Novel Whey Fermented Beverage Enriched with a Mixture of Juice Concentrates: Evaluation of Antimicrobial, Antioxidant, and Angiotensin I Converting Enzyme Inhibitory (ACE) Activities Before and After Simulated Gastrointestinal Digestion
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Samples and Other Ingredients
2.3. Microbial Strains and Growth Conditions
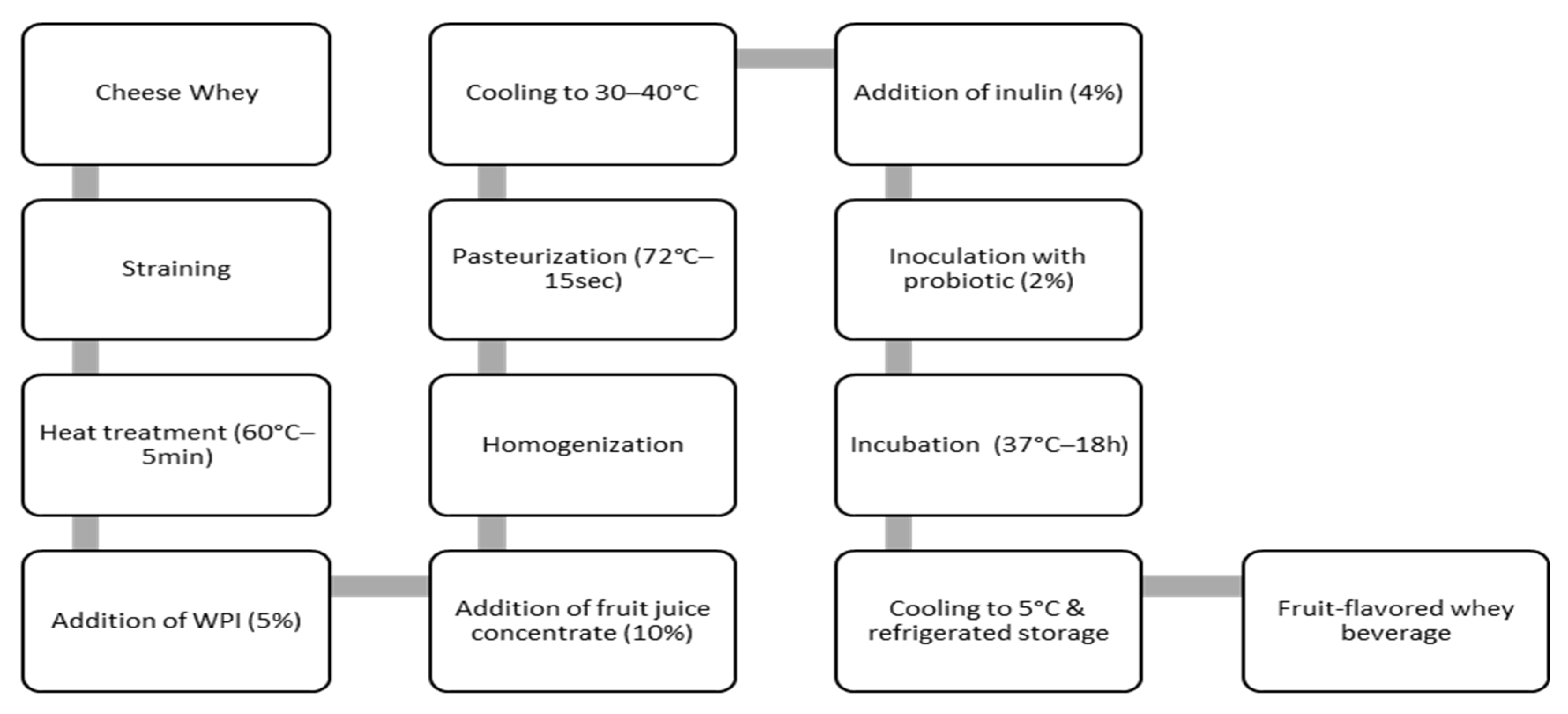
2.4. Preparation of Whey Fermented Beverages
2.5. Nutritional Analysis of the Whey and the Probiotic Whey Drink with Added Juice Concentrates
2.6. Probiotic Viability
2.7. In Vitro Digestion of Whey Fermented Beverages
2.8. Antimicrobial Activity of Whey Fermented Beverages
2.9. Antioxidant Activity Assays of Whey Fermented Beverages
2.9.1. DPPH (2, 2 Diphenyl-1-Picryl Hydrazyl) Assay
2.9.2. Ferric Reducing Antioxidant Power (FRAP) Assay
2.10. Angiotensin-I-Converting Enzyme (ACE)-Inhibitory Activity Assay
2.11. Statistical Analysis
3. Results and Discussion
3.1. Nutritional Analysis of the Whey and the Probiotic Whey Drink with Added Juice Concentrates
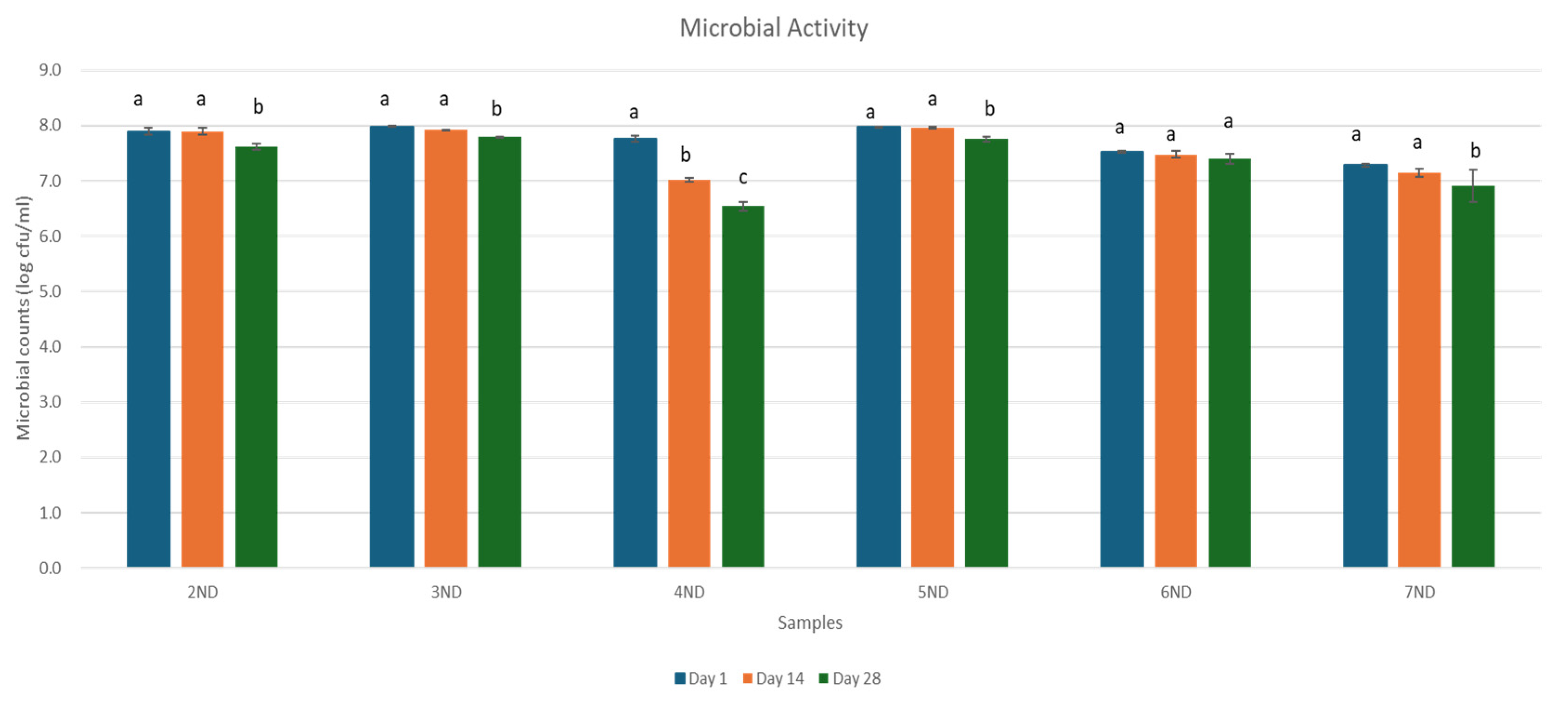
3.2. Probiotic Viability
3.3. Antimicrobial Activity
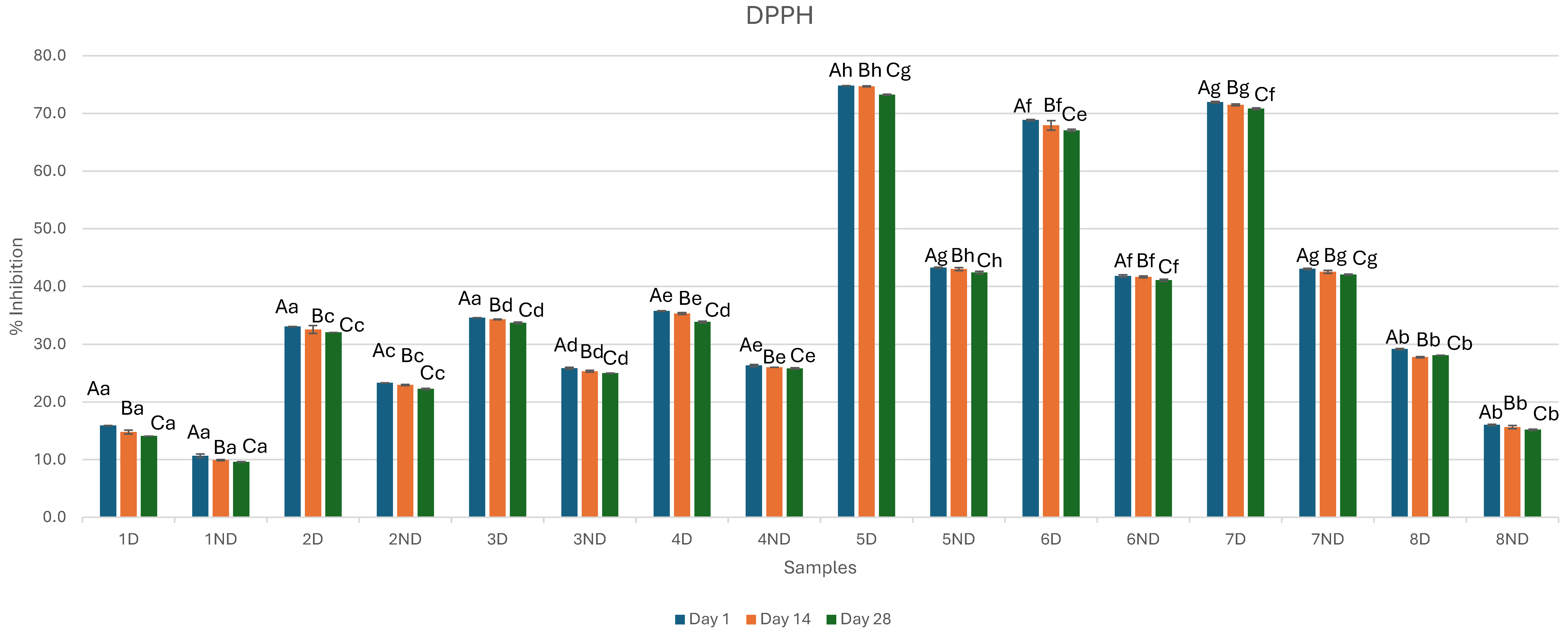
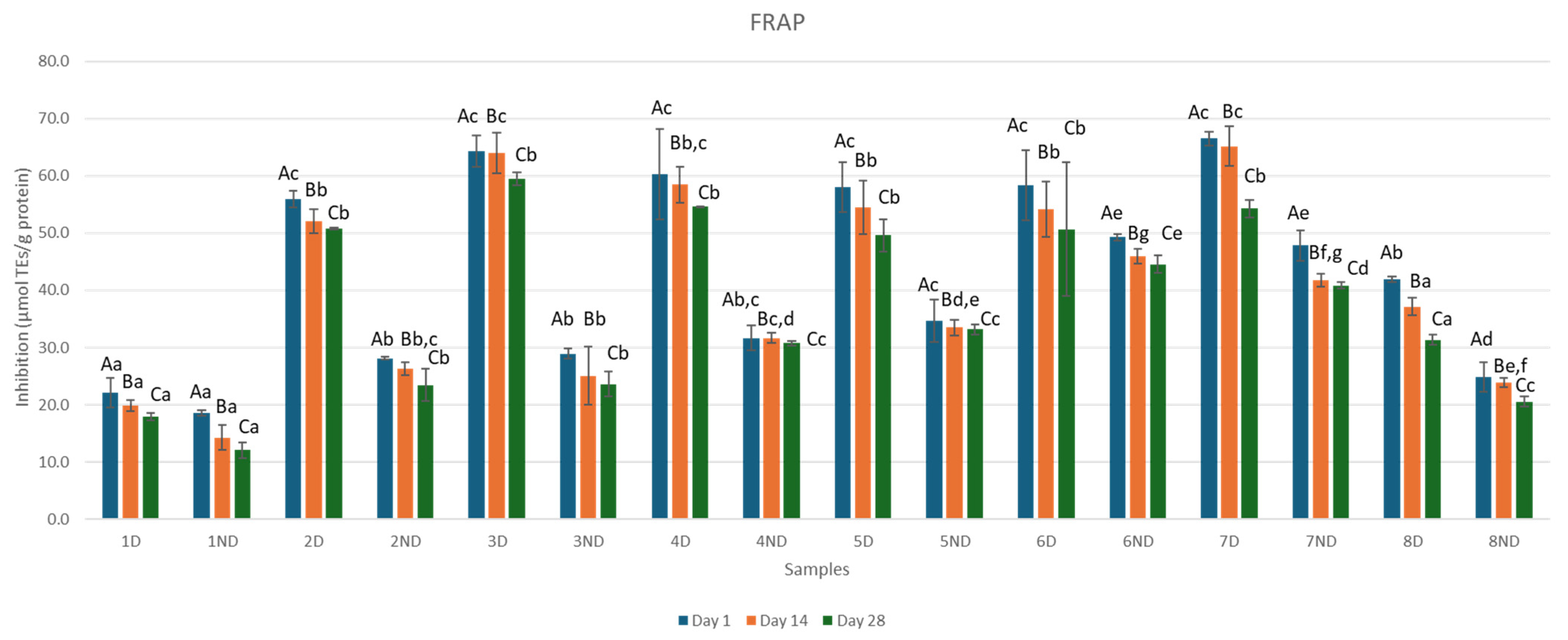
3.4. Antioxidant Activity
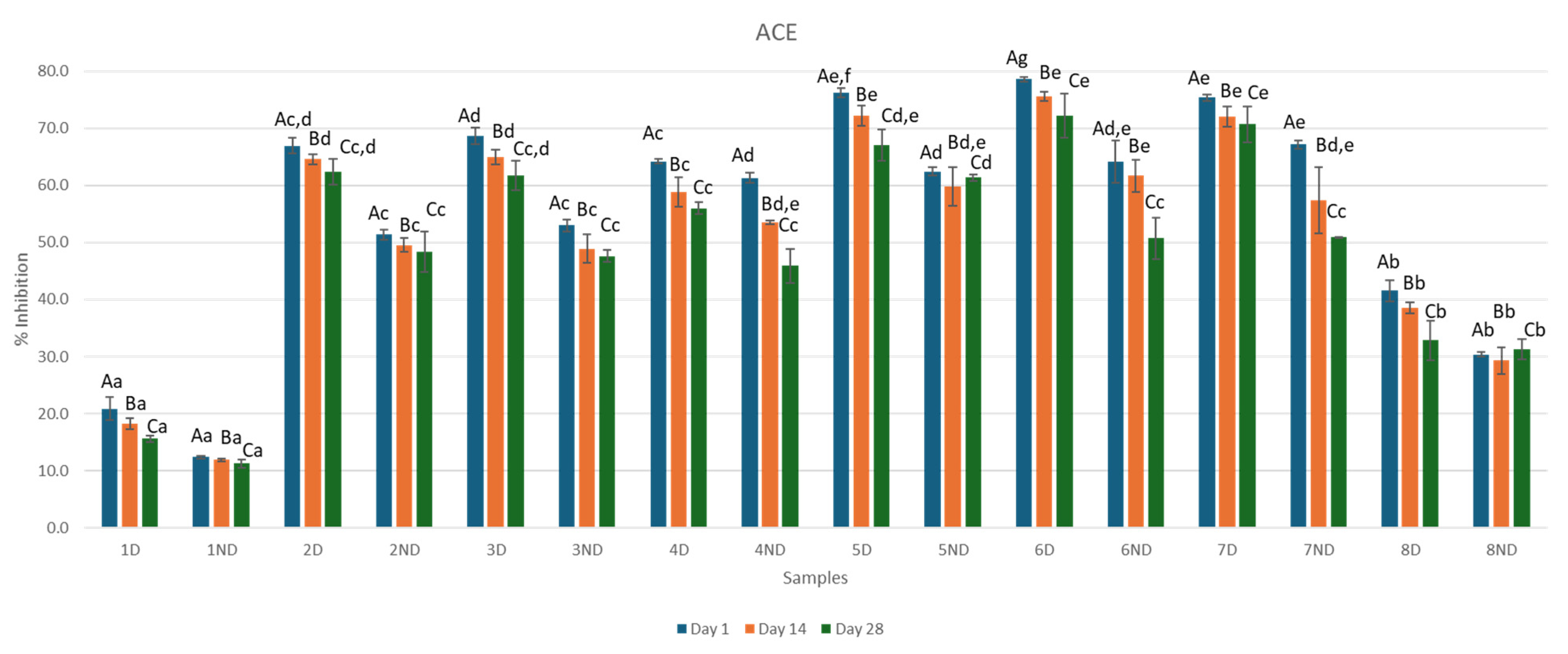
3.5. ACE Inhibitory Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Skryplonek, K.; Dmytrów, I.; Mituniewicz-Małek, A. Probiotic fermented beverages based on acid whey. J. Dairy Sci. 2019, 102, 7773–7780. [Google Scholar] [CrossRef] [PubMed]
- Smithers, G.W. Whey and whey proteins-from ‘gutter-to-gold’. Int. Dairy J. 2008, 18, 695–704. [Google Scholar] [CrossRef]
- Sady, M.; Jaworska, G.; Grega, T.; Bernaś, E.; Domagaĺa, J. Application of acid whey in orange drink production. Food Technol. Biotechnol. 2013, 51, 266–277. [Google Scholar]
- Macwan, S.R.; Dabhi, B.K.; Parmar, S.C.; Aparnathi, K.D. Whey and its Utilization. Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 134–155. [Google Scholar] [CrossRef]
