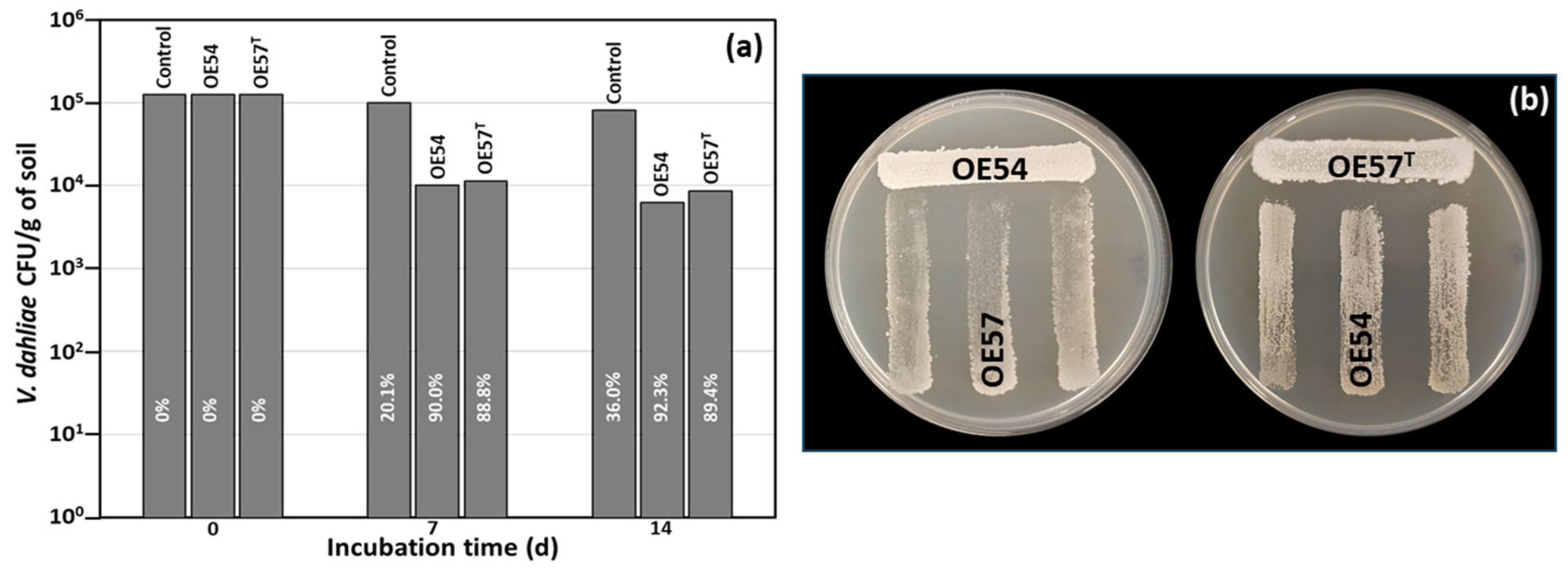
While many microbial isolates exhibit strong AF activity in in vitro assays, their efficacy under real soil conditions remains uncertain. This trait is crucial for their potential application as BCAs in field trials and for promoting root colonization in the case of endophytic strains. To address this, a small-scale in vitro soil experiment was designed to evaluate the AF activity of Streptomyces strains OE54 and OE57T, both individually and in combination, against V. dahliae under soil conditions. For this experiment, both strains were inoculated into sterile soil to eliminate potential interference from other microorganisms present in natural soil. The soil used was classified as loamy according to the USDA standard, with a pH of 7.98, an organic matter content of 6.59%, and a total nitrogen content of 0.32%. V. dahliae was introduced at an initial concentration of 1.25 × 105 colony-forming units (CFUs) per gram of soil. Over time, its viability decreased by 20.1% and 36.0% at 7 and 14 days post-inoculation, respectively. Co-inoculation with strain OE54 led to a significant reduction in V. dahliae survival, with declines of 90.0% and 92.3% at 7 and 14 days, respectively. By the end of the experiment, only 6.20 × 103 CFU/g of V. dahliae remained detectable (Figure 2a). Similarly, co-inoculation with strain resulted in V. dahliae reductions of 88.8% and 89.4% at 7 and 14 days, respectively, with a final viable count of 8.47 × 103 CFU/g of soil (Figure 2a).
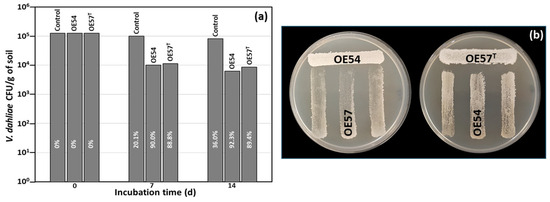
Figure 2.
(a) Small-scale in vitro soil assay to test the antifungal activity of isolates OE54 and OE57T against V. dahliae. The values shown are the average of two independent experiments made by duplicate. The inhibition percentages corresponding to each bar are shown in white letters. (b) Analysis of the cross interaction between both strains. Each strain was challenged with the other on a Petri dish co-culture bioassay 3 times (n = 9).
3.3. Strain-Specific Inhibition Patterns in Streptomyces–Streptomyces Interaction Bioassays
Considering the strong performance of both strains in the small-scale soil assay, we aimed to assess the potential negative interactions between OE54 and OE57T that could hinder their combined application in future field trials. To this end, a co-culture bioassay was conducted (Figure 2b). The results indicated that strain OE54 exerted a minimal inhibitory effect on strain OE57 T, reducing its growth by only 2.0% (±0.3). In contrast, strain OE57T exhibited a slightly higher inhibitory effect on OE54, with a reduction of 9.58% (±2.0) (Figure 2b).
3.4. Molecular Identification of Isolates OE54 and OE57T Using 16S rRNA Analysis, Multilocus Sequence Analysis (MLSA), and Comparative Genomics
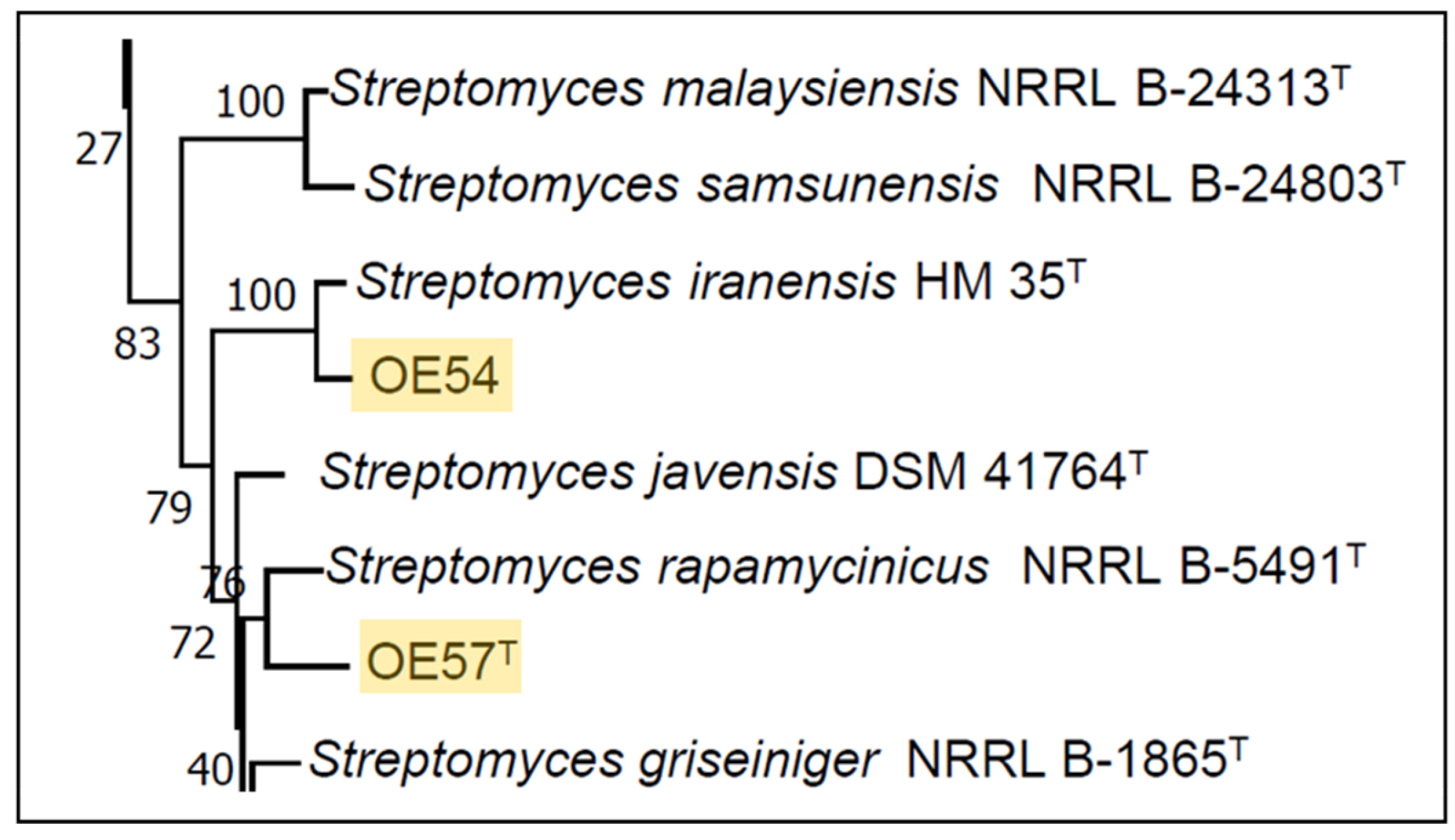
A partial sequencing of the 16S rRNA gene from strain OE54 revealed a sequence similarity exceeding 99.00% with multiple Streptomyces species, including S. iranensis (99.93%), S. rapamycinicus (99.21%), S. yogyakartensis (99.07%), S. javensis (99.07%), S. violaceusniger (99.07%), and S. demainii (99.00%). Similarly, strain OE57T exhibited a >99.00% sequence similarity with S. yogyakartensis (99.22%), S. javensis (99.22%), S. violaceusniger (99.22%), and S. albiflaviniger (99.14%). These findings confirmed that both isolates belong to the Streptomyces genus. However, accurate species-level identification was not possible, as all similarity values exceed the 98.65% threshold for prokaryotic species delineation based on 16S rRNA gene sequences [65]. Given the limitations of 16S rRNA for discriminating closely related Streptomyces species, an MLSA was conducted using five housekeeping genes (atpD, gyrB, recA, rpoB, and trpB). This analysis placed strain OE54 within a subclade with S. iranensis (Figure 3), with a Kimura 2-Parameter MLSA distance of 0.005 (Table 2), supporting its classification as S. iranensis, since strains with MLSA distances ≤ 0.007 are considered conspecific [38]. Conversely, strain OE57T clustered within a subclade that included S. rapamycinicus (Figure 3), but showed a genetic distance of 0.011 (Table 2), suggesting it represents a distinct species.
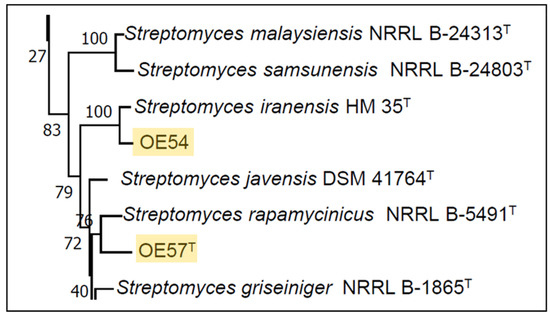
Figure 3.
Partial view of the Neighbor-Joining tree derived from the MLSA analysis, illustrating the phylogenetic placement of Streptomyces strains OE54 and OE57T (highlighted with yellow background). The complete tree is available in Supplementary Information (Figure S1).

Table 2.
Identification of Streptomyces strains OE54 and OE57T based on MLSA analysis and their MLSA evolutionary distances from phylogenetically related strains.
The discovery of two phylogenetically related Streptomyces strains residing as endophytes in olive roots prompted further investigation through whole-genome sequencing. Strain OE57T was sequenced at 94× coverage, yielding a genome size of 12.39 Mbp with a G + C content of 70.8%, 10,904 coding sequences, and 82 RNA genes, while strain OE54 was sequenced at 52× coverage, revealing a genome size of 11.71 Mbp with a G + C content of 71.0%, 10,077 coding sequences, and 82 RNA genes. The genomic features of both strains were consistent with those characteristics of the Streptomyces genus [66].
