Fluoroquinolone Residues in Piglet Viscera and Their Impact on Intestinal Microbiota Resistance: A One Health Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Escherichia coli and Enterococcus spp. Isolation
2.2. Identification of Enterococcus Species
2.3. Antimicrobial Susceptibility Assays
2.4. Quantification of Antimicrobial Residues
2.5. Statistical Analysis
3. Results
3.1. Occurrence of Fluoroquinolone Residues in Piglets’ Liver and Kidney
3.2. Escherichia coli and Antibiotic Resistance
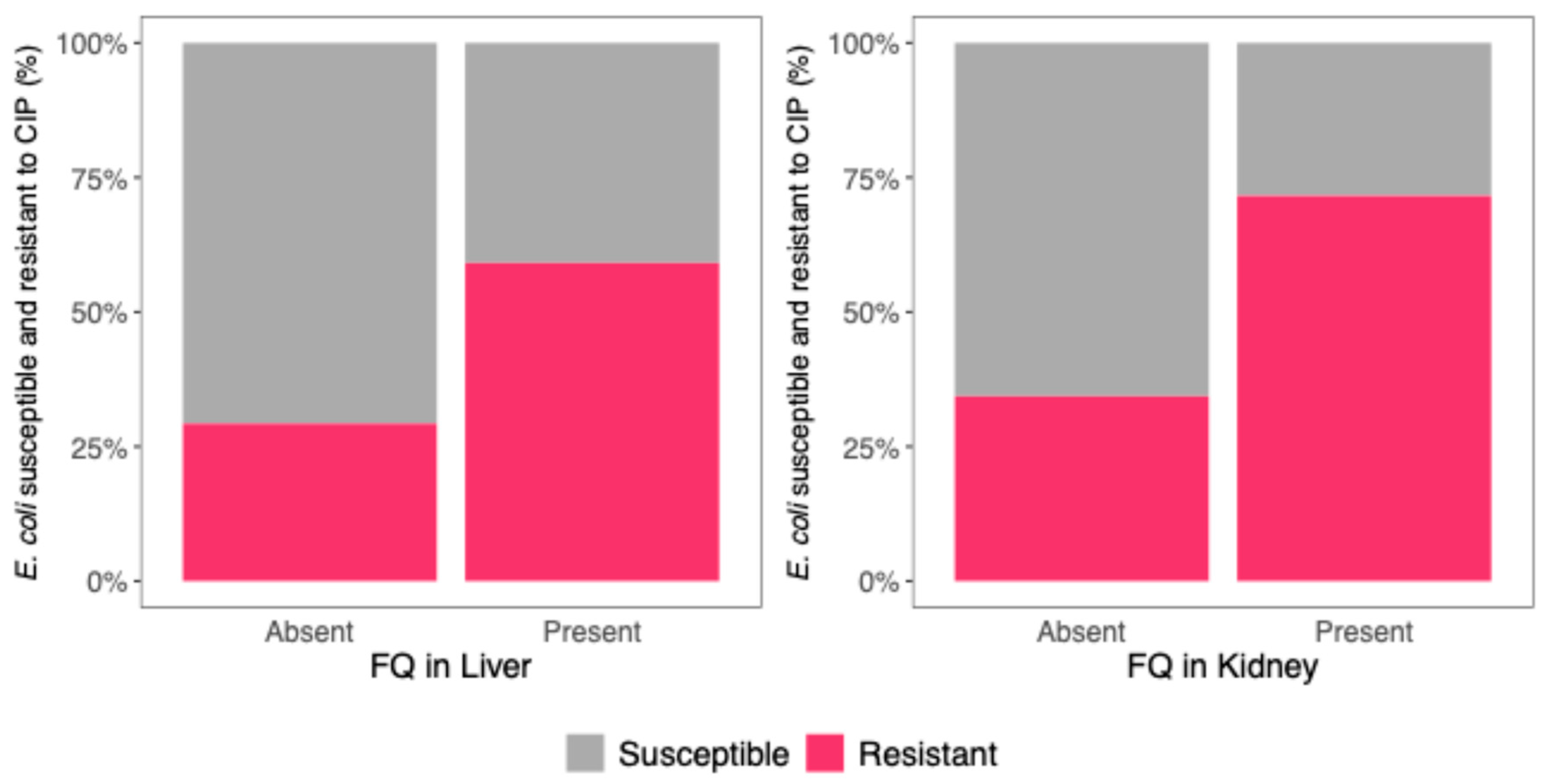
3.3. Association Between FQ Residues in Viscera and E. coli CIP Resistance
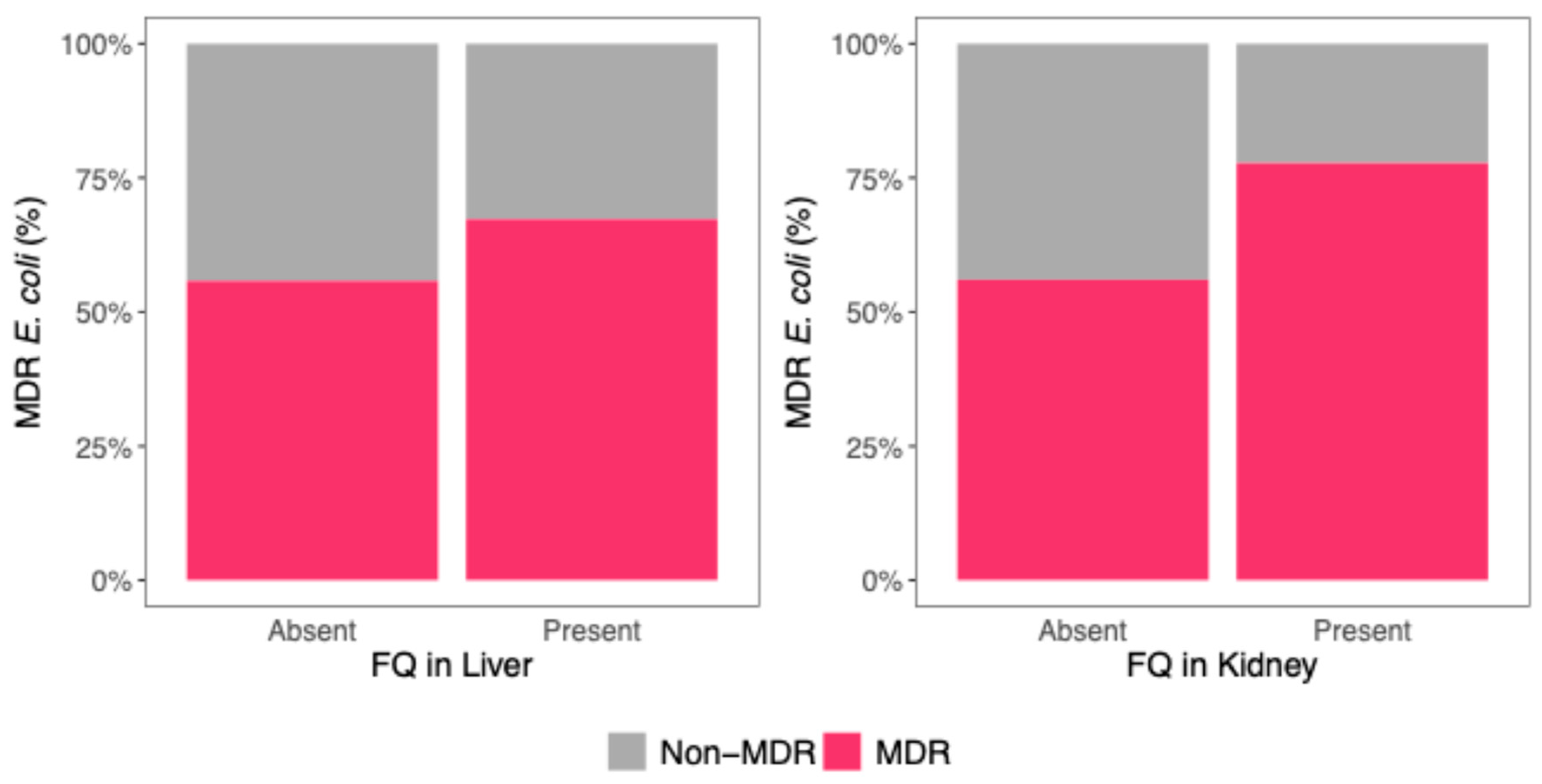
3.4. Association Between FQ Residues and Multidrug Resistance in E. coli
3.5. Enterococcus Species Identification and Antibiotic Resistance
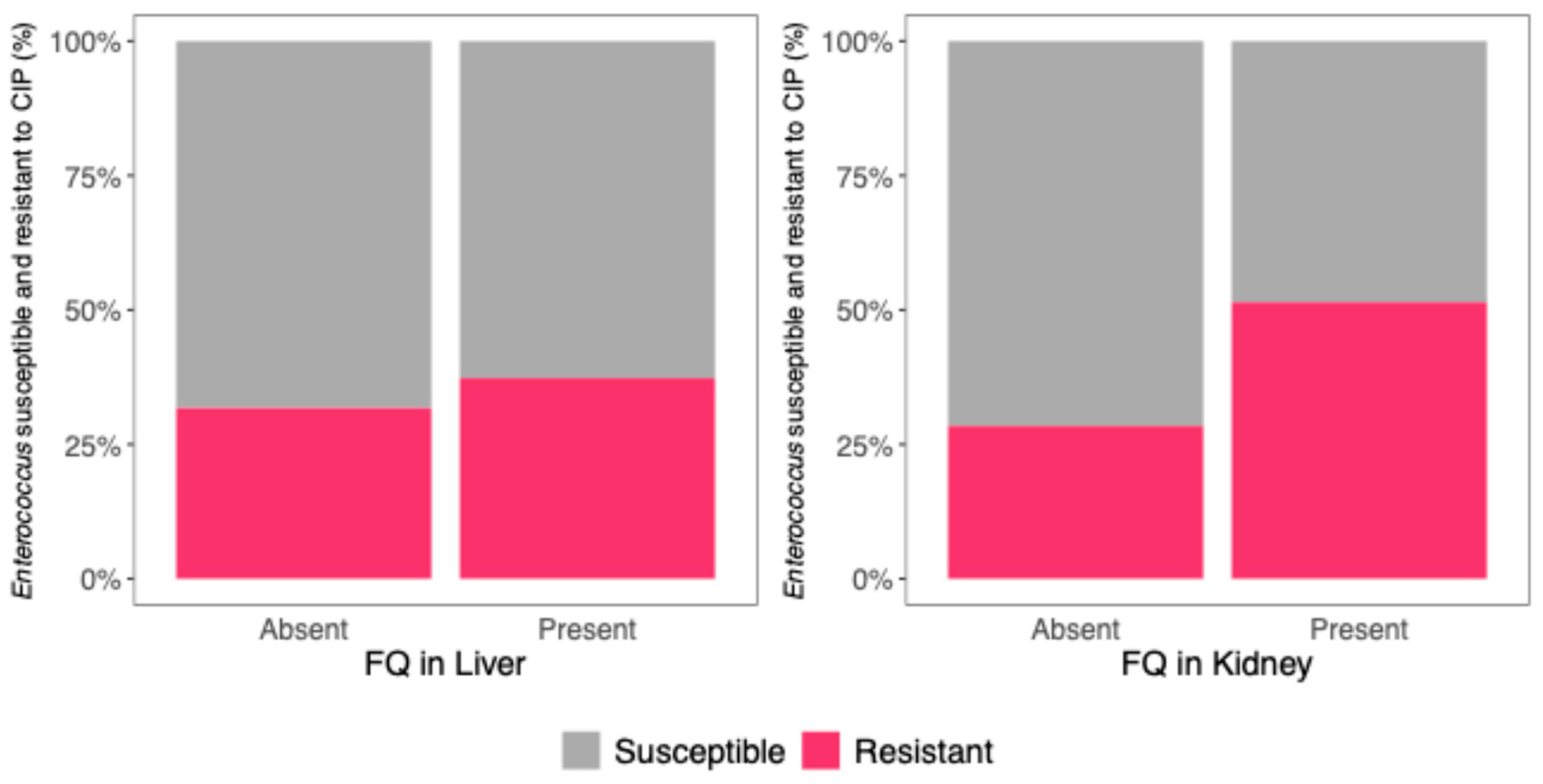
3.6. Association Between FQ Residues in Viscera and Enterococci CIP Resistance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- White, A.; Hughes, J.M. Critical Importance of a One Health Approach to Antimicrobial Resistance. Ecohealth 2019, 16, 404–409. [Google Scholar] [CrossRef]
