Antimicrobial Photodynamic Therapy for Superficial, Skin, and Mucosal Fungal Infections: An Update
Abstract
1. Introduction
2. PDT and aPDT
2.1. aPDT for the Treatment of Dermatophytosis
2.1.1. Methylene Blue
2.1.2. 5-Aminolevulinic Acid
2.1.3. Hypericin
2.1.4. Other Photosensitizers
2.2. aPDT for the Treatment of Superficial and Cutaneous Infections Caused by Candida spp.
2.2.1. MB
2.2.2. Toluidine Blue
2.2.3. ALA
2.2.4. Photodithazine
2.2.5. Other PSs
2.3. aPDT for the Treatment of Infections Caused by Superficial and Cutaneous Agents Other than Dermatophytes and Candida spp.
2.3.1. ALA
2.3.2. MB
2.3.3. Other PSs
2.4. Limitations and Future Directions for aPDT
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Denning, D.W. Global Incidence and Mortality of Severe Fungal Disease. Lancet Infect. Dis. 2024, 24, e428–e438. [Google Scholar] [CrossRef] [PubMed]
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and Multi-National Prevalence of Fungal Diseases-Estimate Precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef] [PubMed]
- Benedict, K.; Whitham, H.K.; Jackson, B.R. Economic Burden of Fungal Diseases in the United States. Open Forum Infect. Dis. 2022, 9, ofac097. [Google Scholar] [CrossRef]
- Konkel Neabore, L. Wake-up Call: Rapid Increase in Human Fungal Diseases under Climate Change. Environ. Health Perspect. 2024, 132, 42001. [Google Scholar] [CrossRef]
- Roberds, A.; Bobrov, A.G.; Rautemaa-Richardson, R.; Walsh, T.J. Invasive Fungal Diseases of Combat Wounds: Burden, Epidemiology, and Mycology. Mycopathologia 2024, 189, 102. [Google Scholar] [CrossRef] [PubMed]
- Badali, H.; Al-Hatmi, A.M.S.; Fakhim, H.; Moghaddasi, A.; Khodavaisy, S.; Vaezi, A.; Ahangarkani, F.; de Hoog, G.S.; Meis, J.F. In Vitro Activity of Nine Antifungal Agents against a Global Collection of Hortaea Werneckii Isolates, the Agent of Tinea Nigra. Int. J. Antimicrob. Agents 2019, 54, 95–98. [Google Scholar] [CrossRef]
- Chen, S.; Luo, Y.; Wu, H.; Zhang, J.; Li, W. Photodynamic Therapy Combined with Voriconazole of Extensive Ulcer Caused by Trichosporon Asahii. Photodiagnosis Photodyn. Ther. 2024, 49, 104313. [Google Scholar] [CrossRef]
- Cui, Z.; Zhang, M.; Geng, S.; Niu, X.; Wang, X.; Zhu, Y.; Ye, F.; Liu, C. Antifungal Effect of Antimicrobial Photodynamic Therapy Mediated by Haematoporphyrin Monomethyl Ether and Aloe Emodin on Malassezia Furfur. Front. Microbiol. 2021, 12, 749106. [Google Scholar] [CrossRef]
- Almeida Junior, H.L.d.; Assis, T.M.d.; Faria, E.C.; Costa, L.R.K.; Ibaldo, B.M. Piedraia Hortae: Biofilm Formation and Its Importance in the Pathogenesis of Piedra Nigra (Black Piedra). Bras. Dermatol. 2024, 99, 863–868. [Google Scholar] [CrossRef]
- Andrés, T.-S.; Alexandro, B. Candida Onychomycosis: An Old Problem in Modern Times. Curr. Fungal Infect. Rep. 2020, 14, 209–216. [Google Scholar] [CrossRef]
- Frazier, W.T.; Santiago-Delgado, Z.M.; Stupka, K.C., 2nd. Onychomycosis: Rapid Evidence Review. Am. Fam. Physician 2021, 104, 359–367. [Google Scholar] [PubMed]
- Sharma, B.; Nonzom, S. Superficial Mycoses, a Matter of Concern: Global and Indian Scenario-an Updated Analysis. Mycoses 2021, 64, 890–908. [Google Scholar] [CrossRef]
- Havlickova, B.; Czaika, V.A.; Friedrich, M. Epidemiological Trends in Skin Mycoses Worldwide. Mycoses 2008, 51, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Narang, T.; Bhattacharjee, R.; Singh, S.; Jha, K.; Kavita; Mahajan, R.; Dogra, S. Quality of Life and Psychological Morbidity in Patients with Superficial Cutaneous Dermatophytosis. Mycoses 2019, 62, 680–685. [Google Scholar] [CrossRef]
- Mada, P.K.; Saldaña Koppel, D.A.; Al Shaarani, M.; Joel Chandranesan, A.S. Primary Cutaneous Aspergillus fumigatus Infection in Immunocompetent Host. BMJ Case Rep. 2020, 13, e233020. [Google Scholar] [CrossRef]
- Wang, X.; Qu, Y.; Yang, Z.; Zhang, T.; Feng, Y.; Li, D.; Yan, H.; Shi, D. Surgery plus Photodynamic Therapy for a Diabetic Patient with Cutaneous Infectious Granuloma Caused by Curvularia Lunata. Photodiagnosis Photodyn. Ther. 2023, 41, 103253. [Google Scholar] [CrossRef]
- Hay, R. Therapy of Skin, Hair and Nail Fungal Infections. J. Fungi 2018, 4, 99. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Venkataraman, M. Antifungal Resistance in Superficial Mycoses. J. Dermatol. Treat. 2021, 33, 1888–1895. [Google Scholar] [CrossRef]
- Roemer, T.; Krysan, D.J. Antifungal Drug Development: Challenges, Unmet Clinical Needs, and New Approaches. Cold Spring Harb. Perspect. Med. 2014, 4, a019703. [Google Scholar] [CrossRef]
- Hossain, C.M.; Ryan, L.K.; Gera, M.; Choudhuri, S.; Lyle, N.; Ali, K.A.; Diamond, G. Antifungals and Drug Resistance. Encyclopedia 2022, 2, 1722–1737. [Google Scholar] [CrossRef]
