Diagnostic Approaches for Candida auris: A Comprehensive Review of Screening, Identification, and Susceptibility Testing
Abstract
1. Introduction
2. Clinical Diagnostic Approaches of C. auris
3. Screening Guidelines and Protocols of C. auris
3.1. Importance of Screening for C. auris Colonization
3.2. Identifying Individuals for Screening
3.3. Screening Methods, Timing, and Laboratory Protocols
4. Diagnostic Methods for C. auris Detection
4.1. Culture-Based Methods
4.1.1. Differential and Selective Media
4.1.2. Chromogenic Media
4.1.3. Conclusion of Culture-Based Methods
4.2. Biochemical Assimilation Tests
4.3. Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry (MALDI-TOF MS)
4.3.1. The Evolution of MALDI-TOF on Detecting C. auris
4.3.2. Sample Preparation and Database Considerations
4.4. Molecular Methods for C. auris Detection
4.4.1. Overview of DNA-Based Detection
4.4.2. PCR-Based Detection
4.4.3. Loop-Mediated Isothermal Amplification (LAMP)
4.4.4. T2 Magnetic Resonance (T2MR) Assay
4.4.5. Summary for Molecular Testing
5. Genomic Typing and Outbreak Investigation in C. auris
6. Antifungal Susceptibility Testing (AFST) for C. auris
6.1. Multidrug Resistance in C. auris
6.2. Reference Methods and Interpretive Challenges
6.3. Performance of Commercial Testing Platforms
6.4. Genotypic Resistance Mechanisms and Molecular Testing
6.5. MALDI-TOF MS-Based AFST: Emerging Innovations
6.6. Summary for AFST
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AFST | Antifungal susceptibility testing |
| AFLP | Amplified fragment length polymorphism |
| AMB | Amphotericin B |
| API 20C AUX | Analytical Profile Index 20C Auxanographic Yeast Identification System |
| API ID 32C | Analytical Profile Index ID 32C Yeast Identification System |
| ASTRA | Antibiotic susceptibility test rapid assay |
| BDG | Beta-D-glucan |
| BMD | Broth microdilution |
| BCID | Blood culture identification |
| CA System | Chromogenic agar system (Bruker’s MALDI-TOF MS software) |
| CDC | Centers for Disease Control and Prevention |
| CCI | Composite correlation index |
| CE-IVD | Conformité Européenne—In Vitro Diagnostic |
| CFU | Colony-forming unit |
| CHROMagar™ | Chromogenic agar (commercial medium) |
| CLSI | Clinical and Laboratory Standards Institute |
| DNA | Deoxyribonucleic acid |
| DOAJ | Directory of Open Access Journals |
| ECV | Epidemiologic cutoff value |
| FDA | U.S. Food and Drug Administration |
| GPI | Glycosylphosphatidylinositol |
| HIV | Human immunodeficiency virus |
| ICU | Intensive care unit |
| ITS | Internal transcribed spacer |
| IVD | In Vitro Diagnostic |
| LAMP | Loop-mediated isothermal amplification |
| LD | Linear dichroism |
| MALDI-TOF MS | Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry |
| MBT ASTRA | MALDI Biotyper antibiotic susceptibility test rapid assay |
| MDPI | Multidisciplinary Digital Publishing Institute |
| MIC | Minimum inhibitory concentration |
| MLST | Multilocus sequence typing |
| nad5 | NADH dehydrogenase subunit 5 |
| NCBI | National Center for Biotechnology Information |
| PCR | Polymerase chain reaction |
| qPCR | Quantitative polymerase chain reaction |
| RAPID Yeast Plus | Enzyme-based yeast identification panel (Remel/Thermo Fisher) |
| RUO | Research use only |
| SCA | Specific Candida auris medium |
| SDA | Sabouraud dextrose agar |
| Se | Sensitivity |
| SNP | Single-nucleotide polymorphism |
| Sp | Specificity |
| STR | Short tandem repeat |
| SYO | Sensititre YeastOne |
| T2MR | T2 magnetic resonance |
| TLA | Three-letter acronym |
| VITEK 2 | VITEK 2 Biochemical Identification System |
| vSNF | Ventilator-capable skilled nursing facility |
| WHO | World Health Organization |
| WGS | Whole genome sequencing |
| YST | Yeast susceptibility testing |
References
- Eix, E.F.; Nett, J.E. Candida auris: Epidemiology and Antifungal Strategy. Annu. Rev. Med. 2025, 76, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Nelson, R. Emergence of resistant Candida auris. Lancet Microbe 2023, 4, e396. [Google Scholar] [CrossRef]
- Lyman, M.; Forsberg, K.; Sexton, D.J.; Chow, N.A.; Lockhart, S.R.; Jackson, B.R.; Chiller, T. Worsening Spread of Candida auris in the United States, 2019 to 2021. Ann. Intern. Med. 2023, 176, 489–495. [Google Scholar] [CrossRef]
- Kohlenberg, A.; Monnet, D.L.; Plachouras, D.; Candida auris Survey Collaborative Group. Increasing number of cases and outbreaks caused by Candida auris in the EU/EEA, 2020 to 2021. Eurosurveillance 2022, 27, 2200846. [Google Scholar] [CrossRef]
- Sticchi, C.; Raso, R.; Ferrara, L.; Vecchi, E.; Ferrero, L.; Filippi, D.; Finotto, G.; Frassinelli, E.; Silvestre, C.; Zozzoli, S.; et al. Increasing Number of Cases Due to Candida auris in North Italy, July 2019–December 2022. J. Clin. Med. 2023, 12, 1912. [Google Scholar] [CrossRef]
- Ahmad, S.; Alfouzan, W. Candida auris: Epidemiology, Diagnosis, Pathogenesis, Antifungal Susceptibility, and Infection Control Measures to Combat the Spread of Infections in Healthcare Facilities. Microorganisms 2021, 9, 807. [Google Scholar] [CrossRef] [PubMed]
- Shastri, P.S.; Shankarnarayan, S.A.; Oberoi, J.; Rudramurthy, S.M.; Wattal, C.; Chakrabarti, A. Candida auris candidaemia in an intensive care unit—Prospective observational study to evaluate epidemiology, risk factors, and outcome. J. Crit. Care 2020, 57, 42–48. [Google Scholar] [CrossRef]
- Shaukat, A.; Al Ansari, N.; Al Wali, W.; Karic, E.; El Madhoun, I.; Mitwally, H.; Hamed, M.; Alutra-Visan, F. Experience of treating Candida auris cases at a general hospital in the state of Qatar. IDCases 2021, 23, e01007. [Google Scholar] [CrossRef] [PubMed]
- Al-Rashdi, A.; Al-Maani, A.; Al-Wahaibi, A.; Alqayoudhi, A.; Al-Jardani, A.; Al-Abri, S. Characteristics, Risk Factors, and Survival Analysis of Candida auris Cases: Results of One-Year National Surveillance Data from Oman. J. Fungi 2021, 7, 31. [Google Scholar] [CrossRef]
