1. Introduction
Microalgae are unicellular, eukaryotic, and photosynthetic organisms that have gained increasing attention due to their ability to produce biomass rich in bioactive compounds such as proteins, lipids, carbohydrates, and antioxidants. These characteristics make them promising candidates for applications in the food, pharmaceutical, nutraceutical, cosmetic, and biofuel industries, owing to their high contents of proteins, essential amino acids, fatty acids, pigments, and other functional metabolites. Additionally, their rapid growth rates and capacity for cultivation under controlled conditions contribute to both the sustainability and efficiency of biomass production [
1].
The genus
Tetradesmus obliquus (Turpin) M.J. Wynne, previously classified under the genus
Scenedesmus, is a green microalga widely distributed in freshwater environments such as lakes and rivers. This species is particularly valued for its high lipid accumulation, ease of cultivation, and tolerance to a wide range of temperatures and pH levels. Moreover,
T. obliquus is effective in removing heavy metals, nitrogen, and phosphorus from aquatic systems and displays resilience under high carbon dioxide (CO
2) concentrations, resulting in high biomass productivity. These attributes make it a strong candidate for applications in bioenergy and wastewater treatment [
2,
3].
The cultivation of microalgae in industrial photobioreactors enables improvement in growth parameters, including light intensity, temperature, and nutrient availability, leading to enhanced productivity and biomass quality. This approach also facilitates process standardization and scalability for commercial applications [
4].
Several microalgal species have demonstrated significant antioxidant activity, primarily attributed to the presence of compounds such as pigments (e.g., carotenoids), polyphenols, and vitamins. These natural antioxidants are of particular interest to the food and pharmaceutical industries, providing viable alternatives to synthetic antioxidants. The evaluation of antioxidant capacity using assays such as DPPH (2,2-diphenyl-1-picrylhydrazyl) and ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid)) is essential for detecting and quantifying such bioactive compounds [
5].
Beyond its wide industrial relevance and role in sustainable production systems,
T. obliquus is particularly noted for its diverse biological activities, especially its ability to synthesize bioactive compounds with therapeutic potential. This species is a rich source of essential fatty acids (EFAs), including polyunsaturated fatty acids (PUFAs), which are crucial for maintaining human health and managing various medical conditions [
6].
As these fatty acids cannot be synthesized endogenously,
T. obliquus represents a valuable dietary alternative. Moreover, this microalga is abundant in amino acids essential for protein biosynthesis. Under mixotrophic cultivation, it demonstrates significantly enhanced protein yields compared to under conventional cultivation methods. Its ability to regulate metabolic pathways and improve amino acid profiles further supports its potential use in the food and animal feed industries, where its biomass may serve as a high-value nutritional input [
7].
Antioxidants are critical molecules that protect cells from oxidative damage caused by free radicals—highly reactive species generated during normal metabolism or in response to environmental factors such as pollution, radiation, and smoking. Free radical accumulation induces oxidative stress, damaging lipids, proteins, and DNA, and contributing to cellular ageing and the development of chronic diseases, including cancer, cardiovascular disorders, and neurodegenerative conditions. Recent studies have shown that
T. obliquus, a green microalga, exhibits strong antioxidant potential due to its content of phenolic compounds, flavonoids, and pigments such as carotenoids. Additionally, it demonstrates noteworthy antitumor and antimicrobial activities [
8].
In parallel, the nutritional profile of this microalga is enhanced by its high protein concentration, with soluble protein levels exceeding 28% of its dry biomass [
7], in addition to its capacity to stabilize food emulsions as a functional protein source. The combination of antioxidant activity and nutritional value positions
T. obliquus as a strong candidate for incorporation into functional formulations within the food, pharmaceutical, and cosmetic industries [
9]. These synergistic characteristics further support its potential use across a wide range of industrial sectors.
Among microalgal species with notable nutritional value,
T. obliquus stands out for its high protein content, with values ranging from 50% to 56% of its dry biomass, positioning it among the richest known microalgal protein sources. This profile is comparable to, or even exceeds, traditional protein sources such as skimmed milk powder (36%) and beef (43%). Its nutritional profile has been highlighted as particularly relevant for sustainable diets, especially in contexts requiring alternatives to animal-derived proteins, thereby expanding its potential in functional food formulations and nutritional supplements. The versatility of
T. obliquus biomass is attributed not only to its protein composition but also to its content of micronutrients, fatty acids, and antioxidant compounds, reinforcing its role as a promising functional ingredient for a wide range of industrial applications [
10].