- Durdu, M.; Kandemir, H.; Karakoyun, A.S.; Ilkit, M.; Tang, C.; de Hoog, S. First Terbinafine-Resistant Trichophyton Indotineae Isolates with Phe397Leu and/or Thr414His Mutations in Turkey. Mycopathologia 2023, 188, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Egger, N.B.; Kainz, K.; Schulze, A.; Bauer, M.A.; Madeo, F.; Carmona-Gutierrez, D. The Rise of Candida Auris: From Unique Traits to Co-Infection Potential. Microb. Cell 2022, 9, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Bila, N.M.; Costa-Orlandi, C.B.; Vaso, C.O.; Bonatti, J.L.C.; de Assis, L.R.; Regasini, L.O.; Fontana, C.R.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J.S. 2-Hydroxychalcone as a Potent Compound and Photosensitizer Against Dermatophyte Biofilms. Front. Cell Infect. Microbiol. 2021, 11, 679470. [Google Scholar] [CrossRef] [PubMed]
- de Arriba, M.; Borel, N.; LeibundGut-Landmann, S. Water-Filtered Infrared A Irradiation Exerts Antifungal Effects on the Skin Fungus Malassezia. J. Photochem. Photobiol. B 2024, 255, 112909. [Google Scholar] [CrossRef]
- Alvarez, N.; Sevilla, A. Current Advances in Photodynamic Therapy (PDT) and the Future Potential of PDT-Combinatorial Cancer Therapies. Int. J. Mol. Sci. 2024, 25, 1023. [Google Scholar] [CrossRef]
- Polat, E.; Kang, K. Natural Photosensitizers in Antimicrobial Photodynamic Therapy. Biomedicines 2021, 9, 584. [Google Scholar] [CrossRef]
- Kolarikova, M.; Hosikova, B.; Dilenko, H.; Barton-Tomankova, K.; Valkova, L.; Bajgar, R.; Malina, L.; Kolarova, H. Photodynamic Therapy: Innovative Approaches for Antibacterial and Anticancer Treatments. Med. Res. Rev. 2023, 43, 717–774. [Google Scholar] [CrossRef] [PubMed]
- Gunaydin, G.; Gedik, M.E.; Ayan, S. Photodynamic Therapy—Current Limitations and Novel Approaches. Front. Chem. 2021, 9, 691697. [Google Scholar] [CrossRef]
- Oruba, Z.; Łabuz, P.; Macyk, W.; Chomyszyn-Gajewska, M. Antimicrobial Photodynamic Therapy—A Discovery Originating from the Pre-Antibiotic Era in a Novel Periodontal Therapy. Photodiagnosis Photodyn. Ther. 2015, 12, 612–618. [Google Scholar] [CrossRef]
- Alberdi, E.; Gómez, C. Successful Treatment of Pityriasis Versicolor by Photodynamic Therapy Mediated by Methylene Blue. Photodermatol. Photoimmunol. Photomed. 2020, 36, 308–312. [Google Scholar] [CrossRef]
- Carvalho, G.G.; Felipe, M.P.; Costa, M.S. The Photodynamic Effect of Methylene Blue and Toluidine Blue on Candida Albicans Is Dependent on Medium Conditions. J. Microbiol. 2009, 47, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wang, S.; Li, D.; Feng, Y.; Fu, H.; Li, J.; Yan, H.; Shi, D. 5-Aminolevulinic Acid-Photodynamic Therapy as a Potential Approach for Kerion. Photodiagnosis Photodyn. Ther. 2022, 38, 102855. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.; Gomes, I.B.; Saavedra, M.J.; Simões, M. Photodynamic Therapy and Combinatory Treatments for the Control of Biofilm-Associated Infections. Lett. Appl. Microbiol. 2022, 75, 548–564. [Google Scholar] [CrossRef] [PubMed]
- Jusuf, S.; Dong, P.-T. Chromophore-Targeting Precision Antimicrobial Phototherapy. Cells 2023, 12, 2664. [Google Scholar] [CrossRef]
- Niculescu, A.-G.; Grumezescu, A.M. Photodynamic Therapy—An Up-to-Date Review. Appl. Sci. 2021, 11, 3626. [Google Scholar] [CrossRef]
- Kim, M.M.; Darafsheh, A. Light Sources and Dosimetry Techniques for Photodynamic Therapy. Photochem. Photobiol. 2020, 96, 280–294. [Google Scholar] [CrossRef]
- Roa-Tort, K.; Saavedra, Y.; Villanueva-Martínez, A.; Ganem-Rondero, A.; Pérez-Carranza, L.A.; de la Rosa-Vázquez, J.M.; Ugalde-Femat, G.; Molina-Alejandre, O.; Becerril-Osnaya, A.A.; Rivera-Fernández, J.D. In Vitro Antimicrobial Photodynamic Therapy for Pseudomonas Aeruginosa (P. aeruginosa) and Methicillin-Resistant Staphylococcus Aureus (MRSA) Inhibition Using a Green Light Source. Pharmaceutics 2024, 16, 518. [Google Scholar] [CrossRef]
- Li, D.; Liu, P.; Tan, Y.; Zhang, Z.; Kang, M.; Wang, D.; Tang, B.Z. Type I Photosensitizers Based on Aggregation-Induced Emission: A Rising Star in Photodynamic Therapy. Biosensors 2022, 12, 722. [Google Scholar] [CrossRef]
- Warrier, A.; Mazumder, N.; Prabhu, S.; Satyamoorthy, K.; Murali, T.S. Photodynamic Therapy to Control Microbial Biofilms. Photodiagnosis Photodyn. Ther. 2021, 33, 102090. [Google Scholar] [CrossRef]
- Mathur, A.; Parihar, A.S.; Modi, S.; Kalra, A. Photodynamic Therapy for ESKAPE Pathogens: An Emerging Approach to Combat Antimicrobial Resistance (AMR). In Microbial Pathogenesis; Academic Press: Cambridge, MA, USA, 2023. [Google Scholar] [CrossRef]
- Hamblin, M.R.; Abrahamse, H. Oxygen-Independent Antimicrobial Photoinactivation: Type III Photochemical Mechanism? Antibiotics 2020, 9, 53. [Google Scholar] [CrossRef]
- Rapacka-Zdończyk, A.; Woźniak, A.; Michalska, K.; Pierański, M.; Ogonowska, P.; Grinholc, M.; Nakonieczna, J. Factors Determining the Susceptibility of Bacteria to Antibacterial Photodynamic Inactivation. Front. Med. 2021, 8, 642609. [Google Scholar] [CrossRef] [PubMed]
- Gholami, L.; Shahabi, S.; Jazaeri, M.; Hadilou, M.; Fekrazad, R. Clinical Applications of Antimicrobial Photodynamic Therapy in Dentistry. Front. Microbiol. 2023, 13, 1020995. [Google Scholar] [CrossRef] [PubMed]