- Panghal, A.; Kumar, V.; Dhull, S.B.; Gat, Y.; Chhikara, N. Utilization of Dairy Industry Waste-Whey in Formulation of Papaya RTS Beverage. Curr. Res. Nutr. Food. Sci. 2017, 5, 168–174. [Google Scholar] [CrossRef]
- Panghal, A.; Patidar, R.; Jaglan, S.; Chhikara, N.; Khatkar, S.K.; Gat, Y.; Sindhu, N. Whey valorization: Current options and future scenario—A critical review. Nutr. Food Sci. 2018, 48, 520–535. [Google Scholar] [CrossRef]
- Yamahata, N.; Toyotake, Y.; Kunieda, S.; Wakayama, M. Optimal Fermentation Conditions and Storage Period of Fermented Beverages Made from Demineralized Whey Using Kluyveromyces marxians. J. Food Sci. Nutr. Res. 2020, 3, 1–17. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, A.; Sharma, S.; Kant, A.; Sevda, S.; Taherzadeh, M.J.; Garlapati, V.K. Functional foods as a formulation ingredients in beverages: Technological advancements and constraints. Bioengineered 2021, 12, 11055–11075. [Google Scholar] [CrossRef]
- Lech, M. Optimisation of protein-free waste whey supplementation used for the industrial microbiological production of lactic acid. Biochem. Eng. J. 2019, 157, 107531. [Google Scholar] [CrossRef]
- Mauriello, G.; Moio, L.; Moschetti, G.; Piombino, P.; Addeo, F.; Coppola, S. Characterization of lactic acid bacteria strains on the basis of neutral volatile compounds produced in whey. J. Appl. Microbiol. 2001, 90, 928–942. [Google Scholar] [CrossRef]
- Miraballes, M.; Hodos, N.; Gámbaro, A. Application of a Pivot Profile Variant Using CATA Questions in the Development of a Whey-Based Fermented Beverage. Beverages 2018, 4, 11. [Google Scholar] [CrossRef]
- Aghda, S.K.; Nasirpour, A.; Nasrabadi, M.N. Effect of acidification rate, acidification temperature, final pH, and stabilizer content on colloidal stability of whey-based pomegranate beverage. J. Dispers. Sci. Technol. 2017, 38, 58–64. [Google Scholar] [CrossRef]
- Bandara, T.A.; Munasinghe-Arachchige, S.P.; Gamlath, C.J. Fermented whey beverages: A review of process fundamentals, recent developments and nutritional potential. Int. J. Dairy Technol. 2023, 76, 737–757. [Google Scholar] [CrossRef]
