Optimizing Cocoa Productivity Through Soil Health and Microbiome Enhancement: Insights from Organic Amendments and a Locally Derived Biofertilizer
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design
2.3. Sample Collection and Processing
2.4. Microbiome Sequencing and Data Processing
2.5. Data Analysis
3. Results
3.1. Soil Physical and Chemical Properties
3.2. Soil Microbial Communities
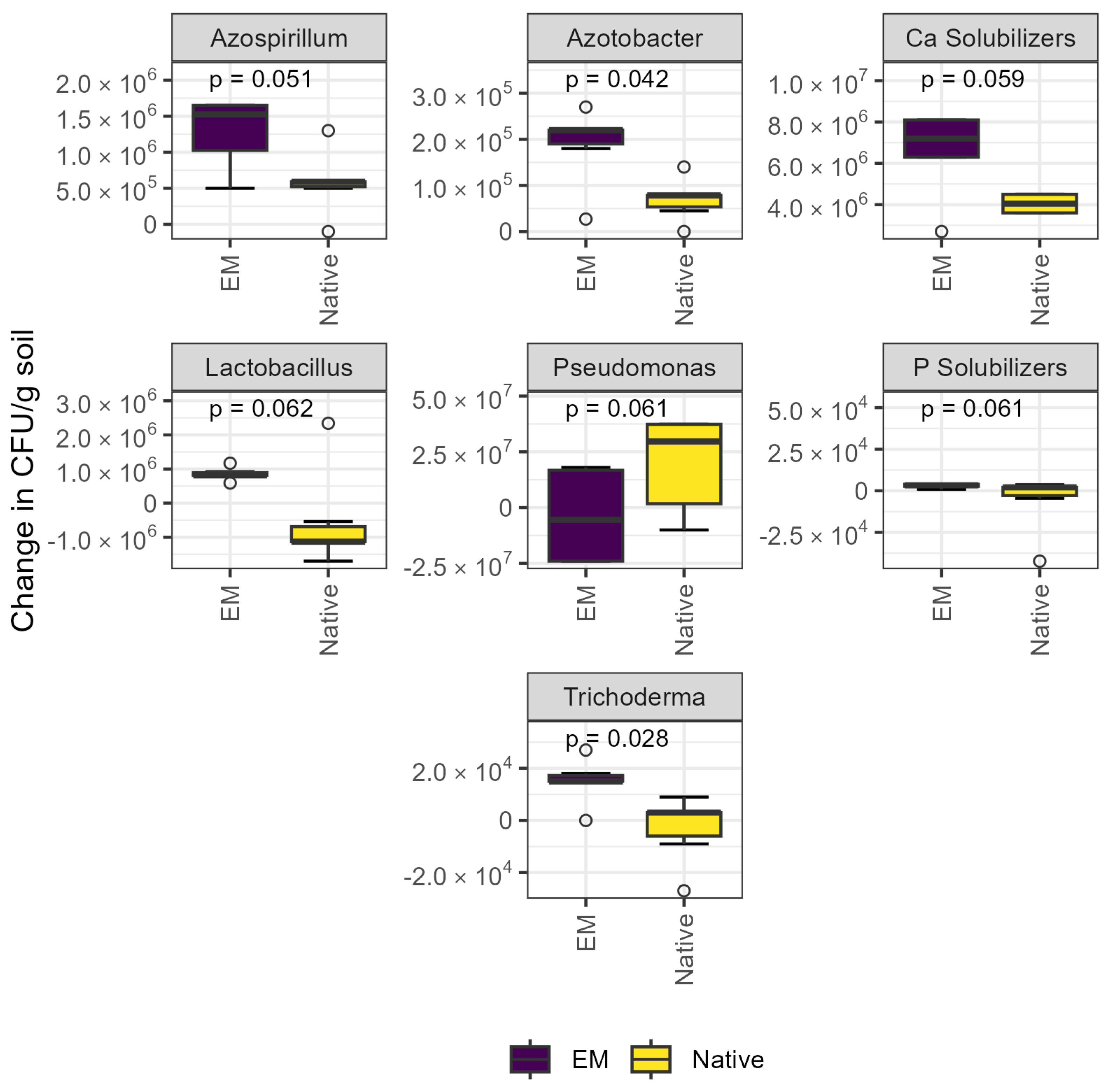
3.2.1. Culture-Dependent Quantification
3.2.2. Beta Diversity
3.2.3. Alpha Diversity
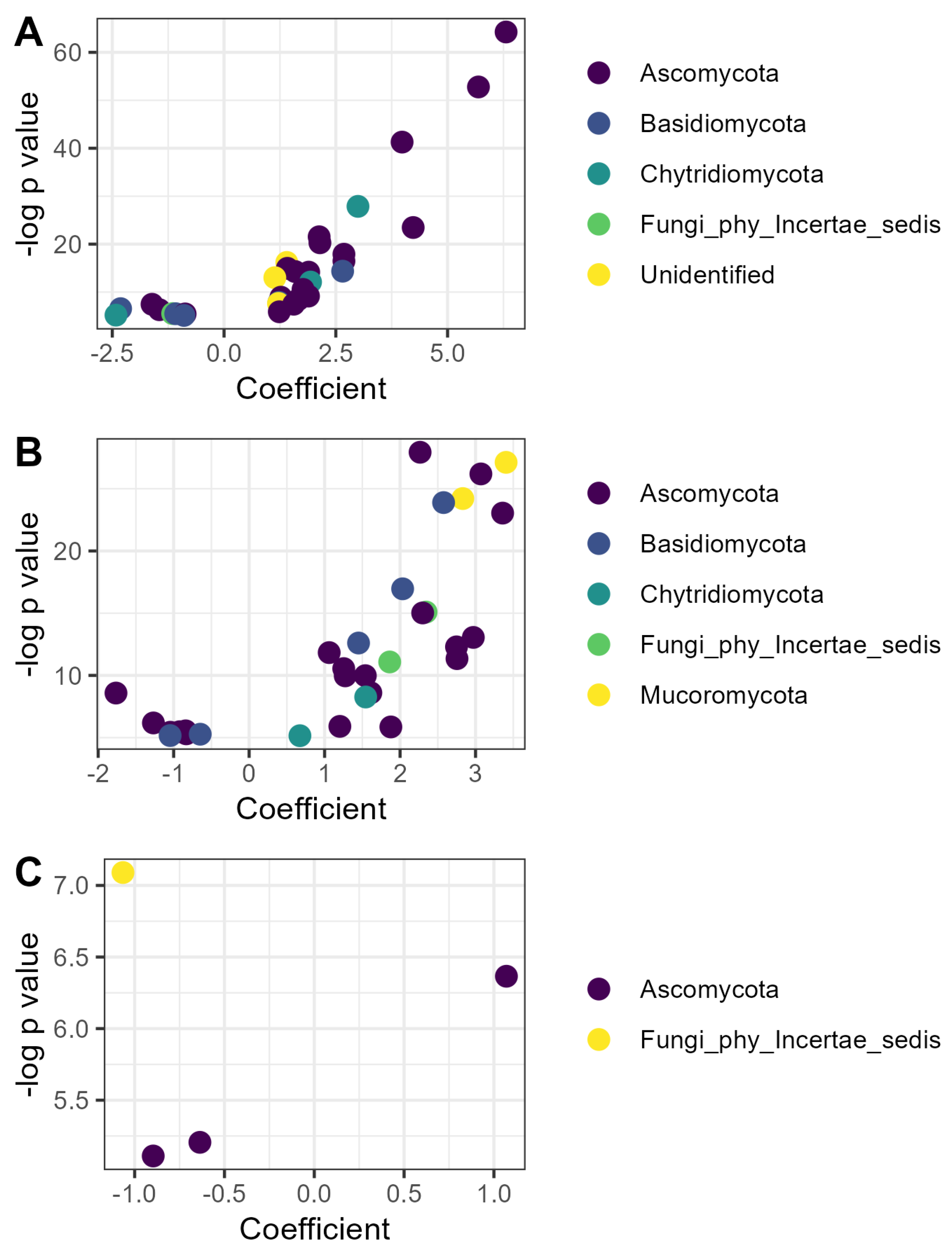
3.2.4. Microbiome-Specific Linear Models
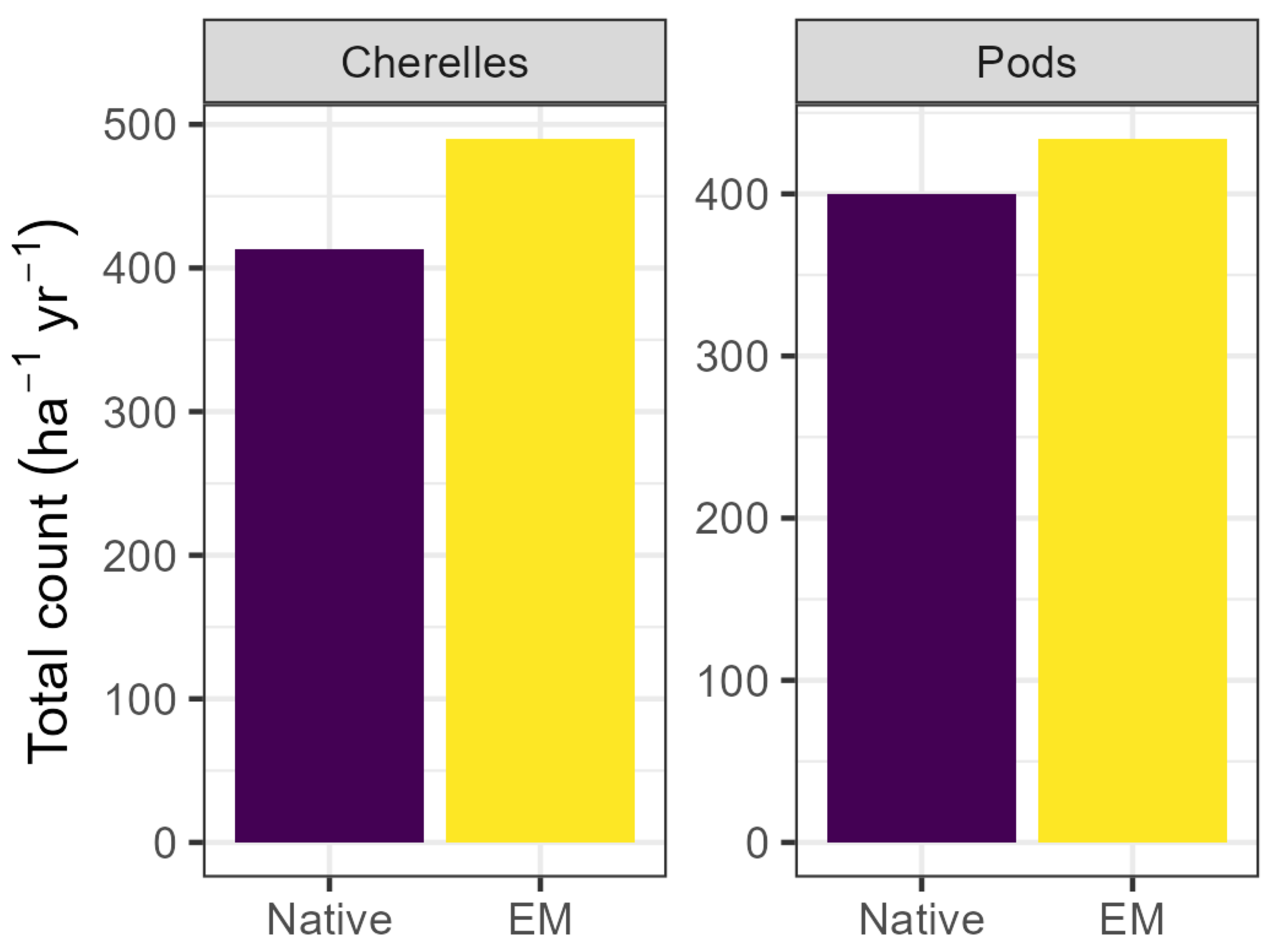
3.3. Flowering, Fruit Set, and Yield
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| ASV | Amplicon sequence variant |
| BD | Bulk density |
| CAP | Constrained analysis of principal coordinates |
| CFU | Colony-forming units |
| EC | Electrical conductivity |
| ESP | Exchangeable sodium percentage |
| OM | Organic matter |
| PERMANOVA | Permutational analysis of variance |
| SOC | Soil organic carbon |
| SOM | Soil organic matter |
Appendix A
| Amplicon | Index | Test | EM | OM |
|---|---|---|---|---|
| Rarefied data | ||||
| 16S | Chao1 | K-W | Χ21,136 = 0.056, p > 0.05 | Χ22,135 = 0.55, p > 0.05 |
| Shannon | K-W | Χ21,136 = 0.00045, p > 0.05 | Χ22,135 = 0.28, p > 0.05 | |
| Simpson | K-W | Χ21,136 = 0.0015, p > 0.05 | Χ22,135 = 0.77, p > 0.05 | |
| ITS | Chao1 | ANOVA | Χ21,138 = 1.16, p > 0.05 | Χ22,137 = 1.10, p > 0.05 |
| Shannon | ANOVA | Χ21,138 = 1.12, p > 0.05 | Χ22,137 = 0.56, p > 0.05 | |
| Simpson | K-W | Χ21,138 = 0.35, p > 0.05 | Χ22,137 = 0.0088, p > 0.05 | |
| Non-rarefied data | ||||
| 16S | Chao1 | K-W | Χ21,138 = 0.074, p > 0.05 | Χ22,137 = 0.54, p > 0.05 |
| Shannon | K-W | Χ21,138 = 0.018, p > 0.05 | Χ22,137 = 0.26, p > 0.05 | |
| Simpson | K-W | Χ21,138 = 0.036, p > 0.05 | Χ22,137 = 0.60, p > 0.05 | |
| ITS | Chao1 | ANOVA | Χ21,138 = 0.036, p > 0.05 | Χ22,137 = 2.20, p > 0.05 |
| Shannon | ANOVA | Χ21,138 = 1.06, p > 0.05 | Χ22,137 = 0.64, p > 0.05 | |
| Simpson | K-W | Χ21,138 = 0.31, p > 0.05 | Χ22,137 = 0.0088, p > 0.05 | |
References
- Wortman, S.E.; Holmes, A.A.; Miernicki, E.; Knoche, K.; Pittelkow, C.M. First-Season Crop Yield Response to Organic Soil Amendments: A Meta-Analysis. Agron. J. 2017, 109, 1210–1217. [Google Scholar] [CrossRef]