- de Lapena, S.A.B.; Terra-Garcia, M.; Ward, R.A.d.C.; Rossoni, R.D.; Melo, V.M.M.; Junqueira, J.C. Enhancing Effect of Chitosan on Methylene Blue-Mediated Photodynamic Therapy against C. Albicans: A Study in Planktonic Growth, Biofilms, and Persister Cells. Photodiagnosis Photodyn. Ther. 2022, 38, 102837. [Google Scholar] [CrossRef]
- Fabio, G.B.; Martin, B.A.; Dalmolin, L.F.; Lopez, R.F.V. Antimicrobial Photodynamic Therapy and the Advances Impacted by the Association with Nanoparticles. J. Drug Deliv. Sci. Technol. 2023, 80, 104147. [Google Scholar] [CrossRef]
- Youf, R.; Müller, M.; Balasini, A.; Thétiot, F.; Müller, M.; Hascoët, A.; Jonas, U.; Schönherr, H.; Lemercier, G.; Montier, T.; et al. Antimicrobial Photodynamic Therapy: Latest Developments with a Focus on Combinatory Strategies. Pharmaceutics 2021, 13, 1995. [Google Scholar] [CrossRef]
- Pinto, S.C.; Acunha, T.V.; Santurio, J.M.; Denardi, L.B.; Iglesias, B.A. Investigation of Powerful Fungicidal Activity of Tetra-Cationic Platinum(II) and Palladium(II) Porphyrins by Antimicrobial Photodynamic Therapy Assays. Photodiagnosis Photodyn. Ther. 2021, 36, 102550. [Google Scholar] [CrossRef]
- Terra Garcia, M.; Castro Pedroso, L.L.; Do Carmo, P.H.F.; da Silva, L.A.N.; Bueno, T.L.; dos Santos, V.G.R.; Fraga, A.S.; Nagai de Lima, P.M.; Soares da Silva, N.; de Paula, L.R.; et al. Functionalization of Cationic Porphyrins with Peripheral Platinum(II) Complexes to Optimize Photodynamic Therapy against Candida-Associated Infections: A Focus on Denture Stomatitis and Burn Wounds. Mycology 2025, 1–20. [Google Scholar] [CrossRef]
- de Oliveira Pereira, F.; Gomes, S.M.; Lima da Silva, S.; Paula de Castro Teixeira, A.; Lima, I.O. The Prevalence of Dermatophytoses in Brazil: A Systematic Review. J. Med. Microbiol. 2021, 70, 001321. [Google Scholar] [CrossRef]
- Rouzaud, C.; Chosidow, O.; Brocard, A.; Fraitag, S.; Scemla, A.; Anglicheau, D.; Bouaziz, J.; Dupin, N.; Bougnoux, M.; Hay, R.; et al. Severe Dermatophytosis in Solid Organ Transplant Recipients: A French Retrospective Series and Literature Review. Transpl. Infect. Dis. 2018, 20, e12799. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Rossi, N.M.; Peres, N.T.A.; Bitencourt, T.A.; Martins, M.P.; Rossi, A. State-of-the-Art Dermatophyte Infections: Epidemiology Aspects, Pathophysiology, and Resistance Mechanisms. J. Fungi 2021, 7, 629. [Google Scholar] [CrossRef]
- Shukla, P.; Verma, P.; Suvirya, S.; Pathania, S.; Kapoor, D. Cutaneous Dermatophytosis: A Problem Deeper than We Perceive—A Cross Sectional Prospective Study on Quality of Life in 385 Patients. Clin. Epidemiol. Glob. Health 2022, 17, 101115. [Google Scholar] [CrossRef]
- Kruithoff, C.; Gamal, A.; McCormick, T.S.; Ghannoum, M.A. Dermatophyte Infections Worldwide: Increase in Incidence and Associated Antifungal Resistance. Life 2023, 14, 1. [Google Scholar] [CrossRef]
- de Hoog, G.S.; Dukik, K.; Monod, M.; Packeu, A.; Stubbe, D.; Hendrickx, M.; Kupsch, C.; Stielow, J.B.; Freeke, J.; Göker, M.; et al. Toward a Novel Multilocus Phylogenetic Taxonomy for the Dermatophytes. Mycopathologia 2017, 182, 5–31. [Google Scholar] [CrossRef]
- Jartarkar, S.R.; Patil, A.; Goldust, Y.; Cockerell, C.J.; Schwartz, R.A.; Grabbe, S.; Goldust, M. Pathogenesis, Immunology and Management of Dermatophytosis. J. Fungi 2021, 8, 39. [Google Scholar] [CrossRef]
- Khurana, A.; Sardana, K.; Chowdhary, A. Antifungal Resistance in Dermatophytes: Recent Trends and Therapeutic Implications. Fungal Genet. Biol. 2019, 132, 103255. [Google Scholar] [CrossRef]
- Chen, B.; Sun, Y.; Zhang, J.; Chen, R.; Zhong, X.; Wu, X.; Zheng, L.; Zhao, J. In Vitro Evaluation of Photodynamic Effects Against Biofilms of Dermatophytes Involved in Onychomycosis. Front. Microbiol. 2019, 10, 1228. [Google Scholar] [CrossRef]
- Alberdi, E.; Gómez, C. Methylene Blue vs Methyl Aminolevulinate Photodynamic Therapy in Combination with Oral Terbinafine in the Treatment of Severe Dermatophytic Toenail Onychomycosis: Short- and Long-term Effects. Mycoses 2020, 63, 859–868. [Google Scholar] [CrossRef]
- Gnat, S.; Łagowski, D.; Dyląg, M.; Zielinski, J.; Nowakiewicz, A. In Vitro Evaluation of Photodynamic Activity of Methylene Blue against Trichophyton verrucosum Azole-susceptible and -resistant Strains. J. Biophotonics 2021, 14, e202100150. [Google Scholar] [CrossRef]
- Shen, J.J.; Arendrup, M.C.; Jemec, G.B.E.; Saunte, D.M.L. Photodynamic Therapy: A Treatment Option for Terbinafine Resistant Trichophyton Species. Photodiagnosis Photodyn. Ther. 2021, 33, 102169. [Google Scholar] [CrossRef] [PubMed]
- Askari, R.; Zaboli, F.; Pordeli, H.; Kaboosi, H. Investigation of Photodynamic and Rhamnolipid Inhibition on the Dermatophyte Biofilm. Indian. J. Microbiol. 2024, 64, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Zhang, J.; Li, J.; Qian, Y.; Huang, B.; Wu, X. Comparative Transcriptome Analysis of T. Rubrum, T. Mentagrophytes, and M. Gypseum Dermatophyte Biofilms in Response to Photodynamic Therapy. Mycopathologia 2024, 189, 59. [Google Scholar] [CrossRef]