- Yang, F.; Han, B.; Gu, Y.; Zhang, K. Swine Liquid Manure: A Hotspot of Mobile Genetic Elements and Antibiotic Resistance Genes. Sci. Rep. 2020, 10, 15037. [Google Scholar] [CrossRef] [PubMed]
- Hayer, S.S.; Casanova-Higes, A.; Paladino, E.; Elnekave, E.; Nault, A.; Johnson, T.; Bender, J.; Perez, A.; Alvarez, J. Global Distribution of Fluoroquinolone and Colistin Resistance and Associated Resistance Markers in Escherichia coli of Swine Origin—A Systematic Review and Meta-Analysis. Front. Microbiol. 2022, 13, 834793. [Google Scholar] [CrossRef]
- Fei, Z.; Song, S.; Yang, X.; Jiang, D.; Gao, J.; Yang, D. Occurrence and Risk Assessment of Fluoroquinolone Residues in Chicken and Pork in China. Antibiotics 2022, 11, 1292. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Technical specifications for a EU-wide baseline survey of antimicrobial resistance in bacteria from aquaculture animals. EFSA J. 2024, 22, e8928. [Google Scholar] [CrossRef]
- Jaleta, M.; Junker, V.; Kolte, B.; Börger, M.; Werner, D.; Dolsdorf, C.; Schwenker, J.; Hölzel, C.; Zentek, J.; Amon, T.; et al. Improvements of Weaned Pigs Barn Hygiene to Reduce the Spread of Antimicrobial Resistance. Front. Microbiol. 2024, 15, 1393923. [Google Scholar] [CrossRef]