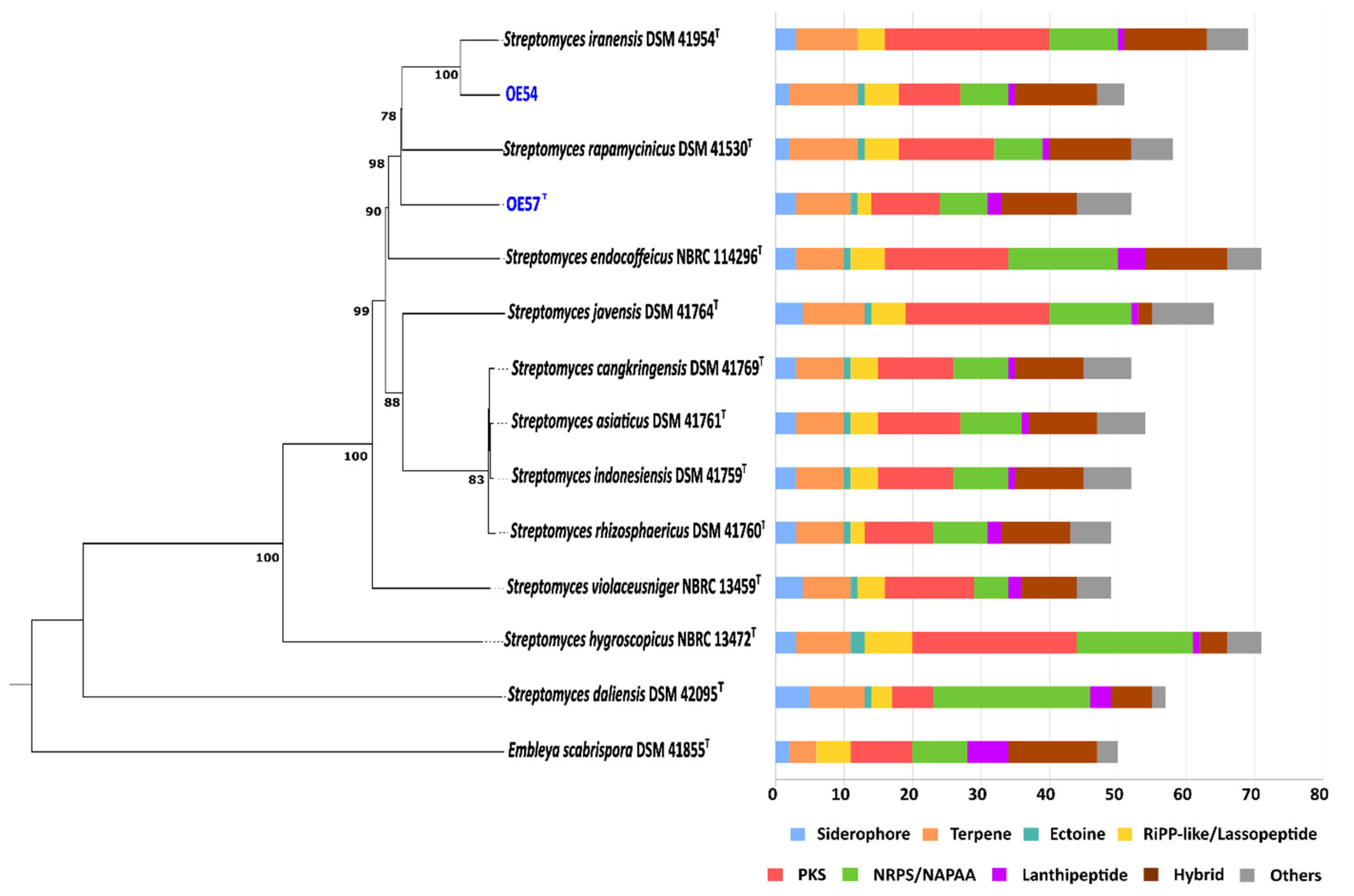
A whole-genome-based phylogenetic classification of strains OE54 and OE57T, along with their closest relatives, revealed a distinct grouping within a subclade that also includes S. iranensis and S. rapamycinicus (Figure 4). Consistent with previous MLSA and 16S rRNA phylogenetic analyses, this whole-genome phylogeny confirmed that strain OE54 belongs to the S. iranensis species (Figure 5). In contrast, while strain OE57 clustered within the same subclade, it represents a distinct and novel Streptomyces species.
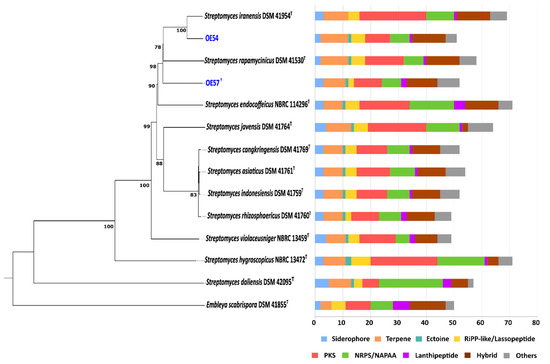
Figure 4.
Whole-genome sequence-based phylogenetic tree of strains OE54 and OE57T (highlighted in blue color) and their closest related species, constructed using the TYGS web server. The tree was inferred using FastME based on genome-to-genome distances computed with the Genome Blast Distance Phylogeny (GBDP) method, employing the d4 distance formula. Branch lengths are scaled according to GBDP distance values. The biosynthetic gene cluster (BGC) composition of each strain is displayed on the right side of the tree, with BGC classes color-coded according to the legend below.
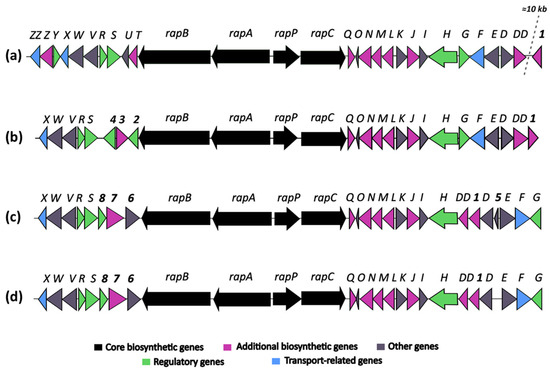
Figure 5.
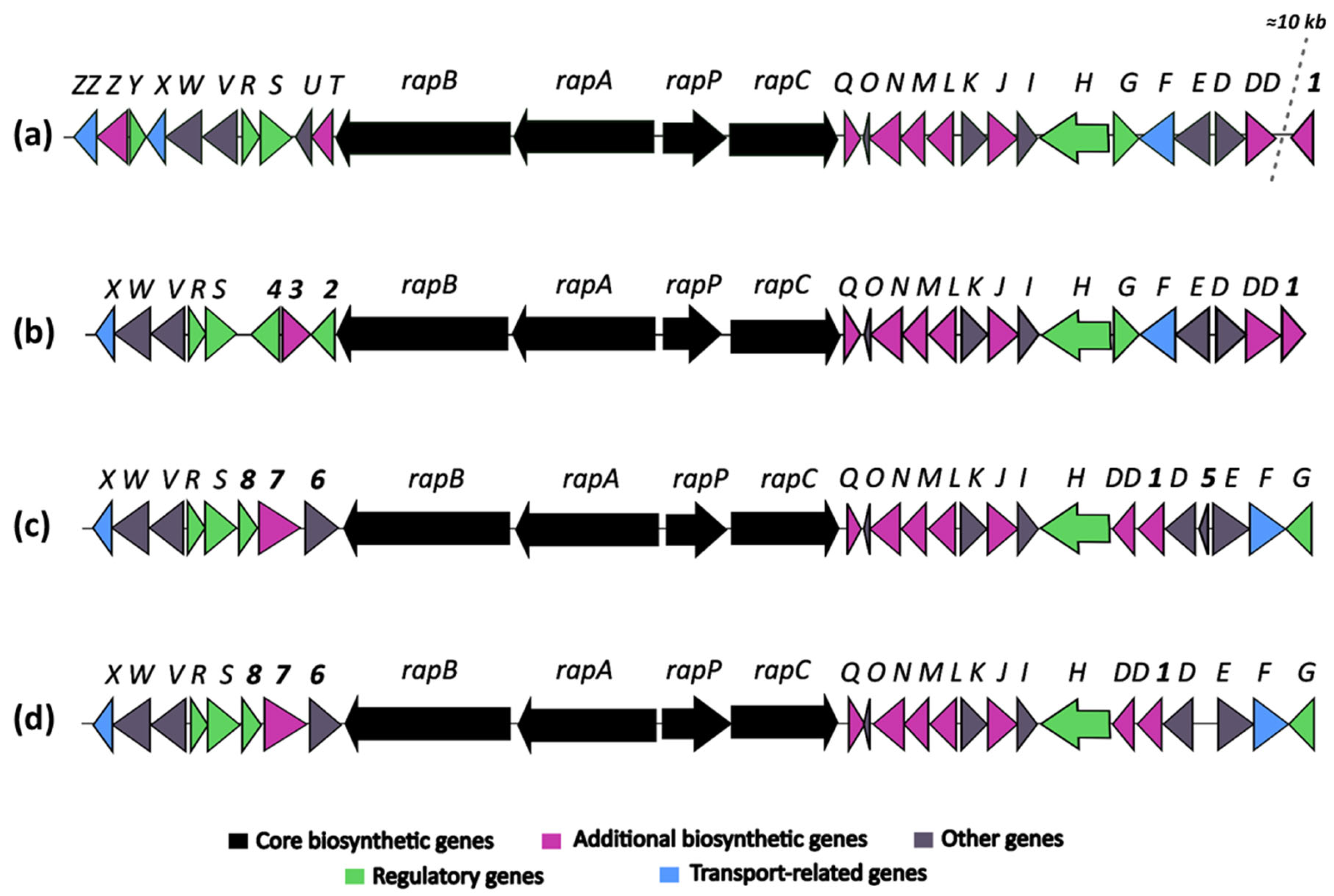
Comparative analysis of rapamycin biosynthetic gene clusters (BGCs) from the following: (a) Streptomyces rapamycinicus NRRL 5491T; (b) Streptomyces sp. OE57T; (c) Streptomyces iranensis DSM 41954T; and (d) Streptomyces iranensis OE54.
Digital DNA–DNA hybridization (dDDH) values further corroborated these results. Strain OE54 showed a 81.7% similarity with S. iranensis DSM 41954T, exceeding the 70% threshold for species delineation [67], thereby confirming its classification as S. iranensis. On the other hand, OE57T showed dDDH values of 60.3% with S. iranensis DSM 41954T, 60.0% with S. rapamycinicus DSM 41530T, and 56.3% with S. endocoffeicus NBRC 114296T. The dDDH similarity between OE54 and OE57T was 59.8%. These dDDH values were well below the 70% threshold established for species delineation, supporting the recognition of strain OE57T as a distinct Streptomyces species.
In addition, the full-length 16S rRNA gene sequences retrieved from the genome assemblies were used to construct a phylogenetic tree (Supplementary Figure S2), which corroborated the previous results. OE54 clustered with S. iranensis, while OE57T grouped with species of the S. violaceusniger clade, particularly S. javensis and S. violaceusniger. Although these associations reflect close evolutionary relationships, they also underscore the limited resolution of the 16S rRNA gene for species-level discrimination within the Streptomyces genus.
3.5. In Silico Screening for Secondary Metabolite Production in Strains OE54 and OE57T
The biosynthetic potential of both strains was assessed through a bioinformatic analysis of their genome sequences using the antiSMASH software. In the genome of strain OE54, a total of 51 predicted BGCs were identified (Figure 4). Among these, eight BGCs were predicted to encode compounds with AF activity. Notably, four BGCs exhibited a high sequence identity (>60%) to known AF biosynthetic pathways. These included the following: (i) a BGC with a 96% sequence identity to neomediomycin B, a polyketide-class aminopolyol compound that is an analog of mediomycin (also known as clethramycin), a known AF agent [68]; (ii) a BGC with a 95% sequence identity to azalomycin F3a, a 36-membered polyhydroxyl macrolide with demonstrated AF activity against various fungal phytopathogens [69]; (iii) a BGC with a 82% sequence identity to rapamycin, a potent AF compound effective against multiple fungal phytopathogens [70,71], including V. dahliae, which exhibits hypersensitivity to this compound [72] (S. iranensis is one of only two known Streptomyces species capable of producing rapamycin [73]); and (iv) a BGC with a 66% sequence identity to nigericin, a polyether compound with weak AF activity that, nonetheless, enhances the AF activity of rapamycin [74]. Additional putative BGCs associated with AF activity were identified, albeit with lower sequence identity. These included the following: rustmicin (33%), a 14-membered macrolide that inhibits sphingolipid synthesis [75]; the polyene macrolactam BE-14106 (28%) [76]; and notonesomycin (11%) [77].
A total of 52 predicted biosynthetic gene clusters (BGCs) were identified in the genome of strain OE57T (Figure 4), five of which were predicted to encode potential AF compounds. Among these, three BGCs exhibited a sequence similarity greater than 60% to known AF biosynthetic pathways: azalomycin F3a (86%), rapamycin (82%), and nigericin (66%). Additional putative BGCs with a lower sequence similarity included toyocamycin (30%) [78] and notonesomycin (11%).
The identification of a putative rapamycin-encoding BGC in the genome of strain OE57T was particularly noteworthy, as the production of this compound has only been experimentally confirmed in two Streptomyces species: S. iranensis and S. rapamycinicus. To further investigate this finding, a comparative analysis was conducted to examine the organization of the rapamycin BGC among S. rapamycinicus NRRL 5491T [79], S. iranensis DSM 41954T (complete genome annotation from October 2024, available at https://www.ncbi.nlm.nih.gov/datasets/genome/GCF_042466515.1/), and the OE54 and OE57T strains (Figure 5).
The comparative analysis of rapamycin BGCs from Streptomyces rapamycinicus (A), Streptomyces iranensis (C), and the strains OE57T (B) and OE54 (D) (Figure 5) revealed both conserved and strain-specific features, potentially reflecting evolutionary adaptations that influence rapamycin biosynthesis. The overall organization of the rapamycin BGC is highly conserved across the four strains, with the core genes rapB, rapA, and rapC, encoding the multifunctional polyketide synthase (PKS), as well as the NRPS-like gene rapP, maintaining a consistent arrangement.