- Shi, L.; Wu, Q.; Yang, J.; Tan, J.; Yang, H.; Hu, C.; Zhang, Y.; Zhang, H.; Zhang, L.; Liu, Y.; et al. ALA-PDT Successfully Treated Majocchi’s Granuloma by Directly Killing Trichophyton Tonsurans and Recruiting T Lymphocytes. Photodiagnosis Photodyn. Ther. 2021, 35, 102328. [Google Scholar] [CrossRef]
- Ji, J.; Liang, C.; Zhong, J.; Kong, X.; Xu, H.; Xu, C.; Fu, M. 5-Aminolevulinic Acid-Based Photodynamic Therapy in Combination with Antifungal Agents for Adult Kerion and Facial Ulcer Caused by Trichophyton Rubrum. Photodiagnosis Photodyn. Ther. 2024, 45, 103954. [Google Scholar] [CrossRef]
- Conrado, P.C.V.; Vaine, A.A.; Arita, G.S.; Sakita, K.M.; Gonçalves, R.S.; Caetano, W.; de Souza, M.; Baesso, M.L.; Malacarne, L.C.; Razzolini, E.; et al. Promising Onychomycosis Treatment with Hypericin-Mediated Photodynamic Therapy: Case Reports. Photodiagnosis Photodyn. Ther. 2023, 42, 103498. [Google Scholar] [CrossRef]
- Fernandes, J.A.; Conrado, P.C.V.; Perina, B.S.; de Oliveira, A.C.V.; Arita, G.S.; Capoci, I.R.G.; Gonçalves, R.S.; Caetano, W.; Svidzinski, T.I.E.; Cotica, E.S.K.; et al. Photodynamic Inactivation by Hypericin-P123 on Azole-Resistant Isolates of the Trichophyton Rubrum Complex as Planktonic Cells and Biofilm. Photodiagnosis Photodyn. Ther. 2023, 44, 103875. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, M.; Cui, Z.; Wang, X.; Niu, X.; Zhu, Y.; Yao, Z.; Ye, F.; Geng, S.; Liu, C. Aloe-emodin-mediated Antimicrobial Photodynamic Therapy against Dermatophytosis Caused by Trichophyton rubrum. Microb. Biotechnol. 2022, 15, 499–512. [Google Scholar] [CrossRef]
- Kuang, Y.; Zhang, X.; Zhou, S. Adsorption of Methylene Blue in Water onto Activated Carbon by Surfactant Modification. Water 2020, 12, 587. [Google Scholar] [CrossRef]
- Markantonatou, A.-M.; Samaras, K.; Vyzantiadis, T.-A. Dermatophytic Biofilms: Characteristics, Significance and Treatment Approaches. J. Fungi 2023, 9, 228. [Google Scholar] [CrossRef]
- Burkharta, C.N.; Burkhart, C.G.; Gupta, A.K. Dermatophytoma: Recalcitrance to Treatment Because of Existence of Fungal Biofilm. J. Am. Acad. Dermatol. 2002, 47, 629–631. [Google Scholar] [CrossRef]
- Zdubek, A.; Maliszewska, I. On the Possibility of Using 5-Aminolevulinic Acid in the Light-Induced Destruction of Microorganisms. Int. J. Mol. Sci. 2024, 25, 3590. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Yi, Y.-C.; Lin, Y.-C.; Chen, C.-C.; Hung, J.-H.; Lin, J.-Y.; Ng, I.-S. Purification and Biofabrication of 5-Aminolevulinic Acid for Photodynamic Therapy against Pathogens and Cancer Cells. Bioresour. Bioprocess. 2022, 9, 68. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, J.; Wang, Y.; Yao, L. Majocchi’s Granuloma. Am. J. Med. Sci. 2024, 368, e59–e60. [Google Scholar] [CrossRef]
- Galinari, C.B.; Biachi, T.d.P.; Gonçalves, R.S.; Cesar, G.B.; Bergmann, E.V.; Malacarne, L.C.; Kioshima Cotica, É.S.; Bonfim-Mendonça, P.d.S.; Svidzinski, T.I.E. Photoactivity of Hypericin: From Natural Product to Antifungal Application. Crit. Rev. Microbiol. 2023, 49, 38–56. [Google Scholar] [CrossRef]
- Lin, H.-D.; Li, K.-T.; Duan, Q.-Q.; Chen, Q.; Tian, S.; Chu, E.S.M.; Bai, D.-Q. The Effect of Aloe-Emodin-Induced Photodynamic Activity on the Apoptosis of Human Gastric Cancer Cells: A Pilot Study. Oncol. Lett. 2017, 13, 3431–3436. [Google Scholar] [CrossRef]
- Karimi-Sales, E.; Mohaddes, G.; Alipour, M.R. Chalcones as Putative Hepatoprotective Agents: Preclinical Evidence and Molecular Mechanisms. Pharmacol. Res. 2018, 129, 177–187. [Google Scholar] [CrossRef]
- Černáková, L.; Líšková, A.; Lengyelová, L.; Rodrigues, C.F. Prevalence and Antifungal Susceptibility Profile of Oral Candida Spp. Isolates from a Hospital in Slovakia. Medicina 2022, 58, 576. [Google Scholar] [CrossRef]
- Huang, S.-H.; Hsu, H.-C.; Lee, T.-F.; Fan, H.-M.; Tseng, C.-W.; Chen, I.-H.; Shen, H.; Lee, C.-Y.; Tai, H.-T.; Hsu, H.-M.; et al. Prevalence, Associated Factors, and Appropriateness of Empirical Treatment of Trichomoniasis, Bacterial Vaginosis, and Vulvovaginal Candidiasis among Women with Vaginitis. Microbiol. Spectr. 2023, 11, e0016123. [Google Scholar] [CrossRef]
- Bhosale, V.B.; Koparde, A.A.; Thorat, V.M. Vulvovaginal Candidiasis-an Overview of Current Trends and the Latest Treatment Strategies. Microb. Pathog. 2025, 200, 107359. [Google Scholar] [CrossRef]
- Acosta-Mosquera, Y.; Tapia, J.C.; Armas-González, R.; Cáceres-Valdiviezo, M.J.; Fernández-Cadena, J.C.; Andrade-Molina, D. Prevalence and Species Distribution of Candida Clinical Isolates in a Tertiary Care Hospital in Ecuador Tested from January 2019 to February 2020. J. Fungi 2024, 10, 304. [Google Scholar] [CrossRef]
- Reda, N.M.; Hassan, R.M.; Salem, S.T.; Yousef, R.H.A. Prevalence and Species Distribution of Candida Bloodstream Infection in Children and Adults in Two Teaching University Hospitals in Egypt: First Report of Candida Kefyr. Infection 2023, 51, 389–395. [Google Scholar] [CrossRef]
- Tajane, S.B.; Pawar, S.; Patil, S. Revisiting the History of Candidiasis. Cureus 2025, 17, e78878. [Google Scholar] [CrossRef]