- Pandya, N.; Cag, Y.; Pandak, N.; Pekok, A.U.; Poojary, A.; Ayoade, F.; Fasciana, T.; Giammanco, A.; Caskurlu, H.; Rajani, D.P.; et al. International Multicentre Study of Candida auris Infections. J. Fungi 2021, 7, 878. [Google Scholar] [CrossRef]
- Chen, J.; Tian, S.; Han, X.; Chu, Y.; Wang, Q.; Zhou, B.; Shang, H. Is the superbug fungus really so scary? A systematic review and meta-analysis of global epidemiology and mortality of Candida auris. BMC Infect. Dis. 2020, 20, 827. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Bustos, V.; Cabanero-Navalon, M.D.; Ruiz-Saurí, A.; Ruiz-Gaitán, A.C.; Salavert, M.; Tormo, M.; Pemán, J. What Do We Know about Candida auris? State of the Art, Knowledge Gaps, and Future Directions. Microorganisms 2021, 9, 2177. [Google Scholar] [CrossRef]
- Fasciana, T.; Cortegiani, A.; Ippolito, M.; Giarratano, A.; Di Quattro, O.; Lipari, D.; Graceffa, D.; Giammanco, A. Candida auris: An Overview of How to Screen, Detect, Test and Control This Emerging Pathogen. Antibiotics 2020, 9, 778. [Google Scholar] [CrossRef]
- Welker, M.; Van Belkum, A.; Girard, V.; Charrier, J.P.; Pincus, D. An update on the routine application of MALDI-TOF MS in clinical microbiology. Expert Rev. Proteom. 2019, 16, 695–710. [Google Scholar] [CrossRef]
- Das, S.; Singh, S.; Tawde, Y.; Chakrabarti, A.; Rudramurthy, S.M.; Kaur, H.; Shankarnarayan, S.A.; Ghosh, A. A Selective Medium for Isolation and Detection of Candida auris, an Emerging Pathogen. J. Clin. Microbiol. 2021, 59, e00326-20. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.; Peyclit, L.; Abdallah, R.; Khelaifia, S.; Chamieh, A.; Rolain, J.M.; Bittar, F. SCA Medium: A New Culture Medium for the Isolation of All Candida auris Clades. J. Fungi 2021, 7, 433. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sachu, A.; Mohan, K.; Vinod, V.; Dinesh, K.; Karim, S. Simple low cost differentiation of Candida auris from Candida haemulonii complex using CHROMagar Candida medium supplemented with Pal’s medium. Rev. Iberoam. Micol. 2017, 34, 109–111. [Google Scholar] [CrossRef]
- Mulet Bayona, J.V.; Salvador García, C.; Tormo Palop, N.; Gimeno Cardona, C. Evaluation of a novel chromogenic medium for Candida spp. identification and comparison with CHROMagar™ Candida for the detection of Candida auris in surveillance samples. Diagn. Microbiol. Infect. Dis. 2020, 98, 115168. [Google Scholar] [CrossRef]
- Borman, A.M.; Fraser, M.; Johnson, E.M. CHROMagarTM Candida Plus: A novel chromogenic agar that permits the rapid identification of Candida auris. Med. Mycol. 2021, 59, 253–258. [Google Scholar] [CrossRef]
- de Jong, A.W.; Dieleman, C.; Carbia, M.; Mohd Tap, R.; Hagen, F. Performance of Two Novel Chromogenic Media for the Identification of Multidrug-Resistant Candida auris Compared with Other Commercially Available Formulations. J. Clin. Microbiol. 2021, 59, e03220-20. [Google Scholar] [CrossRef]
- CDC. Algorithm to Identify Candida auris Based on Phenotypic Laboratory Method and Initial Species Identification. 2019. Available online: https://www.cdc.gov/candida-auris/media/pdfs/Testing-algorithm_by-Method_508.pdf (accessed on 30 April 2025).
- Kathuria, S.; Singh, P.K.; Sharma, C.; Prakash, A.; Masih, A.; Kumar, A.; Meis, J.F.; Chowdhary, A. Multidrug-Resistant Candida auris Misidentified as Candida haemulonii: Characterization by Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry and DNA Sequencing and Its Antifungal Susceptibility Profile Variability by Vitek 2, CLSI Broth Microdilution, and Etest Method. J. Clin. Microbiol. 2015, 53, 1823–1830. [Google Scholar] [PubMed]
- Wong, R.C.; Lee, A.L.; Cheung, I.Y.; Chow, V.C.; Ip, M.; Lai, C.K. Current Updates on Molecular Diagnostic Assays Used for Detection of Candida auris: A Systematic Review. Diagnostics 2025, 15, 140. [Google Scholar] [CrossRef]
- Sexton, D.J.; Bentz, M.L.; Welsh, R.M.; Litvintseva, A.P. Evaluation of a new T2 Magnetic Resonance assay for rapid detection of emergent fungal pathogen Candida auris on clinical skin swab samples. Mycoses 2018, 61, 786–790. [Google Scholar] [CrossRef]
- Welsh, R.M.; Misas, E.; Forsberg, K.; Lyman, M.; Chow, N.A. Candida auris Whole-Genome Sequence Benchmark Dataset for Phylogenomic Pipelines. J. Fungi 2021, 7, 214. [Google Scholar] [CrossRef]
- de Groot, T.; Puts, Y.; Berrio, I.; Chowdhary, A.; Meis, J.F. Development of Candida auris Short Tandem Repeat Typing and Its Application to a Global Collection of Isolates. mBio 2020, 11, e02971-19. [Google Scholar] [CrossRef] [PubMed]
- Sathi, F.A.; Aung, M.S.; Paul, S.K.; Nasreen, S.A.; Haque, N.; Roy, S.; Ahmed, S.; Alam, M.M.; Khan, S.; Rabbany, M.A.; et al. Clonal Diversity of Candida auris, Candida blankii, and Kodamaea ohmeri Isolated from Septicemia and Otomycosis in Bangladesh as Determined by Multilocus Sequence Typing. J. Fungi 2023, 9, 658. [Google Scholar] [CrossRef]
- Vatanshenassan, M.; Boekhout, T.; Mauder, N.; Robert, V.; Maier, T.; Meis, J.F.; Berman, J.; Then, E.; Kostrzewa, M.; Hagen, F. Evaluation of Microsatellite Typing, ITS Sequencing, AFLP Fingerprinting, MALDI-TOF MS, and Fourier-Transform Infrared Spectroscopy Analysis of Candida auris. J. Fungi 2020, 6, 146. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.H.; Wissel, M.C.; Shields, R.K.; Salomoni, M.A.; Hao, B.; Press, E.G.; Shields, R.M.; Cheng, S.; Mitsani, D.; Vadnerkar, A.; et al. Performance of Candida real-time polymerase chain reaction, β-D-glucan assay, and blood cultures in the diagnosis of invasive candidiasis. Clin. Infect. Dis. 2012, 54, 1240–1248. [Google Scholar] [CrossRef]
- Wang, K.; Luo, Y.; Zhang, W.; Xie, S.; Yan, P.; Liu, Y.; Li, Y.; Ma, X.; Xiao, K.; Fu, H.; et al. Diagnostic value of Candida mannan antigen and anti-mannan IgG and IgM antibodies for Candida infection. Mycoses 2020, 63, 181–188. [Google Scholar] [CrossRef]
- CDC. C. auris Screening Recommendations for Healthcare Facilities. 2024. Available online: https://www.cdc.gov/candida-auris/hcp/screening-hcp/index.html (accessed on 30 April 2025).