However, minor structural differences were observed: (a) The BGC organization in S. iranensis and strain OE54 was nearly identical, except for the presence of a small open reading frame, orf5, in S. iranensis (Figure 5). This gene encodes a hypothetical protein and is absent in the OE54 BGC, as well as in the S. rapamycinicus and OE57T clusters. (b) The genes rapO, rapN, rapM, rapL, rapK, rapJ, rapI, rapH, and rapG, which are involved in regulation, precursor synthesis, and macrolactone tailoring, are located downstream of the PKS core region in S. rapamycinicus and strain OE57T. In contrast, in S. iranensis and strain OE54, rapG has been repositioned to the right end of the cluster (Figure 5). (c) The most pronounced differences were observed upstream of the PKS core region. In S. rapamycinicus and OE57T, the genes rapS, rapR, rapV, rapW, and rapX are organized in an identical manner. However, the rapY, rapZ, and rapZZ genes, which have unknown functions, are absent in the OE57T cluster. Similarly, the rapT and rapU genes, located between rapB and rapS in S. rapamycinicus, are missing in OE57T. The rapY encodes a protein homologous to antibiotic export repressors, such as ActII in the actinorhodin cluster and TcmR in the tetracenomycin cluster (Supplementary Information Table S1) [80]. It negatively regulates the expression of most rapamycin biosynthetic genes [81]. In contrast, in the OE57T cluster, three open reading frames (orf2, orf3, and orf4) occupy the region between rapB and rapS. orf2 encodes a TetR/AcrR family transcriptional regulator with >99% similarity to homologs found in various Streptomyces species. These regulators typically function as repressors involved in antibiotic resistance and the regulation of small-molecule exporters [82], suggesting that orf2 in OE57 may serve a regulatory role analogous to rapY in S. rapamycinicus. orf3 encodes a protein with >90% similarity to O-methyltransferases, including acetylserotonin O-methyltransferases, while orf4 encodes a hypothetical protein with no database homologs. (d) In S. iranensis and strain OE54, three ORFs (orf6, orf7, and orf8) are located between rapB and rapS. orf6 encodes a protein belonging to the SGNH/GDSL hydrolase family, orf7 encodes a putative FAD-dependent mono-oxygenase, and orf8—similar to orf2 in S. rapamycinicus and OE57—encodes a protein related to TecR/AcrR family regulators. (e) Finally, attention should be drawn to the orf1 gene, which encodes a protein with a high amino acid identity to orfDD. Both genes are predicted to encode a pyridoxal phosphate-dependent cystathionine synthase. In S. rapamycinicus and the OE57T strain, this gene is located outside the rapamycin cluster, with its right boundary defined by orfDD, which, according to Molnár et al. (1996) [83], also lies outside the cluster. In contrast, in Streptomyces iranensis and the OE54 strain, orf1 is positioned between rapD and rapDD (Supplementary Information, Figure S3). In S. rapamycinicus, a homologous orf1 is situated approximately 10 kb downstream of the rapamycin BGC, close to the actinoplanic acid BGC. Notably, in this species, the actinoplanic acid BGC is located immediately downstream of the rapamycin cluster [84]. While actinoplanic acid BGCs are also present in S. iranensis, OE54, and OE57T, in these strains, they are positioned at a considerable genomic distance (~8.5–9 Mb) from the rapamycin cluster (Supplementary Information, Figure S3).
3.6. Identification of Rapamycin as the Main Antifungal Compound Produced by OE54 and OE57T Strains
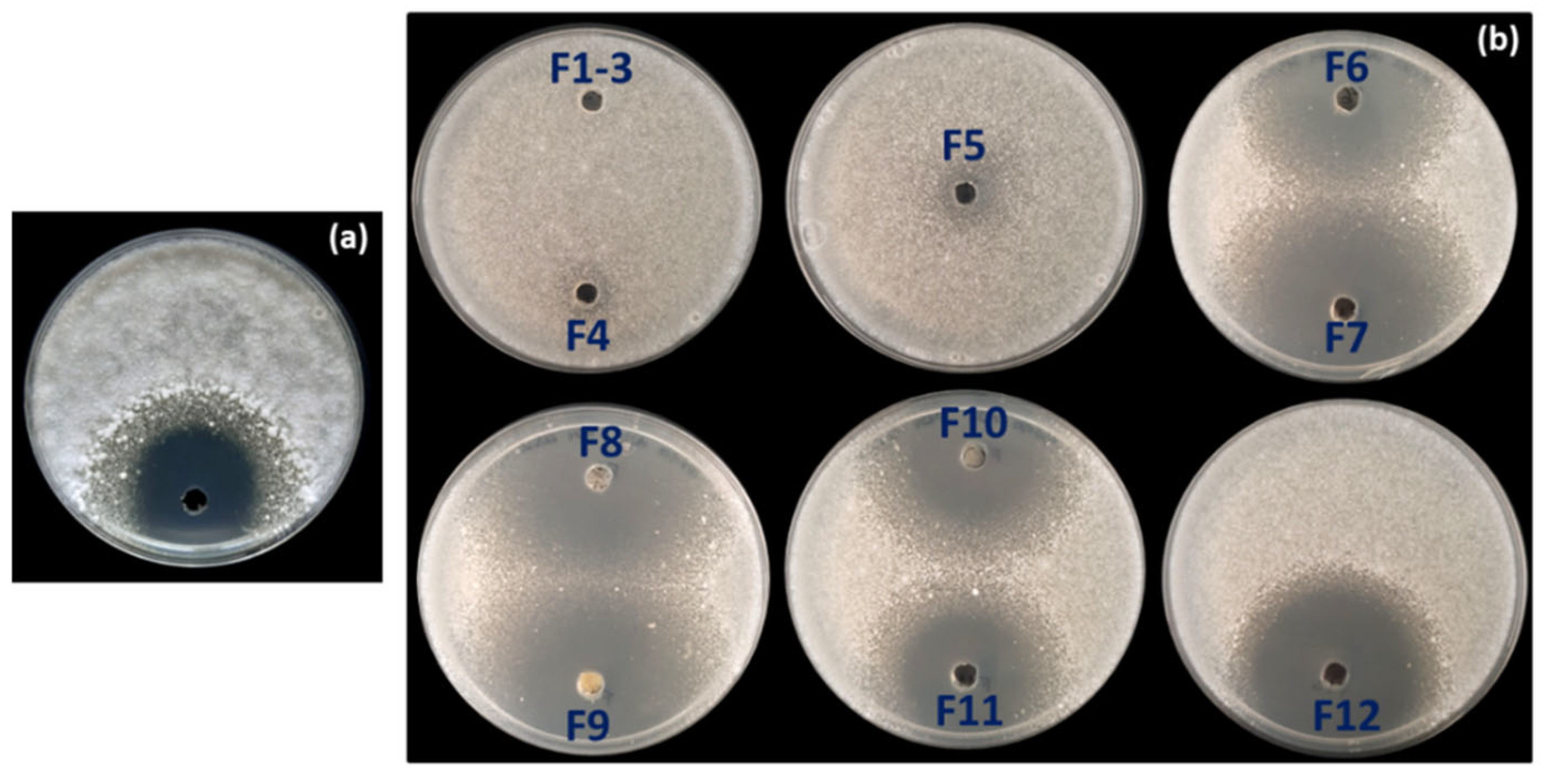
To identify the compound(s) responsible for the AF activity observed in strain OE54, a total of 6.5 L of fermentation broth was obtained using the ISP2 medium following incubation for 72 h at 28 °C and 200 rpm. Optimal AF production was confirmed through a bioassay using the culture broth against Verticillium dahliae (Figure 6a). Subsequently, an activity-guided vacuum flash chromatography (VFC) was performed, yielding 2.90 g of extract. A 2 mg sample of this extract was analyzed by liquid chromatography–mass spectrometry (LC-MS) and tested for AF activity via a plate bioassay. Following VFC, 12 fractions were collected and subjected to AF activity testing using a plate bioassay. AF activity was detected in fractions 6–12, with the most pronounced inhibition observed in fractions 7–9 (Figure 6b).
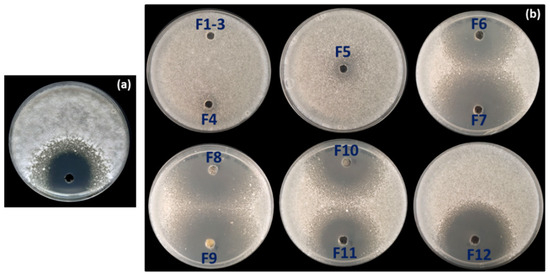
Figure 6.
(a) Antifungal activity against Verticillium dahliae detected in the supernatant of the fermentation broth of strain OE54 after 72 h of growth, and (b) in the 12 fractions (F1–F12) obtained from the initial round of vacuum flash chromatography (VFC) during fractionation of the fermentation broth. Antifungal activity was observed in fractions F6–F12, with the strongest inhibition detected in fractions F7–F9.
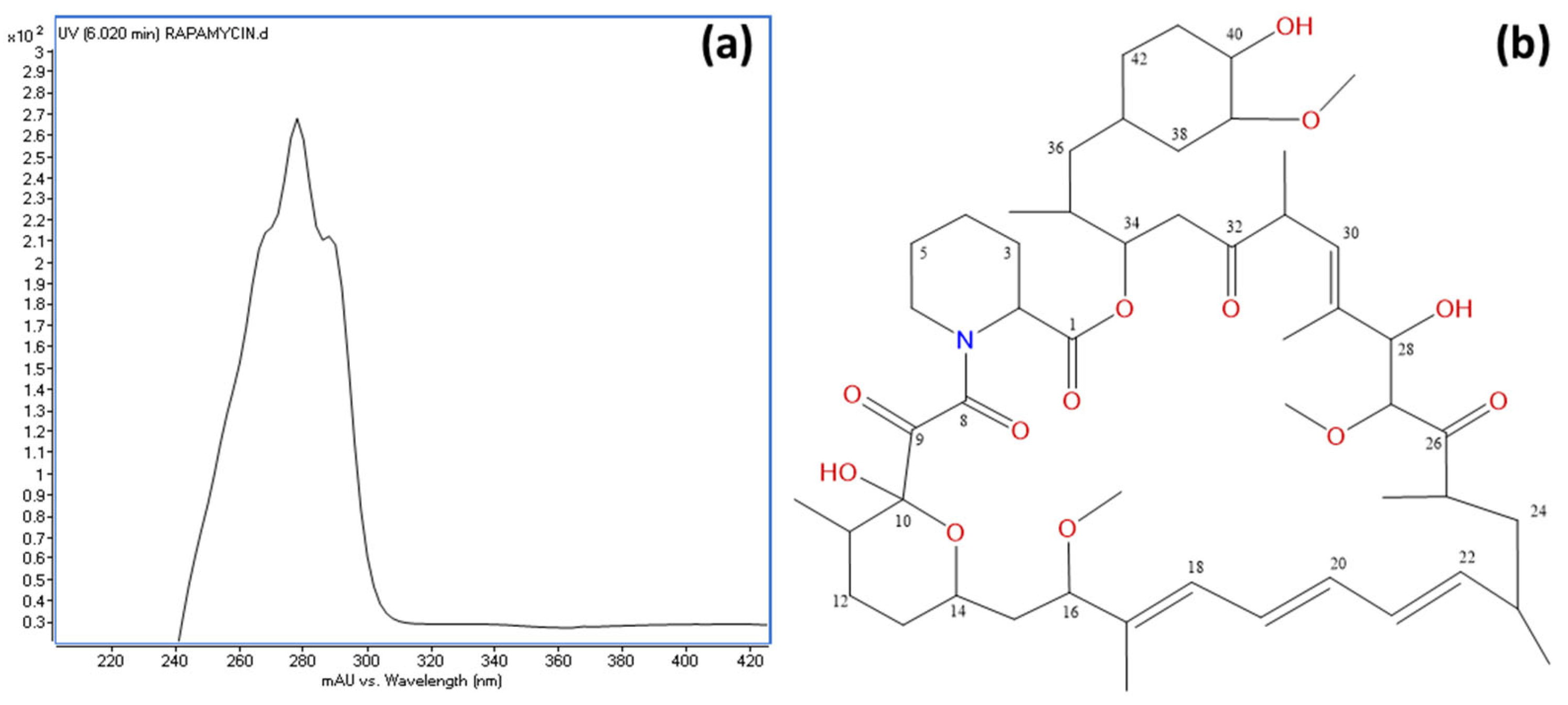
An HPLC and LC/MS analysis of the active fractions revealed a common major peak. This peak was scarcely detected in the diode array detector (DAD) at λ = 220 nm due to the compound’s UV spectrum, which exhibited absorption maxima at 267, 277, and 288 nm (Figure 7a). However, it was clearly visible in the evaporative light-scattering detector (ELSD) chromatogram (Supplementary Information Figure S4). Active fraction 9 (429.0 mg) was further purified using C18 reversed-phase column chromatography, yielding 17.2 mg of a pure active compound. High-resolution electrospray ionization mass spectrometry (HRESIMS) analysis determined the molecular formula as C51H79NO13, indicating 13 degrees of unsaturation (Supplementary Information Figure S4). Structural elucidation was performed using extensive NMR spectroscopy, including 1H NMR, 13C NMR, 1H-1H COSY, gHSQC, and gHMBC experiments, confirming the compound as rapamycin (Figure 7b). The spectroscopic data were consistent with previously reported values [85]. 1H-NMR and 13C-NMR data are provided in Supplementary Information Table S2.
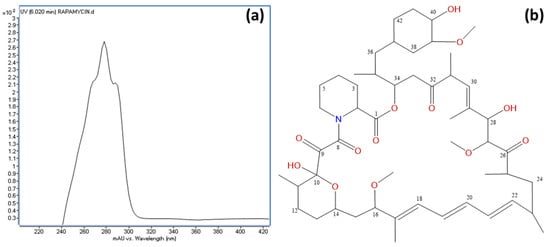
Figure 7.
