Removal of Chemical Oxygen Demand (COD) from Swine Farm Wastewater by Corynebacterium xerosis H1
Abstract
1. Introduction
2. Materials and Methods
2.1. Degradation Capacity
2.2. Strain Identification
2.3. Strain Characterization
2.4. Fourier-Transform Ion Cyclotron Resonance Mass Spectrometry (FT-ICR MS)
2.5. Sequencing Batch Reactor (SBR) Operation and Setup
2.6. Three-Dimensional Fluorescence Spectroscopy (3D-EEM)
2.7. The 16S rRNA Gene Sequencing
2.8. Conventional Index Analysis
2.9. qPCR Validation
2.10. Viable Count of Corynebacterium xerosis
2.11. Statistical Analysis
3. Results and Discussion
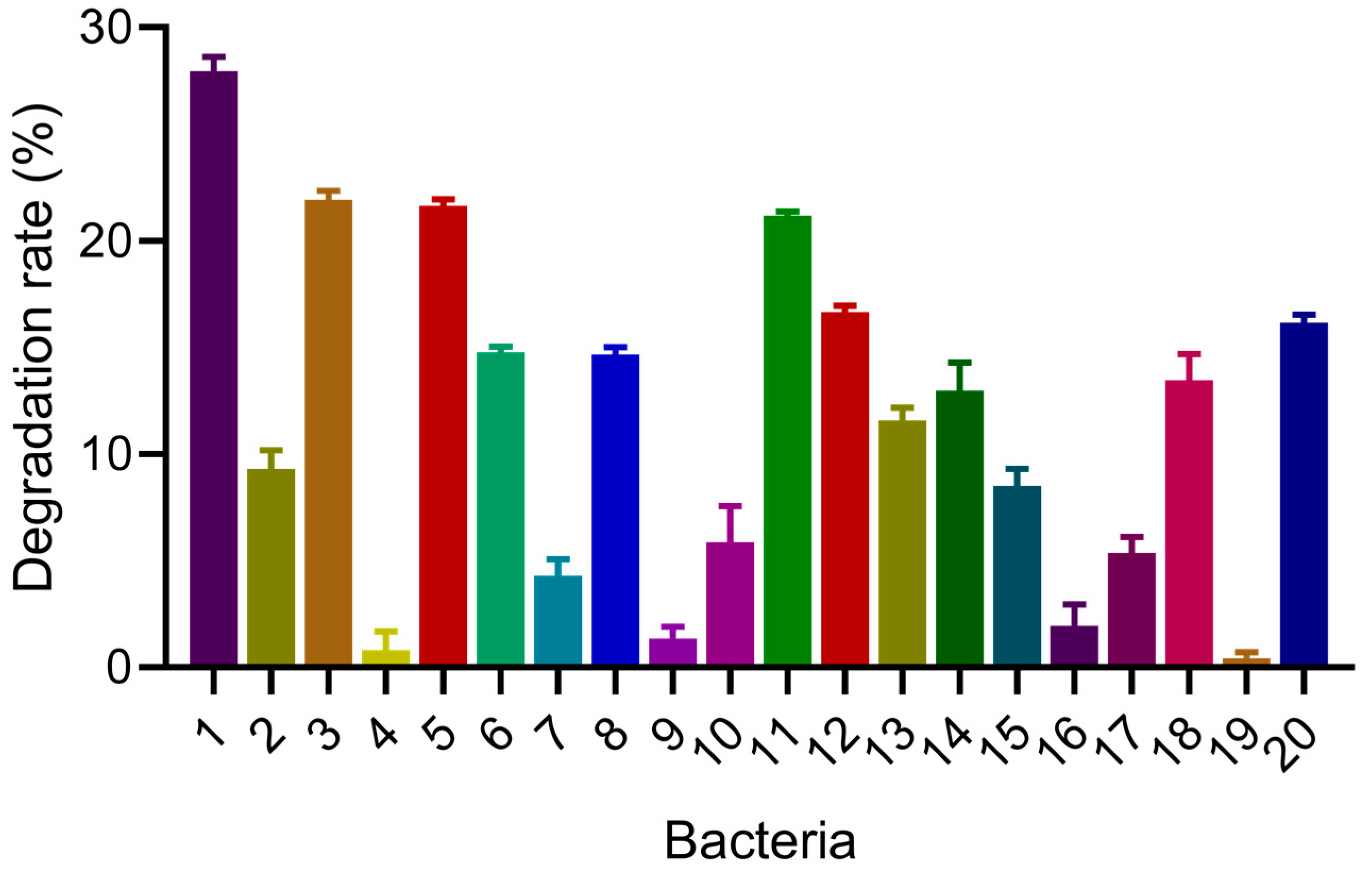
3.1. COD Degradation Ability of Each Strain
3.2. Identification and Characterization of Strain H1
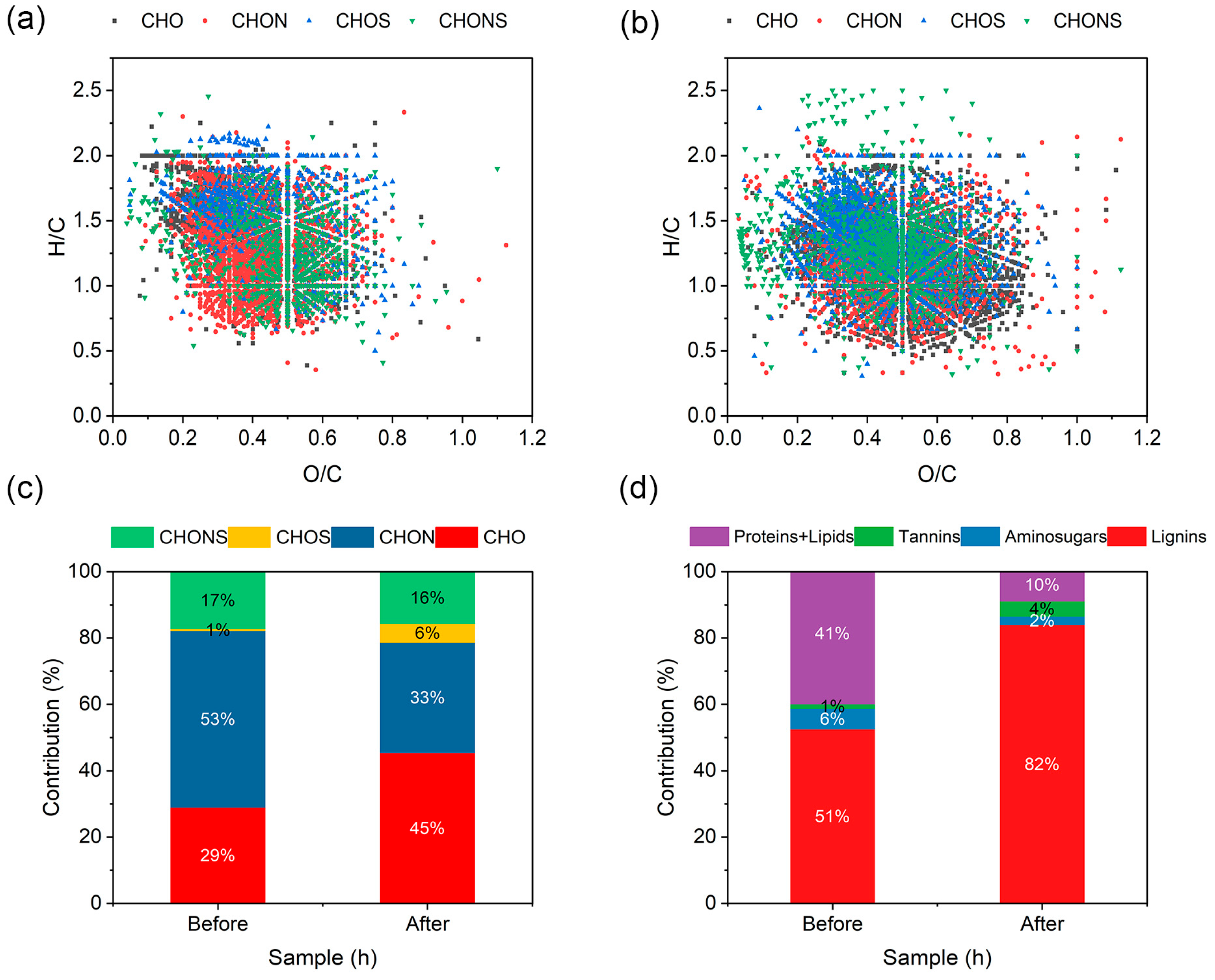
3.3. DOM Molecular Transformation During SW Treatment by Strain H1
3.4. Biofortification Properties of Strain H1 in SBR
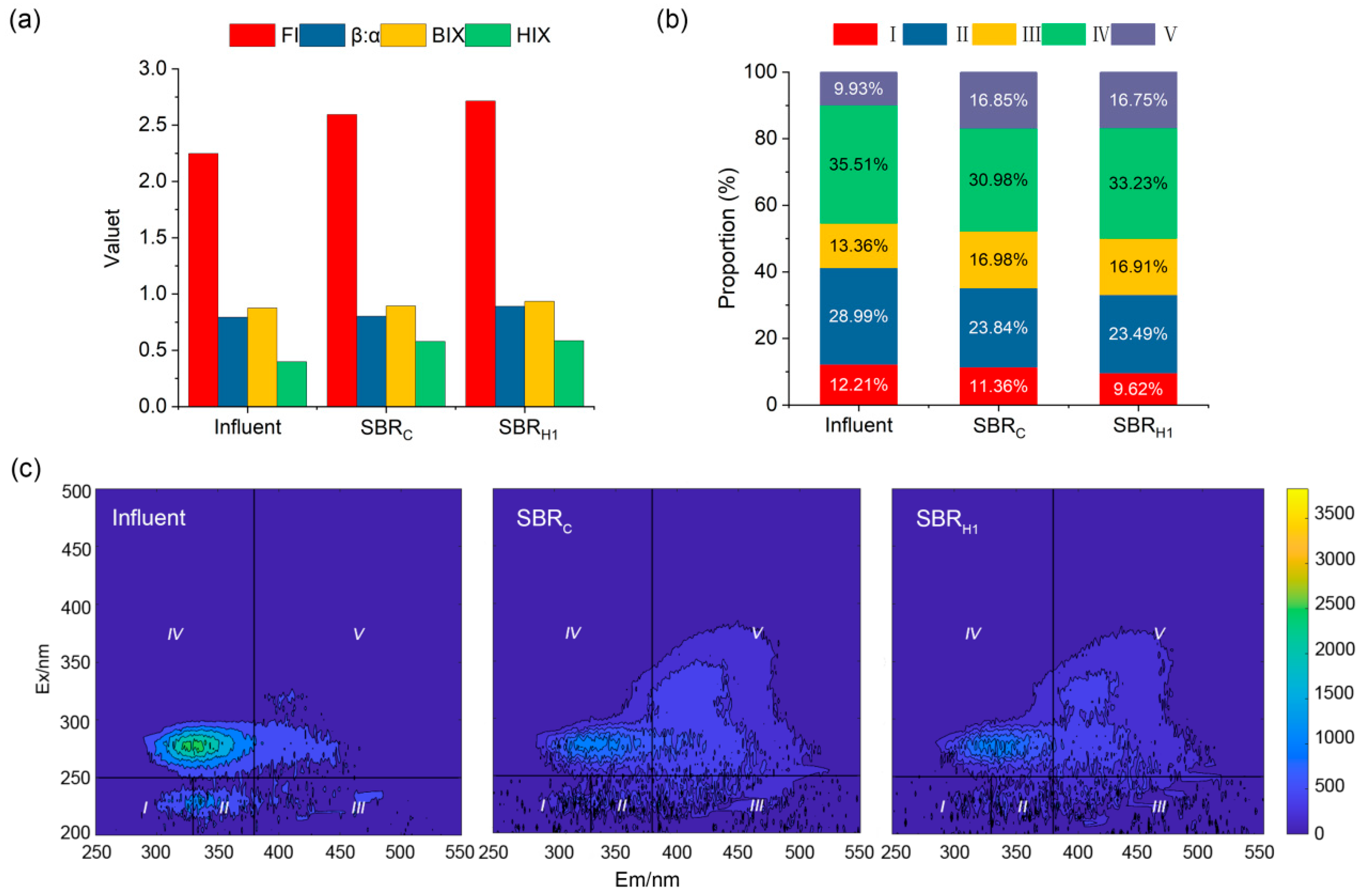
3.5. Spectral Characterization of DOM in Swine Wastewater Treatment Processes
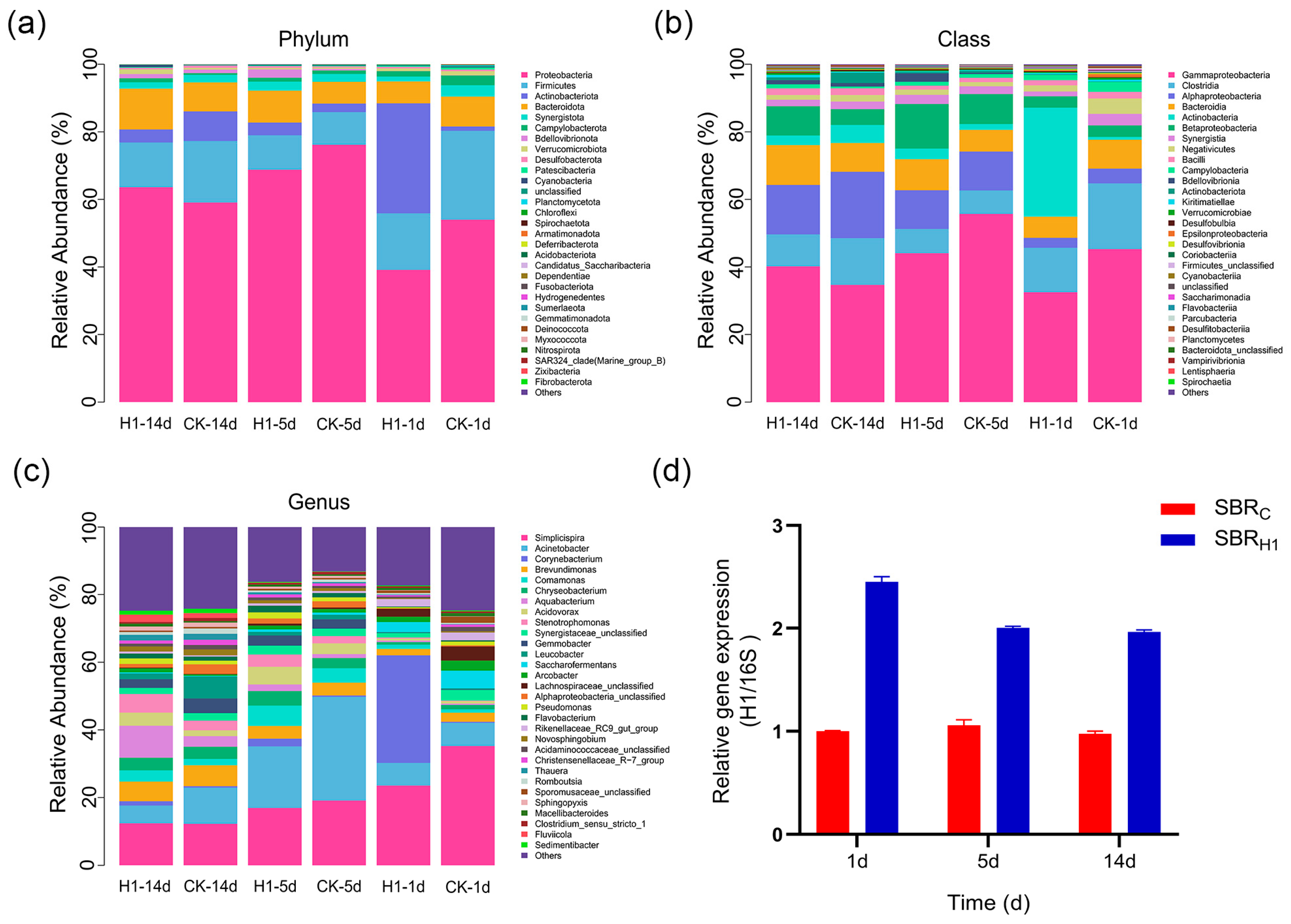
3.6. Changes in Microbial Community Structure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Bureau of Statistics of China. Statistical Communiqué of the People’s Republic of China on the 2023 National Economic and Social Development; China Statistics Press: Beijing, China, 2024. [Google Scholar]
- Wang, H.; Wu, B.; Jiang, N.; Liu, J.; Zhao, Y.; Xu, J.; Wang, H. The effects of influent chemical oxygen demand and strigolactone analog concentration on integral biogas upgrading and pollutants removal from piggery wastewater by different microalgae-based technologies. Bioresour. Technol. 2023, 370, 128483. [Google Scholar] [CrossRef]