- Chowdhary, A.; Prakash, A.; Sharma, C.; Kordalewska, M.; Kumar, A.; Sarma, S.; Tarai, B.; Singh, A.; Upadhyaya, G.; Upadhyay, S.; et al. A multicentre study of antifungal susceptibility patterns among 350 Candida auris isolates (2009–17) in India: Role of the ERG11 and FKS1 genes in azole and echinocandin resistance. J. Antimicrob. Chemother. 2018, 73, 891–899. [Google Scholar] [CrossRef]
- Mikulska, M.; Calandra, T.; Sanguinetti, M.; Poulain, D.; Viscoli, C.; Third European Conference on Infections in Leukemia Group. The use of mannan antigen and anti-mannan antibodies in the diagnosis of invasive candidiasis: Recommendations from the Third European Conference on Infections in Leukemia. Crit. Care 2010, 14, R222. [Google Scholar] [CrossRef] [PubMed]
- Mikulska, M.; Ullah, N.; Magnasco, L.; Codda, G.; Bartalucci, C.; Miletich, F.; Sepulcri, C.; Willison, E.; Vena, A.; Giacobbe, D.R.; et al. Lower (1,3)-beta-d-glucan sensitivity and in vitro levels in Candida auris and Candida parapsilosis strains. Clin. Microbiol. Infect. 2024, 30, 822–827. [Google Scholar] [CrossRef]
- Piedrahita, C.T.; Cadnum, J.L.; Jencson, A.L.; Shaikh, A.A.; Ghannoum, M.A.; Donskey, C.J. Environmental Surfaces in Healthcare Facilities are a Potential Source for Transmission of Candida auris and Other Candida Species. Infect. Control Hosp. Epidemiol. 2017, 38, 1107–1109. [Google Scholar] [CrossRef] [PubMed]
- Welsh, R.M.; Bentz, M.L.; Shams, A.; Houston, H.; Lyons, A.; Rose, L.J.; Litvintseva, A.P. Survival, Persistence, and Isolation of the Emerging Multidrug-Resistant Pathogenic Yeast Candida auris on a Plastic Health Care Surface. J. Clin. Microbiol. 2017, 55, 2996–3005. [Google Scholar] [CrossRef] [PubMed]
- Biswal, M.; Rudramurthy, S.M.; Jain, N.; Shamanth, A.S.; Sharma, D.; Jain, K.; Yaddanapudi, L.N.; Chakrabarti, A. Controlling a possible outbreak of Candida auris infection: Lessons learnt from multiple interventions. J. Hosp. Infect. 2017, 97, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Tharp, B.; Zheng, R.; Bryak, G.; Litvintseva, A.P.; Hayden, M.K.; Chowdhary, A.; Thangamani, S. Role of Microbiota in the Skin Colonization of Candida auris. mSphere 2023, 8, e0062322. [Google Scholar] [CrossRef]
- Kumar, J.; Eilertson, B.; Cadnum, J.L.; Whitlow, C.S.; Jencson, A.L.; Safdar, N.; Krein, S.L.; Tanner, W.D.; Mayer, J.; Samore, M.H.; et al. Environmental Contamination with Candida Species in Multiple Hospitals Including a Tertiary Care Hospital with a Candida auris Outbreak. Pathog. Immun. 2019, 4, 260–270. [Google Scholar] [CrossRef]
- Magnasco, L.; Mikulska, M.; Sepulcri, C.; Ullah, N.; Giacobbe, D.R.; Vena, A.; Di Pilato, V.; Willison, E.; Orsi, A.; Icardi, G.; et al. Frequency of Detection of Candida auris Colonization Outside a Highly Endemic Setting: What Is the Optimal Strategy for Screening of Carriage? J. Fungi 2023, 10, 26. [Google Scholar] [CrossRef]
- Chowdhary, A.; Sharma, C.; Meis, J.F. Candida auris: A rapidly emerging cause of hospital-acquired multidrug-resistant fungal infections globally. PLoS Pathog. 2017, 13, e1006290. [Google Scholar] [CrossRef]
- Bergeron, G.; Bloch, D.; Murray, K.; Kratz, M.; Parton, H.; Ackelsberg, J.; Antwi, M.; Del Rosso, P.; Dorsinville, M.; Kubinson, H.; et al. Candida auris Colonization After Discharge to a Community Setting: New York City, 2017–2019. Open Forum Infect. Dis. 2021, 8, ofaa620. [Google Scholar] [CrossRef]
- Schwartz, I.S.; Smith, S.W.; Dingle, T.C. Something wicked this way comes: What health care providers need to know about Candida auris. Can. Commun. Dis. Rep. 2018, 44, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Southwick, K.; Adams, E.H.; Greenko, J. 2039. New York State 2016–2018: Progression from Candida auris Colonization to Bloodstream Infection. Open Forum Infect. Dis. 2018, 5, S594–S595. [Google Scholar] [CrossRef]
- Silva, I.; Miranda, I.M.; Costa-de-Oliveira, S. Potential Environmental Reservoirs of Candida auris: A Systematic Review. J. Fungi 2024, 10, 336. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhu, R.; Luan, Z.; Ma, X. Risk of invasive candidiasis with prolonged duration of ICU stay: A systematic review and meta-analysis. BMJ Open 2020, 10, e036452. [Google Scholar] [CrossRef] [PubMed]
- Eyre, D.W. Infection prevention and control insights from a decade of pathogen whole-genome sequencing. J. Hosp. Infect. 2022, 122, 180–186. [Google Scholar] [CrossRef]
- Sabino, R.; Veríssimo, C.; Pereira, Á.A.; Antunes, F. Candida auris, an Agent of Hospital-Associated Outbreaks: Which Challenging Issues Do We Need to Have in Mind? Microorganisms 2020, 8, 181. [Google Scholar] [CrossRef]
- Sharp, A.; Muller-Pebody, B.; Charlett, A.; Patel, B.; Gorton, R.; Lambourne, J.; Cummins, M.; Alcolea-Medina, A.; Wilks, M.; Smith, R.; et al. Screening for Candida auris in patients admitted to eight intensive care units in England, 2017 to 2018. Eurosurveillance 2021, 26, 1900730. [Google Scholar] [CrossRef]
- Forsberg, K.; Woodworth, K.; Walters, M.; Berkow, E.L.; Jackson, B.; Chiller, T.; Vallabhaneni, S. Candida auris: The recent emergence of a multidrug-resistant fungal pathogen. Med. Mycol. 2019, 57, 1–12. [Google Scholar] [CrossRef]
- Yadav, A.; Singh, A.; Wang, Y.; Haren, M.H.V.; de Groot, T.; Meis, J.F.; Xu, J.; Chowdhary, A. Colonisation and Transmission Dynamics of Candida auris among Chronic Respiratory Diseases Patients Hospitalised in a Chest Hospital, Delhi, India: A Comparative Analysis of Whole Genome Sequencing and Microsatellite Typing. J. Fungi 2021, 7, 81. [Google Scholar] [CrossRef]
- Tsay, S.; Kallen, A.; Jackson, B.R.; Chiller, T.M.; Vallabhaneni, S. Approach to the Investigation and Management of Patients with Candida auris, an Emerging Multidrug-Resistant Yeast. Clin. Infect. Dis. 2018, 66, 306–311. [Google Scholar] [CrossRef]
- Ruiz-Gaitán, A.; Moret, A.M.; Tasias-Pitarch, M.; Aleixandre-López, A.I.; Martínez-Morel, H.; Calabuig, E.; Salavert-Lletí, M.; Ramírez, P.; López-Hontangas, J.L.; Hagen, F.; et al. An outbreak due to Candida auris with prolonged colonisation and candidaemia in a tertiary care European hospital. Mycoses 2018, 61, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Caceres, D.H.; Forsberg, K.; Welsh, R.M.; Sexton, D.J.; Lockhart, S.R.; Jackson, B.R.; Chiller, T. Candida auris: A Review of Recommendations for Detection and Control in Healthcare Settings. J. Fungi 2019, 5, 111. [Google Scholar] [CrossRef]
- Thomas-Rüddel, D.O.; Schlattmann, P.; Pletz, M.; Kurzai, O.; Bloos, F. Risk Factors for Invasive Candida Infection in Critically Ill Patients: A Systematic Review and Meta-analysis. Chest 2022, 161, 345–355. [Google Scholar] [CrossRef]
- Southwick, K.; Ostrowsky, B.; Greenko, J.; Adams, E.; Lutterloh, E.; Denis, R.J.; Patel, R.; Erazo, R.; Fernandez, R.; Bucher, C.; et al. A description of the first Candida auris-colonized individuals in New York State, 2016–2017. Am. J. Infect. Control. 2022, 50, 358–360. [Google Scholar] [CrossRef]
- Eyre, D.W.; Sheppard, A.E.; Madder, H.; Moir, I.; Moroney, R.; Quan, T.P.; Griffiths, D.; George, S.; Butcher, L.; Morgan, M.; et al. A Candida auris Outbreak and Its Control in an Intensive Care Setting. N. Engl. J. Med. 2018, 379, 1322–1331. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; O’Brien, B.; Leach, L.; Clarke, A.; Bates, M.; Adams, E.; Ostrowsky, B.; Quinn, M.; Dufort, E.; Southwick, K.; et al. Laboratory Analysis of an Outbreak of Candida auris in New York from 2016 to 2018: Impact and Lessons Learned. J. Clin. Microbiol. 2020, 58, e01503-19. [Google Scholar] [CrossRef]
- Proctor, D.M.; Dangana, T.; Sexton, D.J.; Fukuda, C.; Yelin, R.D.; Stanley, M.; Bell, P.B.; Baskaran, S.; Deming, C.; Chen, Q.; et al. Integrated genomic, epidemiologic investigation of Candida auris skin colonization in a skilled nursing facility. Nat. Med. 2021, 27, 1401–1409. [Google Scholar] [CrossRef]
- CDC. C. auris Screening: Patient Swab Collection; CDC: Atlanta, GA, USA, 2024.