- Talapko, J.; Juzbašić, M.; Matijević, T.; Pustijanac, E.; Bekić, S.; Kotris, I.; Škrlec, I. Candida Albicans—The Virulence Factors and Clinical Manifestations of Infection. J. Fungi 2021, 7, 79. [Google Scholar] [CrossRef]
- Cavalheiro, M.; Teixeira, M.C. Candida Biofilms: Threats, Challenges, and Promising Strategies. Front. Med. 2018, 5, 28. [Google Scholar] [CrossRef]
- Kaur, J.; Nobile, C.J. Antifungal Drug-Resistance Mechanisms in Candida Biofilms. Curr. Opin. Microbiol. 2023, 71, 102237. [Google Scholar] [CrossRef]
- Štefánek, M.; Černáková, L.; Dekkerová, J.; Bujdáková, H. Photodynamic Inactivation Effectively Eradicates Candida Auris Biofilm despite Its Interference with the Upregulation of CDR1 and MDR1 Efflux Genes. J. Fungi 2022, 8, 1137. [Google Scholar] [CrossRef]
- Al-Aali, K.A.; Alqahtani, A.S.; AlZaid, A.A.; Almujel, S.H.; Alsaloum, M.; Alanazi, K.K. Efficacy of Adjunct Photodynamic Therapy on Candida Growth and Oral Health Quality of Life in Denture Stomatitis Patients with Type 2 Diabetes Mellitus Wearing Implant-Retained Overdentures: A Randomized Clinical Study. Photodiagnosis Photodyn. Ther. 2023, 42, 103630. [Google Scholar] [CrossRef]
- Soares, J.C.M.; Luiz, M.T.; Oshiro Junior, J.A.; Besegato, J.F.; de Melo, P.B.G.; Rastelli, A.N.d.S.; Chorilli, M. Antimicrobial Photodynamic Therapy Mediated by Methylene Blue-Loaded Polymeric Micelles against Streptococcus Mutans and Candida Albicans Biofilms. Photodiagnosis Photodyn. Ther. 2023, 41, 103285. [Google Scholar] [CrossRef]
- Rodrigues, A.B.F.; Passos, J.C.d.S.; Costa, M.S. Effect of Antimicrobial Photodynamic Therapy Using Toluidine Blue on Dual-Species Biofilms of Candida Albicans and Candida Krusei. Photodiagnosis Photodyn. Ther. 2023, 42, 103600. [Google Scholar] [CrossRef]
- Afrasiabi, S.; Benedicenti, S.; Signore, A.; Arshad, M.; Chiniforush, N. Simultaneous Dual-Wavelength Laser Irradiation against Implant-Adherent Biofilms of Staphylococcus Aureus, Escherichia Coli, and Candida Albicans for Improved Antimicrobial Photodynamic Therapy. Bioengineering 2024, 11, 48. [Google Scholar] [CrossRef]
- Shi, H.; Li, J.; Peng, C.; Xu, B.; Sun, H. The Inhibitory Activity of 5-Aminolevulinic Acid Photodynamic Therapy (ALA-PDT) on Candida Albicans Biofilms. Photodiagnosis Photodyn. Ther. 2021, 34, 102271. [Google Scholar] [CrossRef]
- He, X.; Lu, Y. Successful Combined Treatment with Surgery and ALA-PDT for Cutaneous Infection by Candida Tropicalis: A Case Report and Literature Review. Photodiagnosis Photodyn. Ther. 2024, 49, 104303. [Google Scholar] [CrossRef]
- Wang, L.; Feng, Y.; Wang, S.; Shi, L.; Ren, Y.; Yang, Z.; Shi, D. Successful Management of Recurrent Cutaneous Granulomas Caused by Candida Albicans Using Aminolevulinic Acid Photodynamic Therapy Post-Surgery: A Case Report. Photodiagnosis Photodyn. Ther. 2025, 51, 104466. [Google Scholar] [CrossRef]
- Dias, L.M.; Klein, M.I.; Jordão, C.C.; Carmello, J.C.; Bellini, A.; Pavarina, A.C. Successive Applications of Antimicrobial Photodynamic Therapy Effects the Susceptibility of Candida Albicans Grown in Medium with or without Fluconazole. Photodiagnosis Photodyn. Ther. 2020, 32, 102018. [Google Scholar] [CrossRef]
- Jordão, C.C.; Viana de Sousa, T.; Inêz Klein, M.; Mendonça Dias, L.; Pavarina, A.C.; Carmello, J.C. Antimicrobial Photodynamic Therapy Reduces Gene Expression of Candida Albicans in Biofilms. Photodiagnosis Photodyn. Ther. 2020, 31, 101825. [Google Scholar] [CrossRef]
- Ma, W.; Liu, C.; Li, J.; Hao, M.; Ji, Y.; Zeng, X. The Effects of Aloe Emodin-Mediated Antimicrobial Photodynamic Therapy on Drug-Sensitive and Resistant Candida Albicans. Photochem. Photobiol. Sci. 2020, 19, 485–494. [Google Scholar] [CrossRef]
- Mardani, M.; Kamrani, O. Effectiveness of Antimicrobial Photodynamic Therapy with Indocyanine Green against the Standard and Fluconazole-Resistant Candida Albicans. Lasers Med. Sci. 2021, 36, 1971–1977. [Google Scholar] [CrossRef]
- Alshehri, A.H. Mechanical and Antimicrobial Effects of Riboflavin-Mediated Photosensitization of in Vitro C. Albicans Formed on Polymethyl Methacrylate Resin. Photodiagnosis Photodyn. Ther. 2021, 36, 102488. [Google Scholar] [CrossRef]
- Perić, M.; Miličić, B.; Kuzmanović Pfićer, J.; Živković, R.; Arsić Arsenijević, V. A Systematic Review of Denture Stomatitis: Predisposing Factors, Clinical Features, Etiology, and Global Candida Spp. Distribution. J. Fungi 2024, 10, 328. [Google Scholar] [CrossRef]
- Iyer, K.R.; Camara, K.; Daniel-Ivad, M.; Trilles, R.; Pimentel-Elardo, S.M.; Fossen, J.L.; Marchillo, K.; Liu, Z.; Singh, S.; Muñoz, J.F.; et al. An Oxindole Efflux Inhibitor Potentiates Azoles and Impairs Virulence in the Fungal Pathogen Candida Auris. Nat. Commun. 2020, 11, 6429. [Google Scholar] [CrossRef]
- Kotta, S.; Aldawsari, H.M.; Badr-Eldin, S.M.; Nair, A.B.; YT, K. Progress in Polymeric Micelles for Drug Delivery Applications. Pharmaceutics 2022, 14, 1636. [Google Scholar] [CrossRef]