- Chen, Y.; Camps-Arbestain, M.; Shen, Q.; Singh, B.; Cayuela, M.L. The Long-Term Role of Organic Amendments in Building Soil Nutrient Fertility: A Meta-Analysis and Review. Nutr. Cycl. Agroecosyst. 2018, 111, 103–125. [Google Scholar] [CrossRef]
- Sierra, J.; Desfontaines, L.; Faverial, J.; Loranger-Merciris, G.; Boval, M. Composting and Vermicomposting of Cattle Manure and Green Wastes under Tropical Conditions: Carbon and Nutrient Balances and End-Product Quality. Soil Res. 2013, 51, 142–151. [Google Scholar] [CrossRef]
- Tiefenbacher, A.; Sandén, T.; Haslmayr, H.P.; Miloczki, J.; Wenzel, W.; Spiegel, H. Optimizing Carbon Sequestration in Croplands: A Synthesis. Agronomy 2021, 11, 882. [Google Scholar] [CrossRef]
- Alvarenga, P.; Carneiro, J.P.; Fangueiro, D.; Cordovil, C.M.d.S.; Bernal, M.P. Managing Organic Amendments in Agroecosystems to Enhance Soil Carbon Storage and Mitigate Climate Change. In Climate Change and Soil Interactions; Elsevier: Amsterdam, The Netherlands, 2020; pp. 89–141. [Google Scholar] [CrossRef]
- Hijbeek, R.; van Ittersum, M.K.; ten Berge, H.F.M.; Gort, G.; Spiegel, H.; Whitmore, A.P. Do Organic Inputs Matter—A Meta-Analysis of Additional Yield Effects for Arable Crops in Europe. Plant Soil 2017, 411, 293–303. [Google Scholar] [CrossRef]
- Vanhove, W.; Vanhoudt, N.; Van Damme, P. Effect of Shade Tree Planting and Soil Management on Rehabilitation Success of a 22-Year-Old Degraded Cocoa (Theobroma cacao L.) Plantation. Agric. Ecosyst. Environ. 2016, 219, 14–25. [Google Scholar] [CrossRef]
- Fidelis, C.; Rajashekhar Rao, B.K. Enriched Cocoa Pod Composts and Their Fertilizing Effects on Hybrid Cocoa Seedlings. Int. J. Recycl. Org. Waste Agric. 2017, 6, 99–106. [Google Scholar] [CrossRef]
- Shamshuddin, J.; Anda, M.; Fauziah, C.I.; Omar, S.S.R. Growth of Cocoa Planted on Highly Weathered Soil as Affected by Application of Basalt and/or Compost. Commun. Soil Sci. Plant Anal. 2011, 42, 2751–2766. [Google Scholar] [CrossRef]
- Mulia, S.; McMahon, P.J.; Purwantara, A.; Bin Purung, H.; Djufry, F.; Lambert, S.; Keane, P.J.; Guest, D.I. Effect of Organic and Inorganic Amendments on Productivity of Cocoa on a Marginal Soil in Sulawesi, Indonesia. Exp. Agric. 2019, 55, 1–20. [Google Scholar] [CrossRef]
- Doungous, O.; Minyaka, E.; Longue, E.A.M.; Nkengafac, N.J. Potentials of Cocoa Pod Husk-Based Compost on Phytophthora Pod Rot Disease Suppression, Soil Fertility, and Theobroma cacao L. Growth. Environ. Sci. Pollut. Res. Int. 2018, 25, 25327–25335. [Google Scholar] [CrossRef]