- Cheng, H.-H.; Narindri, B.; Chu, H.; Whang, L. Recent advancement on biological technologies and strategies for resource recovery from swine wastewater. Bioresour. Technol. 2020, 303, 122861. [Google Scholar] [CrossRef]
- Yu, J.; Hu, H.; Wu, X.; Zhou, T.; Liu, Y.; Ruan, R.; Zheng, H. Coupling of biochar-mediated absorption and algal-bacterial system to enhance nutrients recovery from swine wastewater. Sci. Total Environ. 2020, 701, 134935. [Google Scholar] [CrossRef]
- Huang, D.; Tian, Y.; Yu, S.; Wen, X.; Chen, S.; Gao, X.; Ren, L.; Zhen, J.; Chen, X. Inversion prediction of COD in wastewater based on hyperspectral technology. J. Clean. Prod. 2023, 385, 135681. [Google Scholar] [CrossRef]
- Cai, Y.; Yang, H.; Liu, J.; Zuo, D.; Deng, L. Sequencing batch reactor (SBR) and anoxic and oxic process (A/O) display opposite performance for pollutant removal in treating digested effluent of swine wastewater with low and high COD/N ratios. J. Clean. Prod. 2022, 372, 133643. [Google Scholar] [CrossRef]
- Zhou, L.; Liang, M.; Zhang, D.; Niu, X.; Li, K.; Lin, Z.; Luo, X.; Huang, Y. Recent advances in swine wastewater treatment technologies for resource recovery: A comprehensive review. Sci. Total Environ. 2024, 924, 171557. [Google Scholar] [CrossRef]
- Vaishnav, S.; Saini, T.; Chauhan, A.; Gaur, G.K.; Tiwari, R.; Dutt, T.; Tarafdar, A. Livestock and poultry farm wastewater treatment and its valorization for generating value-added products: Recent updates and way forward. Bioresour. Technol. 2023, 382, 129170. [Google Scholar] [CrossRef]
- Pan, Y.; Ma, Z.; Shen, J.; Liang, J.; Yuan, Y.; Lian, X.; Sun, Y. Biotreatment of swine wastewater by mixotrophic Galdieria sulphuraria. J. Environ. Chem. Eng. 2024, 12, 111858. [Google Scholar] [CrossRef]
- Cheng, D.; Ngo, H.; Guo, W.; Chang, S.; Nguyen, D.; Kumar, S.; Du, B.; Wei, Q.; Wei, D. Problematic effects of antibiotics on anaerobic treatment of swine wastewater. Bioresour. Technol. 2018, 263, 642–653. [Google Scholar] [CrossRef]