- Passos, J.C.d.S.; Calvi, G.d.S.; Rodrigues, A.B.F.; Costa, M.S. The Inhibitory Effect of Photodynamic Therapy on Dual-Species Biofilms of Candida Albicans and Candida Krusei Can Be Determined by Candida Albicans/Candida Krusei Ratio. Photodiagnosis Photodyn. Ther. 2023, 44, 103787. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, Q.; Zhang, Y.; Wang, Y.; Liu, N.; Liu, Q. Rapid Inactivation of Mixed Biofilms of Candida Albicans and Candida Tropicalis Using Antibacterial Photodynamic Therapy: Based on PADTM Plus. Heliyon 2023, 9, e15396. [Google Scholar] [CrossRef] [PubMed]
- Stranadko, E.P.; Ponomarev, G.V.; Mechkov, V.M.; Ryabov, M.V.; Ivanov, A.V.; Reshetnickov, A.V.; Koraboyev, U.M.; Dougherty, T.J. First Experience of Photodithazine Clinical Application for Photodynamic Therapy of Malignant Tumors. In Optical Methods for Tumor Treatment and Detection: Mechanisms and Techniques in Photodynamic Therapy IX; Dougherty, T.J., Ed.; SPIE: Bellingham, WA, USA, 2000; pp. 138–144. [Google Scholar] [CrossRef]
- Ferreira, J.; Menezes, P.F.C.; Kurachi, C.; Sibata, C.; Allison, R.R.; Bagnato, V.S. Photostability of Different Chlorine Photosensitizers. Laser Phys. Lett. 2008, 5, 156–161. [Google Scholar] [CrossRef]
- Staab, J.F.; Ferrer, C.A.; Sundstrom, P. Developmental Expression of a Tandemly Repeated, Proline- and Glutamine-Rich Amino Acid Motif on Hyphal Surfaces of Candida Albicans. J. Biol. Chem. 1996, 271, 6298–6305. [Google Scholar] [CrossRef]
- Finkel, J.S.; Mitchell, A.P. Genetic Control of Candida Albicans Biofilm Development. Nat. Rev. Microbiol. 2011, 9, 109–118. [Google Scholar] [CrossRef]
- Zafar, M.S. Prosthodontic Applications of Polymethyl Methacrylate (PMMA): An Update. Polymers 2020, 12, 2299. [Google Scholar] [CrossRef]
- Lockhart, S.R.; Guarner, J. Emerging and Reemerging Fungal Infections. Semin. Diagn. Pathol. 2019, 36, 177–181. [Google Scholar] [CrossRef]
- Mazi, P.B.; Rauseo, A.M.; Spec, A. Blastomycosis. Infect. Dis. Clin. N. Am. 2021, 35, 515–530. [Google Scholar] [CrossRef] [PubMed]
- Queiroz-Telles, F.; de Hoog, S.; Santos, D.W.C.L.; Salgado, C.G.; Vicente, V.A.; Bonifaz, A.; Roilides, E.; Xi, L.; Azevedo, C.d.M.P.eS.; da Silva, M.B.; et al. Chromoblastomycosis. Clin. Microbiol. Rev. 2017, 30, 233–276. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J.; Chen, Y.; Xue, R.; Zeng, W.; Xi, L.; Chen, Y. Phaeohyphomycosis Due to Exophiala Spinifera Greatly Improved by ALA-PDT: A Case Report. Photodiagnosis Photodyn. Ther. 2019, 28, 297–299. [Google Scholar] [CrossRef]
- Lan, Y.; Lu, S.; Zheng, B.; Tang, Z.; Li, J.; Zhang, J. Combinatory Effect of ALA-PDT and Itraconazole Treatment for Trichosporon asahii. Lasers Surg. Med. 2021, 53, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhu, M.; An, L.; Chen, F.; Zhang, X. Fungicidal Efficacy of Photodynamic Therapy Using Methylene Blue against Sporothrix Globosa in Vivo and in Vivo. Eur. J. Dermatol. 2019, 29, 160–166. [Google Scholar] [CrossRef]
- Kodedová, M.; Liška, V.; Mosinger, J.; Sychrová, H. Light-Induced Antifungal Activity of Nanoparticles with an Encapsulated Porphyrin Photosensitizer. Microbiol. Res. 2023, 269, 127303. [Google Scholar] [CrossRef]
- Gupta, A.K.; Lyons, D.C. Pityriasis Versicolor: An Update on Pharmacological Treatment Options. Expert. Opin. Pharmacother. 2014, 15, 1707–1713. [Google Scholar] [CrossRef]
- Mfouo-Tynga, I.S.; Dias, L.D.; Inada, N.M.; Kurachi, C. Features of Third Generation Photosensitizers Used in Anticancer Photodynamic Therapy: Review. Photodiagnosis Photodyn. Ther. 2021, 34, 102091. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, A.R.; Saalmann, M.R.; Nicolay, N.H.; Grosu, A.-L.; Vaupel, P. Improved Oxygenation of Human Skin, Subcutis and Superficial Cancers Upon Mild Hyperthermia Delivered by WIRA-Irradiation; Springer: Berlin/Heidelberg, Germany, 2022; pp. 255–261. [Google Scholar] [CrossRef]
- Johnson, B.Z.; Stevenson, A.W.; Prêle, C.M.; Fear, M.W.; Wood, F.M. The Role of IL-6 in Skin Fibrosis and Cutaneous Wound Healing. Biomedicines 2020, 8, 101. [Google Scholar] [CrossRef]
- Shen, J.J.; Jemec, G.B.E.; Arendrup, M.C.; Saunte, D.M.L. Photodynamic Therapy Treatment of Superficial Fungal Infections: A Systematic Review. Photodiagnosis Photodyn. Ther. 2020, 31, 101774. [Google Scholar] [CrossRef] [PubMed]
- Gilaberte, Y.; Robres, M.P.; Frías, M.P.; García-Doval, I.; Rezusta, A.; Aspiroz, C. Methyl Aminolevulinate Photodynamic Therapy for Onychomycosis: A Multicentre, Randomized, Controlled Clinical Trial. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 347–354. [Google Scholar] [CrossRef]
- Hu, X.; Shou, B.; Yang, L.; Li, L.; Ren, H.-T.; Lin, J.-H.; Lou, C.-W.; Li, T.-T. Antimicrobial Photodynamic Therapy Encapsulation Technology: Frontier Exploration and Application Prospects of Novel Antimicrobial Technology. Chem. Eng. J. 2023, 477, 146773. [Google Scholar] [CrossRef]