- Khatoon, Z.; Huang, S.; Rafique, M.; Fakhar, A.; Kamran, M.A.; Santoyo, G. Unlocking the Potential of Plant Growth-Promoting Rhizobacteria on Soil Health and the Sustainability of Agricultural Systems. J. Environ. Manag. 2020, 273, 111118. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.S.; Pandey, V.C.; Singh, D.P. Efficient Soil Microorganisms: A New Dimension for Sustainable Agriculture and Environmental Development. Agric. Ecosyst. Environ. 2011, 140, 339–353. [Google Scholar] [CrossRef]
- Alzate Zuluaga, M.Y.; Fattorini, R.; Cesco, S.; Pii, Y. Plant-Microbe Interactions in the Rhizosphere for Smarter and More Sustainable Crop Fertilization: The Case of PGPR-Based Biofertilizers. Front. Microbiol. 2024, 15, 1440978. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.E.; DuVal, A.; Isaac, M.E.; Hohmann, P. At the Roots of Chocolate: Understanding and Optimizing the Cacao Root-Associated Microbiome for Ecosystem Services. A Review. Agron. Sustain. Dev. 2022, 42, 14. [Google Scholar] [CrossRef]
- Chulan, A.; Martin, K. The Vesicular-Arbuscular (VA) Mycorrhiza and Its Effects on Growth of Vegetatively Propagated Theobroma cacao L. Plant Soil 1992, 144, 227–233. [Google Scholar] [CrossRef]
- Cuenca, G.; Herrera, R.; Meneses, E. Effects of VA Mycorrhiza on the Growth of Cacao Seedlings under Nursery Conditions in Venezuela. Plant Soil 1990, 126, 71–78. [Google Scholar] [CrossRef]
- Bae, H.; Sicher, R.C.; Kim, M.S.; Kim, S.-H.; Strem, M.D.; Melnick, R.L.; Bailey, B.A. The Beneficial Endophyte Trichoderma hamatum Isolate DIS 219b Promotes Growth and Delays the Onset of the Drought Response in Theobroma cacao. J. Exp. Bot. 2009, 60, 3279–3295. [Google Scholar] [CrossRef]
- Falcäo, L.L.; Silva-Werneck, J.O.; Vilarinho, B.R.; da Silva, J.P.; Pomella, A.W.V.; Marcellino, L.H. Antimicrobial and Plant Growth-Promoting Properties of the Cacao Endophyte Bacillus subtilis ALB629. J. Appl. Microbiol. 2014, 116, 1584–1592. [Google Scholar] [CrossRef]
- Gupta, A.; Gopal, M.; Thomas, G. V Efficacy of Rhizospheric Bacillus spp. for Growth Promotion in Theobroma cacao L. Seedlings. J. Plant. Crops 2011, 39, 19–25. [Google Scholar]
- Leite, H.A.C.; Silva, A.B.; Gomes, F.P.; Gramacho, K.P.; Faria, J.C.; de Souza, J.T.; Loguercio, L.L. Bacillus subtilis and Enterobacter cloacae Endophytes from Healthy Theobroma cacao L. Trees Can Systemically Colonize Seedlings and Promote Growth. Appl. Microbiol. Biotechnol. 2013, 97, 2639–2651. [Google Scholar] [CrossRef]