- Elkot, W.F.; Elmahdy, A.; El-Sawah, T.H.; Alghamdia, O.A.; Alhag, S.K.; Al-Shahari, E.A.; Al-Farga, A.; Ismail, H.A. Development and characterization of a novel flavored functional fermented whey-based sports beverage fortified with Spirulina platensis. Int. J. Biol. Macromol. 2023, 258 Pt 2, 128999. [Google Scholar] [CrossRef]
- Gimhani, I.; Liyanage, C. Development and Quality Evaluation of Ready to Drink Fruit Flavoured Whey Beverage. Int. J. Sci. Res. Publ. 2019, 9, 92107. [Google Scholar] [CrossRef]
- M’Hir, S.; Mejri, A.; Atrous, H.; Ayed, L. Optimization of Parameters Using Response Surface Methodology to Develop a Novel Kefir-Like Functional Beverage from Cheese Whey Enriched with Myrtle Juice. J. Chem. 2021, 2021, 2984470. [Google Scholar] [CrossRef]
- Merlet, M.; Flutto, T.; Valentini, S. Formulation, Biochemical Characterization and Shelf-Life Study of YoAlp® Whey Based Beverage Containing Local Fruits Juices. J. Nat. Prod. Tradit. Med. 2024, 3, 1. [Google Scholar]
- Morya, S.; Sagar, B.S.; Tiwari, S.; Ed, A.; Amoah, D.; Singh, N. A New Generation of Utilizing Dairy by-products: Whey Based Beverages. Adv. Life Sci. 2016, 5, 8105–8106. [Google Scholar]
- Saglam, H.; Sarioglu, T.; Karahan, A.; Oner, Z. An investigation for the development of whey-based probiotic beverages. Rom. Biotechnol. Lett. 2019, 24, 1097–1106. [Google Scholar] [CrossRef]
- Sharma, R.; Choudhary, R.; Thakur, N.S.; Bishist, R.; Thakur, A. Optimization of Fructooligosaccharide Fortified Low Calorie Apple-Whey Based RTS Beverage and Its Quality Evaluation during Storage. Curr. J. Appl. Sci. Technol. 2020, 39, 17–28. [Google Scholar] [CrossRef]
- Ozcan, T.; Eroglu, E. Effect of stevia and inulin interactions on fermentation profile and short-chain fatty acid production of Lactobacillus acidophilus in milk and in vitro systems. Int. J. Dairy Technol. 2022, 75, 171–181. [Google Scholar] [CrossRef]
