1. Introduction
Powdered oil ingredients are widely used across the food, nutrition, and personal care industries as convenient, shelf-stable alternatives to liquid oils [
1]. In food and beverage applications, oil powders enable the inclusion of lipids in dry products, such as powdered soups, instant sauces, baking mixes, and protein shakes, where the presence of liquid oil would be technically incompatible or logistically impractical. In specialized nutrition, including sports nutrition, medical foods, and infant formula, powdered oils allow for accurate dosage, ease of transport, and the extended shelf life of sensitive nutrients [
1,
2,
3]. In all these applications, oxidative stability, lipid load, solubility, and label acceptability are critical parameters.
Despite their broad utility, most commercial oil powders are produced via encapsulation technologies, typically by spray drying emulsified oil blends with carrier matrices composed of maltodextrins, modified starches, milk proteins, or gums [
1,
4,
5]. While effective in generating oil-in-powder formats, these systems generally incorporate multiple additives, such as emulsifiers, stabilizers, and sometimes preservatives, that limit clean-label potential. Moreover, encapsulation yields a lipid distribution where the oil is physically entrapped rather than structurally integrated, making such powders prone to phase separation, oil leakage, cacking, and oxidative degradation over time. These constraints are particularly challenging in applications requiring high fat load, additive-free formulation, or stability under elevated temperatures and humidity.
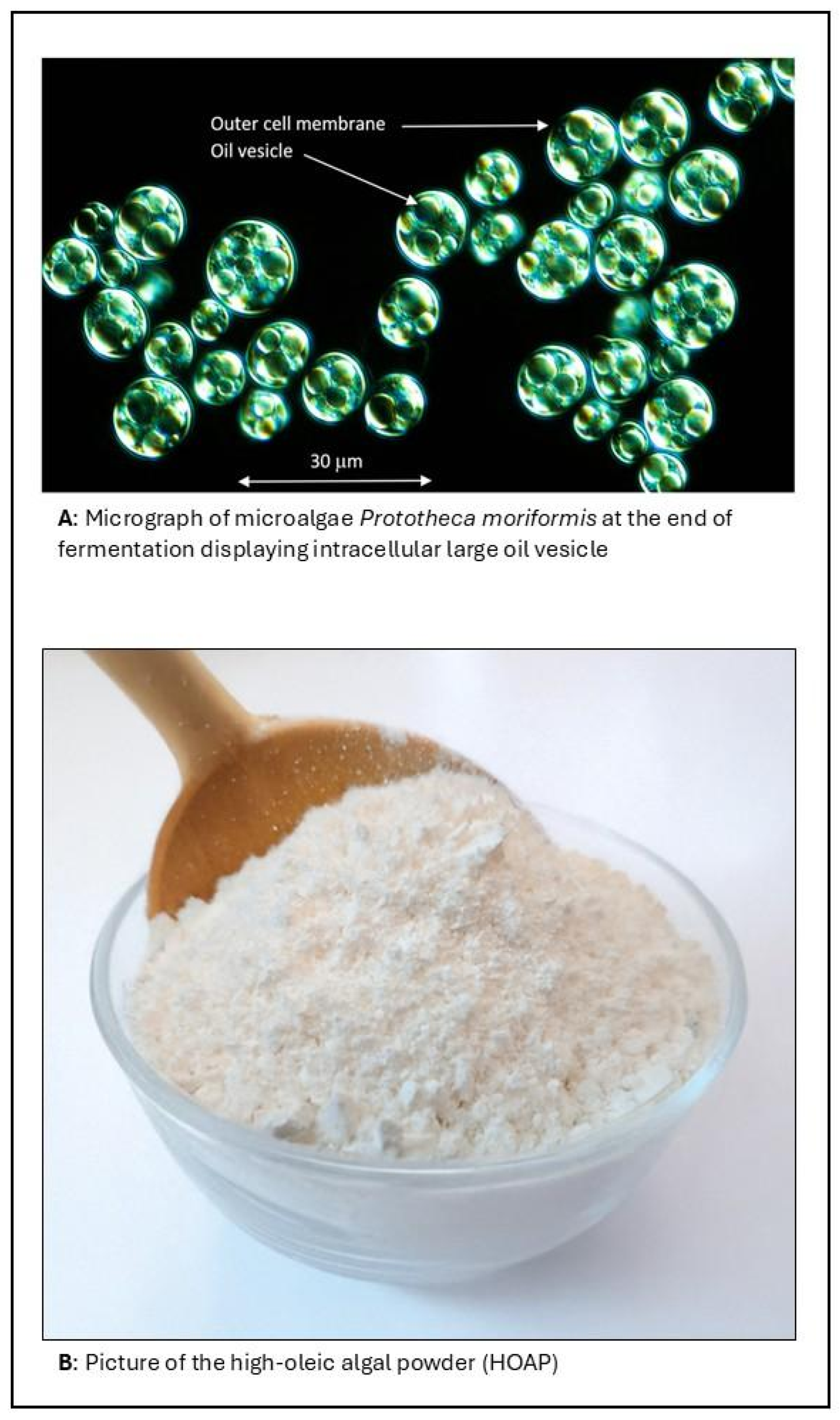
To address these limitations, we developed high-oleic algal powder (HOAP), a lipid-rich powder derived directly from the biomass of a heterotrophically grown microalga. HOAP is not an encapsulated system, but a whole cell with intrinsically high lipid content (>60%), particularly enriched in monounsaturated oleic acid (~89% of total fatty acids). This configuration allows the oil to be stably integrated within a protein–fiber–lipid matrix, providing structural integrity of the powder.
Importantly, HOAP also presents unique potential as a naturally structured delivery system for lipophilic compounds and bioactive fatty acids. Its high oil content, combined with the presence of emulsifying cell wall and membrane components in a fibrous matrix, may enhance the dispersibility and bioavailability of incorporated molecules in nutritional products. This could be advantageous for applications seeking to deliver functional lipids (e.g., omega-3 or fat-soluble vitamins) or other hydrophobic nutraceuticals within stable, powder-based systems. Unlike synthetic encapsulates, HOAP’s native biological matrix offers a simplified and potentially more bioavailable/digestible vehicle for lipid-associated bioactives.