- Furtado, G.S.; Paschoal, M.A.B.; Santos Grenho, L.d.C.; Lago, A.D.N. Does Pre-Irradiation Time Influence the Efficacy of Antimicrobial Photodynamic Therapy? Photodiagnosis Photodyn. Ther. 2020, 31, 101884. [Google Scholar] [CrossRef]
- Güneri, P.; Epstein, J.B.; Ergün, S.; Boyacioğlu, H. Toluidine Blue Color Perception in Identification of Oral Mucosal Lesions. Clin. Oral. Investig. 2011, 15, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Abraham, L.; Rai, A.; Burde, K.; Naikmasur, V. Methylene Blue as a Diagnostic Aid in the Early Detection of Potentially Malignant and Malignant Lesions of Oral Mucosa. Ethiop. J. Health Sci. 2016, 26, 201. [Google Scholar] [CrossRef] [PubMed]
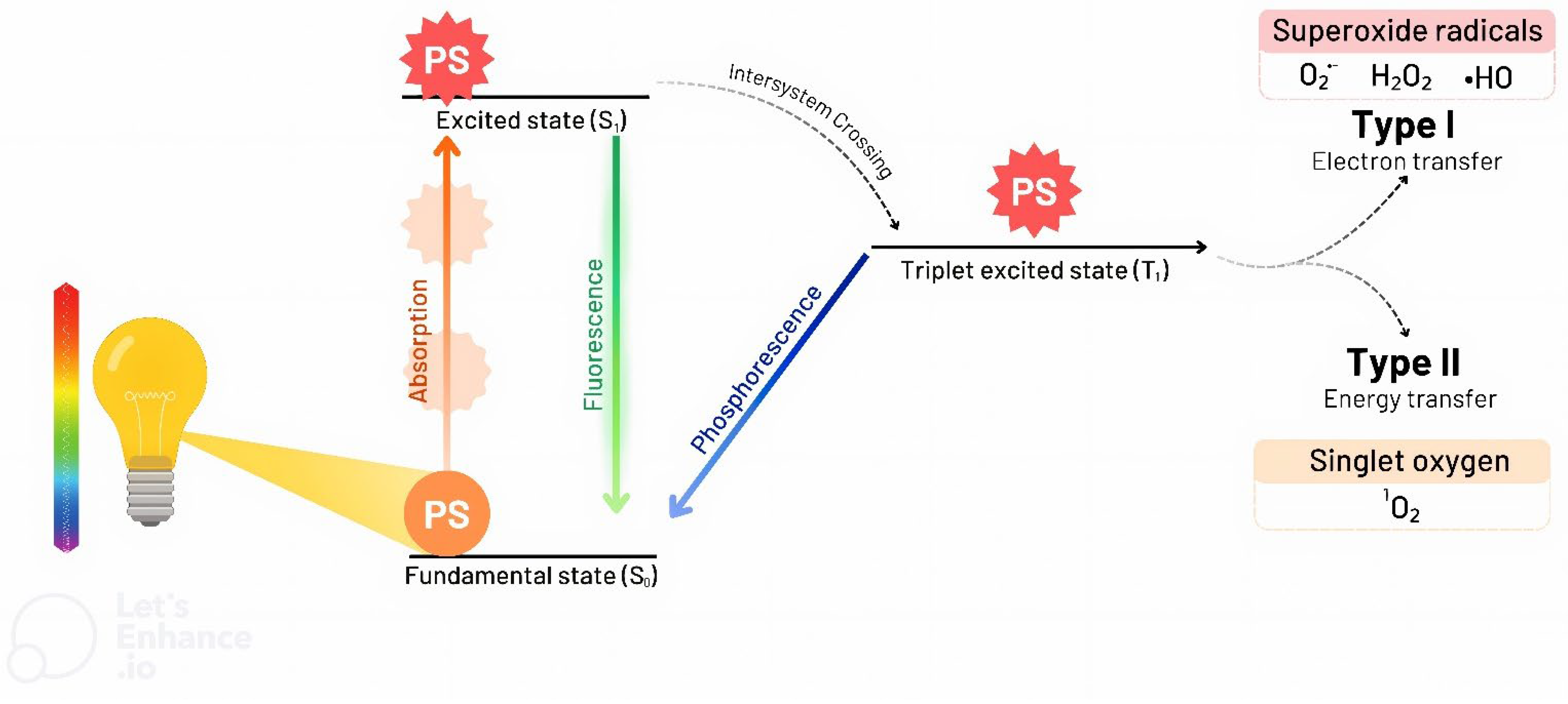
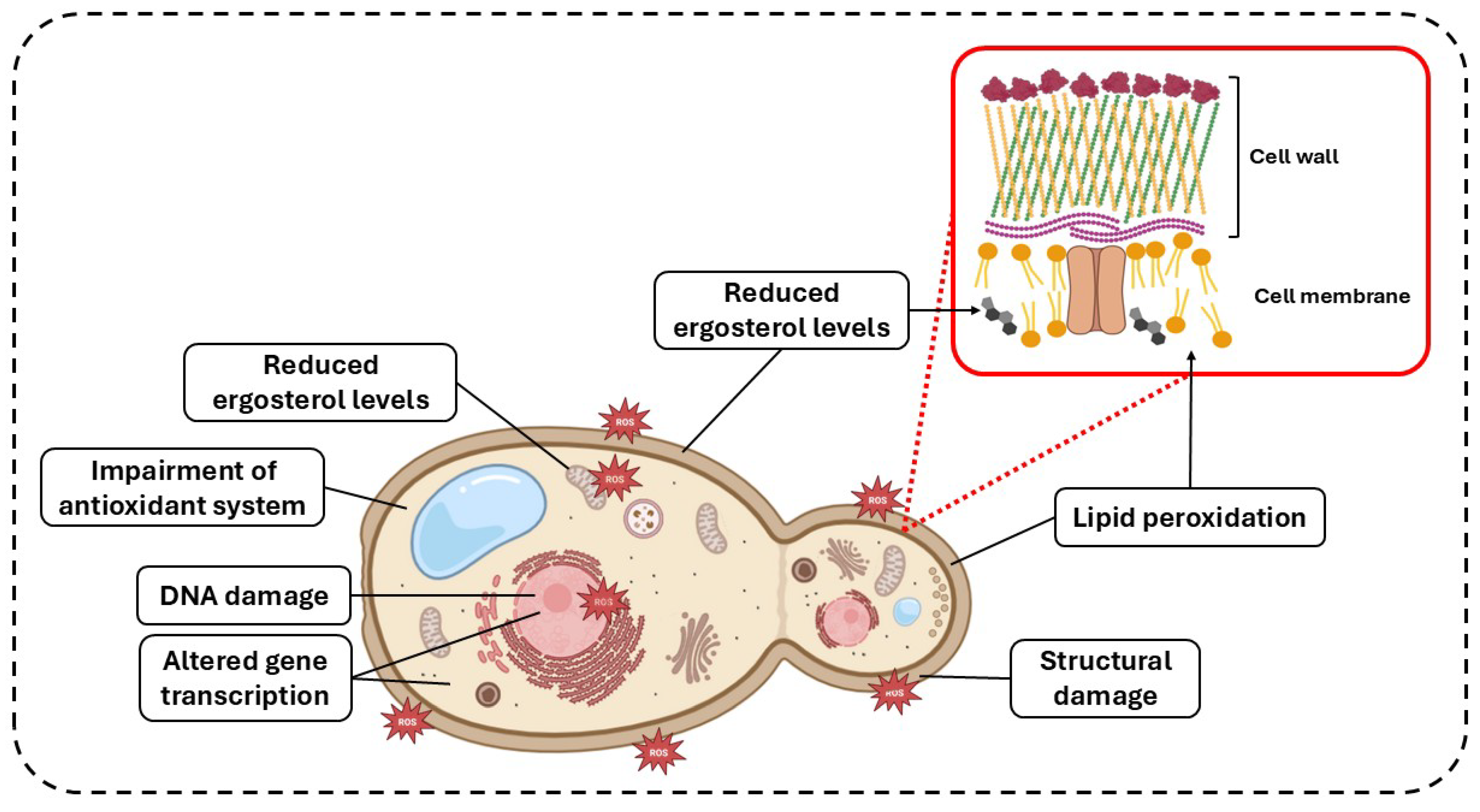
| Reference | Species | Photosensitizer | Irradiation Parameters | Main Results |
|---|---|---|---|---|
| Chen et al. [57] | Trichophyton rubrum, Trichophyton mentagrophytes, and Nannizzia gypsea | Methylene blue (MB) | Pre-irradiation time (PIT): 2 h 635 nm, 100 mW/cm2, 60 J/cm2, 10 min | Antifungal activity against conidia and biofilms Structural disruption of biofilms |
| Alberdi and Gómez [58] | Not specified | MB | PIT: 3 min 635 nm, 62 mW/cm2, 37 J/cm2, 10 min | Significant clinical improvement Synergistic effects with terbinafine Well-tolerated treatment |
| Gnat et al. [59] | Trichophyton verrucosum | MB | PIT: 10 min 635 nm, 58 mW/cm2, 17 to 1750 s, 1–100 J/cm2 | Antifungal effects on both sensitive and resistant strains Antibiofilm effects |
| Shen et al. [60] | T. rubrum and Trichophyton interdigitale | MB | PIT: 3 min, 30 min and 3 h 635 nm, 37 J/cm2, 485 s | Inhibition of conidia growth Antifungal effects on both terbinafine-sensitive and -resistant strains |
| Askari et al. [61] | T. mentagrophytes, T. rubrum, T. verrucosum, Microsporum canis, and N. gypsea | MB | PIT: 3 h 600 and 635 nm, 100 MW/cm2, 10 min | Inhibitory effects on biofilms Structural damage to biofilms Synergism with rhamnolipid |
| Chen et al. [62] | T. rubrum, T. mentagrophytes, and N. gypsea | MB | PIT: 3 h 635 ± 10 nm, 100 mW/cm2, 60 J/cm2, 10 min | Antibiofilm effects Hyphal disruption and structural damage in biofilms |
| Shi et al. [63] | Trichophyton tonsurans | 5-aminolevulinic acid (ALA) | PIT: 5 h 633 nm, 40 mW/cm2, 200 J/cm2 | Antifungal activity Improvement of ulcerative lesions Mycological cure |
| Zhang et al. [32] | M. canis | ALA | PIT: 3 h 630–635 nm, 200–300 mW/cm2, 60–80 J/cm2, 10 to 25 min | Fungal eradication Lesion improvement and regrowth of hair No recurrence Synergistic activity with antifungals |
| Ji et al. [64] | T. rubrum | ALA | PIT: 3 h 635 nm, 60 and 90 mW/cm2, 72 and 108 J/cm2 | Complete lesion healing Effective in recurrent infections Mild adverse effects |
| Conrado et al. [65] | T. rubrum | Hypericin (Hyp) | PIT: 30 m 400–800 nm, 30 and 67 mW/cm2, 37.8 and 66 J/cm2 | Antifungal activity Mycological cure No adverse effects |
| Fernandes et al. [66] | T. rubrum | Hyp | PIT: 2 h 596–600 nm, 37.9 J/cm2 | Antifungal activity Antibiofilm effects Inhibition of cell adhesion |
| Ma et al. [67] | T. rubrum | Aloe-emodin | PIT: 1, 2 and 3 h 435 ± 10 nm, 40 mW/cm2, 2.4–72 J/cm2 | Antifungal activity on both conidia and hyphae Effective in treating both tinea corporis and tinea unguium Healing and hair regrowth post-treatment |
| Bila et al. [23] | T. rubrum and T. mentagrophytes | 2-hydroxychalcone | PIT: 10 min 455–492 nm, 58 mW/cm2, 150 J/cm2 | Antifungal activity Activity against mature biofilms Biocompatibility with mammalian cells |
| Pinto et al. [47] | T. rubrum, T. tonsurans, T. mentagrophytes, M. canis, N. gypsea, M. nanum, and E. floccosum | Tetra-cationic porphyrins with peripheral platinum (II) and palladium (II) complexes | 400–800 nm, 25 mW/cm2, 180 J/cm2, 120 min | Antifungal activity Increased ROS generation Synergism with itraconazole |
| Reference | Species | Photosensitizer | Irradiation Parameters | Main Results |
|---|---|---|---|---|
| Štefánek et al. [86] | Candidozyma auris | Methylene blue (MB) | Pre-irradiation time (PIT): 1 h 660 nm, 190 mW/cm2, 15, 23, and 58 J/cm2, 79, 120, and 300 s | Upregulation of efflux pumps genes (CDR1 and MDR1) Antibiofilm effects |
| Al-Aali et al. [87] | Candida spp. | MB | PIT: 5 min 600 nm, 100 mW, 3527 mW/cm2, 9 J | Antifungal activity Synergism with miconazole Resolution of palatal inflammation |
| Soares et al. [88] | Candida albicans | MB | PIT: 5, 15 and 30 min 660 nm, 19 mW/cm2, 15 J/cm2, 13 min and 16 s | Decreased fungal viability Inhibition of biofilm formation |
| Rodrigues et al. [89] | C. albicans and P. kudriavzevii | Toluidine Blue O (TBO) | PIT: 10 min 630 nm, 0.069 W, 30 J/cm2, 165 s | Decreased viability of dual-species biofilms |
| Afrasiabi et al. [90] | C. albicans | TBO | 635 and 980 nm, 200 and 800 mW, 6 and 24 J/cm2, 60 s | Reduction of fungal viability Increased reactive oxygen species (ROS) levels |
| Shi et al. [91] | C. albicans | 5-aminolevulinic acid (ALA) | PIT: 5 h 635 nm, 100 mW/cm2, 300 J/cm2, 50 min | Structural damage to biofilms Induced cell apoptosis Downregulation of biofilm-associated genes |