- CDC. Identification of C. auris. 2024. Available online: https://www.cdc.gov/candida-auris/hcp/laboratories/identification-of-c-auris.html (accessed on 30 April 2025).
- Cortegiani, A.; Misseri, G.; Fasciana, T.; Giammanco, A.; Giarratano, A.; Chowdhary, A. Epidemiology, clinical characteristics, resistance, and treatment of infections by Candida auris. J. Intensive Care 2018, 6, 69. [Google Scholar] [CrossRef] [PubMed]
- Hata, D.J.; Humphries, R.; Lockhart, S.R.; College of American Pathologists Microbiology Committee. Candida auris: An Emerging Yeast Pathogen Posing Distinct Challenges for Laboratory Diagnostics, Treatment, and Infection Prevention. Arch. Pathol. Lab. Med. 2020, 144, 107–114. [Google Scholar] [CrossRef]
- Osei Sekyere, J. Candida auris: A systematic review and meta-analysis of current updates on an emerging multidrug-resistant pathogen. Microbiologyopen 2018, 7, e00578. [Google Scholar] [CrossRef]
- Bentz, M.L.; Sexton, D.J.; Welsh, R.M.; Litvintseva, A.P. Phenotypic switching in newly emerged multidrug-resistant pathogen Candida auris. Med. Mycol. 2019, 57, 636–638. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Bing, J.; Hu, T.; Ennis, C.L.; Nobile, C.J.; Huang, G. Candida auris: Epidemiology, biology, antifungal resistance, and virulence. PLoS Pathog. 2020, 16, e1008921. [Google Scholar] [CrossRef]
- Satoh, K.; Makimura, K.; Hasumi, Y.; Nishiyama, Y.; Uchida, K.; Yamaguchi, H. Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol. Immunol. 2009, 53, 41–44. [Google Scholar] [CrossRef]
- Mahmoudi, S.; Agha Kuchak Afshari, S.; Aghaei Gharehbolagh, S.; Mirhendi, H.; Makimura, K. Methods for identification of Candida auris, the yeast of global public health concern: A review. J. Mycol. Med. 2019, 29, 174–179. [Google Scholar] [CrossRef]
- Zerrouki, H.; Ibrahim, A.; Rebiahi, S.A.; Elhabiri, Y.; Benhaddouche, D.E.; de Groot, T.; Meis, J.F.; Rolain, J.M.; Bittar, F. Emergence of Candida auris in intensive care units in Algeria. Mycoses 2022, 65, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Gaitán, A.; Sigona-Giangreco, I.; Pérez-Royo, J.M.; Garcia-Bustos, V.; García-Hita, M.; Valentín-Gómez, E.; Almaraz, S.G.; de Groot, P.W.J.; Pemán, J. Usefulness of Chromogenic Media with Fluconazole Supplementation for Presumptive Identification of Candida auris. Diagnostics 2023, 13, 231. [Google Scholar] [CrossRef] [PubMed]
- Komorowski, A.S.; Aftanas, P.; Porter, V.; Katz, K.; Kozak, R.A.; Li, X.X. Verification, Analytical Sensitivity, Cost-effectiveness, and Comparison of 4 Candida auris Screening Methods. Open Forum Infect. Dis. 2024, 11, ofae017. [Google Scholar] [CrossRef]
- Marathe, A.; Zhu, Y.; Chaturvedi, V.; Chaturvedi, S. Utility of CHROMagar™ Candida Plus for presumptive identification of Candida auris from surveillance samples. Mycopathologia 2022, 187, 527–534. [Google Scholar] [CrossRef]
- Tamura, T.; Alshahni, M.M.; Makimura, K. Evaluation of CHROMagar™ Candida Plus chromogenic agar for the presumptive identification of Candida auris. Microbiol. Immunol. 2022, 66, 292–298. [Google Scholar] [CrossRef]
- Keighley, C.; Garnham, K.; Harch, S.A.J.; Robertson, M.; Chaw, K.; Teng, J.C.; Chen, S.C. Candida auris: Diagnostic Challenges and Emerging Opportunities for the Clinical Microbiology Laboratory. Curr. Fungal Infect. Rep. 2021, 15, 116–126. [Google Scholar] [CrossRef]
- Snayd, M.; Dias, F.; Ryan, R.W.; Clout, D.; Banach, D.B. Misidentification of Candida auris by RapID Yeast Plus, a Commercial, Biochemical Enzyme-Based Manual Rapid Identification System. J. Clin. Microbiol. 2018, 56, e00080-18. [Google Scholar] [CrossRef] [PubMed]
- Mizusawa, M.; Miller, H.; Green, R.; Lee, R.; Durante, M.; Perkins, R.; Hewitt, C.; Simner, P.J.; Carroll, K.C.; Hayden, R.T.; et al. Can Multidrug-Resistant Candida auris Be Reliably Identified in Clinical Microbiology Laboratories? J. Clin. Microbiol. 2017, 55, 638–640. [Google Scholar] [CrossRef]
- Lee, W.G.; Shin, J.H.; Uh, Y.; Kang, M.G.; Kim, S.H.; Park, K.H.; Jang, H.C. First three reported cases of nosocomial fungemia caused by Candida auris. J. Clin. Microbiol. 2011, 49, 3139–3142. [Google Scholar] [CrossRef]
- Ruiz Gaitán, A.C.; Moret, A.; López Hontangas, J.L.; Molina, J.M.; Aleixandre López, A.I.; Cabezas, A.H.; Mollar Maseres, J.; Arcas, R.C.; Gómez Ruiz, M.D.; Chiveli, M.; et al. Nosocomial fungemia by Candida auris: First four reported cases in continental Europe. Rev. Iberoam. Micol. 2017, 34, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.H.; Situ, S.F.; Steven, A.; Razak, M.F.A. The Pitfall of Utilizing a Commercial Biochemical Yeast Identification Kit to Detect Candida auris. Ann. Clin. Lab. Sci. 2019, 49, 546–549. [Google Scholar] [PubMed]
- Ambaraghassi, G.; Dufresne, P.J.; Dufresne, S.F.; Vallières, É.; Muñoz, J.F.; Cuomo, C.A.; Berkow, E.L.; Lockhart, S.R.; Luong, M.L. Identification of Candida auris by Use of the Updated Vitek 2 Yeast Identification System, Version 8.01: A Multilaboratory Evaluation Study. J. Clin. Microbiol. 2019, 57, e00884-19. [Google Scholar] [CrossRef]