- Kumar, L.; Chugh, M.; Kumar, S.; Kumar, K.; Sharma, J.; Bharadvaja, N.; Protection, E. Remediation of petrorefinery wastewater contaminants: A review on physicochemical and bioremediation strategies. Process Saf. Environ. Prot. 2022, 159, 362–375. [Google Scholar] [CrossRef]
- Guo, J.; Chen, C.; Chen, W.; Jiang, J.; Chen, B.; Zheng, F. Effective immobilization of Bacillus subtilis in chitosan-sodium alginate composite carrier for ammonia removal from anaerobically digested swine wastewater. Chemosphere 2021, 284, 131266. [Google Scholar] [CrossRef]
- Li, S.; Chu, Y.; Xie, P.; Xie, Y.; Chang, H.; Ho, S.-H. Insights into the microalgae-bacteria consortia treating swine wastewater: Symbiotic mechanism and resistance genes analysis. Bioresour. Technol. 2022, 349, 126892. [Google Scholar] [CrossRef]
- Gomes, J.; Domingues, E.; Fernandes, E.; Castro, L.; Martins, R.C.; Quinta-Ferreira, R.M. Coagulation and biofiltration by Corbicula fluminea for COD and toxicity reduction of swine wastewater. J. Water Process Eng. 2021, 42, 102145. [Google Scholar] [CrossRef]
- Palafox-Sola, M.F.; Yebra-Montes, C.; Orozco-Nunnelly, D.A.; Carrillo-Nieves, D.; González-López, M.E.; Gradilla-Hernández, M.S. Modeling growth kinetics and community interactions in microalgal cultures for bioremediation of anaerobically digested swine wastewater. Algal Res. 2023, 70, 102981. [Google Scholar] [CrossRef]
- Wang, Y.; Li, B.; Li, Y.; Chen, X. Research progress on enhancing the performance of autotrophic nitrogen removal systems using microbial immobilization technology-ScienceDirect. Sci. Total Environ. 2021, 774, 145136. [Google Scholar] [CrossRef]