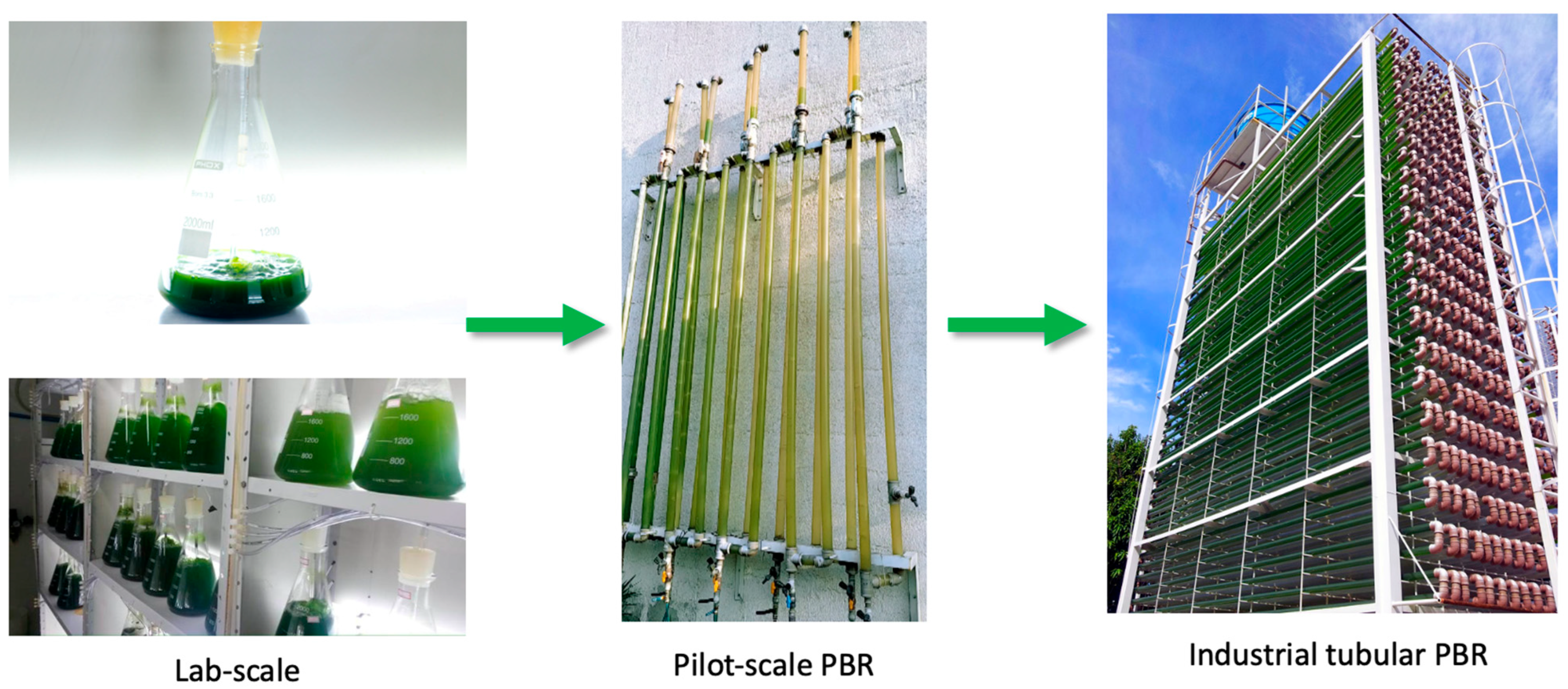
Realizing the biotechnological potential of microalgae for commercial applications requires overcoming key challenges related to large-scale cultivation. Although microalgae possess vast potential, their commercial viability still hinges on the efficient transition from laboratory-scale processes to pilot- and industrial-scale operations. This transition is particularly critical in the context of photobioreactors (PBRs), which provide tightly controlled conditions for microalgal growth. PBRs allow for the precise regulation of variables such as light intensity, temperature, and nutrient concentrations, thereby improving biomass production. Additionally, these systems offer key logistical advantages, including reduced land area requirements and higher productivity, with shorter and more efficient cultivation cycles [
11,
12].