The strain used for HOAP production has been previously documented and has been developed through classical mutagenesis and adaptive selection for enhanced lipid accumulation and oleic acid content [
6]. This strain has previously been used to produce a high-quality refined oil extensively characterized for use in food, personal care, and industrial formulations [
6]. Building on this foundation, the current work describes the production process and physicochemical characterization of HOAP and evaluates its batch-to-batch reproducibility, compositional profile, microbiological and allergenic safety, and storage stability. HOAP offers a differentiated solution for formulators seeking additive-free, high-fat, shelf-stable ingredients with clean-label appeal. This manuscript aims to provide a comprehensive technical and safety profile of HOAP and to support its potential use as a next-generation lipid structuring ingredient for a wide range of applications in food, nutrition, and beyond.
2. Materials and Methods
Strain development. Classical strain improvement was initiated on wild-type
Prototheca moriformis strain isolate UTEX 1533 displaying levels of 28% and 56–60% of palmitic and oleic acids, respectively, as previously described [
6]. Briefly, classical strain improvement regimes included repeated rounds of UV (8000–20,000 μJoules) and chemical (270 mM ethyl methane sulfonate (EMS), 45 min @ 32 °C; 4-nitroquinoline 1-oxide-4-NQO, at concentrations, exposure times, and temperatures ranging from 2.7–60 μM, 5–30 min, and 28–32 °C, respectively) mutagenesis. Enrichment strategies performed after mutagenesis included both chemical (cerulenin, a beta-keto-acyl-ACP synthase inhibitor at 7–50 μM; AZD-8055 an mTOR kinase inhibitor at 26–75 μM and clomiphene, an inhibitor of sterol biosynthesis at 12–100 μM) and physical (buoyant density centrifugation) means. The iterative methodology involved the creation of mutant libraries followed by clonal isolation, assessments of feedstock utilization and growth, lipid accumulation, and the validation of oil composition. Screening these libraries led to the identification of isolates with the ability to produce elevated levels of oleic acid, aligning with the targets for high-oleic acid substitutes. Detailed conditions for the production of the high-oleic algae strain are provided elsewhere [
6,
7].