- Boudjeko, T.; Tchinda, R.A.M.; Zitouni, M.; Nana, J.A.V.T.; Lerat, S.; Beaulieu, C. Streptomyces cameroonensis sp. nov., a Geldanamycin Producer That Promotes Theobroma cacao Growth. Microbes Environ. 2017, 32, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Hipólito-Romero, E.; Carcaño-Montiel, M.G.; Ramos-Prado, J.M.; Vázquez-Cabañas, E.A.; López-Reyes, L.; Ricaño-Rodríguez, J. Effect of Mixed Edaphic Bacterial Inoculants in the Early Development of Improved Cocoa Cultivars (Theobroma cacao L.) in a Traditional Agroforestry System of Oaxaca, Mexico. Rev. Argent. Microbiol. 2017, 49, 356–365. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, W.N.; Brito, N.F.; Felsemburgh, C.A.; Vieira, T.A.; Lustosa, D.C. Evaluation of Trichoderma spp. Isolates in Cocoa Seed Treatment and Seedling Production. Plants 2021, 10, 1964. [Google Scholar] [CrossRef] [PubMed]
- Simarmata, R.; Widowati, T.; Dewi, T.K.; Lekatompessy, S.J.R.; Antonius, S. Isolation, Screening and Identification of Plant Growth-Promoting Endophytic Bacteria from Theobroma cacao. Biosaintifika J. Biol. Biol. Educ. 2020, 12, 155–162. [Google Scholar] [CrossRef]
- Argüello-Navarro, A.Z.; Moreno-Rozo, L.Y. Evaluación Del Potencial Biofertilizante de Bacterias Diazótrofas Aisladas de Suelos Con Cultivo de Cacao (Theobroma cacao L.). Acta Agron. 2014, 63, 238–245. [Google Scholar] [CrossRef]
- Sanders, E.R. Aseptic Laboratory Techniques: Plating Methods. J. Vis. Exp. 2012, e3064. [Google Scholar] [CrossRef]
- DSMZ. 65. GYM STREPTOMYCES MEDIUM. 2007. Available online: https://mediadive.dsmz.de/medium/65 (accessed on 4 April 2024).
- Subba Rao, N.S. Soil Microorganisms and Plant Growth; Oxford and IBH Publishing Co.: New Delhi, India, 1977. [Google Scholar]
- Sheng, X.F. Growth Promotion and Increased Potassium Uptake of Cotton and Rape by a Potassium Releasing Strain of Bacillus edaphicus. Soil Biol. Biochem. 2005, 37, 1918–1922. [Google Scholar] [CrossRef]
- Caceres, E.A.R. Improved Medium for Isolation of Azospirillum spp. Appl. Environ. Microbiol. 1982, 44, 990–991. [Google Scholar] [CrossRef]
- Herlemann, D.P.R.; Labrenz, M.; Jürgens, K.; Bertilsson, S.; Waniek, J.J.; Andersson, A.F. Transitions in Bacterial Communities along the 2000 Km Salinity Gradient of the Baltic Sea. ISME J. 2011, 5, 1571–1579. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J.W. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press, Inc.: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Turenne, C.Y.; Sanche, S.E.; Hoban, D.J.; Karlowsky, J.A.; Kabani, A.M. Rapid Identification of Fungi by Using the ITS2 Genetic Region and an Automated Fluorescent Capillary Electrophoresis System. J. Clin. Microbiol. 1999, 37, 1846–1851. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt Removes Adapter Sequences from High-Throughput Sequencing Reads. EMBnet J. 2011, 17, 10. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Yilmaz, P.; Parfrey, L.W.; Yarza, P.; Gerken, J.; Pruesse, E.; Quast, C.; Schweer, T.; Peplies, J.; Ludwig, W.; Glöckner, F.O. The SILVA and “All-Species Living Tree Project (LTP)” Taxonomic Frameworks. Nucleic Acids Res. 2014, 42, D643–D648. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Abarenkov, K.; Zirk, A.; Piirmann, T.; Pöhönen, R.; Ivanov, F.; Nilsson, R.H.; Kõljalg, U. UNITE General FASTA Release for Fungi 2024. Available online: https://unite.ut.ee/repository.php (accessed on 4 April 2024).
- Oksanen, J.; Simpson, G.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.; Solymos, P.; Stevens, M.; Szoecs, E.; et al. Vegan: Community Ecology Package 2024. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 5 December 2024).
- McMurdie, P.J.; Holmes, S. Waste Not, Want Not: Why Rarefying Microbiome Data Is Inadmissible. PLoS Comput. Biol. 2014, 10, e1003531. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y. Statistical Normalization Methods in Microbiome Data with Application to Microbiome Cancer Research. Gut Microbes 2023, 15, 2244139. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.; Xu, Z.Z.; Peddada, S.; Amir, A.; Bittinger, K.; Gonzalez, A.; Lozupone, C.; Zaneveld, J.R.; Vázquez-Baeza, Y.; Birmingham, A.; et al. Normalization and Microbial Differential Abundance Strategies Depend upon Data Characteristics. Microbiome 2017, 5, 27. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2024. [Google Scholar]
- Pinheiro, J.; Bates, D. Nlme: Linear and Nonlinear Mixed Effects Models. Available online: https://cran.r-project.org/web/packages/nlme/index.html (accessed on 14 August 2024).
- Lahti, L.; Shetty, S. Microbiome R Package. 2019. Available online: https://www.bioconductor.org/packages/release/bioc/html/microbiome.html (accessed on 14 August 2024).
- Mallick, H.; Rahnavard, A.; McIver, L. MaAsLin 2: Multivariable Association in Population-Scale Meta-Omics Studies. 2020. Available online: https://huttenhower.sph.harvard.edu/maaslin/ (accessed on 14 August 2024).