- Ribeiro, J.E.S.; da Silva Sant’Ana, A.M.; da Silva, F.L.H.; Filho, E.M.B. Use of water-soluble soy extract and inulin as ingredients to produce a fermented dairy beverage made from caprine milk. Food Sci. Technol. 2023, 43, e102122. [Google Scholar] [CrossRef]
- Meyer, D.; Bayarri, S.; Tárrega, A.; Costell, E. Inulin as texture modifier in dairy products. Food Hydrocoll. 2011, 25, 1881–1890. [Google Scholar] [CrossRef]
- Onverti, A.C. The effect of inulin as a prebiotic on the production of probiotic fibre-enriched fermented milk. Int. J. Dairy Technol. 2009, 62, 195–203. [Google Scholar] [CrossRef]
- Kheto, A.; Adhikary, U.; Dhua, S.; Sarkar, A.; Kumar, Y.; Vashishth, R.; Shrestha, B.B.; Saxena, D.C. A review on advancements in emerging processing of whey protein: Enhancing functional and nutritional properties for functional food applications. Food Saf. Health 2024, 3, 23–45. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food-an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Field, D.; Connor, P.M.O.; Cotter, P.D.; Hill, C.; Ross, R.P. The generation of nisin variants with enhanced activity against specific Gram-positive pathogens. Mol. Microbiol. 2008, 69, 218–230. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, L.; Rui, X.; Li, W.; Chen, X.; Jiang, M.; Dong, M. Enhancement of the antioxidant capacity of soy whey by fermentation with Lactobacillus plantarum B1–6. J. Funct. Foods 2015, 12, 33–44. [Google Scholar] [CrossRef]
- Li, G.H.; Liu, H.; Shi, Y.H.; Le, G.W. Direct spectrophotometric measurement of angiotensin I-converting enzyme inhibitory activity for screening bioactive peptides. J. Pharm. Biomed. Anal. 2005, 37, 219–224. [Google Scholar] [CrossRef]
- Tamime, A.Y. Dairy Powders and Concentrated Products; Blackwell Publishing: Hoboken, NJ, USA, 2009; pp. 1–380. [Google Scholar] [CrossRef]