Culture media composition. Cultured conditions and media used has been described in a previous paper [
6,
7]. Briefly, vegetative growth medium was comprised of macronutrients including NaH
2PO
4, K
2HPO
4, citric acid monohydrate, magnesium sulfate heptahydrate, calcium chloride dihydrate, and dextrose at 1.64, 1.98, 1.05, 1.23, 0.02, and 40 g/L, respectively. Ammonium sulfate served as the sole nitrogen source at 1.0 g/L. Anti-foam (Sigma 204, Sigma-Aldrich, St Louis, MO, USA) was added to a final concentration of 0.225 mL/L. Trace minerals were prepared as a 1000 × stock solution comprised of boric acid, zinc sulfate heptahydrate, manganese sulfate monohydrate, sodium molybdate dihydrate, nickel nitrate heptahydrate, citric acid monohydrate, copper (II) sulfate pentahydrate, and iron (II) sulfate heptahydrate at 0.91, 1.76, 1.23, 0.05, 0.04, 20.49, 0.05, and 0.75 g/L, respectively. A 1000 × vitamin stock was comprised of thiamine HCl, D-pantothenic acid hemicalcium salt, biotin, cyanocobalamin, riboflavin, and pyridoxine HCl at 3.0, 0.16, 0.0048, 0.00034, 0.015, and 0.0078 g/L, respectively. Lipid production medium was identical to vegetative growth medium except that ammonium sulfate was supplied at 0.2 g/L. All solutions were filter sterilized prior to use.
Production process. The production process was performed at the 20 L reactor scale essentially as described previously [
6,
7]. The seed train for each run was initiated from a single cryovial from a master cell bank of the HOAP strain, which was used to inoculate 50 mL of lipid production medium in a 250 mL baffle flask grown at 28 °C with shaking (200 rpm). This culture was transferred once it reached an A750 of 10–15 to two, 1 L baffle flasks, each containing 200 mL of lipid production medium. These flasks were grown with shaking (200 rpm) at 28 °C until they also reached an A750 of 10–15, at which point their contents were sterilely transferred to a 20 L fermentation vessel, supplying a 10% inoculum (20 L M-Series, Solaris Biotech, Porto Mantovano (MN) Italy. Fermentation conditions included an inoculation volume of 0.25–0.3 of the fermenter volume, with pH and dissolved oxygen (DO) setpoints of 5.5–6.0 and 30%, respectively. The operating temperature was 28 °C throughout with aeration and agitation managed to maintain DO. Fermentation medium was fortified to support higher cell density as follows. Medium composition remained the same; however, trace metals and vitamin solutions were increased 15 and 10-fold, respectively. Macronutrients (sodium phosphate, potassium phosphate, citric acid monohydrate, magnesium sulfate heptahydrate, and calcium chloride dihydrate) were increased to 7.13, 9.25, 2.1, 17.33, and 0.8 g/L, respectively. Glucose feed consisted of a 55% wt.: wt. sterile solution. At the conclusion of the fermentation, the resulting fermentation broth was pasteurized at 65 °C for 30 min, prior to being dried on a double drum dryer (Buflovak Model ADDD operating at 70 psig steam, 1200 rpm; Buflovak, Tonawanda, NY, USA) such that the final moisture content was <2%.