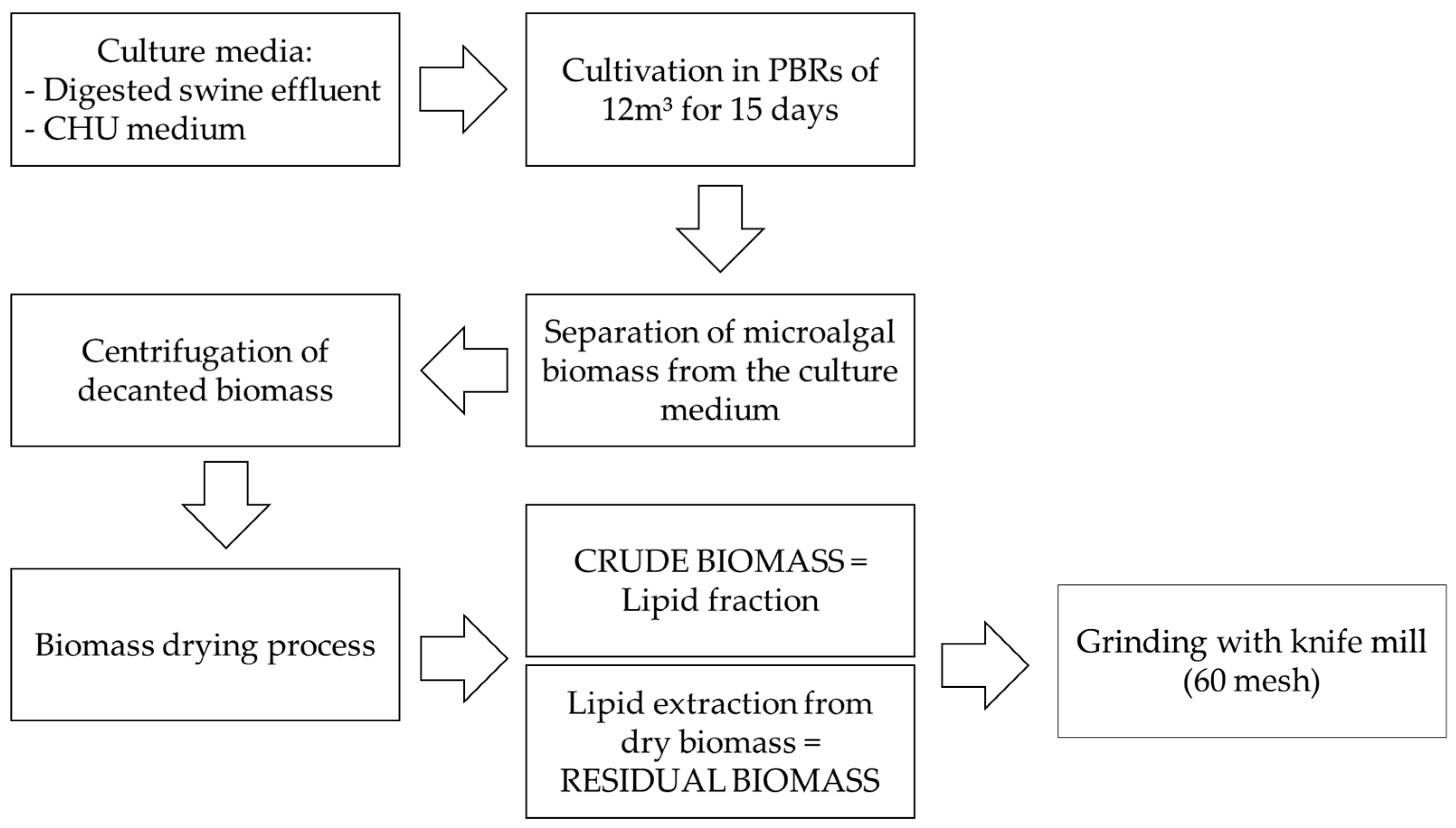
Given this context, the present study aimed to perform a comprehensive multiparametric characterization of T. obliquus biomass, focusing on its physicochemical, nutritional, thermochemical, and antioxidant properties. This characterization was conducted to evaluate its potential as an innovative ingredient for industrial applications, particularly within the food, pharmaceutical, and bioenergy sectors. To this end, dry biomass samples produced by the Sustainable Energy Research and Development Center (NPDEAS) research group were analyzed.