- Tan, Y.E.; Teo, J.Q.; Rahman, N.B.A.; Ng, O.T.; Kalisvar, M.; Tan, A.L.; Koh, T.H.; Ong, R.T.H. Candida auris in Singapore: Genomic epidemiology, antifungal drug resistance, and identification using the updated 8.01 VITEK®2 system. Int. J. Antimicrob. Agents 2019, 54, 709–715. [Google Scholar] [CrossRef]
- Arnold, R.J.; Reilly, J.P. Fingerprint matching of E. coli strains with matrix-assisted laser desorption/ionization time-of-flight mass spectrometry of whole cells using a modified correlation approach. Rapid Commun. Mass Spectrom. 1998, 12, 630–636. [Google Scholar] [CrossRef]
- Marklein, G.; Josten, M.; Klanke, U.; Müller, E.; Horré, R.; Maier, T.; Wenzel, T.; Kostrzewa, M.; Bierbaum, G.; Hoerauf, A.; et al. Matrix-assisted laser desorption ionization-time of flight mass spectrometry for fast and reliable identification of clinical yeast isolates. J. Clin. Microbiol. 2009, 47, 2912–2917. [Google Scholar] [CrossRef]
- Bader, O.; Weig, M.; Taverne-Ghadwal, L.; Lugert, R.; Gross, U.; Kuhns, M. Improved clinical laboratory identification of human pathogenic yeasts by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Clin. Microbiol. Infect. 2011, 17, 1359–1365. [Google Scholar] [CrossRef]
- Vatanshenassan, M.; Boekhout, T.; Meis, J.F.; Berman, J.; Chowdhary, A.; Ben-Ami, R.; Sparbier, K.; Kostrzewa, M. Candida auris Identification and Rapid Antifungal Susceptibility Testing Against Echinocandins by MALDI-TOF MS. Front. Cell. Infect. Microbiol. 2019, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.A.; Liu, Y.L.; Liang, C.; Huang, Y.Y.; Li, J.W.; Li, Z.W.; Fan, S.J.; Chen, J.T.; Xia, Y.; Li, X.Y.; et al. Accuracy of matrix-assisted LASER desorption ionization-time of flight mass spectrometry for identification of Candida. Biosci. Rep. 2019, 39, BSR20190859. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Spencer, J.E.; Lockhart, S.R.; Singleton, S.; Petway, D.J.; Bagarozzi, D.A., Jr.; Herzegh, O.T. A high-throughput and rapid method for accurate identification of emerging multidrug-resistant Candida auris. Mycoses 2019, 62, 513–518. [Google Scholar] [CrossRef]
- Sterkel, A.; Bateman, A.; Valley, A.; Warshauer, D. Viability of Candida auris and Other Candida Species after Various Matrix-Assisted Laser Desorption Ionization-Time of Flight (MALDI-TOF) Mass Spectrometry-Based Extraction Protocols. J. Clin. Microbiol. 2018, 56, e00886-18. [Google Scholar] [CrossRef] [PubMed]
- Normand, A.C.; Gabriel, F.; Riat, A.; Cassagne, C.; Bourgeois, N.; Huguenin, A.; Chauvin, P.; De Geyter, D.; Bexkens, M.; Rubio, E.; et al. Optimization of MALDI-ToF mass spectrometry for yeast identification: A multicenter study. Med. Mycol. 2020, 58, 639–649. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Paul, S.; Sood, P.; Rudramurthy, S.M.; Rajbanshi, A.; Jillwin, T.J.; Chakrabarti, A. Matrix-assisted laser desorption ionization time-of-flight mass spectrometry for the rapid identification of yeasts causing bloodstream infections. Clin. Microbiol. Infect. 2015, 21, 372–378. [Google Scholar] [CrossRef]
- CDC. MicrobeNet. 2024. Available online: https://www.cdc.gov/microbenet/php/about/index.html (accessed on 30 April 2025).
- Ceballos-Garzon, A.; Amado, D.; Vélez, N.; Jiménez, A.M.; Rodríguez, C.; Parra-Giraldo, C.M. Development and Validation of an in-House Library of Colombian Candida auris Strains with MALDI-TOF MS to Improve Yeast Identification. J. Fungi 2020, 6, 72. [Google Scholar] [CrossRef]
- Leach, L.; Zhu, Y.; Chaturvedi, S. Development and Validation of a Real-Time PCR Assay for Rapid Detection of Candida auris from Surveillance Samples. J. Clin. Microbiol. 2018, 56, e01223-17. [Google Scholar] [CrossRef]
- Kordalewska, M.; Zhao, Y.; Lockhart, S.R.; Chowdhary, A.; Berrio, I.; Perlin, D.S. Rapid and Accurate Molecular Identification of the Emerging Multidrug-Resistant Pathogen Candida auris. J. Clin. Microbiol. 2017, 55, 2445–2452. [Google Scholar] [CrossRef]
- Hernández Felices, F.J.; Tormo Palop, N.; Salvador García, C.; Mulet Bayona, J.V.; Guna Serrano, M.R.; Gimeno Cardona, C. Evaluation of Eazyplex® LAMP test for fast Candida auris direct detection of colonized patients. Mycoses 2024, 67, e13665. [Google Scholar] [CrossRef]
- Ruiz-Gaitán, A.C.; Fernández-Pereira, J.; Valentin, E.; Tormo-Mas, M.A.; Eraso, E.; Pemán, J.; de Groot, P.W.J. Molecular identification of Candida auris by PCR amplification of species-specific GPI protein-encoding genes. Int. J. Med. Microbiol. 2018, 308, 812–818. [Google Scholar] [CrossRef]
- Ibrahim, A.; Baron, S.A.; Yousfi, H.; Hadjadj, L.; Lalaoui, R.; Morand, S.; Rolain, J.M.; Bittar, F. Development and standardization of a specific real-time PCR assay for the rapid detection of Candida auris. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 1547–1551. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, M.; Bartolomé Álvarez, J.; Lockhart, S.R.; Valentín, E.; Ruiz-Gaitán, A.C.; Eraso, E.; de Groot, P.W.J. Identification of Candida auris and related species by multiplex PCR based on unique GPI protein-encoding genes. Mycoses 2021, 64, 194–202. [Google Scholar] [CrossRef]
- CDC. Real-Time PCR Based Identification. 2024. Available online: https://www.cdc.gov/candida-auris/hcp/laboratories/real-time-pcr-identification.html (accessed on 30 April 2025).