(a) UV spectrum of rapamycin, displaying absorption maxima at 267, 277, and 288 nm; and (b) chemical structure of rapamycin with carbon positions numbered as determined by NMR analysis.
The presence of a putative rapamycin BGC in the genome of strain OE57T suggested that the strong AF activity observed against V. dahliae could be attributed to the production of this compound. To test this hypothesis, liquid cultures were grown in ISP2 medium, and the culture broth was extracted with ethyl acetate. The solvent was then evaporated, and the residue was dissolved in 80% methanol for HPLC analysis. The HPLC chromatogram revealed a peak with a retention time of 16.3 min, identical to that of a pure rapamycin standard. Additionally, the UV absorption spectrum of the detected compound matched that of rapamycin. These results confirm that rapamycin is responsible for most of the AF activity exhibited by strain OE57T.
3.7. Identification of the Epoxy Cinnamoyl-Containing Nonribosomal Peptide Epoxinnamide Produced by OE54
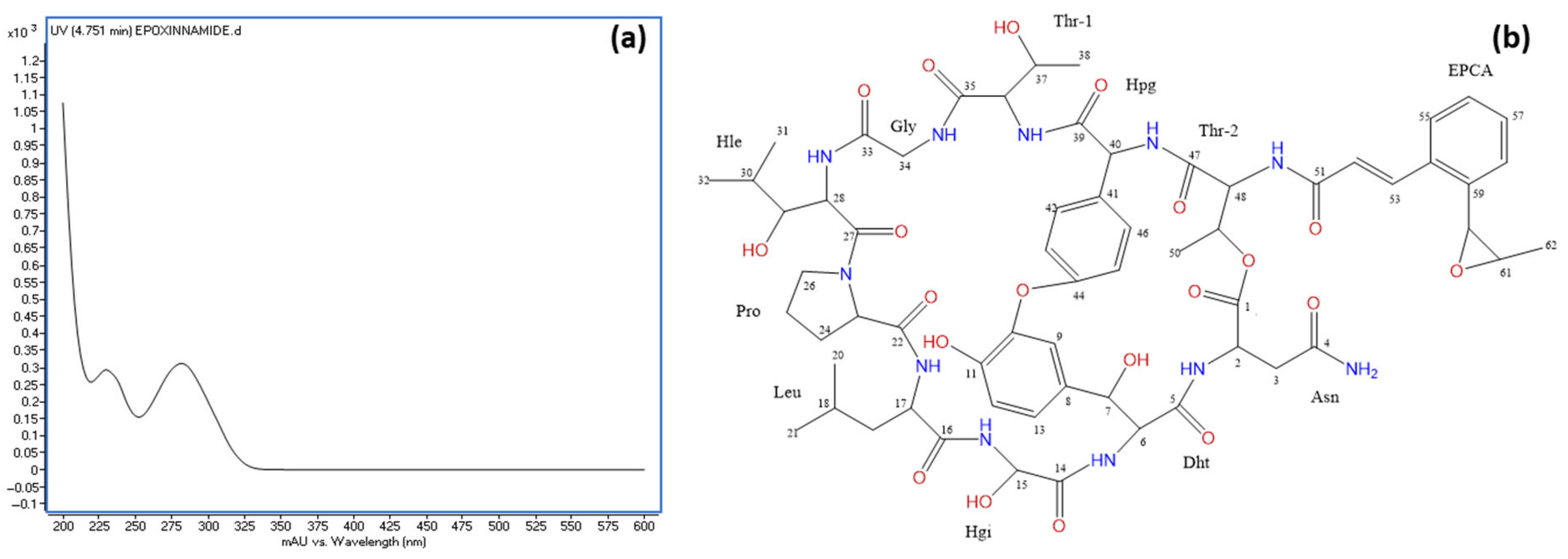
During the purification of rapamycin from the fermentation broth, a second round of VFC was applied to the combined fractions F10–F12 (see Figure 6b). Notably, fraction F8′ contained 14.7 mg of a highly pure compound, as confirmed by HPLC with ELSD detection (Supplementary Information Figure S5). This compound, identified as epoxinnamide, exhibited no AF activity and displayed a UV absorption peak at 280 nm (Figure 8a). An HRESIMS analysis determined the molecular formula of epoxinnamide as C62H79N11O20, corresponding to 29 degrees of unsaturation (Supplementary Information Figure S5). Structural elucidation using 1H and 13C NMR spectroscopy (Figure 8b) confirmed its identity, with spectroscopic data consistent with previously reported values [86]. The corresponding 1H-NMR and 13C-NMR data are provided in Supplementary Information Table S3.
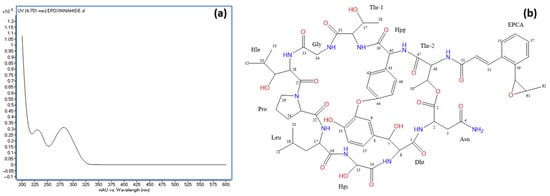
Figure 8.
(a) UV spectrum of epoxinnamide and (b) chemical structure of epoxinnamide with carbon positions labeled as determined by NMR analysis. Abbreviations: Dht, 3β-dihydroxytyrosine; Hgy, α-hydroxyglycine; Hle, β-hydroxyleucine; Hpg, hydroxyphenylglycine; EPCA, O-1,2-epoxypropyl cinnamic acid.
Epoxinnamide is an epoxy cinnamoyl-containing nonribosomal peptide (NRP) that has, to date, only been reported in a Streptomyces sp. strain isolated from intertidal mudflats in Oido, Republic of Korea. This finding establishes strain OE54 as the second known producer of this compound. Furthermore, epoxinnamide BGCs have been identified in the genomes of S. iranensis, S. rapamycinicus, OE54, and OE57T. The overall organization of the epoxinnamide BGC is highly conserved in Streptomyces sp. OID44, where it was first described [86], as well as in S. rapamycinicus and strain OE57T. However, notable differences in the gene arrangement were observed in S. iranensis and strain OE54 BGCs (Supplementary Information Figure S6).
3.8. Effect of Rapamycin on V. dahliae Conidiospore Germination, Radial Growth Inhibition, and MIC Determination
Rapamycin effectively inhibited the germination of Verticillium dahliae V937I conidiospores within the tested concentration range of 5–125 μg/mL. A probit analysis of the germination data produced a regression equation of y = 0.6649x + 3.7187 (R2 = 0.9175), allowing for the calculation of the LC50 (lethal concentration for 50% inhibition) as 87.36 μg/mL (Supplementary Information Figure S7).
Rapamycin exhibited significantly greater potency in inhibiting the radial growth of V. dahliae, with detectable effects at concentrations as low as 2 ng/mL. The IC50 (half-maximal inhibitory concentration) was determined to be 3.91 ng/mL.
3.9. Cultural, Morphological, and Phenotypic Properties of OE54 and OE57 Strains
Strains OE54 and OE57T exhibit phenotypic and morphological features consistent with their classification within the genus Streptomyces [66]. As summarized in Supplementary Information Table S4, OE54 and S. iranensis demonstrated growth within a temperature range of 15–37 °C, whereas S. rapamycinicus and OE57T were capable of growth at temperatures ranging from 15 °C to 42 °C. Regarding pH tolerance, S. iranensis and OE57T were able to grow in a pH range of 5–10, while OE54 and S. rapamycinicus exhibited a broader tolerance of pH 5–12, though the growth of S. rapamycinicus was weak at pH 12. Differences in salt tolerance were also observed: S. iranensis and OE54 exhibited low salt tolerance, growing only in the presence of 2.5% NaCl, whereas S. rapamycinicus and OE57T were able to grow in up to 5.0% NaCl. None of the strains were capable of growth at 7.5% NaCl.
The strains also displayed distinct growth characteristics on various culture media (Supplementary Information Table S4). All strains exhibited moderate to good growth on ISP 1, ISP 2, ISP 3, ISP 4, Bennet, R5, and GYM agar media (growth characteristics of S. iranensis and S. rapamycinicus type strains are available at https://www.dsmz.de/collection/catalogue). Growth on TSA was weak for all strains except for OE57T, which exhibited moderate growth. In ISP 5, S. iranensis demonstrated moderate growth, whereas all other strains displayed weak growth. In ISP 6, all strains exhibited weak growth.
Biochemical and enzymatic properties further differentiate these strains from one another and from their closest phylogenomic relatives (Supplementary Information Table S5). Notably, OE54 and S. iranensis differ in traits such as urea degradation and the production of esterase C4 and α-glucosidase, which are absent in the reference strain S. iranensis DSM 41954T. Given that phylogenetic analyses clearly classify OE54 within the S. iranensis species, these differences may reflect environmental adaptation. While the S. iranensis type strain was isolated from rhizosphere soil at a depth of 10 cm with no indication of the associated plant [87], OE54 was isolated as an endophyte from the interior of olive roots. Similarly, OE57T can be distinguished from the reference strains of S. iranensis DSM 41954T and S. rapamycinicus DSM 41530T by the absence of enzymatic activities such as C8 and C14 lipases, α-galactosidase, and α-mannosidase (Supplementary Information Table S5).
3.10. Chemotaxonomic Features
The chemotaxonomic feature of strain OE57T were in concordance with those of the genus Streptomyces [66]. Whole-organism hydrolysates of strain OE57T and its close phylogenomic neighbor Streptomyces rapamycinicus DSM 41530T were rich in LL-diaminopimelic acid in the cell-wall peptidoglycan and have glucose and ribose as whole-cell sugars. The strain contained diphosphatidylglycerol (DPG), phosphatidylethanolamine (PE), hydroxy phosphatidylethanolamine (OH-PE), phosphatidylinositol (PI), phospholipid (PL), aminophospholipid (APL), and unidentified lipids (Ls), while strain DSM 41530T had phosphatidylmethylethanolamine (PME) and glycophospholipid (Supplementary Information Figure S8). The major fatty acids (>10%) of strain OE57T contained iso-C15:0, C16:0, and iso-C17:0, while strain DSM 41530T showed iso-C15:0, anteiso-C15:0, iso-C16:0, and C16:0 (Supplementary Information Table S6). The predominant menaquinone (>5%) of strains OE57T and DSM 41530T consisted of MK9-(H4), MK9-(H6), MK9-(H8), and MK-10(H6).
3.11. Description of Streptomyces lacaronensis sp. nov.
Streptomyces lacaronensis (la.ca.ro.nen’sis N.L. gen. N. lacaronensis, referring to river Lacarón, on whose bank the type strain was isolated from the roots of an olive tree, Extremadura region, Spain):
The strain is a Gram-stain-positive, aerobic, non-motile bacterium that forms light grey aerial mycelium on oat meal (ISP 3) agar and signal white aerial mycelium in starch-mineral (ISP 4), ISP 1, ISP 2, ISP 5, ISP 6, NA, and GYM agars. A salmon range diffusible pigment is produced in ISP 7 media at 28 °C. The strain is able to grow from 15 °C to 42 °C, optimally at 28 °C, and from pH 5.0–10.0, optimally at pH 7.0. Additional cultural and morphological properties are mentioned in Supplementary Tables S4 and S5.
The strain has LL-diaminopimelic acid of the cell wall peptidoglycan and glucose and ribose as whole cell sugars. It contained diphosphatidylglycerol (DPG), phosphatidylethanolamine (PE), hydroxy phosphatidylethanolamine (OH-PE), phosphatidylinositol (PI), phospholipid (PL), aminophospholipid (APL), and unidentified lipids (Ls). The fatty acid profile consists of (>5%) iso-C15:0, anteiso-C15:0, C16:1 cis 9, iso-C16:0, C16:0, iso-C17:1 cis 9, and iso-C17:0. It has MK9-(H4), MK9-(H6), MK9-(H8), and MK-10(H6) as predominant menaquinone.
The genome size of the strain is 12.29 Mbp, and its in silico G + C content is 70.9%. The type strain OE57T (=DSM 118741T = CECT 31164T) was isolated from inside a sample of an olive root collected from a tree exhibiting visual symptoms compatible with Verticillium wilt that was located on a commercial plot located in the town of La Garrovilla (Spain) and at geographic coordinates 38°55′08.1” N 6°31′27.7” W. The genome sequence of OE57T strain has been deposited in the GenBank database under the accession number JBLHDK000000000. The BioProject accession number is PRJNA1185486.
4. Discussion
The genus Streptomyces represents one of the most complex and diverse bacterial taxa characterized to date, comprising over 755 validly named species (https://lpsn.dsmz.de/genus/streptomyces; accessed on 1 June 2025), with this number continually increasing as new species are discovered. A defining evolutionary feature of Streptomyces species is their large genome size relative to other bacterial groups, which encodes the biosynthetic pathways for numerous secondary metabolites with significant industrial applications. Among these metabolites, many exhibit potent AF properties, making Streptomyces particularly attractive candidates for use as BCAs in the management of fungal phytopathogens affecting a wide range of cultivated plants.
In a screening study aimed to isolate Streptomyces strains from the interior root tissues of olive plants, two strains, OE54 and OE57T, were identified based on their strong in vitro AF activity against V. dahliae. Molecular, physiological, and growth analyses confirmed that strain OE54 belongs to S. iranensis, whereas strain OE57T represents a novel species, herein designated S. lacaronensis. The discovery of a new Streptomyces species is inherently valuable, as it contributes to a more comprehensive understanding of the genus. However, a particularly noteworthy finding of this study was that both S. iranensis OE54 and S. lacaronensis OE57T exhibited pronounced AF activity attributable to rapamycin production.