The biomass was cultivated in a patented industrial-scale PBR system integrated with a residue incinerator, enabling large-scale production under controlled engineering conditions. This sustainable production process aligns with several United Nations Sustainable Development Goals (SDGs), including Goal 2—Zero Hunger; Goal 3—Good Health and Well-being; Goal 7—Affordable and Clean Energy; Goal 9—Industry, Innovation and Infrastructure; Goal 11—Sustainable Cities and Communities; Goal 12—Responsible Consumption and Production; and Goal 13—Climate Action.
4. Discussion
The characterization of microalgal biomass produced in patented industrial photobioreactors contributes to ongoing efforts to explore new sources of energy generation and other industrial biotechnological applications. Microalgae have become key cultures for the global food and beverage industries, aquaculture, and both animal and human nutrition. This relevance is attributed to (i) their high content of proteins, essential amino acids, vitamins, antioxidants, omega-3 fatty acids, and minerals; (ii) their long-term sustainability, due to lower carbon, water, and arable land footprints compared to conventional crops; (iii) their role in environmental pollution remediation; and (iv) their higher productivity relative to terrestrial crops and traditional animal feed [
26].
The use of microalgae as a protein source has gained increasing attention due to their high photosynthetic efficiency and nutritional profile. Studies have shown that microalgae can be incorporated into a wide range of food products, such as dietary supplements, meat substitutes, baked goods, and beverages, providing additional nutritional benefits such as dietary fiber and natural antioxidants [
10]. Moreover, microalgae have emerged as a sustainable alternative for the agribusiness sector, particularly in the production of biostimulants and biofertilizers [
27].
Tetradesmus obliquus is recognized for its potential in biofuel production due to its high lipid content and photosynthetic efficiency [
28], reinforcing the growing interest in microalgae as a promising source for bioenergy and other biotechnological applications.
The thermochemical properties of microalgae are directly influenced by their VM, A, and FC content, which are essential parameters for evaluating their potential as biofuels. Volatile matter, in particular, has a significant impact on the HHV, as higher VM content tends to correlate with greater energy output [
18]. The results of this study are consistent with the existing literature on dried microalgal biomass. Zakaria et al. (2022) [
29] reported that the biomass of
Limnospira (formerly
Spirulina) sp. (Cyanobacteria) exhibited 68.15% VM, 8.06% A, and 12.57% FC.
Elemental analysis revealed significant variations in the chemical composition of the samples cultivated under different conditions. The carbon content was higher in the crude biomass samples, particularly in the 2018 batch, indicating a greater energy potential compared to the residual biomass. The increased hydrogen content observed in the samples cultivated with swine effluent in 2023 suggests a favorable contribution to the calorific value, despite a slight increase in the oxygen content, which may reduce the energy density of the biomass [
30].
The HHV of biomass is positively correlated with its carbon and hydrogen contents and negatively correlated with its oxygen content. This implies that biomass with a higher oxygen content tends to exhibit a lower HHV [
30,
31]. Moreover, the distinction between crude and residual biomass highlights the impact of lipid extraction on energy content, with the crude biomass consistently exhibiting higher values, although the differences were not statistically significant.
These results provide a foundation for understanding the functional behavior of the biomass in industrial applications. Such characteristics, associated with the presence of organic and inorganic compounds, may directly influence oxidative stability and the preservation of bioactive fractions during biomass processing. In this context, the evaluation of the antioxidant activity of this biomass represents a complementary and strategic step, enabling the exploration of not only its energy value but also its functional potential for various applications in industrial sectors such as food, cosmetics, and pharmaceuticals [
32].