- Ahmed, T.; Sabuz, A.A.; Mohaldar, A.; Fardows, H.M.S.; Inbaraj, B.S.; Sharma, M.; Rana, R.; Sridhar, K. Development of Novel Whey-Mango Based Mixed Beverage: Effect of Storage on Physicochemical, Microbiological, and Sensory Analysis. Foods 2023, 12, 237. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Y.; Zhang, W.; Sun, M.; Zhang, Z. Hypoglycemic effect of inulin combined with Ganoderma lucidum polysaccharides in T2DM rats. J. Funct. Foods 2019, 55, 381–390. [Google Scholar] [CrossRef]
- Bassan, J.C.; Goulart, A.J.; Nasser, A.L.M.; Bezerra, T.M.S.; Garrido, S.S.; Rustiguel, C.B.; Guimarães, L.H.S.; Monti, R. Buffalo Cheese Whey Proteins, Identification of a 24 KDa Protein and Characterization of Their Hydrolysates: In Vitro Gastrointestinal Digestion. PLoS ONE 2015, 10, 1–18. [Google Scholar] [CrossRef]
- Messaoui, H.; Roudj, S.; Karam, N.-E. Antioxidant and antibacterial activities of bovine whey proteins hydrolysed with selected Lactobacillus strains. Food Environ. Saf. 2020, XIX, 25–39. [Google Scholar] [CrossRef]
- Brandelli, A.; Daroit, D.J.; Corrêa, A.P.F. Whey as a source of peptides with remarkable biological activities. Food Res. Int. 2015, 73, 149–161. [Google Scholar] [CrossRef]
- Kadyan, S.; Rashmi, H.M.; Pradhan, D.; Kumari, A.; Chaudhari, A.; Deshwal, G.K. Effect of lactic acid bacteria and yeast fermentation on antimicrobial, antioxidative and metabolomic profile of naturally carbonated probiotic whey drink. LWT-Food Sci. Technol. 2021, 142, 111059. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; FitzGerald, R.J. Bioactive properties of milk proteins in humans: A review. Peptides 2015, 73, 20–34. [Google Scholar] [CrossRef]
- Rai, A.K.; Sanjukta, S.; Jeyaram, K. Production of angiotensin I converting enzyme inhibitory (ACE-I) peptides during milk fermentation and their role in reducing hypertension. Crit. Rev. Food Sci. Nutr. 2017, 57, 2789–2800. [Google Scholar] [CrossRef]
| Bacterial Strain | Origin/Characteristics | Microbiological Media | Incubation Conditions |
|---|---|---|---|
| Indicator Strains | |||
| Listeria monocytogenes 33423 | UCC Culture Collection/Food Isolate | BHI | 37 °C/48 h |