- Wu, S.; Hao, P.; Lv, Z.; Zhang, X.; Wang, L.; Basang, W.; Zhu, Y.; Gao, Y. Construction of Magnetic Composite Bacterial Carrier and Application in 17 β -Estradiol Degradation. Molecules 2022, 27, 5807. [Google Scholar] [CrossRef]
- Hou, L.; Hu, K.; Huang, F.; Pan, Z.; Jia, X.; Liu, W.; Yao, X.; Yang, Z.; Tang, P.; Li, J. Advances in immobilized microbial technology and its application to wastewater treatment: A review. Bioresour. Technol. 2024, 413, 131518. [Google Scholar] [CrossRef]
- Mehrotra, T.; Dev, S.; Banerjee, A.; Chatterjee, A.; Singh, R.; Aggarwal, S. Use of Immobilized Bacteria for Environmental Bioremediation: A Review. J. Environ. Chem. Eng. 2021, 9, 105920. [Google Scholar] [CrossRef]
- Smith, D.F.; Podgorski, D.C.; Rodgers, R.P.; Blakney, G.T.; Hendrickson, C.L. 21 tesla FT-ICR mass spectrometer for ultrahigh-resolution analysis of complex organic mixtures. Anal. Chem. 2018, 90, 2041–2047. [Google Scholar] [CrossRef]
- Gan, S.; Wu, P.; Song, Y.; Guo, P.; Cai, N.; Yuan, F.; Yang, Q.; Wu, Y.; Liu, N.; Pan, J. Non-targeted characterization of dissolved organic matter from a wastewater treatment plant by FT-ICR-MS: A case study of hospital sewage. J. Water Process Eng. 2022, 48, 102834. [Google Scholar] [CrossRef]
- Martin, K.R.; Robey, N.M.; Ma, S.; Powers, L.C.; Heyes, A.; Schmitt-Kopplin, P.; Cooper, W.J.; Townsend, T.G.; Gonsior, M. Characterization of landfill leachate molecular composition using ultrahigh resolution mass spectrometry. Environ. Sci. Water Res. Technol. 2021, 7, 1250–1266. [Google Scholar] [CrossRef]
- Wang, W.; Qi, L.; Zhang, P.; Luo, J.; Li, J. Removal of COD in wastewater by magnetic coagulant prepared from modified fly ash. Environ. Sci. Pollut. Res. Int. 2022, 29, 52175–52188. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, Y. Generation and characterization of DOM in wastewater treatment processes. Chemosphere 2018, 201, 96–109. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Hao, P.; Gou, C.; Zhang, X.; Wang, L.; Basang, W.; Zhu, Y.; Gao, Y. Lysinibacillus sp. GG242 from Cattle Slurries Degrades 17 β-Estradiol and Possible 2 Transformation Routes. Microorganisms 2022, 10, 1745. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Hao, P.; Duan, X.; Gao, Y.; Liang, X. Cellulosimicrobium sp. Strain L1: A Study on the Optimization of the Conditions and Performance of a Combined Biological Trickling Filter for Hydrogen Sulfide Degradation. Microorganisms 2024, 12, 1513. [Google Scholar] [CrossRef]
- Zhang, H.; Zheng, Y.; Wang, X.C.; Zhang, Q.; Dzakpasu, M. Photochemical behavior of constructed wetlands-derived dissolved organic matter and its effects on Bisphenol A photodegradation in secondary treated wastewater. Sci. Total Environ. 2022, 845, 157300. [Google Scholar] [CrossRef]
- Guo, L.; Lu, M.; Li, Q.; Zhang, W.; Zong, Y.; She, L. Three-dimensional fluorescence excitation-emission matrix (EEM) spectroscopy with regional integration analysis for assessing waste sludge hydrolysis treated with multi-enzyme and thermophilic bacteria. Bioresour. Technol. 2014, 171, 22–28. [Google Scholar] [CrossRef]
- Administration, S.E.P. Water and Wastewater Monitoring and Analysis Method; China Environmental Science Press: Beijing, China, 2002. [Google Scholar]
- Sun, K.; Sun, Y.; Jia, Y.; Duan, X.; Ma, Z.; Zhang, X.; Wang, L.; Zhu, Y.; Gao, Y.; Basang, W. MicroRNA miR-212-5p Regulates the MEK/ERK Signaling Pathway by Targeting A-Raf proto-oncogene serine/threonine-protein kinase (ARAF) to Regulate Cowshed PM 2.5 -Induced NR8383 Apoptosis. Toxics 2023, 11, 981. [Google Scholar] [CrossRef]
- Li, W.; Jia, X.; Deng, J.; Wang, H.; Lin, L.; Liu, C.; Wang, S.; Tang, X.; Zeng, X.; Ma, L. Isolation, genetic identification and degradation characteristics of COD-degrading bacterial strain in slaughter wastewater. Saudi J. Biol. Sci. 2018, 25, 1800–1805. [Google Scholar] [CrossRef]