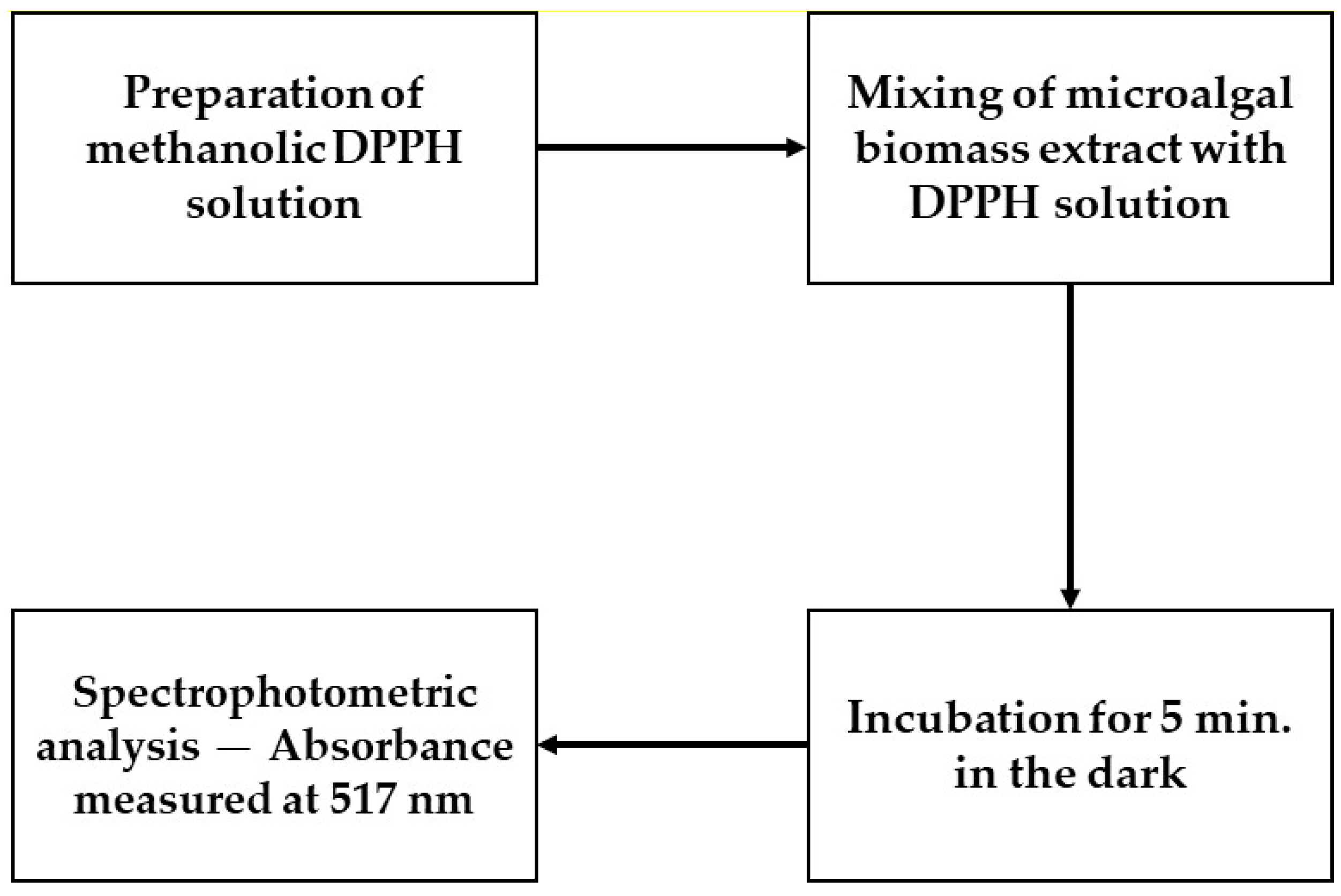
The antioxidant activity of the samples was evaluated using the DPPH method, which assesses the capacity of substances to neutralize free radicals. This analysis is instrumental in determining the antioxidant potential of materials, with significant implications in various sectors, including pharmacology and food [
33].