- Zaheer, R.; Cook, S.R.; Barbieri, R.; Goji, N.; Cameron, A.; Petkau, A.; Polo, R.O.; Tymensen, L.; Stamm, C.; Song, J.; et al. Surveillance of Enterococcus spp. Reveals Distinct Species and Antimicrobial Resistance Diversity across a One-Health Continuum. Sci. Rep. 2020, 10, 3937. [Google Scholar] [CrossRef]
- Ladyhina, V.; Sternberg-Lewerin, S.; Andersson, L.; Rajala, E. Antimicrobial Resistance among Indicator Enterococcus faecium and Escherichia coli in Swedish Pig Farms. Acta Vet. Scand. 2024, 66, 34. [Google Scholar] [CrossRef] [PubMed]
- Rasschaert, G.; Van Elst, D.; Colson, L.; Herman, L.; de Carvalho Ferreira, H.C.; Dewulf, J.; Decrop, J.; Meirlaen, J.; Heyndrickx, M.; Daeseleire, E. Antibiotic Residues and Antibiotic-Resistant Bacteria in Pig Slurry Used to Fertilize Agricultural Fields. Antibiotics 2020, 9, 34. [Google Scholar] [CrossRef]
- Donato, M.M.; Assis, G.; Cardoso, O.; Oliveiros, B.; Freitas, A.; Ramos, F. Assessment of Zn and Cu in Piglets’ Liver and Kidney: Impact in Fecal Enterococcus spp.? Environ. Sci. Pollut. Res. 2024, 31, 20941–20952. [Google Scholar] [CrossRef]