The simultaneous isolation of two distinct rapamycin-producing Streptomyces species from the same plant tissue is highly unusual, as the production of this compound has been reported in only a limited number of bacterial species to date. This finding suggests that olive trees affected by Verticillium wilt may recruit rapamycin-producing Streptomyces strains from the rhizosphere, enabling their establishment as endophytes. Such recruitment could confer advantages to the host plant, including the production of antifungal compounds that may mitigate the impact of pathogenic infections. Previous research has indicated that the rhizosphere of hop plants affected by Verticillium wilt undergoes significant shifts in microbial composition compared to that of healthy plants, specifically characterized by the recruitment of putatively beneficial fungi [88]. This phenomenon of attracting potentially beneficial microorganisms to the rhizosphere of plants afflicted by phytopathogenic fungi may facilitate subsequent colonization by those capable of adopting an endophytic lifestyle.
Prior to this study, rapamycin biosynthesis had been documented exclusively in Streptomyces rapamycinicus (formerly S. hygroscopicus) [89], S. iranensis [73], and Actinoplanes sp. strain N902-109 [90]. The detection of rapamycin in the fermentation broths of S. iranensis OE54 and S. lacaronensis OE57T expands this previously limited list, underscoring the potential ecological significance of rapamycin production in endophytic Streptomyces strains. This finding is particularly noteworthy, as rapamycin—often referred to as a “billion-dollar molecule”—is the second most widely used immunosuppressant of microbial origin, following cyclosporine [91]. Initially regarded as a low-toxicity AF compound, its potent immunosuppressive properties were identified in 1977 [92], leading to its widespread clinical application in organ transplantation and autoimmune disease management. Moreover, rapamycin has since been recognized for its broad therapeutic potential, including antitumor activity [93], neuroprotective effects in neurodegenerative diseases [94,95], and its possible role in lifespan extension [96].
The molecular target of rapamycin in eukaryotic cells is well-established. Rapamycin interacts with FK506-binding protein 12 (FKBP12), forming a complex that inhibits the activity of the Target of Rapamycin (TOR) kinase. TOR is an evolutionarily conserved phosphoinositide 3-kinase (PI3K)-related protein kinase that regulates numerous cellular processes in response to diverse extracellular and intracellular signals [72,97]. In this study, we demonstrate that V. dahliae exhibits a high degree of sensitivity to rapamycin, which markedly suppresses hyphal growth and significantly inhibited conidiospore germination. These findings align with the results of Li et al. (2019) [72], who reported that rapamycin effectively inhibits ribosomal biogenesis, RNA polymerase II transcription factors, and various metabolic pathways, including the transcription of genes encoding cell-wall-degrading enzymes in V. dahliae-treated cells. Moreover, their study confirmed that rapamycin substantially reduces the pathogenicity of V. dahliae, thereby preventing the onset of Verticillium wilt [72]. Collectively, these findings suggest that rapamycin may serve as a promising biofungicide, offering a viable alternative to conventional chemical fungicides for the management of Verticillium wilt. Furthermore, rapamycin-producing Streptomyces spp. strains, such as those identified in this study, hold significant potential as effective BCAs.
A comparative analysis of the BGCs associated with rapamycin production in S. rapamycinicus, S. iranensis, and strains OE54 and OE57T reveals both conserved and strain-specific features, reflecting potential evolutionary adaptations that influence rapamycin biosynthesis. As illustrated in Figure 5, the BGCs of S. iranensis and strain OE54 exhibit a high degree of similarity, with the sole distinction being the presence of a small open reading frame (orf5) located between rapD and rapE in S. iranensis, which is absent in the OE54 cluster. orf5 encodes a small hypothetical protein with no significant homologs in existing databases, suggesting that its presence or absence may stem from a sequencing artifact in one of the genomes. The close similarity between these BGCs further supports the classification of strain OE54 as S. iranensis.
The core biosynthetic genes (rapA, rapB, rapC, and rapP) are highly conserved across all four clusters, underscoring their essential role in rapamycin biosynthesis. Additionally, the downstream flanking region encompassing rapQ, rapO, rapN, rapM, rapL, rapK, rapJ, rapI, and rapH exhibits substantial conservation, suggesting that these genes play crucial roles in rapamycin production (see Supplementary Table S1 for a description of their putative functions). Previous studies have demonstrated that rapG and rapH function as positive regulators of rapamycin biosynthesis in S. rapamycinicus [98]. Furthermore, rapI, rapM, and rapQ encode S-adenosylmethionine (SAM)-dependent O-methyltransferases [83], which are likely responsible for the O-methylation of positions C16, C27, and C39 of the rapamycin molecule (Figure 7). In contrast, the products of rapJ and rapN (encoding cytochrome P450 mono-oxygenases) and rapO (encoding a ferredoxin-like protein) [83,99] are predicted to mediate the formation of keto groups at positions C9, C27, and, potentially, C32 (Figure 7). Lastly, rapL encodes a lysine cyclodeaminase, an enzyme that catalyzes the cyclization of lysine to generate L-pipecolate, a direct precursor in rapamycin biosynthesis [83,100].
A notable degree of variability is observed in the organization of genes located downstream of rapH, using the S. rapamycinicus BGC as a reference. In both S. rapamycinicus and strain OE57T, this region maintains a conserved gene arrangement, comprising rapG, rapF, orfE, and orfD, with orfDD positioned outside the cluster, as reported by Molnár et al. (1996) [83]. In contrast, S. iranensis and strain OE54 exhibit an inverted gene organization, with orfDD, orf1, orfD, orfE, rapF, and rapG positioned downstream of rapH. This rearrangement suggests a potential inversion event affecting a DNA fragment containing these genes. The presence of orf1 within the S. iranensis, and OE54 and OE57 BGCs is particularly noteworthy. This gene encodes a protein exhibiting a 92.12% amino acid identity with orfDD. An InterPro analysis indicates that both orf1 and orfDD contain conserved domains characteristic of cysteine synthase/cystathionine β-synthase (IPR050214) (see Supplementary Table S1). Additionally, orf1 shares a homology with a 2,3-diaminopropionate biosynthesis protein (SbnA), a homologous enzyme identified in multiple Streptomyces species. The biological function of SbnA has been extensively studied in Staphylococcus aureus, where it participates in the biosynthesis of L-2,3-diaminopropionic acid (L-DAP), a precursor for siderophores and antibiotics [101]. Specifically, SbnA catalyzes the condensation of O-phospho-L-serine and L-glutamate to produce N-(1-amino-1-carboxyl-2-ethyl)-glutamic acid, a direct precursor of L-DAP. Interestingly, in S. rapamycinicus, orf1 is located approximately 10 kb downstream from the right boundary of the rapamycin BGC (see Supplementary Figure S2). Despite its genomic proximity, no direct evidence currently supports its involvement in rapamycin biosynthesis. However, its presence within or near this biosynthetic cluster, along with its high sequence similarity to orfDD and its conserved domain architecture, raises intriguing questions regarding its potential evolutionary, regulatory, or functional significance. Further investigations are warranted to elucidate its precise role in rapamycin biosynthesis and its broader physiological implications.
The genomic region upstream of rapB, which encompasses regulatory and accessory genes associated with rapamycin biosynthesis, exhibits considerable variation among the analyzed strains. In Streptomyces rapamycinicus, this region includes rapT, orfU, rapS, rapR, orfV, orfW, rapX, rapY, and rapZ, with orfZZ positioned outside the cluster, as previously described by Molnár et al. (1996) [83]. Notably, rapS, rapR, orfV, orfW, and rapX are universally conserved across all four strains (Figure 5). The co-occurrence of orfV, orfW, and rapX suggests that these genes may form an operon involved in rapamycin export, given their homology with known transport proteins (see Supplementary Table S1) [83]. However, rapT and orfU are absent in S. iranensis and strains OE54 and OE57T, indicating that these genes may not be essential for rapamycin biosynthesis. This finding is particularly significant given that rapT encodes a putative ketoreductase implicated in the synthesis of the dihydroxycyclohexane carboxylic acid starter unit of rapamycin [83]. Similarly, rapY and rapZ are absent from the S. iranensis, OE54, and OE57T clusters. The absence of rapY is especially notable, as this gene has been identified as a negative regulator of rapamycin biosynthesis, alongside rapR and rapS [81]. In S. rapamycinicus, the overexpression of rapY, rapR, or rapS results in a marked reduction in rapamycin production, further confirming their role as negative regulators [81]. Interestingly, rapY encodes a protein with a high sequence identity to members of the TetR family of transcriptional regulators. Its regulatory function may be compensated by orf2 in strain OE57T and orf8 in S. iranensis and OE54, as these genes encode putative TetR/AcrR family transcriptional regulators. Additionally, the absence of orfZ, which encodes a hypothetical protein with no significant sequence similarity to known functional domains (see Supplementary Table S1), suggests that it may not play a crucial role in rapamycin biosynthesis.
The OE57T strain harbors three unique genes within its rapamycin BGC —orf2, orf3, and orf4— that are absent in the other analyzed clusters. Notably, orf3 encodes a protein with homology to various methyltransferases (see Supplementary Table S1), suggesting a potential role in the site-specific methylation of the rapamycin structure. Meanwhile, orf2 encodes a putative transcriptional regulator belonging to the TetR/AcrR family, which may influence rapamycin biosynthesis. The orf4 gene product exhibits a similarity to protein kinase domain-containing proteins, implying a possible role in regulatory pathways controlling rapamycin production. Similarly, the S. iranensis and OE54 clusters contain three unique genes orf6, orf7, and orf8. orf8 encodes another TetR/AcrR family regulator, likely contributing to the transcriptional control of rapamycin biosynthesis in these strains. The orf7 gene product shares a similarity with FAD-dependent mono-oxygenases, suggesting a potential involvement in hydroxylation reactions that could modify the rapamycin structure. Lastly, orf6 encodes a protein belonging to the SGNH/GDSL hydrolase family, which is known to participate in diverse biological processes, including lipid metabolism and secondary metabolite biosynthesis. The considerable genetic variability observed in the left-flanking region of the rapamycin BGC across these strains suggests that these additional genes may contribute to the biosynthesis of structurally distinct rapamycin analogs (rapalogs) or modulate biosynthetic regulatory mechanisms. These variations likely reflect diverse ecological adaptations among rapamycin-producing Streptomyces species and warrant further investigation to elucidate their functional significance.
Finally, we would like to emphasize that this comparative genomic analysis provides valuable insights into the conservation and diversification of rapamycin biosynthesis among different Streptomyces species and highlights the genetic basis underlying its production in strains OE54 and OE57T. These variations in gene content and organization highlight potential regulatory adaptations in rapamycin-producing Streptomyces species and suggest functional redundancy, or alternative regulatory mechanisms that warrant further investigation.
Beyond the rapamycin BGC, we analyzed the clusters responsible for actinoplanic acid and epoxinnamide biosynthesis. Notably, neither cluster was directly identified by antiSMASH. Instead, their classification was inferred based on literature comparisons and detailed analyses of sequence homology and synteny. Our findings indicate that the epoxinnamide cluster belongs to the non-ribosomal peptide synthetase (NRPS) family with arylpolyene-like features, exhibiting 50% similarity to kitacinnamycin. Meanwhile, the actinoplanic acid cluster is classified as NRPS-like, contains a Type I polyketide synthase (T1PKS) module, and shares 20% similarity with lydicamycin.
The genetic organization of the epoxinnamide BGC revealed a conserved core region (epcA–epcE) across all analyzed strains, though substantial differences were observed in the flanking biosynthetic, regulatory, and transport-associated genes. In S. rapamycinicus and OE57T, the cluster retains a compact and continuous structure, with regulatory genes (epcB and epcC) and transport-related genes (epcF and epcG) positioned adjacent to the core biosynthetic genes. In contrast, S. iranensis and OE54 display notable gene losses and rearrangements, particularly in the upstream region, where genes 1–5 are either absent or fragmented (Supplementary Information Figure S6). Furthermore, while the only previously published study on epoxinnamide described two separate core genes (epcD and epcE), our analysis of two newly sequenced strains alongside two reference strains consistently identified a single gene in this region, which we have designated epcDE (Supplementary Information Figure S6). This discrepancy may arise from differences in the annotation criteria, the natural variation in gene organization, or potential sequencing and assembly artifacts. Additionally, the observed variation in regulatory gene organization across these strains suggests potential differences in expression control mechanisms, which could influence metabolite production.
A notable feature of the actinoplanic acid BGC is its genomic context across the analyzed Streptomyces strains. In S. rapamycinicus, the APL cluster is co-localized with the rapamycin BGC, suggesting a potential evolutionary and functional interdependence between the two pathways [84]. However, in S. iranensis, OE54, and OE57T, the APL and rapamycin clusters are situated in opposite chromosomal regions. Despite this spatial separation, the APL cluster remains highly conserved in both gene content and organization across these strains (Supplementary Information Figure S3). This genomic dispersion, previously reported in S. iranensis [84], extends to OE54 and OE57T, suggesting that such rearrangements are not isolated events but may represent a broader evolutionary trend. Interestingly, actinoplanic acid was not detected in the chemical analyses of OE54 and OE57T fermentation broths. While the genomic separation of regulatory elements, such as aplR, from the core biosynthetic genes might suggest transcriptional uncoupling, Mrak et al. (2018) [84] demonstrated that aplR deletion in S. rapamycinicus did not abolish APL production. This finding could imply the existence of alternative regulatory mechanisms that maintain pathway expression despite genomic separation. Moreover, the mere presence of biosynthetic genes does not necessarily indicate active expression, as regulatory control is complex and influenced by strain-specific and environmental factors. The absence of APL production under the tested conditions may reflect these intricate regulatory dynamics.