| Listeria monocytogenes 33413 | UCC Culture Collection/Food Isolate United Kingdom outbreak (L. mono Ts45) | BHI | 37 °C/48 h |
| Staphylococcus aureus SA 113 | UCC Culture Collection/Representative staphylococcal organism in model virulence studies | BHI | 37 °C/48 h |
| Staphylococcus aureus Newman | UCC Culture Collection | BHI | 37 °C/48 h |
| Bacillus cereus DPC 6089 | DPC culture Collection | BHI | 37 °C/48 h |
| Escherichia coli NCTC 9001 | NCTC Culture Collection | BHI | 37 °C/48 h |
| Salmonella Enteritidis NCTC 6676 | NCTC Culture Collection | BHI | 37 °C/48 h |
| LAB used for the production of fermented whey beverages | |||
| Lacticaseibacillus rhamnosus (LGG) | MRS | 37 °C/24 h | |
| Lacticaseibacillus casei (431) | MRS | 37 °C/24 h | |
| Lactobacillus helveticus (R0052) | MRS | 37 °C/24 h | |
| Sample Number | Probiotic | Inulin | Whey Protein Isolate (WPI) | Juice Concentrate *1 |
|---|---|---|---|---|
| 1 | No | No | No | No |
| 2 | Lactobacillus rhamnosus (LGG)—2% | 4% | 5% | No |
| 3 | Lacticaseibacillus casei (L. casei)—2% | 4% | 5% | No |
| 4 | Lactobacillus helveticus (R0052) 2% | 4% | 5% | No |
| 5 | Lactobacillus rhamnosus (LGG) 2% | 4% | 5% | 10% |
| 6 | Lacticaseibacillus casei (L. casei) 2% | 4% | 5% | 10% |
| 7 | Lactobacillus helveticus (R0052) 2% | 4% | 5% | 10% |
| 8 | No | 4% | 5% | No |
| Parameter | Probiotic Whey Drink |
|---|---|
| Fat % | 0.09 ± 0.01 |
| Protein % | 3.89 ± 0.11 |
| Ash % | 1.18 ± 0.19 |
| Total Solids % | 21.35 ± 0.35 |
| Calcium mg/L | 511.80 ± 53.02 |
| Phosphorus mg/L | 437.17 ± 69.08 |
| Potassium mg/L | 2039.69 ± 204.59 |
| Sodium mg/L | 2943.03 ± 683.96 |
| pH | 3.65 |
| Indicator Strains | Days | Sample 1D | Sample 2D | Sample 3D | Sample 4D | Sample 5D | Sample 6D | Sample 7D | Sample 8D |
|---|---|---|---|---|---|---|---|---|---|
| Listeria monocytogenes 33423 | 1 | 27.92 ± 0.04 a | 28.14 ± 0.06 a.b | 28.57 ± 0.06 b | 29.46 ± 0.06 c | 28.32 ± 0.06 a.b | 29.20 ± 0.05 c | 28.45 ± 0.63 a.b | 28.49 ± 0.04 a.b |
| 14 | 24.05 ± 0.05 a | 26.41 ± 0.05 c | 24.13 ± 0.04 a | 26.09 ± 0.04 b. | 26.36 ± 0.06 c | 27.86 ± 0.04 e | 27.62 ± 0.10 d | 27.52 ± 0.03 d | |