- Igbinosa, E.O.; Beshiru, A. Antimicrobial Resistance, Virulence Determinants, and Biofilm Formation of Enterococcus Species from Ready-to-Eat Seafood. Front. Microbiol. 2019, 10, 728. [Google Scholar] [CrossRef] [PubMed]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters. Version 09.0. 2019. Available online: https://www.eucast.org (accessed on 9 May 2025).
- Gamboa-Cruz, C.; Barros, S.; Vila Pouca, A.S.; Barbosa, J.; Freitas, A.; Ramos, F. Assessing Antibiotic Residues in Piglet Liver and Kidney Samples: How to Manage the Results Obtained. Food Control 2021, 122, 107819. [Google Scholar] [CrossRef]
- Sodhi, K.K.; Singh, D.K. Insight into the Fluoroquinolone Resistance, Sources, Ecotoxicity, and Degradation with Special Emphasis on Ciprofloxacin. J. Water Process Eng. 2021, 43, 102218. [Google Scholar] [CrossRef]
- Hoelzer, K.; Wong, N.; Thomas, J.; Talkington, K.; Jungman, E.; Coukell, A. Antimicrobial Drug Use in Food-Producing Animals and Associated Human Health Risks: What, and How Strong, Is the Evidence? BMC Vet. Res. 2017, 13, 211. [Google Scholar] [CrossRef]
- Antimicrobial Resistance Surveillance in Europe 2023–2021 Data. Stockholm: European Centre for Disease Prevention and Control and World Health Organization; 2023. Cataloguing-in-Publication (CIP) Data. CIP Data. Available online: http://apps.who.int/iris (accessed on 9 May 2025).