- Sayer, E.J.; Lopez-Sangil, L.; Crawford, J.A.; Bréchet, L.M.; Birkett, A.J.; Baxendale, C.; Castro, B.; Rodtassana, C.; Garnett, M.H.; Weiss, L.; et al. Tropical Forest Soil Carbon Stocks Do Not Increase despite 15 Years of Doubled Litter Inputs. Sci. Rep. 2019, 9, 18030. [Google Scholar] [CrossRef]
- Nijmeijer, A.; Lauri, P.É.; Harmand, J.M.; Saj, S. Carbon Dynamics in Cocoa Agroforestry Systems in Central Cameroon: Afforestation of Savannah as a Sequestration Opportunity. Agrofor. Syst. 2019, 93, 851–868. [Google Scholar] [CrossRef]
- Van Vliet, J.A.; Slingerland, M.; Giller, K.E. Mineral Nutrition of Cocoa: A Review. Adv. Agron. 2017, 141, 185–270. [Google Scholar] [CrossRef]
- Vuolo, F.; Novello, G.; Bona, E.; Gorrasi, S.; Gamalero, E. Impact of Plant-Beneficial Bacterial Inocula on the Resident Bacteriome: Current Knowledge and Future Perspectives. Microorganisms 2022, 10, 2462. [Google Scholar] [CrossRef]
- Nurmayulis, N.; Sodiq, A.H.; Eris, F.R.; Hastuti, D.; Denny, Y.R.; Susilowati, D.N. Molecular Identification of Microbes from the Soil Rhizosphere of Cocoa as A Potential Biofertilizer. AGRIVITA J. Agric. Sci. 2023, 45, 124–130. [Google Scholar] [CrossRef]
- Gurikar, C.; Naik, M.K.; Sreenivasa, M.Y. Azotobacter: PGPR Activities with Special Reference to Effect of Pesticides and Biodegradation. In Microbial Inoculants in Sustainable Agricultural Productivity: Vol. 1: Research Perspectives; Springer: New Delhi, India, 2016; Volume 1, pp. 229–244. [Google Scholar] [CrossRef]
- Tchameni, S.N.; Ngonkeu, M.E.L.; Begoude, B.A.D.; Nana, L.W.; Fokom, R.; Owona, A.D.; Mbarga, J.B.; Tchana, T.; Tondje, P.R.; Etoa, F.X.; et al. Effect of Trichoderma asperellum and Arbuscular Mycorrhizal Fungi on Cacao Growth and Resistance against Black Pod Disease. Crop Prot. 2011, 30, 1321–1327. [Google Scholar] [CrossRef]
- Bailey, B.A.; Bae, H.; Melnick, R.L.; Crozier, J. The Endophytic Trichoderma hamatum Isolate DIS 219b Enhances Seedling Growth and Delays the Onset of Drought Stress in Theobroma cacao. In Endophytes of Forest Trees: Biology and Applications; Springer: Dordrecht, The Netherlands, 2011; pp. 157–172. [Google Scholar]
- Leiva, S.; Oliva, M.; Hernández, E.; Chuquibala, B.; Rubio, K.; García, F.; de la Cruz, M.T. Assessment of the Potential of Trichoderma spp. Strains Native to Bagua (Amazonas, Peru) in the Biocontrol of Frosty Pod Rot (Moniliophthora roreri). Agronomy 2020, 10, 1376. [Google Scholar] [CrossRef]
- Loguercio, L.L.; De Souza, J.; Pomella, A.; Loguercio, L.L.; De Carvalho, A.C.; Niella, G.R.; De Souza, J.T.; Pomella, A.W.V. Selection of Trichoderma stromaticum Isolates for Efficient Biological Control of Witches’ Broom Disease in Cacao. Biol. Control 2009, 51, 130–139. [Google Scholar] [CrossRef]
- De Souza, J.T.; Bailey, B.A.; Pomella, A.W.V.; Erbe, E.F.; Murphy, C.A.; Bae, H.; Hebbar, P.K. Colonization of Cacao Seedlings by Trichoderma stromaticum, a Mycoparasite of the Witches’ Broom Pathogen, and Its Influence on Plant Growth and Resistance. Biol. Control 2008, 46, 36–45. [Google Scholar] [CrossRef]
- Bailey, B.A.; Bae, H.; Strem, M.D.; Roberts, D.P.; Thomas, S.E.; Crozier, J.; Samuels, G.J.; Choi, I.Y.; Holmes, K.A. Fungal and Plant Gene Expression during the Colonization of Cacao Seedlings by Endophytic Isolates of Four Trichoderma Species. Planta 2006, 224, 1449–1464. [Google Scholar] [CrossRef]
- Adedeji, A.R.; Odebode, A.C.; Agbeniyi, S.O. Bioassay of Five Trichoderma Strains against Phytophthora megakarya (Cacao Pod-Rot) in Nigeria. Sci. Res. Essay 2008, 3, 390–394. [Google Scholar]
- Li, X.; Li, B.; Liu, Y.; Xu, J. Rhizospheric Lactobacillus spp. Contribute to the High Cd-Accumulating Characteristics of Phytolacca spp. in Acidic Cd-Contaminated Soil. Environ. Res. 2023, 238, 117270. [Google Scholar] [CrossRef]