The microalga
Tetradesmus obliquus (formerly
Scenedesmus obliquus) (Chlorophyta) has been studied as a source of antioxidant bioactive compounds due to its rapid growth and resilience under various environmental conditions. In the DPPH method, the capacity of the extracts to reduce the DPPH radical (which has a purple color) to its neutral yellow form is measured, typically by quantifying the decrease in absorbance around 515–517 nm. Lower IC
50 values (the extract concentration required to reduce 50% of the DPPH radical) indicate greater antioxidant potency [
33,
34].
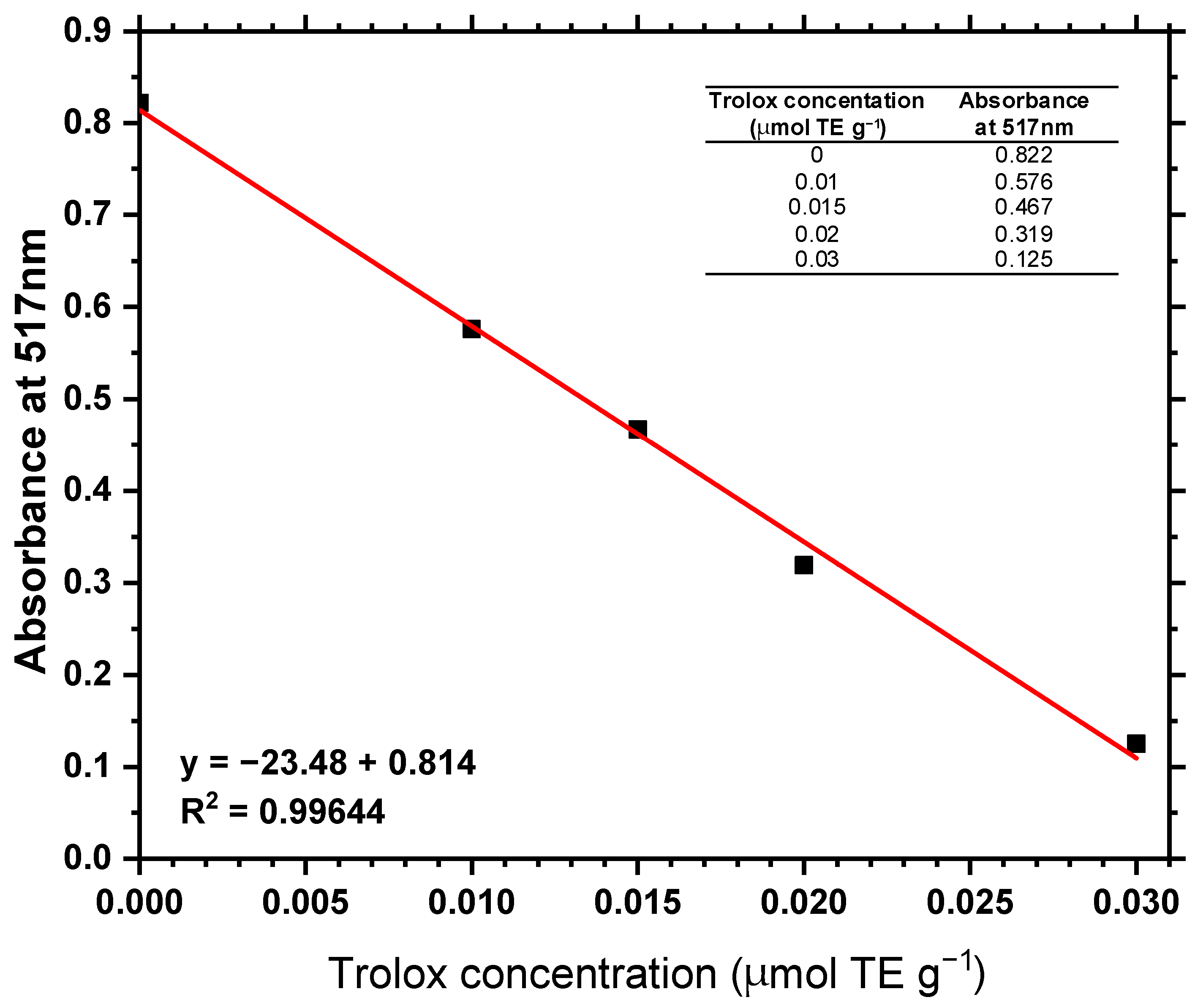
According to the observations in
Table 5, no antioxidant activity was expressed in terms of TE in any of the samples evaluated, with no indicative signs of such activity under the experimental conditions assessed. The samples were prepared according to the conditions described and all exhibited absorbance values similar to those of the negative control, indicating the absence of compounds with antioxidant activity.