- Deng, J.; Chen, Q.; Hu, B.; Li, W.; Jia, M.; Shi, Y.; Xiong, S.; Bai, J.; Yin, H. Synergic effect of adsorption and biodegradation by microsphere immobilizing Bacillus velezensis for enhanced removal organics in slaughter wastewater. Processes 2021, 9, 1145. [Google Scholar] [CrossRef]
- Gibbons, R.E.B.N.R. Bergey’s Manual of Systematic Bacteriology. Nature 1984, 162, 833. [Google Scholar] [CrossRef]
- Li, L.; Ai, J.; He, H.; Hu, A.; Su, P.; Zhou, H.; Wang, D.; Zhang, W. Molecular-level insights into the transformation and degradation pathways of dissolved organic matter during full-scale swine wastewater treatment. Sci. Total Environ. 2024, 909, 168604. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, B.; Zhang, P.; Chen, Y.; Chen, P. Improvement in nitrogen removal and changes in community structure in a sequencing batch reactor bioaugmented with P. stutzeri strain XL-2. Bioresour. Technol. 2020, 317, 123976. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, J.; Luo, X.; Zhu, Y.; Xia, L.; Luo, L. Simultaneous ammonia and Cr (VI) removal by Pseudomonas aeruginosa LX in wastewater. Biochem. Eng. J. 2020, 157, 107551. [Google Scholar] [CrossRef]
- Chen, T.; Yang, X.; Sun, Q.; Hu, A.; Qin, D.; Li, J.; Wang, Y.; Yu, C. Changes in wastewater treatment performance and the microbial community during the bioaugmentation of a denitrifying Pseudomonas strain in the low carbon–nitrogen ratio sequencing batch reactor. Water 2022, 14, 540. [Google Scholar] [CrossRef]
- GB/T18596-2001; Discharge Standards of Pollutants for Livestock and Poultry Breeding. General Administration of Quality Supervision. Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2001.