These findings underscore the complexity and plasticity of secondary metabolism in Streptomyces. The structural variability observed among these BGCs, likely driven by genomic rearrangements, highlights the limitations of annotation tools such as antiSMASH when analyzing clusters not well-represented in reference databases. Consequently, integrating comparative genomics with experimental validation is essential for fully exploring the biosynthetic potential of these strains. Future studies employing transcriptomic and metabolomic approaches will be crucial to elucidating how these genetic differences translate into metabolite diversity, potentially leading to the discovery of novel bioactive compounds.
5. Conclusions
Root tissues from olive trees affected by Verticillium wilt have proven to be a valuable reservoir for the isolation of endophytic Streptomyces strains. More than 25% of the isolates exhibited some antifungal activity against V. dahliae. Among these, two isolates—Streptomyces iranensis OE54 and Streptomyces lacaronensis OE57—exhibited the highest levels of growth inhibition. Both strains were able to show strong antifungal activity against V. dahliae in both in vitro and small-scale soil bioassays. This activity could be attributed to the production of rapamycin, a compound that effectively inhibited conidial germination and was particularly potent in suppressing the mycelial growth of V. dahliae at concentrations within the nanograms per mililiter range. Streptomyces lacaronensis sp. nov. OE57T has been formally described as a new species within the genus Streptomyces and recognized as a new producer of rapamycin.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms13071622/s1, Figure S1: Streptomyces phylogenetic tree inferred from concatenated partial sequences of the housekeeping genes (atpD, gyrB, recA, rpoB, and trpB) of endophytic OE54 and OE57T strains with type strains obtained from the ARS Microbial Genomic Sequence Database server; Figure S2: Maximum likelihood phylogenetic tree based on 16S rRNA gene sequences, showing the relationships between the OE54 and OE57T strains and their closely related species; Figure S3: Genetic organization of the rapamycin and actinoplanic acid biosynthetic gene clusters (BGCs) in S. rapamycinicus DSM 41530T (a), OE57T (b), S. iranensis DSM 41954T (c), and OE54 (d) genomes; Figure S4: High-performance liquid chromatography (HPLC) chromatogram of fraction F9, obtained via vacuum flash chromatography from the fermentation broth of strain OE54, using an evaporative light scattering detector (ELSD) (a) and high-resolution electrospray ionization time-of-flight mass spectrometry (HR ESI-TOF-MS) spectrum of the corresponding compound, identified as rapamycin, with a molecular ion at m/z 936.5197 [M + Na]+ (b); Figure S5: High-performance liquid chromatography (HPLC) chromatogram of fraction F8, obtained after a second round of flash chromatography from the fermentation broth of strain OE54, using an evaporative light scattering detector (ELSD) (a) and high-resolution electrospray ionization time-of-flight mass spectrometry (HR ESI-TOF-MS) spectrum of the corresponding compound, identified as epoxinnamide, with a molecular ion at m/z 1298.5188 [M + H]+ (b); Figure S6: Genetic organization of the epoxinnamide cluster in Streptomyces sp. OID44 (a), S. rapamycinicus DSM 41530T (b), OE57T strain (c), S. iranensis DSM 41954T (d), and OE54 strain (e); Figure S7: Probit analysis for germination of conidispores of V. dahliae V937I strain after treatment with commercial rapamycin; Figure S8: Two-dimensional TLC plate of polar lipids of strains OE57T and its close phylogenomic relative Streptomyces rapamycinicus DSM 41530T stained with molybdatophosphoric acid. Table S1: Inferred functions of rap genes and ORFs located in the rapamycin biosynthetic clusters of S. rapamycinicus NRRL 5491, S. iranensis DSM 41954, OE54, and OE57T strains; Table S2. 13C, 1H Nuclear Magnetic Resonance (NMR) spectral data of rapamycin [δ (ppm), CDCl3]; Table S3: 13C, 1H Nuclear Magnetic Resonance (NMR) Spectral Data of Eppoxinnamide [δ (ppm), JHH (Hz); CDCl3]; Table S4: Cultural and growth characteristics of strains OE54 and OE57T, along with their closest phylogenomic relatives; Table S5: Phenotypic characteristics of strains OE54 and OE57T, along with their closest phylogenomic relatives, as determined by API 20NE and API ZYM assays; Table S6: Fatty acids profiles of the Streptomyces lacaronensis sp. nov. strain OE57T (=DSM 118741T) and its closest phylogenomic neighbor Streptomyces rapamycinicus DSM 41530T. References [81,83,98,99,100,102] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, I.N., R.C. and J.J.R.C.; methodology, C.C.-P., M.R.-M., S.K., M.N.-S., J.M.S.-L. and S.G.; bioinformatics analysis, M.R.-M.; investigation, C.C.-P., M.R.-M., S.K., M.N.-S., J.M.S.-L. and S.G.; writing—original draft preparation, M.R.-M., I.N., J.M.S.-L., R.C. and J.J.R.C.; writing—review and editing, I.N., J.M.S.-L., R.C. and J.J.R.C.; project administration, J.J.R.C.; funding acquisition, J.J.R.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Rio Lacarón S.L. (La Garrovilla, Badajoz, Spain). Carla Calvo-Peña (EDU/601/2020) was supported by a predoctoral contract from the Junta de Castilla y León and the European Social Fund. Marina Ruiz-Muñoz was supported by a postdoctoral contract financed by the Ministry of Science and Innovation (MCIN), the State Investigation Agency (AEI) (DOI/10.13039/501100011033), and the European Union “NextGenerationEU”/Recovery Plant, Transformation and Resilience (PRTR) (Project PCI2022-132966).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding authors.
Acknowledgments
We thank Diego Díaz García (Río Lacarón S.L.) for his valuable help in collecting samples in the field. We are also grateful to Jesús Mercado Blanco (Estación Experimental del Zaidín, Spanish National Research Council) for kindly providing V. dahliae V937I strain, and to Birgti Grün, Gesa Martens, Gabriele Pötter, and Marlen Jando (DSMZ—German Collection of Microorganisms and Cell Cultures) for excellent technical assistance.
Conflicts of Interest
José María Sánchez-López was employed by the company Biomar Microbial Technologies. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Inderbitzin, P.; Subbarao, K.V. Verticillium Systematics and Evolution: How Confusion Impedes Verticillium Wilt Management and How to Resolve It. Phytopathology 2014, 104, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Klosterman, S.J.; Atallah, Z.K.; Vallad, G.E.; Subbarao, K.V. Diversity, Pathogenicity, and Management of Verticillium Species. Annu. Rev. Phytopathol. 2009, 47, 39–62. [Google Scholar] [CrossRef]
- Keykhasaber, M.; Thomma, B.P.H.J.; Hiemstra, J.A. Verticillium Wilt Caused by Verticillium dahliae in Woody Plants with Emphasis on Olive and Shade Trees. Eur. J. Plant Pathol. 2018, 150, 21–37. [Google Scholar] [CrossRef]
- Kunej, U.; Jakše, J.; Radišek, S.; Štajner, N. Identification and Characterization of Verticillium nonalfalfae-Responsive MicroRNAs in the Roots of Resistant and Susceptible Hop Cultivars. Plants 2021, 10, 1883. [Google Scholar] [CrossRef]
- López-Escudero, F.J.; Mercado-Blanco, J. Verticillium Wilt of Olive: A Case Study to Implement an Integrated Strategy to Control a Soil-Borne Pathogen. Plant Soil 2011, 344, 1–50. [Google Scholar] [CrossRef]
- Coque, J.J.R.; Álvarez-Pérez, J.M.; Cobos, R.; González-García, S.; Ibáñez, A.M.; Diez Galán, A.; Calvo-Peña, C. Advances in the Control of Phytopathogenic Fungi That Infect Crops through Their Root System. In Advances in Applied Microbiology; Gadd, G.M., Sariaslani, S., Eds.; Academic Press Inc.: Cambridge, MA, USA, 2020; Volume 111, pp. 123–170. [Google Scholar]
- Mulero-Aparicio, A.; Agustí-Brisach, C.; Varo, Á.; López-Escudero, F.J.; Trapero, A. A Non-Pathogenic Strain of Fusarium oxysporum as a Potential Biocontrol Agent against Verticillium Wilt of Olive. Biol. Control 2019, 139, 104045. [Google Scholar] [CrossRef]
- Mulero-Aparicio, A.; Cernava, T.; Turrà, D.; Schaefer, A.; Di Pietro, A.; López-Escudero, F.J.; Trapero, A.; Berg, G. The Role of Volatile Organic Compounds and Rhizosphere Competence in Mode of Action of the Non-Pathogenic Fusarium oxysporum FO12 toward Verticillium Wilt. Front. Microbiol. 2019, 10, 1808. [Google Scholar] [CrossRef]
- Varo, A.; Raya-Ortega, M.C.; Trapero, A. Selection and Evaluation of Micro-Organisms for Biocontrol of Verticillium dahliae in Olive. J. Appl. Microbiol. 2016, 121, 767–777. [Google Scholar] [CrossRef]
- Carrero-Carrón, I.; Trapero-Casas, J.L.; Olivares-García, C.; Monte, E.; Hermosa, R.; Jiménez-Díaz, R.M. Trichoderma asperellum Is Effective for Biocontrol of Verticillium Wilt in Olive Caused by the Defoliating Pathotype of Verticillium dahliae. Crop Prot. 2016, 88, 45–52. [Google Scholar] [CrossRef]
- Lozano-Tovar, M.D.; Garrido-Jurado, I.; Quesada-Moraga, E.; Raya-Ortega, M.C.; Trapero-Casas, A. Metarhizium brunneum and Beauveria bassiana Release Secondary Metabolites with Antagonistic Activity against Verticillium dahliae and Phytophthora megasperma Olive Pathogens. Crop Prot. 2017, 100, 186–195. [Google Scholar] [CrossRef]
- Gómez-Lama Cabanás, C.; Sesmero, R.; Valverde-Corredor, A.; López-Escudero, F.J.; Mercado-Blanco, J. A Split-Root System to Assess Biocontrol Effectiveness and Defense-Related Genetic Responses in above-Ground Tissues during the Tripartite Interaction Verticillium dahliae-Olive-Pseudomonas fluorescens PICF7 in Roots. Plant Soil 2017, 417, 433–452. [Google Scholar] [CrossRef]
- Gómez-Lama Cabanás, C.; Legarda, G.; Ruano-Rosa, D.; Pizarro-Tobías, P.; Valverde-Corredor, A.; Niqui, J.L.; Triviño, J.C.; Roca, A.; Mercado-Blanco, J. Indigenous Pseudomonas spp. Strains from the Olive (Olea europaea L.) Rhizosphere as Effective Biocontrol Agents against Verticillium dahliae: From the Host Roots to the Bacterial Genomes. Front. Microbiol. 2018, 9, 277. [Google Scholar] [CrossRef]
- Markakis, E.A.; Tjamos, S.E.; Antoniou, P.P.; Paplomatas, E.J.; Tjamos, E.C. Biological Control of Verticillium Wilt of Olive by Paenibacillus alvei, Strain K165. BioControl 2016, 61, 293–303. [Google Scholar] [CrossRef]
- Cheffi Azabou, M.; Gharbi, Y.; Medhioub, I.; Ennouri, K.; Barham, H.; Tounsi, S.; Triki, M.A. The Endophytic Strain Bacillus velezensis OEE1: An Efficient Biocontrol Agent against Verticillium Wilt of Olive and a Potential Plant Growth Promoting Bacteria. Biol. Control 2020, 142, 104168. [Google Scholar] [CrossRef]
- Calvo-Peña, C.; Cobos, R.; Sánchez-López, J.M.; Ibáñez, A.; Coque, J.J.R. Albocycline Is the Main Bioactive Antifungal Compound Produced by Streptomyces sp. OR6 against Verticillium dahliae. Plants 2023, 12, 3612. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Díaz, M.; Antón-Domínguez, B.I.; Raya, M.C.; Bernal-Cabrera, A.; Medina-Marrero, R.; Trapero, A.; Agustí-Brisach, C. Streptomyces spp. Strains as Potential Biological Control Agents against Verticillium Wilt of Olive. J. Fungi 2024, 10, 138. [Google Scholar] [CrossRef]