- Berlau, A.; Stoll, S.; Edel, B.; Löffler, B.; Rödel, J. Evaluation of the Eazyplex®Candida ID LAMP Assay for the Rapid Diagnosis of Positive Blood Cultures. Diagnostics 2024, 14, 2125. [Google Scholar] [CrossRef]
- Lin, Z.Z.; Chen, J.C.; Li, Q.Y.; Zheng, G.Y.; Li, Y.Q.; Zhu, G.D.; Guo, X.G. A Pooled Analysis of the PCR for the Detection of Candida auris. Clin. Lab. 2022, 68, 1369. [Google Scholar] [CrossRef] [PubMed]
- Caméléna, F.; Péan de Ponfilly, G.; Pailhoriès, H.; Bonzon, L.; Alanio, A.; Poncin, T.; Lafaurie, M.; Dépret, F.; Cambau, E.; Godreuil, S.; et al. Multicenter Evaluation of the FilmArray Blood Culture Identification 2 Panel for Pathogen Detection in Bloodstream Infections. Microbiol. Spectr. 2023, 11, e0254722. [Google Scholar] [CrossRef]
- Zhang, S.X.; Carroll, K.C.; Lewis, S.; Totten, M.; Mead, P.; Samuel, L.; Steed, L.L.; Nolte, F.S.; Thornberg, A.; Reid, J.L.; et al. Multicenter Evaluation of a PCR-Based Digital Microfluidics and Electrochemical Detection System for the Rapid Identification of 15 Fungal Pathogens Directly from Positive Blood Cultures. J. Clin. Microbiol. 2020, 58, e02096-19. [Google Scholar] [CrossRef]
- Franco, L.C.; Ahmed, M.; Kendra, C.G.; Sperling, R.M.; Van Benten, K.; Lavik, J.P.; Emery, C.L.; Relich, R.F.; Gavina, K. Validation of a qualitative real-time PCR assay for the detection of Candida auris in hospital inpatient screening. J. Clin. Microbiol. 2024, 62, e0015824. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, J.D.; Wang, C.Y.; Bolton, D.; Liggayu, B.; Schaefer, S.; Patel, G.; Javaid, W.; Cordon-Cardo, C.; Firpo-Betancourt, A.; Sordillo, E.M.; et al. Molecular Detection of Candida auris Using DiaSorin Molecular Simplexa® Detection Kit: A Diagnostic Performance Evaluation. J. Fungi 2023, 9, 849. [Google Scholar] [CrossRef]
- Rosa, R.; Jimenez, A.; Andrews, D.; Dinh, H.; Parra, K.; Martinez, O.; Abbo, L.M. Impact of In-house Candida auris Polymerase Chain Reaction Screening on Admission on the Incidence Rates of Surveillance and Blood Cultures with C. auris and Associated Cost Savings. Open Forum Infect. Dis. 2023, 10, ofad567. [Google Scholar] [CrossRef]
- Sattler, J.; Noster, J.; Brunke, A.; Plum, G.; Wiegel, P.; Kurzai, O.; Meis, J.F.; Hamprecht, A. Comparison of Two Commercially Available qPCR Kits for the Detection of Candida auris. J. Fungi 2021, 7, 154. [Google Scholar] [CrossRef] [PubMed]
- Mulet Bayona, J.V.; Salvador García, C.; Tormo Palop, N.; Gimeno Cardona, C. Validation and implementation of a commercial real-time PCR assay for direct detection of Candida auris from surveillance samples. Mycoses 2021, 64, 612–615. [Google Scholar] [CrossRef]
- Yamamoto, M.; Alshahni, M.M.; Tamura, T.; Satoh, K.; Iguchi, S.; Kikuchi, K.; Mimaki, M.; Makimura, K. Rapid Detection of Candida auris Based on Loop-Mediated Isothermal Amplification (LAMP). J. Clin. Microbiol. 2018, 56, e00591-18. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.H.; Jee, H.; Moon, K.C.; Lim, C.S.; Jang, W.S. Development of a Simple DNA Extraction Method and Candida Pan Loop-Mediated Isothermal Amplification Assay for Diagnosis of Candidemia. Pathogens 2022, 11, 111. [Google Scholar] [CrossRef]
- Narayanan, A.; Selvakumar, P.; Siddharthan, R.; Sanyal, K. ClaID: A Rapid Method of Clade-Level Identification of the Multidrug Resistant Human Fungal Pathogen Candida auris. Microbiol. Spectr. 2022, 10, e0063422. [Google Scholar] [CrossRef]
- Lockhart, S.R.; Etienne, K.A.; Vallabhaneni, S.; Farooqi, J.; Chowdhary, A.; Govender, N.P.; Colombo, A.L.; Calvo, B.; Cuomo, C.A.; Desjardins, C.A.; et al. Simultaneous Emergence of Multidrug-Resistant Candida auris on 3 Continents Confirmed by Whole-Genome Sequencing and Epidemiological Analyses. Clin. Infect. Dis. 2017, 64, 134–140. [Google Scholar] [CrossRef]
- CDC. GITHUB. 2021. Available online: https://github.com/CDCgov/mycosnp (accessed on 30 April 2025).
- de Groot, T.; Spruijtenburg, B.; Parnell, L.A.; Chow, N.A.; Meis, J.F. Optimization and Validation of Candida auris Short Tandem Repeat Analysis. Microbiol. Spectr. 2022, 10, e0264522. [Google Scholar] [CrossRef] [PubMed]
- Magobo, R.; Mhlanga, M.; Corcoran, C.; Govender, N.P. Multilocus sequence typing of azole-resistant Candida auris strains, South Africa. S. Afr. J. Infect. Dis. 2020, 35, a116. [Google Scholar] [CrossRef]
- Curtoni, A.; Pastrone, L.; Cordovana, M.; Bondi, A.; Piccinini, G.; Genco, M.; Bottino, P.; Polizzi, C.; Cavallo, L.; Mandras, N.; et al. Fourier Transform Infrared Spectroscopy Application for Candida auris Outbreak Typing in a Referral Intensive Care Unit: Phylogenetic Analysis and Clustering Cut-Off Definition. Microorganisms 2024, 12, 1312. [Google Scholar] [CrossRef]
- Contreras, D.A.; Morgan, M.A. Surveillance diagnostic algorithm using real-time PCR assay and strain typing method development to assist with the control of C. auris amid COVID-19 pandemic. Front. Cell. Infect. Microbiol. 2022, 12, 887754. [Google Scholar] [CrossRef]
- Biswas, C.; Wang, Q.; van Hal, S.J.; Eyre, D.W.; Hudson, B.; Halliday, C.L.; Mazsewska, K.; Kizny Gordon, A.; Lee, A.; Irinyi, L.; et al. Genetic Heterogeneity of Australian Candida auris Isolates: Insights From a Nonoutbreak Setting Using Whole-Genome Sequencing. Open Forum Infect. Dis. 2020, 7, ofaa158. [Google Scholar] [CrossRef] [PubMed]
- CDC. Antifungal Susceptibility Testing for C. auris. 2024. Available online: https://www.cdc.gov/candida-auris/hcp/laboratories/antifungal-susceptibility-testing.html (accessed on 30 April 2025).