Some probable factors may explain these negative results.
Tetradesmus obliquus may contain antioxidants primarily in the form of trans-carotenoids and other compounds that are less reactive with DPPH [
35]. Moreover, the cultivation conditions may not have favored the synthesis of polyphenols or other potent antioxidant compounds. The analyzed biomass may not contain appreciable amounts of free radical scavengers. For instance, cultures in the exponential growth phase or those grown under nutrient-rich conditions may prioritize growth-related processes (such as protein synthesis and the production of photosynthetic pigments for light capture) over the production of antioxidant secondary metabolites [
36]. Therefore, it is plausible that, under the evaluated conditions, the samples lacked sufficient quantities—or the appropriate type—of antioxidants capable of reacting with DPPH at a detectable level.
Although the DPPH assay does not involve intentional fluorescence excitation, highly pigmented samples may introduce signal noise. Moreover, recent studies have indicated that chlorophyll derivatives exhibit their own absorbance peaks and even some degree of reactivity, which may obscure DPPH results when present at high concentrations. Reports have documented interference between chlorophyll derivatives and the DPPH radical at 515 nm, particularly at elevated pigment levels, which can complicate data interpretation [
37]. Accordingly, it is worth noting that the samples evaluated in this study exhibited an intense green coloration, a factor that supports the findings of Alotaiby et al. (2024) [
37] regarding the DPPH method and spectrophotometric wavelength readings.
The Fluorescence Resonance Energy Transfer (FRET) effect is less commonly observed, yet it cannot be ruled out. Some form of energy transfer may occur between molecules within the extract. Classical FRET requires a fluorescent donor and an acceptor whose absorption band overlaps with the donor’s emission. Chlorophyll’s fluoresce is in the red region (~680 nm); DPPH itself is not fluorescent, but its reduction products or other impurities could potentially interact. Although the literature does not explicitly report FRET between chlorophyll and DPPH, it is reasonable to extrapolate that emission or energy transfer phenomena in highly pigmented samples may indirectly disturb the radical’s equilibrium [
36,
37,
38].
The lack of significant antioxidant activity in the evaluated samples suggests the need for further research. For instance, alternative methods for extracting antioxidant compounds, absorbance measurements at different wavelengths, or testing under varying cultivation conditions, such as analyzing the liquid inoculum before transfer into industrial-scale PBRs, could be explored. Such approaches may help to elucidate the antioxidant potential of this biomass.
The composition of algal biomass is often the primary driver of the economic viability of research and the development of commercial algae-based products. As the field of commercial algae production continues to expand, there is a growing need for standardized terminology between producers and markets with regard to biomass characterization [
39].
Cultivating microalgae in nitrogen-deficient media is considered one of the most effective strategies for increasing their lipid content. Nitrogen limitation enhances lipid accumulation while altering overall biomass productivity. In fact, microalgae are known to modify their biochemical composition in response to changing environmental conditions. The nitrogen concentration in the culture medium directly influences biomass composition, affecting both the growth rate and the proportion of stored macromolecules [
40,
41].
The study by Akgül (2024) [
40] demonstrated that under nitrogen-deficient conditions, a decrease in the specific growth rate and protein content of
Tetradesmus obliquus was observed, while the lipid content increased. Protein levels ranged between 47.62% and 47.82% of dry weight in media containing 75% and 50% of the available nitrogen, respectively. In addition, Vladić et al. (2023) [
42], in research on the application of
T. obliquus for wastewater remediation, demonstrated its ability to absorb nitrogenous compounds and synthesize proteins from these nutrients.
The determination of the protein content in biomass samples, such as microalgae, is commonly carried out using the Kjeldahl and Dumas methods. Both techniques measure the total nitrogen content, but they differ in the analytical procedures employed [
43].