- Seipke, R.F.; Kaltenpoth, M.; Hutchings, M.I. Streptomyces as Symbionts: An Emerging and Widespread Theme? FEMS Microbiol. Rev. 2012, 36, 862–876. [Google Scholar] [CrossRef]
- Janssen, P.H. Identifying the Dominant Soil Bacterial Taxa in Libraries of 16S rRNA and 16S rRNA Genes. Appl. Environ. Microbiol. 2006, 72, 1719–1728. [Google Scholar] [CrossRef]
- Viaene, T.; Langendries, S.; Beirinckx, S.; Maes, M.; Goormachtig, S. Streptomyces as a Plant’s Best Friend? FEMS Microbiol. Ecol. 2016, 92, fiw119. [Google Scholar] [CrossRef]
- Li, J.; Guo-zhen, Z.; Huang, H.; Qin, S.; Zhu, W.-Y.; Zhao, L.-X.; Xu, L.-H.; Zhang, S.; Li, W.-J.; Strobel, G. Isolation and Characterization of Culturable Endophytic Actinobacteria Associated with Artemisia annua L. Antonie Van Leeuwenhoek 2012, 101, 515–527. [Google Scholar] [CrossRef]
- Álvarez-Pérez, J.M.; González-García, S.; Cobos, R.; Olego, M.Á.; Ibañez, A.; Díez-Galán, A.; Garzón-Jimeno, E.; Coque, J.J.R. Use of Endophytic and Rhizosphere Actinobacteria from Grapevine Plants to Reduce Nursery Fungal Graft Infections That Lead to Young Grapevine Decline. Appl. Environ. Microbiol. 2017, 83, e01564-17. [Google Scholar] [CrossRef] [PubMed]
- Ayswaria, R.; Vasu, V.; Krishna, R. Diverse Endophytic Streptomyces Species with Dynamic Metabolites and Their Meritorious Applications: A Critical Review. Crit. Rev. Microbiol. 2020, 46, 750–758. [Google Scholar] [CrossRef]
- Polpass, J.; Maharshi, A.; Jha, B. Actinobacteria in Natural Products Research: Progress and Prospects. Microbiol. Res. 2021, 246, 126708. [Google Scholar] [CrossRef]
- Van Der Heul, H.U.; Bilyk, B.L.; McDowall, K.J.; Seipke, R.F.; Van Wezel, G.P. Regulation of Antibiotic Production in Actinobacteria: New Perspectives from the Post-Genomic Era. Nat. Prod. Rep. 2018, 35, 575–604. [Google Scholar] [CrossRef]
- Chater, K.F.; Biró, S.; Lee, K.J.; Palmer, T.; Schrempf, H. The Complex Extracellular Biology of Streptomyces. FEMS Microbiol. Rev. 2010, 34, 171–198. [Google Scholar] [CrossRef]
- Cuervo, L.; Álvarez-García, S.; Salas, J.A.; Méndez, C.; Olano, C.; Malmierca, M.G. The Volatile Organic Compounds of Streptomyces Spp.: An In-Depth Analysis of Their Antifungal Properties. Microorganisms 2023, 11, 1820. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Bertolli, S.; Park, Y.J.; Tan, Y.; Cutler, K.J.; Srinivas, P.; Asfahl, K.L.; Fonesca-García, C.; Gallagher, L.A.; Li, Y.; et al. Streptomyces Umbrella Toxin Particles Block Hyphal Growth of Competing Species. Nature 2024, 629, 165–173. [Google Scholar] [CrossRef]
- Shirling, E.B.; Gottlieb, D. Methods for the Characterization of Streptomyces Species. Int. J. Syst. Bacteriol. 1966, 16, 313–340. [Google Scholar] [CrossRef]
- Martin, J.F.; McDaniel, L.E. Biosynthesis of Candicidin by Phosphate-Limited Resting Cells of Streptomyces griseus. Eur. J. Appl. Microbiol. 1976, 3, 135–144. [Google Scholar]
- Kieser, T.; Bibb, M.; Buttner, M.; Chater, K.; Hopwood, D. Practical Streptomyces Genetics; John Innes Foundation: Norwich, UK, 2000; ISBN 0708406238. [Google Scholar]
- Collado-Romero, M.; Mercado-Blanco, J.; Olivares-García, C.; Valverde-Corredor, A.; Jiménez-Díaz, R.M. Molecular Variability within and among Verticillium dahliae Vegetative Compatibility Groups Determined by Fluorescent Amplified Fragment Length Polymorphism and Polymerase Chain Reaction Markers. Phytopathology 2006, 96, 485–495. [Google Scholar] [CrossRef]
- Schrey, S.D.; Erkenbrack, E.; Früh, E.; Fengler, S.; Hommel, K.; Horlacher, N.; Schulz, D.; Ecke, M.; Kulik, A.; Fiedler, H.P.; et al. Production of Fungal and Bacterial Growth Modulating Secondary Metabolites Is Widespread among Mycorrhiza-Associated Streptomycetes. BMC Microbiol. 2012, 12, 164. [Google Scholar] [CrossRef]
- Hopwood, D.A.; Bibb, M.J.; Chater, K.F.; Kieser, H.M.; Lydiate, D.J.; Smith, C.P.; Ward, J.M.; Schrempf, H. Genetic Manipulation of Streptomyces: A Laboratory Manual; The John Innes Institute: Norwich, UK, 1985. [Google Scholar]
- Lane, D. 16S/23S rRNA Sequencing. In Nucleic Acid Techniques in Bacterial Systematics; Stackebrandt, E., Goodfellow, M., Eds.; John Wiley and Sons: New York, NY, USA, 1991; pp. 115–175. [Google Scholar]
- Kim, O.S.; Cho, Y.J.; Lee, K.; Yoon, S.H.; Kim, M.; Na, H.; Park, S.C.; Jeon, Y.S.; Lee, J.H.; Yi, H.; et al. Introducing EzTaxon-e: A Prokaryotic 16S RRNA Gene Sequence Database with Phylotypes That Represent Uncultured Species. Int. J. Syst. Evol. Microbiol. 2012, 62, 716–721. [Google Scholar] [CrossRef]
- Kimura, M. A Simple Method for Estimating Evolutionary Rates of Base Substitutions through Comparative Studies of Nucleotide Sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Rong, X.; Huang, Y. Taxonomic Evaluation of the Streptomyces hygroscopicus Clade Using Multilocus Sequence Analysis and DNA-DNA Hybridization, Validating the MLSA Scheme for Systematics of the Whole Genus. Syst. Appl. Microbiol. 2012, 35, 7–18. [Google Scholar] [CrossRef]
- Guo, Y.P.; Zheng, W.; Rong, X.Y.; Huang, Y. A Multilocus Phylogeny of the Streptomyces griseus 16S rRNA Gene Clade: Use of Multilocus Sequence Analysis for Streptomycete Systematics. Int. J. Syst. Evol. Microbiol. 2008, 58, 149–159. [Google Scholar] [CrossRef]
- Rong, X.; Guo, Y.; Huang, Y. Proposal to Reclassify the Streptomyces albidoflavus Clade on the Basis of Multilocus Sequence Analysis and DNA-DNA Hybridization, and Taxonomic Elucidation of Streptomyces griseus subsp. solvifaciens. Syst. Appl. Microbiol. 2009, 32, 314–322. [Google Scholar] [CrossRef]
- Koren, S.; Walenz, B.P.; Berlin, K.; Miller, J.R.; Bergman, N.H.; Phillippy, A.M. Canu: Scalable and Accurate Long-Read Assembly via Adaptive k-Mer Weighting and Repeat Separation. Genome Res. 2017, 27, 722–736. [Google Scholar] [CrossRef] [PubMed]
- Mikheenko, A.; Prjibelski, A.; Saveliev, V.; Antipov, D.; Gurevich, A. Versatile Genome Assembly Evaluation with QUAST-LG. Bioinformatics 2018, 34, i142–i150. [Google Scholar] [CrossRef]
- Overbeek, R.; Olson, R.; Pusch, G.D.; Olsen, G.J.; Davis, J.J.; Disz, T.; Edwards, R.A.; Gerdes, S.; Parrello, B.; Shukla, M.; et al. The SEED and the Rapid Annotation of Microbial Genomes Using Subsystems Technology (RAST). Nucleic Acids Res. 2014, 42, 206–214. [Google Scholar] [CrossRef]
- Schleifer, K.H.; Kandler, O. Peptidoglycan Types of Bacterial Cell Walls and Their Taxonomic Implications. Bacteriol. Rev. 1972, 36, 407–477. [Google Scholar] [CrossRef]
- Lechevalier, M.P.; Lechevalier, H.A. Chemical Composition as a Criterion in the Classification of Aerobic Actinomycetes. Int. J. Syst. Bacteriol. 1970, 20, 435–443. [Google Scholar]
- Minnikin A’, D.E.; O’donnell, A.G.; Goodfellow, M.; Alderson, G.; Athalye, M.; Schaal, A.; Parlett, J.H. An Integrated Procedure for the Extraction of Bacterial Isoprenoid Quinones and Polar Lipids. J. Microbiol. Methods 1984, 2, 233–241. [Google Scholar] [CrossRef]
- Kroppenstedt, R.M.; Goodfellow, M. The Family Thermomonosporaceae: Actinocorallia, Actinomadura, Spirillispora and Thermomonospora. In The Prokaryotes: A Handbook on the Biology of Bacteria. Archaea, Bacteria, Firmicutes, Actinomycetes; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, H.H., Stackebrandt, E., Eds.; Springer: New York, NY, USA, 2006; Volume 3, pp. 682–724. [Google Scholar]
- Sasser, M. MIDI Technical Note 101. Identification of Bacteria by Gas Chromatography of Cellular Fatty Acids. In MIDI Technical Note 101; MIDI Inc: Newark, DE, USA, 1990; pp. 1–7. [Google Scholar]
- Vieira, S.; Huber, K.J.; Neumann-Schaal, M.; Geppert, A.; Luckner, M.; Wanner, G.; Overmann, J. Usitatibacter rugosus Gen. Nov., Sp. Nov. and Usitatibacter palustris Sp. Nov., Novel Members of Usitatibacteraceae Fam. Nov. within the Order Nitrosomonadales Isolated from Soil. Int. J. Syst. Evol. Microbiol. 2021, 71, 1–12. [Google Scholar] [CrossRef]
- Moss, C.W.; Lambert-Fair, M.A. Location of Double Bonds in Monounsaturated Fatty Acids of Campylobacter cryaerophila with Dimethyl Disulfide Derivatives and Combined Gas Chromatography-Mass Spectrometry. J. Clin. Microbiol. 1989, 27, 1467–1470. [Google Scholar] [CrossRef] [PubMed]
- Schumann, P.; Kalensee, F.; Cao, J.; Criscuolo, A.; Clermont, D.; Köhler, J.M.; Meier-Kolthoff, J.P.; Neumann-Schaal, M.; Tindall, B.J.; Pukall, R. Reclassification of Haloactinobacterium glacieicola as Occultella glacieicola Gen. Nov., Comb. Nov., of Haloactinobacterium album as Ruania alba Comb. Nov, with an Emended Description of the Genus Ruania, Recognition That the Genus Names Haloactinobacterium and Ruania Are Heterotypic Synonyms and Description of Occultella aeris Sp. Nov., a Halotolerant Isolate from Surface Soil Sampled at an Ancient Copper Smelter. Int. J. Syst. Evol. Microbiol. 2021, 71, 004769. [Google Scholar] [CrossRef]
- Meier-Kolthoff, J.P.; Auch, A.F.; Klenk, H.-P.; Göker, M.G. Genome Sequence-Based Species Delimitation with Confidence Intervals and Improved Distance Functions. BMC Bioinform. 2013, 14, 60. [Google Scholar] [CrossRef]
- Meier-Kolthoff, J.P.; Carbasse, J.S.; Peinado-Olarte, R.L.; Göker, M. TYGS and LPSN: A Database Tandem for Fast and Reliable Genome-Based Classification and Nomenclature of Prokaryotes. Nucleic Acids Res. 2022, 50, D801–D807. [Google Scholar] [CrossRef]
- Yoon, S.H.; Ha, S.M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing EzBioCloud: A Taxonomically United Database of 16S rRNA Gene Sequences and Whole-Genome Assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613–1617. [Google Scholar] [CrossRef]
- Meier-Kolthoff, J.P.; Göker, M. TYGS Is an Automated High-Throughput Platform for State-of-the-Art Genome-Based Taxonomy. Nat. Commun. 2019, 10, 2182. [Google Scholar] [CrossRef]
- Meier-Kolthoff, J.P.; Göker, M.; Spröer, C.; Klenk, H.P. When Should a DDH Experiment Be Mandatory in Microbial Taxonomy? Arch. Microbiol. 2013, 195, 413–418. [Google Scholar] [CrossRef]
- Blin, K.; Shaw, S.; Augustijn, H.E.; Reitz, Z.L.; Biermann, F.; Alanjary, M.; Fetter, A.; Terlouw, B.R.; Metcalf, W.W.; Helfrich, E.J.N.; et al. AntiSMASH 7.0: New and Improved Predictions for Detection, Regulation, Chemical Structures and Visualisation. Nucleic Acids Res. 2023, 51, W46–W50. [Google Scholar] [CrossRef]
- Medema, M.H.; Kottmann, R.; Yilmaz, P.; Cummings, M.; Biggins, J.B.; Blin, K.; De Bruijn, I.; Chooi, Y.H.; Claesen, J.; Coates, R.C.; et al. Minimum Information about a Biosynthetic Gene Cluster. Nat. Chem. Biol. 2015, 11, 625–631. [Google Scholar] [CrossRef] [PubMed]