- Garcia Gabriel Alejandro, E. Actividad Antagonista de Rizobacterias Promotoras Del Crecimiento Vegetal (PGPR) a Moniliophthora Perniciosa (Escoba de Bruja) En Cacao (Theobroma cacao L.). Bachelor’s Thesis, Universidad Técnica Estatal de Quevedo, Quevedo, Ecuador, 2019. [Google Scholar]
- Santoyo, G.; del Orozco-Mosqueda, M.C.; Govindappa, M. Mechanisms of Biocontrol and Plant Growth-Promoting Activity in Soil Bacterial Species of Bacillus and Pseudomonas: A Review. Biocontrol Sci. Technol. 2012, 22, 855–872. [Google Scholar] [CrossRef]
- Li, C.; Chen, X.; Jia, Z.; Zhai, L.; Zhang, B.; Grüters, U.; Ma, S.; Qian, J.; Liu, X.; Zhang, J.; et al. Meta-Analysis Reveals the Effects of Microbial Inoculants on the Biomass and Diversity of Soil Microbial Communities. Nat. Ecol. Evol. 2024, 8, 1270–1284. [Google Scholar] [CrossRef] [PubMed]
- Cornell, C.; Kokkoris, V.; Richards, A.; Horst, C.; Rosa, D.; Bennett, J.A.; Hart, M.M. Do Bioinoculants Affect Resident Microbial Communities? A Meta-Analysis. Front. Agron. 2021, 3, 753474. [Google Scholar] [CrossRef]
- Gorguette, R.M.-P.; Souza, T.M.; Pereira, J.; Silva, E.F.M.S. Endophytic Fungi from Cacao Trees in “Cabruca” System in South Bahia, Brazil. Obs. Econ. Latinoam. 2025, 23, e9117. [Google Scholar] [CrossRef]
- Hanada, R.E.; Pomella, A.W.V.; Costa, H.S.; Bezerra, J.L.; Loguercio, L.L.; Pereira, J.O. Endophytic Fungal Diversity in Theobroma cacao (Cacao) and T. grandiflorum (Cupuaçu) Trees and Their Potential for Growth Promotion and Biocontrol of Black-Pod Disease. Fungal Biol. 2010, 114, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Rubini, M.R.; Silva-Ribeiro, R.T.; Pomella, A.W.V.; Maki, C.S.; Araújo, W.L.; dos Santos, D.R.; Azevedo, J.L. Diversity of Endophytic Fungal Community of Cacao (Theobroma cacao L.) and Biological Control of Crinipellis Perniciosa, Causal Agent of Witches’ Broom Disease. Int. J. Biol. Sci. 2005, 1, 24–33. [Google Scholar] [CrossRef]
- Liu, Q.; Pang, Z.; Yang, Z.; Nyumah, F.; Hu, C.; Lin, W.; Yuan, Z. Bio-Fertilizer Affects Structural Dynamics, Function, and Network Patterns of the Sugarcane Rhizospheric Microbiota. Microb. Ecol. 2022, 84, 1195–1211. [Google Scholar] [CrossRef]
- Fallah, N.; Pang, Z.; Lin, Z.; Nyimbo, W.J.; Lin, W.; Mbuya, S.N.; Ishimwe, C.; Zhang, H. Sustained Organic Amendments Utilization Enhances Ratoon Crop Growth and Soil Quality by Enriching Beneficial Metabolites and Suppressing Pathogenic Bacteria. Front. Plant Sci. 2023, 14, 1273546. [Google Scholar] [CrossRef]
- Valle, R.R.; De Almeida, A.-A.F.; De O. Leite, R.M. Energy Costs of Flowering, Fruiting, and Cherelle Wilt in Cacao. Tree Physiol. 1990, 6, 329–336. [Google Scholar] [CrossRef]
- Koziol, L.; McKenna, T.P.; Bever, J.D. Meta-Analysis Reveals Globally Sourced Commercial Mycorrhizal Inoculants Fall Short. New Phytol. 2024, 246, 821–827. [Google Scholar] [CrossRef]
- Santos, F.; Melkani, S.; Oliveira-Paiva, C.; Bini, D.; Pavuluri, K.; Gatiboni, L.; Mahmud, A.; Torres, M.; McLamore, E.; Bhadha, J.H. Biofertilizer Use in the United States: Definition, Regulation, and Prospects. Appl. Microbiol. Biotechnol. 2024, 108, 511. [Google Scholar] [CrossRef]
- Boza, E.J.; Motamayor, J.C.; Amores, F.M.; Cedeño-Amador, S.; Tondo, C.L.; Livingstone, D.S.; Schnell, R.J.; Gutiérrez, O.A. Genetic Characterization of the Cacao Cultivar CCN 51: Its Impact and Significance on Global Cacao Improvement and Production. J. Am. Soc. Hortic. Sci. 2014, 139, 219–229. [Google Scholar] [CrossRef]


| Compost | Vermicompost | Litter | |
|---|---|---|---|
| Organic matter (%) | 48.09 | 31.57 | 85.41 |