The Kjeldahl method is widely recognized and involves three main steps: digestion, distillation, and back titration. It is considered the official method for protein determination in food by the Association of Official Analytical Chemists (AOAC) International. This method relies on the conversion of organic nitrogen present in the sample into ammonia, which is then quantified. In contrast, the Dumas method involves the combustion of samples, releasing nitrogen in its gaseous form, which is directly quantified by a thermal conductivity detector after the removal of interfering substances such as CO
2 and water [
43].
A significant advantage of the Dumas method over the Kjeldahl method is its speed and reduced use of hazardous reagents, as well as the requirement for smaller sample quantities. However, both methods may be subject to interference, potentially leading to divergent results, particularly in the presence of non-protein nitrogen compounds, which affect the nitrogen-to-protein conversion factor (k), typically 6.25, and may result in the under- or overestimation of the actual protein content [
43]. It is worth noting that, for the present study, a conversion factor (k) of 5.08 was applied, as recommended by Templeton and Laurens (2015) [
22] for the
Tetradesmus genus.
In the study conducted by Cruz et al. (2018) [
44], the protein content of
Monoraphidium sp. (Chlorophyta) biomass, cultivated in a 100 L photobioreactor under controlled conditions, was determined using the Kjeldahl method following centrifugation and lyophilization. The authors reported a protein content of 34.26% in the evaluated biomass.
In a review on microalgal proteins, Xu et al. (2024) [
45] reported that some species may exhibit protein contents exceeding 60% on a dry weight basis, particularly when cultivated under optimal nitrogen availability. Moreover, other studies have indicated that the protein content of
Tetradesmus obliquus biomass can vary widely depending on nutrient limitation and the extraction strategies employed [
46,
47].
The study conducted by Lane et al. (2021) [
39] investigated the elemental composition and protein content of two microalgae species,
Nannochloropsis salina and
Scenedesmus acutus LRB-AP-0401. The characterization involved determining ash, elemental carbon, and nitrogen, as well as estimating the protein content based on the measured nitrogen levels. The reported protein contents were 40.92% for
S. obliquus and 31.98% for
N. salina. These differences were primarily attributed to the application of different nitrogen-to-protein conversion factors specific to each species, 6.25 for
S. obliquus and 4.78 for
N. salina, taking into account the particularities of the amino acid composition of each microalga.
In summary, the protein averages determined in the present study ranged from 40.02% (Kjeldahl) to 36.66% (Dumas). These values are consistent with the ranges commonly reported in the literature for microalgae. It is important to emphasize that the biomass analyzed in this study was produced on an industrial scale and subjected to various physicochemical stress conditions throughout the production process, including exposure to exhaust gases, climatic fluctuations, flocculation, and high temperatures. Nevertheless, the protein contents obtained remained in line with the literature values, reinforcing the biotechnological and industrial applicability of the biomass. Furthermore, based on the protein determinations presented in this study using both the Kjeldahl and Dumas methods, it is possible to apply the more commonly adopted nitrogen-to-protein conversion factor (6.25) to obtain alternative results. Doing so would yield values even higher than those reported here.
Overall, the variations observed in the biochemical composition of the evaluated biomass can be attributed to the combined effects of nutrient availability and cultivation duration, both of which play a crucial role in regulating cellular metabolism. Under nutrient-limited conditions, microalgae typically shift their metabolic pathways towards the accumulation of storage compounds such as lipids and carbohydrates, often reducing protein synthesis as a physiological response. In contrast, nitrogen-rich environments tend to promote protein accumulation and faster biomass growth. Additionally, the length of the cultivation period influences the specific growth phase reached by the microalgae, with each phase associated with distinct metabolic activity and resource allocation strategies, ultimately shaping the final biochemical profile of the biomass [
41,
47].
It is important to note that the complexity of the cultivation media used in this study, especially those derived from swine manure, introduces additional variability in biomass composition. The swine manure collected over different production cycles inevitably reflects differences in the animal feed composition, growth stages, and physiological conditions of the livestock at the time of waste generation. These factors contribute to variations in the nutrient content and organic load within the culture media, leading to additional metabolic adjustments by the microalgae during growth.