- van den Belt, M.; Gilchrist, C.; Booth, T.J.; Chooi, Y.H.; Medema, M.H.; Alanjary, M. CAGECAT: The CompArative GEne Cluster Analysis Toolbox for Rapid Search and Visualisation of Homologous Gene Clusters. BMC Bioinform. 2023, 24, 181. [Google Scholar] [CrossRef]
- Navarro-Muñoz, J.C.; Selem-Mojica, N.; Mullowney, M.W.; Kautsar, S.A.; Tryon, J.H.; Parkinson, E.I.; De Los Santos, E.L.C.; Yeong, M.; Cruz-Morales, P.; Abubucker, S.; et al. A Computational Framework to Explore Large-Scale Biosynthetic Diversity. Nat. Chem. Biol. 2020, 16, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Romi, W.; Das, R.; Sharma, H.K.; Thakur, D. Antimicrobial Potentiality of Actinobacteria Isolated from Two Microbiologically Unexplored Forest Ecosystems of Northeast India. BMC Microbiol. 2018, 18, 71. [Google Scholar] [CrossRef]
- Awla, H.K.; Rashid, T.S. HPLC Fractionation: A Comparative Analysis of Anti-Fungal Compounds from Different Streptomyces Isolates Inhibiting Colletotrichum acutatum. Biocatal. Agric. Biotechnol. 2020, 27, 101688. [Google Scholar] [CrossRef]
- López-Moral, A.; Agustí-Brisach, C.; Leiva-Egea, F.M.; Trapero, A. Influence of Cultivar and Biocontrol Treatments on the Effect of Olive Stem Extracts on the Viability of Verticillium dahliae Conidia. Plants 2022, 11, 554. [Google Scholar] [CrossRef]
- EUCAST Definitive Document. Methods for the determination of susceptibility of bacteria to antimicrobial agents. Terminology. Clin. Microbiol. Infect. 1998, 4, 291–296. [Google Scholar] [CrossRef]
- Kim, M.; Oh, H.S.; Park, S.C.; Chun, J. Towards a Taxonomic Coherence between Average Nucleotide Identity and 16S rRNA Gene Sequence Similarity for Species Demarcation of Prokaryotes. Int. J. Syst. Evol. Microbiol. 2014, 64, 346–351. [Google Scholar] [CrossRef]
- Kämpfer, P. Genus Streptomyces Waksman and Henrici 1943, 339AL Emend. Witt and Stackebrandt 1990, 370, Emend. Wellington, Stackebrandt, Sanders, Wolstrup and Jorgensen, 1992, 159. In Bergey’s Manual of Systematic Bacteriology; Springer: New York, NY, USA, 2012; Volume 5, pp. 1455–1767. [Google Scholar]
- Wayne, L.G.; Brenner, D.J.; Colwell, R.R.; Grimont, P.A.D.; Kandler, O.; Krichevsky, M.I.; Moore, L.H.; Moore, W.E.C.; Murray, R.G.E.; Stackebrandt, E.; et al. Report of the Ad Hoc Committee on Reconciliation of Approaches to Bacterial Systematics. Int. J. Syst. Bacteriol. 1987, 37, 463–464. [Google Scholar] [CrossRef]
- Sun, F.; Xu, S.; Jiang, F.; Liu, W. Genomic-Driven Discovery of an Amidinohydrolase Involved in the Biosynthesis of Mediomycin A. Appl. Microbiol. Biotechnol. 2018, 102, 2225–2234. [Google Scholar] [CrossRef]
- Cheng, J.; Yang, S.H.; Palaniyandi, S.A.; Han, J.S.; Yoon, T.M.; Kim, T.J.; Suh, J.W. Azalomycin F Complex Is an Antifungal Substance Produced by Streptomyces malaysiensis MJM1968 Isolated from Agricultural Soil. J. Appl. Biol. Chem. 2010, 53, 545–552. [Google Scholar] [CrossRef]
- Bastidas, R.J.; Shertz, C.A.; Lee, S.C.; Heitman, J.; Cardenas, M.E. Rapamycin Exerts Antifungal Activity In Vitro and In Vivo against Mucor circinelloides via FKBP12-Dependent Inhibition of Tor. Eukaryot. Cell 2012, 11, 270. [Google Scholar] [CrossRef]
- Jiang, H.; Rao, Y.; Mei, L.; Wang, Y. Antifungal Activity of Rapamycin on Botryosphaeria dothidea and Its Effect against Chinese Hickory Canker. Pest. Manag. Sci. 2021, 77, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhu, T.; Song, Y.; Luo, X.; Feng, L.; Zhuo, F.; Li, F.; Ren, M. Functional Characterization of Target of Rapamycin Signaling in Verticillium dahliae. Front. Microbiol. 2019, 10, 501. [Google Scholar] [CrossRef]
- Netzker, T.; Schroeckh, V.; Gregory, M.A.; Flak, M.; Krespach, M.K.C.; Leadlay, P.F.; Brakhage, A.A. An Efficient Method to Generate Gene Deletion Mutants of the Rapamycin-Producing Bacterium Streptomyces iranensis HM 35. Appl. Environ. Microbiol. 2016, 82, 3481–3492. [Google Scholar] [CrossRef]
- Fang, A.; Wong, G.K.; Demain, A.L. Enhancement of the Antifungal Activity of Rapamycin by the Coproduced Elaiophylin and Nigerin. J. Antibiot. 2000, 53, 158–162. [Google Scholar]
- Mandala, S.M.; Thornton, R.A.; Milligan, J.; Rosenbach, M.; Garcia-Calvo, M.; Bull, H.G.; Harris, G.; Abruzzo, G.K.; Flattery, A.M.; Gill, C.J.; et al. Rustmicin, a Potent Antifungal Agent, Inhibits Sphingolipid Synthesis at Inositol Phosphoceramide Synthase. J. Biol. Chem. 1998, 273, 14942–14949. [Google Scholar] [CrossRef]
- Fujita, K.; Sugiyama, R.; Nishimura, S.; Ishikawa, N.; Arai, M.A.; Ishibashi, M.; Kakeya, H. Stereochemical Assignment and Biological Evaluation of BE-14106 Unveils the Importance of One Acetate Unit for the Antifungal Activity of Polyene Macrolactams. J. Nat. Prod. 2016, 79, 1877–1880. [Google Scholar] [CrossRef]
- Goh, F.; Zhang, M.M.; Lim, T.R.; Low, K.N.; Nge, C.E.; Heng, E.; Yeo, W.L.; Sirota, F.L.; Crasta, S.; Tan, Z.; et al. Identification and Engineering of 32 Membered Antifungal Macrolactone Notonesomycins. Microb. Cell Fact. 2020, 19, 71. [Google Scholar] [CrossRef]
- Shentu, X.P.; Cao, Z.Y.; Xiao, Y.; Tang, G.; Ochi, K.; Yu, X.P. Substantial Improvement of Toyocamycin Production in Streptomyces diastatochromogenes by Cumulative Drug-Resistance Mutations. PLoS ONE 2018, 13, e0203006. [Google Scholar] [CrossRef]
- Jo, H.G.; Adidjaja, J.J.; Kim, D.K.; Park, B.S.; Lee, N.; Cho, B.K.; Kim, H.U.; Oh, M.K. Comparative Genomic Analysis of Streptomyces rapamycinicus NRRL 5491 and Its Mutant Overproducing Rapamycin. Sci. Rep. 2022, 12, 10302. [Google Scholar] [CrossRef]
- Park, S.R.; Yoo, Y.J.; Ban, Y.H.; Yoon, Y.J. Biosynthesis of Rapamycin and Its Regulation: Past Achievements and Recent Progress. J. Antibiot. 2010, 63, 434–441. [Google Scholar] [CrossRef]
- Yoo, Y.J.; Hwang, J.Y.; Shin, H.L.; Cui, H.; Lee, J.; Yoon, Y.J. Characterization of Negative Regulatory Genes for the Biosynthesis of Rapamycin in Streptomyces rapamycinicus and Its Application for Improved Production. J. Ind. Microbiol. Biotechnol. 2015, 42, 125–135. [Google Scholar] [CrossRef]
- Cuthbertson, L.; Nodwell, J.R. The TetR Family of Regulators. Microbiol. Mol. Biol. Rev. 2013, 77, 440–475. [Google Scholar] [CrossRef] [PubMed]
- Molnár, I.; Aparicio, J.F.; Haydock, S.F.; Khaw, L.; Schwecke, T.; König, A.; Staunton, J.; Leadlay, P.F. Organisation of the Biosynthetic Gene Cluster for Rapamycin in Streptomyces hygroscopicus: Analysis of Genes Flanking the Polyketide Synthase. Gene 1996, 169, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mrak, P.; Krastel, P.; Lukančič, P.P.; Tao, J.; Pistorius, D.; Moore, C.M. Discovery of the Actinoplanic Acid Pathway in Streptomyces rapamycinicus Reveals a Genetically Conserved Synergism with Rapamycin. J. Biol. Chem. 2018, 293, 19982–19995. [Google Scholar] [CrossRef]
- McAlpine, J.B.; Swanson, S.J.; Jackson, M.; Whittern, D.N. Revised NMR Assignments for Rapamycin. J. Antibiot. 1991, 44, 688–690. [Google Scholar] [CrossRef]
- Kang, S.; Han, J.; Jang, S.C.; An, J.S.; Kang, I.; Kwon, Y.; Nam, S.J.; Shim, S.H.; Cho, J.C.; Lee, S.K.; et al. Epoxinnamide: An Epoxy Cinnamoyl-Containing Nonribosomal Peptide from an Intertidal Mudflat-Derived Streptomyces sp. Mar. Drugs 2022, 20, 455. [Google Scholar] [CrossRef]
- Hamedi, J.; Mohammadipanah, F.; Klenk, H.P.; Pötter, G.; Schumann, P.; Spröer, C.; Von Jan, M.; Kroppenstedt, R.M. Streptomyces iranensis Sp. Nov., Isolated from Soil. Int. J. Syst. Evol. Microbiol. 2010, 60, 1504–1509. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Clemente, E.; Moreno-González, V.; Ibáñez, A.; Calvo-Peña, C.; Ghoreshizadeh, S.; Radišek, S.; Cobos, R.; Coque, J.J.R. Changes in the Microbial Composition of the Rhizosphere of Hop Plants Affected by Verticillium Wilt Caused by Verticillium Nonalfalfae. Microorganisms 2023, 11, 1819. [Google Scholar] [CrossRef] [PubMed]
- Vézina, C.; Kudelski, A.; Sehgal, S.N. Rapamycin (AY-22,989), a New Antifungal Antibiotic. J. Antibiot. 1975, 28, 721–726. [Google Scholar] [CrossRef]
- Nishida, H.; Sakakibara, T.; Aoki, F.; Saito, T.; Ichikawa, K.; Inagaki, T.; Kojima, Y.; Yamauchi, Y.; Huangt, L.H.; Guadliana, M.A.; et al. Generation of Novel Rapamycin Structures by Microbial Manipulations. J. Antibiot. 1995, 48, 657–666. [Google Scholar] [CrossRef]
- Trevillian, P. Immunosuppressants-Clinical Applications. Aust. Prescr. 2006, 29, 102–108. [Google Scholar] [CrossRef]
- Martel, R.R.; Klicius, J.; Galet, S. Inhibition of the Immune Response by Aapamycin, a New Antifungal Antibiotic. Can. J. Physiol. Pharmacol. 1977, 55, 48–51. [Google Scholar] [CrossRef]
- Law, B.K. Rapamycin: An Anti-Cancer Immunosuppressant? Crit. Rev. Oncol. Hematol. 2005, 56, 47–60. [Google Scholar] [CrossRef]
- Tain, L.S.; Mortiboys, H.; Tao, R.N.; Ziviani, E.; Bandmann, O.; Whitworth, A.J. Rapamycin Activation of 4E-BP Prevents Parkinsonian Dopaminergic Neuron Loss. Nat. Neurosci. 2009, 12, 1129–1135. [Google Scholar] [CrossRef]
- Malagelada, C.; Jin, Z.H.; Jackson-Lewis, V.; Przedborski, S.; Greene, L.A. Rapamycin Protects against Neuron Death in In Vitro and In Vivo Models of Parkinson’s Disease. J. Neurosci. 2010, 30, 1166–1175. [Google Scholar] [CrossRef]
- Sharp, Z.D.; Strong, R. Rapamycin, the Only Drug That Has Been Consistently Demonstrated to Increase Mammalian Longevity. An Update. Exp. Gerontol. 2023, 176, 112166. [Google Scholar] [CrossRef]
- Yoo, Y.J.; Kim, H.; Park, S.R.; Yoon, Y.J. An Overview of Rapamycin: From Discovery to Future Perspectives. J. Ind. Microbiol. Biotechnol. 2017, 44, 537–553. [Google Scholar] [CrossRef] [PubMed]
- Kuščer, E.; Coates, N.; Challis, I.; Gregory, M.; Wilkinson, B.; Sheridan, R.; Petković, H. Roles of RapH and RapG in Positive Regulation of Rapamycin Biosynthesis in Streptomyces hygroscopicus. J. Bacteriol. 2007, 189, 4756–4763. [Google Scholar] [CrossRef] [PubMed]
- Schwecke, T.; Aparicio, J.F.; Molnar, I.; König, A.; Khaw, L.E.; Haydock, S.F.; Oliynyk, M.; Caffrey, P.; Cortés, J.; Lester, J.B.; et al. The Biosynthetic Gene Cluster for the Polyketide Immunosuppressant Rapamycin. Proc. Natl. Acad. Sci. USA 1995, 92, 7839–7843. [Google Scholar] [CrossRef]
- Gatto, G.J.; Boyne, M.T.; Kelleher, N.L.; Walsh, C.T. Biosynthesis of Pipecolic Acid by RapL, a Lysine Cyclodeaminase Encoded in the Rapamycin Gene Cluster. J. Am. Chem. Soc. 2006, 128, 3838–3847. [Google Scholar] [CrossRef] [PubMed]
- Kobylarz, M.J.; Grigg, J.C.; Takayama, S.I.J.; Rai, D.K.; Heinrichs, D.E.; Murphy, M.E.P. Synthesis of L-2,3-Diaminopropionic Acid, a Siderophore and Antibiotic Precursor. Chem. Biol. 2014, 21, 379–388. [Google Scholar] [CrossRef]
- König, A.; Schwecke, T.; Molnár, I.; Böhm, G.A.; Lowden, P.A.S.; Staunton, J.; Leadlay, P.F. The Pipecolate-Incorporating Enzyme for the Biosynthesis of the Immunosuppressant Rapamycin—Nucleotide Sequence Analysis, Disruption and Heterologous Expression of Rap P from Streptomyces Hygroscopicus. Eur. J. Biochem. 1997, 247, 526–534. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).