- Jiang, X.; Xie, Y.; Liu, M.; Bin, S.; Liu, Y.; Huan, C.; Ji, G.; Wang, X.; Yan, Z.; Lyu, Q. Study on anaerobic co-digestion of municipal sewage sludge and fruit and vegetable wastes: Methane production, microbial community and three-dimension fluorescence excitation-emission matrix analysis. Bioresour. Technol. 2022, 347, 126748. [Google Scholar] [CrossRef]
- Huguet, A.; Vacher, L.; Relexans, S.; Saubusse, S.; Froidefond, J.-M.; Parlanti, E. Properties of fluorescent dissolved organic matter in the Gironde Estuary. Org. Geochem. 2009, 40, 706–719. [Google Scholar] [CrossRef]
- Liu, R.; Hao, X.; van Loosdrecht, M.C.; Zhou, P.; Li, J. Dynamics of humic substance composition during anaerobic digestion of excess activated sludge. Int. Biodeterior. Biodegrad. 2019, 145, 104771. [Google Scholar] [CrossRef]
- Chen, W.; Westerhoff, P.; Leenheer, J.A.; Booksh, K. Fluorescence excitation− emission matrix regional integration to quantify spectra for dissolved organic matter. Environ. Sci. Technol. 2003, 37, 5701–5710. [Google Scholar] [CrossRef]
- Wang, K.; Li, W.; Gong, X.; Li, Y.; Wu, C.; Ren, N. Spectral study of dissolved organic matter in biosolid during the composting process using inorganic bulking agent: UV–vis, GPC, FTIR and EEM. Int. Biodeterior. Biodegrad. 2013, 85, 617–623. [Google Scholar] [CrossRef]
- Chen, F.; Li, G.; Li, X.; Wang, H.; Wu, H.; Li, J.; Li, C.; Li, W.; Zhang, L.; Xi, B. The cotreatment of old landfill leachate and domestic sewage in rural areas by deep subsurface wastewater infiltration system (SWIS): Performance and bacterial community. Environ. Pollut. 2021, 274, 115800. [Google Scholar] [CrossRef]
- Cinà, P.; Bacci, G.; Arancio, W.; Gallo, G.; Fani, R.; Puglia, A.M.; Di Trapani, D.; Mannina, G. Assessment and characterization of the bacterial community structure in advanced activated sludge systems. Bioresour. Technol. 2019, 282, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ming, J.; Yoza, B.; Liang, J.; Li, Q.; Guo, H.; Liu, Z.; Deng, J.; Wang, Q. Characterization of aerobic granular sludge used for the treatment of petroleum wastewater. Bioresour. Technol. 2018, 271, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhu, J.; Duan, W.; Gao, J.; Li, W. Biological nitrogen removal and metabolic characteristics in a full-scale two-staged anoxic-oxic (A/O) system to treat optoelectronic wastewater. Bioresour. Technol. 2020, 300, 122595. [Google Scholar] [CrossRef]
- Li, Y.; Cao, P.; Wang, S.; Xu, X. Research on the treatment mechanism of anthraquinone dye wastewater by algal-bacterial symbiotic system. Bioresour. Technol. 2022, 347, 126691. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.; Wang, X.; Zhang, Q.; Littleton, H. Rapid aerobic granulation in an SBR treating piggery wastewater by seeding sludge from a municipal WWTP. J. Environ. Sci. 2017, 51, 332–341. [Google Scholar] [CrossRef]
- Li, J.; Chen, X.; Yang, Z.; Liu, Z.; Chen, Y.; Wang, Y.; Xie, H. Denitrification performance and mechanism of sequencing batch reactor with a novel iron-polyurethane foam composite carrier. Biochem. Eng. J. 2021, 176, 108209. [Google Scholar] [CrossRef]
- Siddiqi, M.Z.; Sok, W.; Choi, G.; Kim, S.Y.; Wee, J.-H.; Im, W. Simplicispira hankyongi sp. nov., a novel denitrifying bacterium isolated from sludge. Antonie van Leeuwenhoek 2020, 113, 331–338. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Yu, G.; Yao, Y.; Lin, H. Effects of flow pattern, Leersia hexandra, and circuit mode on the Cr (VI) removal capacity, electricity generation performance, and microbial community of constructed wetland-microbial fuel cells. Fuel 2023, 338, 127326. [Google Scholar] [CrossRef]

 influent concentration,
influent concentration,  effluent concentration of SBRC,
effluent concentration of SBRC,  effluent concentration of SBRH1,
effluent concentration of SBRH1,  removal efficiency of SBRC, and
removal efficiency of SBRC, and  removal efficiency of SBRH1.
removal efficiency of SBRH1.
 influent concentration,
influent concentration,  effluent concentration of SBRC,
effluent concentration of SBRC,  effluent concentration of SBRH1,
effluent concentration of SBRH1,  removal efficiency of SBRC, and
removal efficiency of SBRC, and  removal efficiency of SBRH1.