| 28 | 21.86 ± 0.04 a | 22.14 ± 0.04 b | 22.76 ± 0.06 c | 24.47 ± 0.03 f | 23.05 ± 0.05 d | 24.63 ± 0.05 g | 24.51 ± 0.04 f | 24.09 ± 0.05 e | |
| Listeria monocytogenes 33413 | 1 | 19.45 ± 0.06 a | 20.12 ± 0.06 b | 20.68 ± 0.08 c | 20.90 ± 0.08 d | 21.25 ± 0.06 e | 20.70 ± 0.11 c | 21.35 ± 0.08 e | 21.17 ± 0.08 e |
| 14 | 19.34 ± 0.05 a | 19.50 ± 0.06 a.b | 19.9 ± 0.11 d.e | 19.74 ± 0.03 c.d | 19.80 ± 0.11 d | 20.06 ± 0.08 e | 20.79 ± 0.04 f | 19.6 ± 0.04 c.b | |
| 28 | 19.09 ± 0.07 a | 19.14 ± 0.08 a.b | 19.89 ± 0.04 e | 19.29 ± 0.09 b | 19.68 ± 0.10 d | 19.95 ± 0.04 e | 19.95 ± 0.04 e | 19.5 ± 0.10 c | |
| Staphylococcus aureus 113 | 1 | 21.30 ± 0.08 d | 19.73 ± 0.04 b | 19.57 ± 0.08 a | 20.55 ± 0.02 c | 21.84 ± 0.06 f | 23.03 ± 0.04 h | 22.38 ± 0.12 g | 21.52 ± 0.06 e |
| 14 | 19.11 ± 0.06 a | 20.29 ± 0.03 d | 19.5 ± 0.06 a | 20.48 ± 0.06 e | 19.45 ± 0.05 b | 19.34 ± 0.06 b | 20.53 ± 0.04 e | 19.91 ± 0.04 c | |
| 28 | 16.13 ± 0.11 a | 18.32 ± 0.08 e | 17.06 ± 0.08 b | 17.8 ± 0.06 d | 19.20 ± 0.05 g | 17.27 ± 0.04 c | 18.78 ± 0.10 f | 16.25 ± 0.04 a | |
| Staphylococcus aureus Newman | 1 | 24.04 ± 0.05 b | 24.36 ± 0.04 c | 24.36 ± 0.06 c | 23.59 ± 0.02 a | 24.69 ± 0.05 d | 26.59 ± 0.11 g | 25.10 ± 0.06 e | 26.39 ± 0.06 f |
| 14 | 23.49 ± 0.07 a | 23.92 ± 0.04 c | 23.59 ± 0.07 a | 24.23 ± 0.05 d | 24.19 ± 0.05 d | 24.27 ± 0.05 d | 26.11 ± 0.05 e | 23.76 ± 0.08 b | |
| 28 | 21.1 ± 0.04 a | 21.64 ± 0.04 c | 21.24 ± 0.04 b | 22.41 ± 0.06 e | 22.15 ± 0.06 d | 22.47 ± 0.05 f | 22.80 ± 0.06 e | 22.16 ± 0.04 d | |
| Bacillus cereus DPC 6089 | 1 | 20.63 ± 0.04 a | 20.95 ± 0.04 b | 20.86 ± 0.04 b | 22.45 ± 0.05 e | 21.32 ± 0.10 c | 21.27 ± 0.04 c | 21.61 ± 0.10 d | 21.52 ± 0.05 d |
| 14 | 19.92 ± 0.04 a | 20.79 ± 0.08 c | 20.26 ± 0.06 a | 21.82 ± 0.10 e | 21.13 ± 0.06 d | 21.05 ± 0.04 d | 22.40 ± 0.05 f | 21.09 ± 0.12 d | |
| 28 | 19.05 ± 0.06 a | 19.27 ± 0.08 b | 19.68 ± 0.09 c | 19.94 ± 0.04 d.e | 20.11 ± 0.10 e | 19.18 ± 0.11 c.d | 20.33 ± 0.06 f | 19.09 ± 0.06 a.b | |