| He and Lu [92] | C. tropicalis | ALA | PIT: 4 h 635 nm, 177 mW/cm2, 120 J/cm2, 15 min | Synergism with surgical debridement and itraconazole Good clinical outcomes No significant adverse events No recurrence |
| Wang et al. [93] | C. albicans | ALA | PIT: 2 h 633 ± 10 nm, 80 mW/cm2, 25 min | Clinical improvement No significant adverse events No recurrence |
| Dias et al. [94] | C. albicans | Photodithazine (PDZ) | PIT: 20 min 660 nm, 34 mW/cm2, 18 J/cm2, 9 min | Activity on planktonic cells and biofilms Synergism with fluconazole |
| Jordão et al. [95] | C. albicans | PDZ and curcumin | PIT: 20 min PDZ: 660 nm, 71.7 mV/cm2, 37.5 and 50 J/cm2, 9 and 12 min CUR: 450 nm, 30 mV/cm2, 37.5 and 50 J/cm2, 21 and 27 min | Downregulation of biofilm-associated genes Downregulation of genes associated with oxidative stress defense |
| Ma et al. [96] | C. albicans | Aloe emodin | PIT: 30 min 400–780 nm, 80 mW/cm2, 2.4, 4.8, 14.4, and 24 J/cm2, 30, 60, 180, and 300 s PIT: 0, 10, 30 and 60 min 400–780 nm, 80 mW/cm2, 4.8 J/cm2, 1 min | Inhibitory effects on planktonic cells Structural damage of fungal cells |
| Mardani and Kamrani [97] | C. albicans | Indocyanine green | PIT: 30 min 810 nm, 300 mW, 228 J/cm2, 2 min | Antifungal activity Decreased biofilm formation |
| Alshehri et al. [98] | C. albicans | Riboflavin | PIT: 10 min 450 nm, 25 mW/cm2, 15 J/cm2, 10 min | Antifungal activity No deterioration of acrylic denture material |
| Garcia et al. [48] | C. albicans | Tetra-cationic porphyrins with peripheral platinum (II) and palladium (II) complexes | PIT: 5 to 80 s 420 nm, 1250 mW, 0.16 W/cm2, 0.79–12.78 J/cm2 | Activity against planktonic cells and biofilms Reduced fungal filamentation Increased in vivo survival in a burn wound model |
| Reference | Species | Photosensitizer | Irradiation Parameters | Main Results |
|---|---|---|---|---|
| Liu et al. [112] | Exophiala spinifera | 5-aminolevulinic acid (ALA) | Pre-irradiation time (PIT): 4 h 633 nm, 120 mW/cm2, 25 min | Clinical improvement Resolution of papules and nodules Mycological cure No significant adverse events |
| Lan et al. [113] | Trichosporon asahii | ALA | PIT: 1 h 635 ± 10 nm, 60 mW/cm2, 36, 72, and 108 J/cm2 4 h (in vivo) | Reduced planktonic cells Decrease adherence and biofilm formation in vitro Wound healing Mycological cure |
| Wang et al. [16] | Curvularia lunata | ALA | PIT: 4 h 635 nm, 80 mW/cm2, 30 min | Complete resolution of lesions No discomfort or serious adverse effects No recurrence |
| Chen et al. [7] | Trichosporon asahii | ALA | 4 h | Improvement of ulcerative lesions |
| Li et al. [114] | Sporothrix globosa | Methylene blue (MB) | PIT: 30 min 640 nm, 40 J/cm2, 30 min | Antifungal activity against conidia Synergism with itraconazole in vitro Reduction of lesions No systemic dissemination |
| Alberdi and Gomez [30] | Malassezia spp. | MB | PIT: 3 min 630 ± 5 nm, 37 J/cm2, 50 min | Complete cure No recurrence and adverse effects Good cosmetic outcome |
| Cui et al. [8] | Malassezia furfur | Haematoporphyrin monomethyl ether Aloe emodin | PIT: 30 min 400–780 nm, 40 mV/cm2, 72 J/cm2 (15 min) and 96 J/cm2 (20 min) | Antifungal activity Increased reactive oxygen species (ROS) levels Inhibition of enzymatic activity |
| Kodedová et al. [115] | Hortaea werneckii | 5,10,15,20-tetraphenylporphyrin associated with sulfonated polystyrene nanoparticles | 395–630 nm, 23 W | Antifungal activity Reduction of fungal viability Increased ROS levels |
| Arriba et al. [24] | Malassezia furfur | Methyl aminolevulinate | PIT: 0 to 120 min 570–1400 nm, 200 mW/cm2, 30 min to 3 h | Reduction of fungal viability Biocompatibility Anti-inflammatory effects |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, L.B.B.d.; Castilho, I.G.; Souza Silva, F.A.d.; Ghannoum, M.; Garcia, M.T.; Carmo, P.H.F.d. Antimicrobial Photodynamic Therapy for Superficial, Skin, and Mucosal Fungal Infections: An Update. Microorganisms 2025, 13, 1406. https://doi.org/10.3390/microorganisms13061406
Silva LBBd, Castilho IG, Souza Silva FAd, Ghannoum M, Garcia MT, Carmo PHFd. Antimicrobial Photodynamic Therapy for Superficial, Skin, and Mucosal Fungal Infections: An Update. Microorganisms. 2025; 13(6):1406. https://doi.org/10.3390/microorganisms13061406
Chicago/Turabian StyleSilva, Laura Beatriz Borim da, Ivana Giovannetti Castilho, Fabiana Alves de Souza Silva, Mahmoud Ghannoum, Maíra Terra Garcia, and Paulo Henrique Fonseca do Carmo. 2025. "Antimicrobial Photodynamic Therapy for Superficial, Skin, and Mucosal Fungal Infections: An Update" Microorganisms 13, no. 6: 1406. https://doi.org/10.3390/microorganisms13061406
APA StyleSilva, L. B. B. d., Castilho, I. G., Souza Silva, F. A. d., Ghannoum, M., Garcia, M. T., & Carmo, P. H. F. d. (2025). Antimicrobial Photodynamic Therapy for Superficial, Skin, and Mucosal Fungal Infections: An Update. Microorganisms, 13(6), 1406. https://doi.org/10.3390/microorganisms13061406