removal efficiency of SBRH1.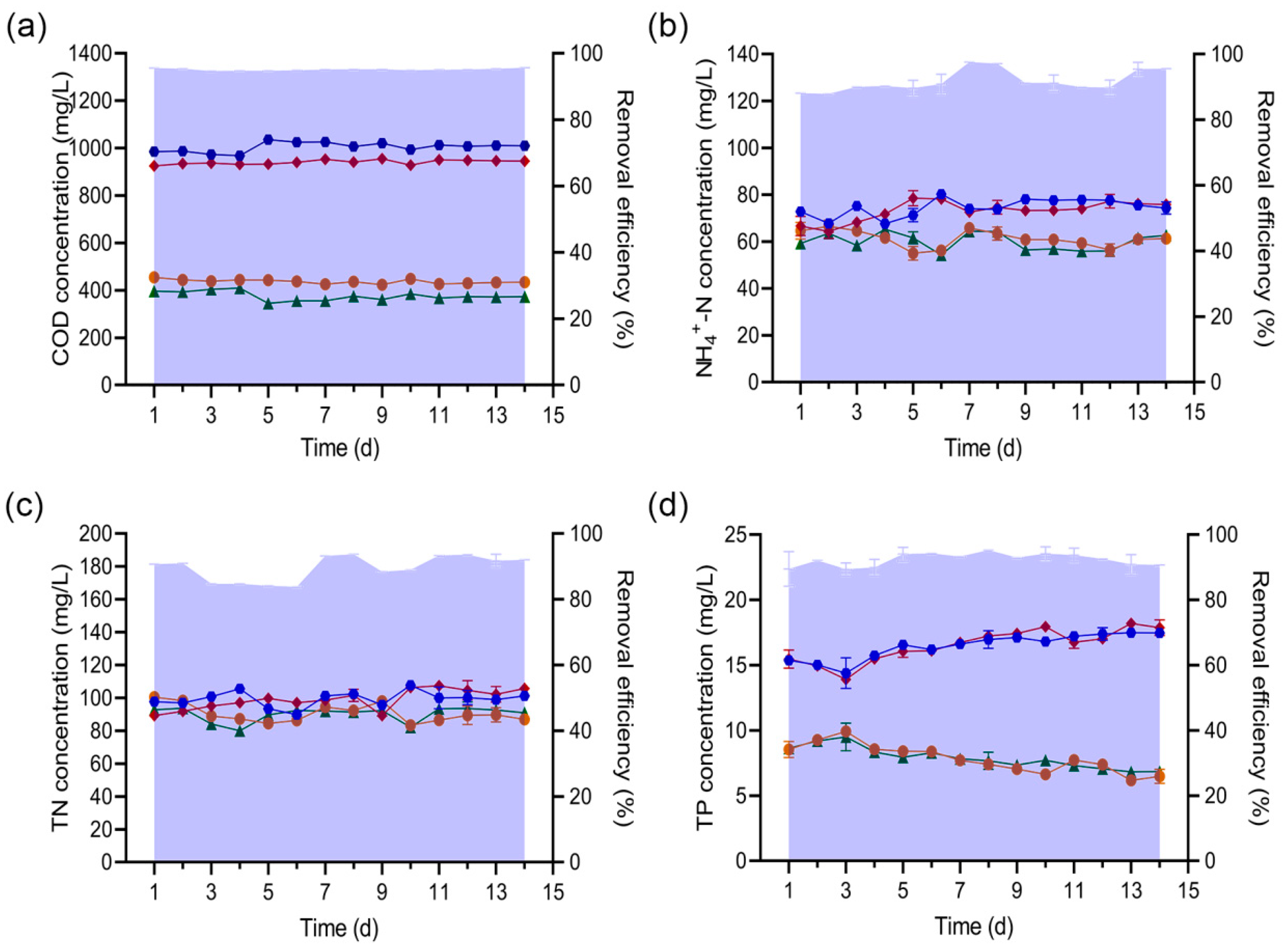
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Liu, M.; Tian, H.; Kong, L.; Yang, W.; Yang, L.; Gao, Y. Removal of Chemical Oxygen Demand (COD) from Swine Farm Wastewater by Corynebacterium xerosis H1. Microorganisms 2025, 13, 1621. https://doi.org/10.3390/microorganisms13071621
Zhang J, Liu M, Tian H, Kong L, Yang W, Yang L, Gao Y. Removal of Chemical Oxygen Demand (COD) from Swine Farm Wastewater by Corynebacterium xerosis H1. Microorganisms. 2025; 13(7):1621. https://doi.org/10.3390/microorganisms13071621
Chicago/Turabian StyleZhang, Jingyi, Meng Liu, Heshi Tian, Lingcong Kong, Wenyan Yang, Lianyu Yang, and Yunhang Gao. 2025. "Removal of Chemical Oxygen Demand (COD) from Swine Farm Wastewater by Corynebacterium xerosis H1" Microorganisms 13, no. 7: 1621. https://doi.org/10.3390/microorganisms13071621
APA StyleZhang, J., Liu, M., Tian, H., Kong, L., Yang, W., Yang, L., & Gao, Y. (2025). Removal of Chemical Oxygen Demand (COD) from Swine Farm Wastewater by Corynebacterium xerosis H1. Microorganisms, 13(7), 1621. https://doi.org/10.3390/microorganisms13071621