- Jones, C.R.; Neill, C.; Borman, A.M.; Budd, E.L.; Cummins, M.; Fry, C.; Guy, R.L.; Jeffery, K.; Johnson, E.M.; Manuel, R.; et al. The laboratory investigation, management, and infection prevention and control of Candida auris: A narrative review to inform the 2024 national guidance update in England. J. Med. Microbiol. 2024, 73, 001820. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Prakash, A.; Meletiadis, J.; Sharma, C.; Chowdhary, A. Comparison of EUCAST and CLSI Reference Microdilution MICs of Eight Antifungal Compounds for Candida auris and Associated Tentative Epidemiological Cutoff Values. Antimicrob. Agents Chemother. 2017, 61, e00485-17. [Google Scholar] [CrossRef] [PubMed]
- Szekely, A.; Borman, A.M.; Johnson, E.M. Candida auris Isolates of the Southern Asian and South African Lineages Exhibit Different Phenotypic and Antifungal Susceptibility Profiles In Vitro. J. Clin. Microbiol. 2019, 57, e02055-18. [Google Scholar] [CrossRef]
- Kordalewska, M.; Lee, A.; Park, S.; Berrio, I.; Chowdhary, A.; Zhao, Y.; Perlin, D.S. Understanding Echinocandin Resistance in the Emerging Pathogen Candida auris. Antimicrob. Agents Chemother. 2018, 62, e00238-18. [Google Scholar] [CrossRef] [PubMed]
- Ceballos-Garzon, A.; Garcia-Effron, G.; Cordoba, S.; Rodriguez, J.Y.; Alvarez-Moreno, C.; Pape, P.L.; Parra-Giraldo, C.M.; Morales-López, S. Head-to-head comparison of CLSI, EUCAST, Etest and VITEK®2 results for Candida auris susceptibility testing. Int. J. Antimicrob. Agents 2022, 59, 106558. [Google Scholar] [CrossRef]
- Siopi, M.; Pachoulis, I.; Leventaki, S.; Spruijtenburg, B.; Meis, J.F.; Pournaras, S.; Vrioni, G.; Tsakris, A.; Meletiadis, J. Evaluation of the Vitek 2 system for antifungal susceptibility testing of Candida auris using a representative international panel of clinical isolates: Overestimation of amphotericin B resistance and underestimation of fluconazole resistance. J. Clin. Microbiol. 2024, 62, e0152823. [Google Scholar] [CrossRef]
- Patwardhan, S.A.; Prayag, P.S.; Soman, R.N.; Purandare, B.D.; Ramya, S.; Dawra, R.; Joshi, R.; Prayag, A.P. Candida auris—Comparison of sensititre YeastOne and Vitek 2 AST systems for antifungal susceptibility testing—A single centre experience. Indian J. Med. Microbiol. 2024, 50, 100618. [Google Scholar] [CrossRef]
- Asadzadeh, M.; Ahmad, S.; Alfouzan, W.; Al-Obaid, I.; Spruijtenburg, B.; Meijer, E.F.J.; Meis, J.F.; Mokaddas, E. Evaluation of Etest and MICRONAUT-AM Assay for Antifungal Susceptibility Testing of Candida auris: Underestimation of Fluconazole Resistance by MICRONAUT-AM and Overestimation of Amphotericin B Resistance by Etest. Antibiotics 2024, 13, 840. [Google Scholar] [CrossRef]
- Frías-De-León, M.G.; Hernández-Castro, R.; Vite-Garín, T.; Arenas, R.; Bonifaz, A.; Castañón-Olivares, L.; Acosta-Altamirano, G.; Martínez-Herrera, E. Antifungal Resistance in Candida auris: Molecular Determinants. Antibiotics 2020, 9, 568. [Google Scholar] [CrossRef]
- Kordalewska, M.; Perlin, D.S. Molecular Diagnostics in the Times of Surveillance for Candida auris. J. Fungi 2019, 5, 77. [Google Scholar] [CrossRef] [PubMed]
- Marinach, C.; Alanio, A.; Palous, M.; Kwasek, S.; Fekkar, A.; Brossas, J.Y.; Brun, S.; Snounou, G.; Hennequin, C.; Sanglard, D.; et al. MALDI-TOF MS-based drug susceptibility testing of pathogens: The example of Candida albicans and fluconazole. Proteomics 2009, 9, 4627–4631. [Google Scholar] [CrossRef] [PubMed]
- Vella, A.; De Carolis, E.; Mello, E.; Perlin, D.S.; Sanglard, D.; Sanguinetti, M.; Posteraro, B. Potential Use of MALDI-ToF Mass Spectrometry for Rapid Detection of Antifungal Resistance in the Human Pathogen Candida glabrata. Sci. Rep. 2017, 7, 9099. [Google Scholar] [CrossRef] [PubMed]
- Theparee, T.; Das, S.; Thomson, R.B., Jr. Total Laboratory Automation and Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry Improve Turnaround Times in the Clinical Microbiology Laboratory: A Retrospective Analysis. J. Clin. Microbiol. 2018, 56, e01242-17. [Google Scholar] [CrossRef]
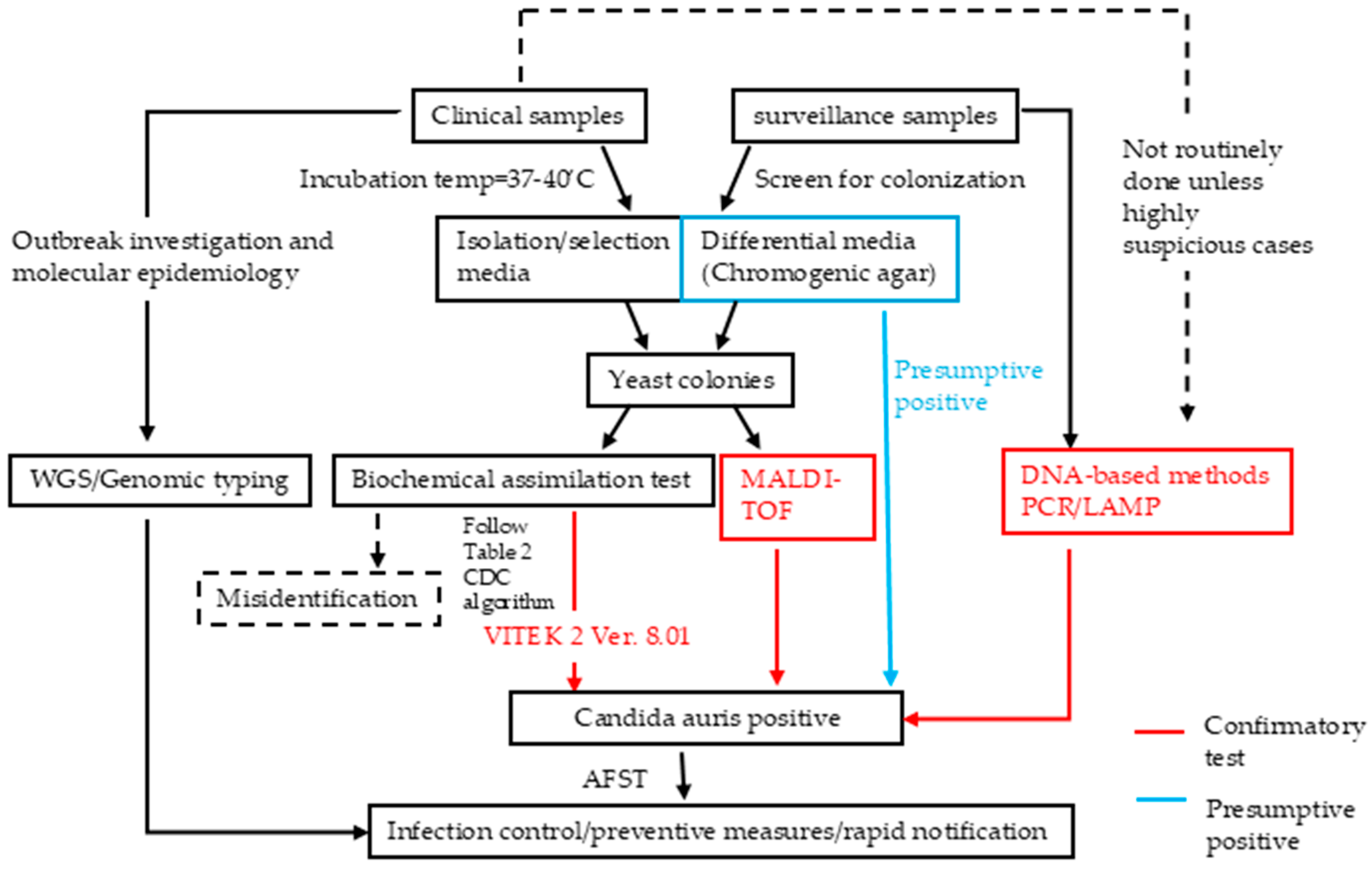
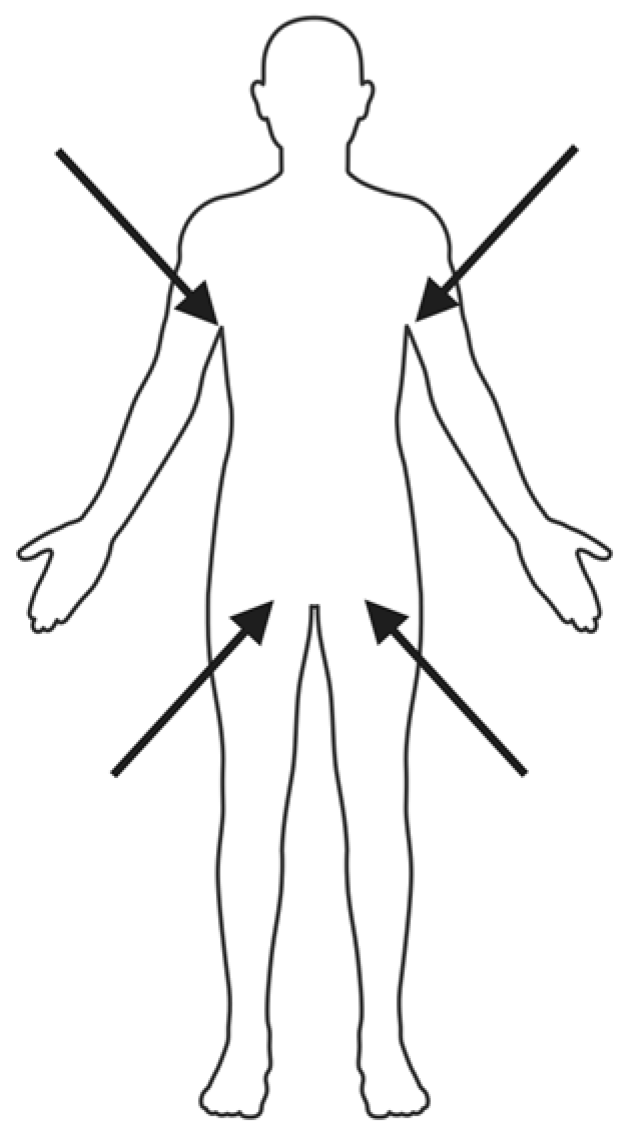


| Category | Diagnostic Method | Mechanism | Highlights | Strengths | Weaknesses/Limitations | References |
|---|---|---|---|---|---|---|
| Culture-Based | Mycological media/Selective media/Chromogenic media | 1. Requires higher incubation temperature(37–40 °C) than routine culture. 2. Adds saline, carbohydrates, or color indicators to differentiate from similar species. | 1. SCA/SAM Media Se/Sp:100%/100%. 2. CHROMagarTM with Pal’s medium Se/Sp: 100%/100%. 3. CHROMagar™ Candida Plus Se/Sp: 90–100%/98–100%. | 1. Basis for culture-based methods (e.g., biochemical assimilation methods and MALDI-TOF). 2. Simple, widely available, and low cost. 3. Gold standard for diagnosis in clinical samples; enables AFST. | 1. Slow (48–72 h). 2. Various accuracy among different media; Non-specific colony morphology, prone to misidentification, and requires additional confirmation (MALDI-TOF MS or molecular methods). | [15,16,17,18,19,20] |