- Aarestrup, F.M.; Duran, C.O.; Burch, D.G.S. Antimicrobial Resistance in Swine Production. Anim. Health Res. Rev. 2008, 9, 135–148. [Google Scholar] [CrossRef]
- Amsler, M.; Zurfluh, K.; Hartnack, S.; Sidler, X.; Stephan, R.; Kümmerlen, D. Occurrence of Escherichia coli Non-Susceptible to Quinolones in Faecal Samples from Fluoroquinolone-Treated, Contact and Control Pigs of Different Ages from 24 Swiss Pig Farms. Porc. Health Manag. 2021, 7, 29. [Google Scholar] [CrossRef]
- Cebeci, T. Species Prevalence, Virulence Genes, and Antibiotic Resistance of Enterococci from Food-Producing Animals at a Slaughterhouse in Turkey. Sci. Rep. 2024, 14, 13191. [Google Scholar] [CrossRef]
- Tang, K.L.; Caffrey, N.P.; Nóbrega, D.B.; Cork, S.C.; Ronksley, P.E.; Barkema, H.W.; Polachek, A.J.; Ganshorn, H.; Sharma, N.; Kellner, J.D.; et al. Restricting the Use of Antibiotics in Food-Producing Animals and Its Associations with Antibiotic Resistance in Food-Producing Animals and Human Beings: A Systematic Review and Meta-Analysis. Lancet Planet. Health 2017, 1, e316–e327. [Google Scholar] [CrossRef]
- Ardakani, Z.; Canali, M.; Aragrande, M.; Tomassone, L.; Simoes, M.; Balzani, A.; Beber, C.L. Evaluating the Contribution of Antimicrobial Use in Farmed Animals to Global Antimicrobial Resistance in Humans. One Health 2023, 17, 100647. [Google Scholar] [CrossRef]
- Li, X.; Hu, H.; Zhu, Y.; Wang, T.; Lu, Y.; Wang, X.; Peng, Z.; Sun, M.; Chen, H.; Zheng, J.; et al. Population Structure and Antibiotic Resistance of Swine Extraintestinal Pathogenic Escherichia coli from China. Nat. Commun. 2024, 15, 5811. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, C.; Yue, L.; Sun, Y.; Ding, H.; Liu, Y. Excretion of Enrofloxacin in Pigs and Its Effect on Ecological Environment. Environ. Toxicol. Pharmacol. 2008, 26, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Hammerum, A.M. Enterococci of Animal Origin and Their Significance for Public Health. Clin. Microbiol. Infect. 2012, 18, 619–625. [Google Scholar] [CrossRef]
- Hammerum, A.M.; Baig, S.; Kamel, Y.; Roer, L.; Pinholt, M.; Gumpert, H.; Holzknecht, B.; Røder, B.; Justesen, U.S.; Samulioniené, J.; et al. Emergence of VanA Enterococcus faecium in Denmark, 2005–2015. J. Antimicrob. Chemother. 2017, 72, 2184–2190. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Moon, D.C.; Kim, S.J.; Mechesso, A.F.; Song, H.J.; Kang, H.Y.; Choi, J.H.; Yoon, S.S.; Lim, S.K. Nationwide Surveillance on Antimicrobial Resistance Profiles of Enterococcus faecium and Enterococcus faecalis Isolated from Healthy Food Animals in South Korea, 2010 to 2019. Microorganisms 2021, 9, 925. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.R.; Munita, J.M.; Arias, C.A. Mechanisms of Antibiotic Resistance in Enterococci. Expert Rev. Anti-Infect. Ther. 2014, 12, 1221–1236. [Google Scholar] [CrossRef]