Analysis of the high-oleic algal oil powder (HOAP). For all analytes measured, the average, standard deviation (SD), and coefficient of variation (CV%) were calculated based on HOAP samples recovered from three non-consecutive production batches. This approach was used to assess the reproducibility and robustness of the production process. The compositional profile of HOAP powder (HOAP) was assessed using standardized and validated analytical methods. Visual appearance was evaluated by macroscopic inspection for consistency in color and texture. Moisture content was determined using an internal gravimetric method based on Standard NF EN ISO 662, and ash content was measured via incineration using an internal method aligned with Standard ISO 6884:2008. Protein content was quantified using the Kjeldahl method (Standard NF V 04-407), while total fat content was extracted and analyzed using the Folch method [
8], which relies on a chloroform–methanol extraction system. Sucrose concentration was determined using an enzymatic colorimetric method. Dietary fiber content was measured following the Standard AOAC Official Method 985.29, which includes enzymatic digestion and the gravimetric determination of both soluble and insoluble fiber fractions. Amino acid analysis was conducted using the following two complementary methods: tryptophan content was quantified in accordance with Standard EU Regulation 152/2009, while all other amino acids were analyzed using the Standard ISO 13903:2005 protocol. Biogenic amines, including histamine, tyramine, putrescine, cadaverine, and spermidine, were quantified using high-performance liquid chromatography (HPLC) with pre-column derivatization, following the procedure described elsewhere [
9]. This method involves the dansyl chloride derivatization of amines and HPLC separation using a C18 column, with detection by UV absorbance at 254 nm. All compositional analyses were performed on three independent production batches of HOAP to assess batch-to-batch variability and ensure data robustness.
Fatty acid (FA) analysis. Fatty acid composition of algae and high-oleic sunflower oil samples were measured as their fatty acid methyl esters (FAMEs) following direct trans-esterification with a sulfuric acid methanol solution [
6]. Samples were injected on an Agilent 8890 gas chromatograph system equipped with a split/split less inlet and a flame ionization detector (Agilent Technologies, Palo Alto, CA, USA). An Agilent DB-WAX column (30 m × 0.32 mm × 0.25 um dimensions) was used for the chromatographic separation of the FAME peaks. A FAME standard mixture purchased from Nu-Chek Prep (Nu-Chek Prep Inc., Elysian, MN, USA) was injected to establish retention times. Response factor corrections were previously determined empirically using various standard mixtures from Nu-Chek Prep. Methyl nonadecanoate (19:0) was used as an internal standard for the quantitation of individual FAMEs. Run-to-run reproducibility was controlled by running an internal reference standard, an algal biomass control sample. As an example, this standard, run over three years and assessing 18 random runs, shows a standard deviation in oil content (g/L) of 0.80 g/L (average of 49.14), oleic acid content of 0.13 area % (average of 83.60), total saturate content of 0.05 area % (average of 7.43), total monounsaturated FA content of 0.14 area % (average of 84.68), and total polyunsaturated FA content of 0.03 area % (average of 7.51). The coefficient of variation (CV%) across these five parameters was 1.62, 0.15, 0.73, 0.17, and 0.40 percent, respectively. Such low analytical variability allows us to pick-up small variations of targeted fatty acid levels when performing the high-throughput screening of classically improved strains.
Microbiological analysis. The microbiological quality and safety of HOAP were assessed using accredited standard methods. Total aerobic plate counts were determined in accordance with Standard NFEN ISO 4833-2, while coliform counts were evaluated using Standard NF V08-050. Escherichia coli (E. coli) detection was performed following the Standard NF ISO 16649-2 protocol. The presence of Salmonella spp. was assessed in 25 g of samples using the method outlined in Standard BKR 23/07-10/11. The enumeration of coagulase-positive Staphylococci was conducted using Standard NF EN ISO 6888-2, and Pseudomonas spp. were quantified using Standard ISO 22717. Fungal contamination was evaluated by separate determination of mold and yeast counts, both performed according to Standard NF V08-036. All microbial analyses were conducted in triplicate on three independent production batches to evaluate compliance with quality standards and to confirm microbiological safety of the final product.
In silico assessment of allergenicity. An in silico assessment of potential allergenicity was performed using the predicted amino acid sequences from the
P. moriformis base strain. Protein sequences were derived from an in-house genome assembly generated using PacBio HiFi sequencing, assembled with Canu [
10], scaffolded with RagOut [
11], and annotated using Augustus [
12] with a model trained on
Chlamydomonas reinhardtii and transcriptome data from
Auxenochlorella protothecoides. The resulting amino acid sequences were compared against the COMPARE allergen database [
13] using BLASTp (NIH, Bethesda, MD, USA) with an E-value threshold of 1 × 10
−5 (
Supplementary Material Table S1 and Figure S1).