| Salmonella Enteritidis NCTC 6676 | 1 | 23.47 ± 0.06 b | 22.8 ± 0.10 a | 23.83 ± 0.06 b | 23.77 ± 0.07 b | 24.18 ± 0.06 c | 24.42 ± 0.07 d | 23.16 ± 0.06 b | 23.87 ± 0.05 b |
| 14 | 23.06 ± 0.08 b | 22.91 ± 0.04 a | 23.8 ± 0.06 f | 23.21 ± 0.06 c | 23.60 ± 0.04 e | 24.08 ± 0.08 g | 23.36 ± 0.06 d | 23.51 ± 0.06 e | |
| 28 | 22.57 ± 0.04 a | 22.77 ± 0.08 b | 23.17 ± 0.03 d.e | 22.60 ± 0.05 a | 22.91 ± 0.03 c | 23.24 ± 0.04 e.f | 23.34 ± 0.04 f | 23.06 ± 0.07 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotsaki, P.; Aspri, M.; Papademas, P. Novel Whey Fermented Beverage Enriched with a Mixture of Juice Concentrates: Evaluation of Antimicrobial, Antioxidant, and Angiotensin I Converting Enzyme Inhibitory (ACE) Activities Before and After Simulated Gastrointestinal Digestion. Microorganisms 2025, 13, 1490. https://doi.org/10.3390/microorganisms13071490
Kotsaki P, Aspri M, Papademas P. Novel Whey Fermented Beverage Enriched with a Mixture of Juice Concentrates: Evaluation of Antimicrobial, Antioxidant, and Angiotensin I Converting Enzyme Inhibitory (ACE) Activities Before and After Simulated Gastrointestinal Digestion. Microorganisms. 2025; 13(7):1490. https://doi.org/10.3390/microorganisms13071490
Chicago/Turabian StyleKotsaki, Paschalia, Maria Aspri, and Photis Papademas. 2025. "Novel Whey Fermented Beverage Enriched with a Mixture of Juice Concentrates: Evaluation of Antimicrobial, Antioxidant, and Angiotensin I Converting Enzyme Inhibitory (ACE) Activities Before and After Simulated Gastrointestinal Digestion" Microorganisms 13, no. 7: 1490. https://doi.org/10.3390/microorganisms13071490
APA StyleKotsaki, P., Aspri, M., & Papademas, P. (2025). Novel Whey Fermented Beverage Enriched with a Mixture of Juice Concentrates: Evaluation of Antimicrobial, Antioxidant, and Angiotensin I Converting Enzyme Inhibitory (ACE) Activities Before and After Simulated Gastrointestinal Digestion. Microorganisms, 13(7), 1490. https://doi.org/10.3390/microorganisms13071490