- de Jong, A.; Simjee, S.; El Garch, F.; Moyaert, H.; Rose, M.; Youala, M.; Dry, M. Antimicrobial Susceptibility of Enterococci Recovered from Healthy Cattle, Pigs and Chickens in Nine EU Countries (EASSA Study) to Critically Important Antibiotics. Vet. Microbiol. 2018, 216, 168–175. [Google Scholar] [CrossRef]
| Species | Primer Sequence | PCR Conditions | Amplicon Size (bp) | Reference |
|---|---|---|---|---|
| Enterococcus spp. | TCA ACC GGG GAG GGT | 95 °C, 10 s; 60 °C, 5 s; 72 °C, 29 s | 733 | [11] |
| ATT ACT AGC GAT TCC GG | ||||
| E. faecalis | ATC AAG TAC AGT TAG TCT | 95 °C, 10 s; 44 °C, 5 s; 72 °C, 38 s | 941 | [11] |
| ACG ATT CAA AGC TAA CTG | ||||
| E. faecium | TTG AGG CAG ACC AGA TTG ACG | 95 °C, 10 s; 55 °C, 5 s; 72 °C, 26 s | 658 | [11] |
| TAT GAC AGC GAC TCC GAT TCC |
| Antibiotic | Total |
|---|---|
| Amikacin (AMK) | 115 (33.8%) |
| Ciprofloxacin (CIP) | 147 (43.2%) |
| Sulfamethoxazole-Trimethoprim (SXT) | 197 (57.9%) |
| Aztreonam (AZT) | 64 (18.8%) |
| Cefoxitin (FOX) | 42 (12.4%) |
| Cefepime (FEP) | 59 (17.4%) |
| Ceftazidime (CAZ) | 39 (11.5%) |
| Amoxicillin + Clavulanic Acid (AMC) | 228 (67.1%) |
| Piperacillin (PIP) | 229 (67.4%) |
| Imipenem (IP) | 9 (2.6%) |
| FQ Residues | ||
|---|---|---|
| Kidney OR (95% CI), p-Value | Liver OR (95% CI, p-Value) | |
| E. coli resistance to CIP | 2.94 (1.57–5.50), p = 0.0013 | 2.07 (1.20–3.57), p = 0.0093 |
| MDR E. coli | 2.70 (1.37–5.47), p = 0.0047 | 1.03 (0.60–1.76), p = 0.930 |
| Enterococcus resistance to CIP | 3.78 (1.34–11.60), p = 0.015 | 0.60 (0.21–1.57), p = 0.316 |
| Antibiotic | E. faecalis (n = 40) | E. faecium (n = 45) | Enterococcus spp. (n = 45) | Total (n = 130) |
|---|---|---|---|---|
| Vancomycin (VAN) | 1 | 1 | 3 | 5 |
| Ciprofloxacin (CIP) | 18 | 7 | 20 | 45 |
| Linezolid (LNZ) | 0 | 1 | 1 | 2 |
| Tigecycline (TIG) | 0 | 0 | 0 | 0 |
| Ampicillin (AMP) | 1 | 2 | 1 | 4 |
| Imipenem (IP) | 6 | 29 | 6 | 41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardoso, O.; Donato, M.M.; Henriques, S.C.; Ramos, F. Fluoroquinolone Residues in Piglet Viscera and Their Impact on Intestinal Microbiota Resistance: A One Health Approach. Microorganisms 2025, 13, 1389. https://doi.org/10.3390/microorganisms13061389
Cardoso O, Donato MM, Henriques SC, Ramos F. Fluoroquinolone Residues in Piglet Viscera and Their Impact on Intestinal Microbiota Resistance: A One Health Approach. Microorganisms. 2025; 13(6):1389. https://doi.org/10.3390/microorganisms13061389
Chicago/Turabian StyleCardoso, Olga, Maria Manuel Donato, Sara Carolina Henriques, and Fernando Ramos. 2025. "Fluoroquinolone Residues in Piglet Viscera and Their Impact on Intestinal Microbiota Resistance: A One Health Approach" Microorganisms 13, no. 6: 1389. https://doi.org/10.3390/microorganisms13061389
APA StyleCardoso, O., Donato, M. M., Henriques, S. C., & Ramos, F. (2025). Fluoroquinolone Residues in Piglet Viscera and Their Impact on Intestinal Microbiota Resistance: A One Health Approach. Microorganisms, 13(6), 1389. https://doi.org/10.3390/microorganisms13061389