| Culture-Based | Biochemical Tests (VITEK, API, and Microscan) | Analysis of metabolic profile through carbohydrate assimilation, nitrogen utilization, and enzymatic activity. | 1. Vitek 2 Version; 8.01 is confirmatory. 2. API 20C AUX or API ID 32C leads to frequent misidentification. 3. MicroScan/BD Pheonix has no C. auris database. | 1. Low cost and easy to use (automated). 2. A rapid AFST tool reliable for azoles. | 1. Requires database updates. 2. Various misidentification rates among different. commercial assays; often requires confirmatory test following CDC algorithm (Table 2). | [21] |
| Culture-Based | MALDI-TOF MS | Analysis of protein profiles and comparing them to reference databases (Figure 4). | 1. Accuracy depends on databases, sample preparation, and instrument calibration. 2. Limited use for AFST with no clinical breakpoint of MPCC. | 1. Rapid (4–5 h post culture). 2. Highly specific (Sp > 90%) when database is updated. 3. Cost effective. | 1. Requires database updates. 2. Expensive equipment and limited access in resource-poor settings. | [22] |
| Culture-Independent | DNA-Based Assays (PCR/LAMP) | DNA amplification using species-specific primers (e.g., ITS and D1/D2 regions). | 1. Both LDTs and commercial assays demonstrate reliable accuracy (Se/Sp > 90%); LAMP has lower sensitivity. 2. Current FDA-approved commercial assays for blood culture: GenMark ePlex BCID-FP and BioFire FilmArray BCID2. | 1. Accurate and rapid, useful for colonization screening and outbreak control in healthcare settings. | 1. Cannot determine antifungal susceptibility and detect DNA from both viable and non-viable C. auris cells. 2. LAMP has lower sensitivity. | [23] |
| Culture-Independent | T2 MR assay | Superparamagnetic nanoparticles bind target DNA/RNA, altering T2 relaxation for MR detection. | Rapid (<3 h) and highly sensitive detection for surveillance and bloodstream infection. | Research use only. | [24] | |
| Culture-Independent | Whole Genome Sequencing (WGS)/genomic typing | High-resolution genetic analysis. | Tracking outbreaks, identifying strains, and analyzing resistance, virulence, and epidemiology; not usually used for individual diagnosis. | WGS: High resolution; gold standard for outbreaks investigation. | Costly, requires specialized analysis. | [25,26,27,28] |
| STR typing: High reproducibility; aligns well with WGS. | Differentiate C. auris strains only if >30 SNP differences. | |||||
| MLST: Differentiates C. auris; supports resistance surveillance. | Low resolution within clades; limited for outbreaks. | |||||
| AFLP: Rapid, cost effective. | Poor reproducibility; inconsistent clustering. | |||||
| Culture-Independent | Beta-D-glucan (BDG) assays | Detection of fungal cell wall components | Non-invasive; useful for early detection for clinical samples. | Limited sensitivity and low specificity for invasive candidiasis. | [29,30] |
| Identification Method | Database/Software, If Applicable | Is Confirmed If Initial Identification Is C. auris. | Is Possible If the Following Initial Identifications Are Given; Further Work-Up Is Needed to Determine If the Isolate Is C. auris |
|---|---|---|---|
| Bruker Biotyper MALDI-TOF | RUO libraries (Versions 2014 [5627] and more recent) | n/a | |
| CA System library (Version Claim 4) | n/a | ||
| bioMérieux VITEK MS MALDI- TOF | RUO library (with Saccharomycetaceae update) | n/a | |
| IVD library (v3.2) | n/a | ||
| Older IVD libraries | n/a | C. haemulonii C. lusitaniae No identification | |
| VITEK 2 YST | Software version 8.01 | C. haemulonii C. duobushaemulonii; Candida spp. not identified | |
| Older versions | n/a | C. haemulonii C. duobushaemulonii; Candida spp. not identified | |
| API 20C | n/a | Rhodotorula glutinis (without characteristic red color) C. sake Candida spp. not identified | |
| API ID 32C | n/a | C. intermedia C. sake Saccharomyces kluyveri | |
| BD Phoenix | n/a | C. catenulata C. haemulonii Candida spp. not identified | |
| MicroScan | n/a | C. lusitaniae C. guilliermondii C. parapsilosis C. famata Candida spp. not identified | |
| RapID Yeast Plus | n/a | C. parapsilosis Candida spp. not identified | |
| GenMark ePlex BCID-FP Panel | n/a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, C.; Yassin, M. Diagnostic Approaches for Candida auris: A Comprehensive Review of Screening, Identification, and Susceptibility Testing. Microorganisms 2025, 13, 1461. https://doi.org/10.3390/microorganisms13071461
Hsu C, Yassin M. Diagnostic Approaches for Candida auris: A Comprehensive Review of Screening, Identification, and Susceptibility Testing. Microorganisms. 2025; 13(7):1461. https://doi.org/10.3390/microorganisms13071461
Chicago/Turabian StyleHsu, Christine, and Mohamed Yassin. 2025. "Diagnostic Approaches for Candida auris: A Comprehensive Review of Screening, Identification, and Susceptibility Testing" Microorganisms 13, no. 7: 1461. https://doi.org/10.3390/microorganisms13071461
APA StyleHsu, C., & Yassin, M. (2025). Diagnostic Approaches for Candida auris: A Comprehensive Review of Screening, Identification, and Susceptibility Testing. Microorganisms, 13(7), 1461. https://doi.org/10.3390/microorganisms13071461