4. Discussion
The development of a HOAP production strain demonstrates the effective application of classical mutagenesis and phenotypic selection to enhance both lipid productivity and oleic acid content. Starting from a well-characterized axenic isolate, sequential rounds of chemical and UV mutagenesis led to the identification of a production strain with markedly improved biosynthetic capacity [
6]. The fermentation process, implemented under tightly regulated conditions, exhibited strong reproducibility. Across three independent 20 L-scale fermentations, key metrics, including oleic acid content, dry cell weight (DCW), oil content, and oil titer, showed minimal variation (CVs < 2.0%), confirming the genetic stability of the production strain and the robustness of the production process (
Table 1).
A compositional analysis of HOAP confirms its designation as a lipid-rich, fiber-containing material with low moisture and sugar content (
Table 2). With an average fat content of 64.5 g/100 g, HOAP lies within the upper range for microbial lipid ingredients and meets the functional requirements for use in food applications demanding high lipid loads (
Table 2). The elevated fiber content (34.4 g/100 g) enhances its potential as a dual-function ingredient, delivering both lipid and dietary fiber. Low moisture content (2.4 g/100 g) further contributes to shelf stability and reduces microbial risk, as confirmed by microbial safety data (
Table 7). The fatty acid profile, dominated by oleic acid (88.8% of total fatty acids), closely resembles that of high-oleic sunflower or canola oils, conferring excellent oxidative and thermal stability. Low levels of saturated (e.g., palmitic and stearic) and polyunsaturated fatty acids (e.g., linoleic and α-linolenic acids) support both health-oriented and formulation-stable applications. The amino acid composition includes all essential amino acids, most notably leucine, lysine, and valine albeit at low concentrations consistent with its primary role as a structuring fat rather than a protein supplement (
Table 4). The detection of spermidine (74.8 mg/kg), a naturally occurring polyamine, provides an additional nutritional benefit (
Table 5), present at levels higher to those found in legumes, cheeses, and soybeans. Spermidine is known to support autophagy, lipid metabolism, and cellular homeostasis [
17,
18,
19], and HOAP may serve as a valuable dietary source of this bioactive compound. These compositional characteristics confirm HOAP’s suitability for use in formulations requiring lipid structuring, oxidative stability, and added nutritional functionality. The stability study presented here further reinforces HOAP’s commercial readiness, demonstrating its resistance to oxidation and physical degradation under both ambient and elevated storage conditions. Fat content remained stable over time, and no changes in appearance were observed, suggesting the matrix integrity of the powder was preserved. These findings underscore HOAP’s potential for use in shelf-stable food systems and support its inclusion in formulations requiring resilience to heat, oxygen, and long-term storage (
Table 6). The stability profile is comparable to those reported for other high-lipid microbial powders. This confirms the utility of antioxidant strategies for extending product shelf life when needed.
The overall safety profile of HOAP is supported by both allergenicity and microbiological assessments. Given the close phylogenetic relationship between
Prototheca and
Auxenochlorella, it may be relevant to reference the published safety study of a
Chlorella-based whole algal flour in a rat model, which demonstrated no adverse effects at high doses [
22], to further support the safety profile of HOAP. In silico analysis of the
P. moriformis base strain proteome revealed only six proteins with moderate sequence similarity (>70%) to known allergens in the COMPARE database (
Table S1). Further screening using AlgPred2 confirmed that none of these candidates possessed IgE-binding epitopes or allergenic motifs. These proteins, including alpha-tubulin and heat shock protein 70 kDa, are commonly identified in BLAST-based screens of microbial genomes and have not been associated with clinically relevant allergic reactions in vivo. Similar findings have been reported for related microalgae, such as
Chlorella variabilis and
A. protothecoides, which share a comparable allergen database profile and have established use in food and feed applications. In parallel, the risk of Protothecal infection is considered negligible. Although
Prototheca species are occasionally implicated in opportunistic infections (protothecosis), such cases are extremely rare and primarily involve
P. wickerhamii in immunocompromised individuals. The production strain used was derived from a
P. moriformis isolate classified under Biosafety Level 1 (BSL-1), indicating no known risk to healthy individuals. Furthermore, the manufacturing process includes a validated thermal inactivation step (>65 °C for at least 30 min), ensuring complete microbial deactivation and preventing the presence of any viable cells in the final product. Collectively, the absence of known allergenic proteins with clinical relevance, the BSL-1 classification of the production organism, and the robust thermal processing step confirm that HOAP presents no significant safety concerns for human consumption.