| Moisture content (%) | 38.63 | 35.13 | 12.94 |
| N (%) | 1.63 | 1.68 | 1.6 |
| P (%) | 1.47 | Trace | Trace |
| K (%) | 2.27 | 1.1 | 0.38 |
| Ca (%) | 3.09 | 2.75 | 3.86 |
| Application rate | 10 MT/ha/yr | 10 MT/ha/yr | 1.6 MT/ha/yr |
| Incorporation method | Rototiller | Rototiller | Mulched on surface |
| EM | OM | ||||
|---|---|---|---|---|---|
| EM | Native | Compost | Litter | Vermicompost | |
| BD (g/cm3) | 1.46 ± 0.01 a | 1.46 ± 0.01 a | 1.46 ± 0.02 a | 1.46 ± 0.02 a | 1.47 ± 0.02 a |
| Ca (meq/100 g) | 24.11 ± 0.37 a | 24.29 ± 0.37 a | 24.00 ± 0.45 a | 24.46 ± 0.45 a | 24.15 ± 0.46 a |
| CEC (meq/100 g) | 32.36 ± 0.59 a | 32.77 ± 0.70 a | 31.91 ± 0.83 a | 32.95 ± 0.78 a | 32.84 ± 0.76 a |
| Cu (ppm) | 10.84 ± 0.77 a | 11.44 ± 0.89 a | 12.99 ± 1.18 a | 10.72 ± 0.95 a | 9.71 ± 0.82 a |
| EC (dS/m) | 0.83 ± 0.05 a | 0.72 ± 0.04 a | 0.79 ± 0.06 a | 0.81 ± 0.05 a | 0.73 ± 0.05 a |
| ESP (%) | 0.96 ± 0.05 a | 1.06 ± 0.07 a | 1.00 ± 0.08 ab | 0.88 ± 0.06 b | 1.15 ± 0.07 a |
| Fe (ppm) | 152.90 ± 15.66 a | 148.50 ± 12.90 a | 140.40 ± 16.2 a | 161.53 ± 21.04 a | 150.17 ± 14.99 a |
| K (meq/100 g) | 1.20 ± 0.03 a | 1.25 ± 0.04 a | 1.32 ± 0.05 a | 1.15 ± 0.03 b | 1.20 ± 0.04 ab |
| Mg (meq/100 g) | 5.01 ± 0.19 a | 5.31 ± 0.23 a | 5.12 ± 0.23 a | 5.18 ± 0.28 a | 5.18 ± 0.26 a |
| Mn (ppm) | 21.05 ± 2.40 a | 27.47 ± 3.96 a | 21.74 ± 3.71 a | 26.34 ± 4.69 a | 24.68 ± 3.75 a |
| Na (meq/100 g) | 0.31 ± 0.02 a | 0.34 ± 0.02 a | 0.32 ± 0.03 ab | 0.29 ± 0.02 b | 0.38 ± 0.02 a |
| P (ppm) | 41.13 ± 3.42 a | 43.52 ± 3.94 a | 49.82 ± 3.54 a | 38.86 ± 5.05 b | 38.30 ± 4.53 b |
| pH | 6.79 ± 0.05 b | 6.94 ± 0.06 a | 6.92 ± 0.05 a | 6.87 ± 0.07 a | 6.80 ± 0.08 a |
| SOC (%) | 1.19 ± 0.03 a | 1.17 ± 0.02 a | 1.19 ± 0.03 a | 1.15 ± 0.03 a | 1.20 ± 0.03 a |
| Zn (ppm) | 12.47 ± 1.10 a | 14.35 ± 1.16 a | 12.09 ± 1.42 a | 14.27 ± 1.48 a | 13.88 ± 1.29 a |
| Treatment | Lot | Yield (t/ha/yr) |
|---|---|---|
| Compost + EM | 1 | 1.88 ± 0.36 |
| Compost + Native | 1.92 ± 0.46 | |
| Litter + EM | 2.26 ± 0.57 | |
| Litter + Native | 1.77 ± 0.13 | |
| Vermicompost + EM | 1.78 ± 0.35 | |
| Vermicompost + Native | 1.94 ± 0.21 | |
| Compost + EM | 22 | 2.39 ± 0.06 |
| Compost + Native | 2.48 ± 0.10 | |
| Litter + EM | 2.48 ± 0.22 | |
| Litter + Native | 2.56 ± 0.16 | |
| Vermicompost + EM | 2.25 ± 0.51 | |
| Vermicompost + Native | 2.61 ± 0.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmidt, J.E.; Flores, J.; Barragan, L.; Amores, F., Jr.; Khalsa, S.D.S. Optimizing Cocoa Productivity Through Soil Health and Microbiome Enhancement: Insights from Organic Amendments and a Locally Derived Biofertilizer. Microorganisms 2025, 13, 1408. https://doi.org/10.3390/microorganisms13061408
Schmidt JE, Flores J, Barragan L, Amores F Jr., Khalsa SDS. Optimizing Cocoa Productivity Through Soil Health and Microbiome Enhancement: Insights from Organic Amendments and a Locally Derived Biofertilizer. Microorganisms. 2025; 13(6):1408. https://doi.org/10.3390/microorganisms13061408
Chicago/Turabian StyleSchmidt, Jennifer E., Julia Flores, Luigy Barragan, Freddy Amores, Jr., and Sat Darshan S. Khalsa. 2025. "Optimizing Cocoa Productivity Through Soil Health and Microbiome Enhancement: Insights from Organic Amendments and a Locally Derived Biofertilizer" Microorganisms 13, no. 6: 1408. https://doi.org/10.3390/microorganisms13061408
APA StyleSchmidt, J. E., Flores, J., Barragan, L., Amores, F., Jr., & Khalsa, S. D. S. (2025). Optimizing Cocoa Productivity Through Soil Health and Microbiome Enhancement: Insights from Organic Amendments and a Locally Derived Biofertilizer. Microorganisms, 13(6), 1408. https://doi.org/10.3390/microorganisms13061408