The unique compositional and functional attributes of HOAP position it as a promising ingredient for applications in the food and nutrition space. Unlike conventional oil powders, which are typically produced through the encapsulation of liquid oils with carrier agents, such as maltodextrins or proteins [
1,
4,
5], HOAP is an intrinsically structured lipid-rich biomass that does not require encapsulation to achieve a dry powder format (
Figure 1B). This provides significant advantages in terms of ingredient simplicity, clean labeling, and process efficiency. The high content of oleic acid confers excellent oxidative stability and a favorable fatty acid profile aligned with current nutritional guidelines promoting monounsaturated fat intake. In addition, the presence of dietary fiber, minimal sugar, and low moisture content enhance its functionality in shelf-stable and health-oriented formulations. HOAP’s natural matrix eliminates the need for emulsifiers or complex encapsulation systems, reducing formulation complexity and improving compatibility with clean-label product strategies.
In addition to its compositional and stability advantages, HOAP exhibits functional properties that are highly relevant for food processing applications. As a naturally structured biomass with intracellular lipid droplets embedded in a protein–fiber matrix, HOAP differs fundamentally from conventional oil powders that rely on emulsifier-based encapsulation systems [
1]. When conventional powdered oils are added to a water continuous phase, the shell usually dissolves, releasing the oil. When added to an oil continuous phase with some shear, the oil dissolves leaving the power dispersed in the oil. HOAP can be added to water or oil continuous systems with shear creating a dispersion, but the oil remains inside the cell. The oil in HOAP is not externally emulsified but instead retained within the intact cellular structure of the microalgae, providing both oxidative protection and processing flexibility. However, to fully access the lipid fraction for formulation purposes, the mechanical disruption of the matrix is often required. Several processing techniques can be employed to facilitate oil release and integration from HOAP into food matrices. High-pressure homogenization (HPH) at pressures up to 1500 bar has proven effective in disrupting the microalgal cell walls, enabling the release of intracellular oil into homogenous emulsions [
23,
24]. This approach is particularly well-suited for applications such as plant-based beverages, infant nutrition, or clinical nutrition formulas, where lipid dispersion and emulsion stability are critical. Bead milling is another effective method for unlocking HOAP’s lipid content. This high-shear mechanical process breaks down the cell wall and disperses the oil within an aqueous phase, yielding a smooth lipid-rich paste. As shown in
Supplementary Figure S2, the conversion from the HOAP to a homogenous paste can be achieved by milling the HOAP. This processing approach is advantageous for applications such as culinary sauces, dairy analogs, or functional spreads. Additionally, HOAP can be incorporated into dry and semi-moist applications through extrusion. The high temperature, pressure, and shear forces encountered during extrusion can be sufficient to disrupt the microalgal matrix, resulting in oil release during processing. This makes HOAP suitable for use in baked goods, extruded snacks, and plant-based meat alternatives, where it can serve both as a lipid source and as a structuring agent.
Furthermore, the robust stability profile under both ambient and elevated temperature conditions supports its use in a wide range of applications, including nutritional bars, plant-based dairy and meat analogues, powdered meal replacements, and bakery products. The combined nutritional, functional, and stability attributes of HOAP offer a differentiated value proposition relative to traditional oil powders, expanding formulation possibilities while aligning with consumer demand for minimally processed and nutritionally beneficial ingredients.