The Intersection of SARS-CoV-2 and Diabetes
Abstract
1. COVID-19
2. Diabetes Has Emerged as an Important Comorbidity That Markedly Increases the Severity of COVID-19
2.1. Meta-Analysis of Diabetes Representation Among Persons with β-CoVs (Epidemiology)
2.2. Why Diabetes Contributes to Worse Outcomes
2.3. Clinical Findings and Indicators Associated with Worse Outcomes (Pathogenesis)
3. The Incidence of Diabetes Is Higher in Individuals After COVID-19
3.1. Potential Mechanisms Proposed for New-Onset Diabetes Post-COVID-19
3.2. SARS-CoV-2 Targets Mitochondrial Function
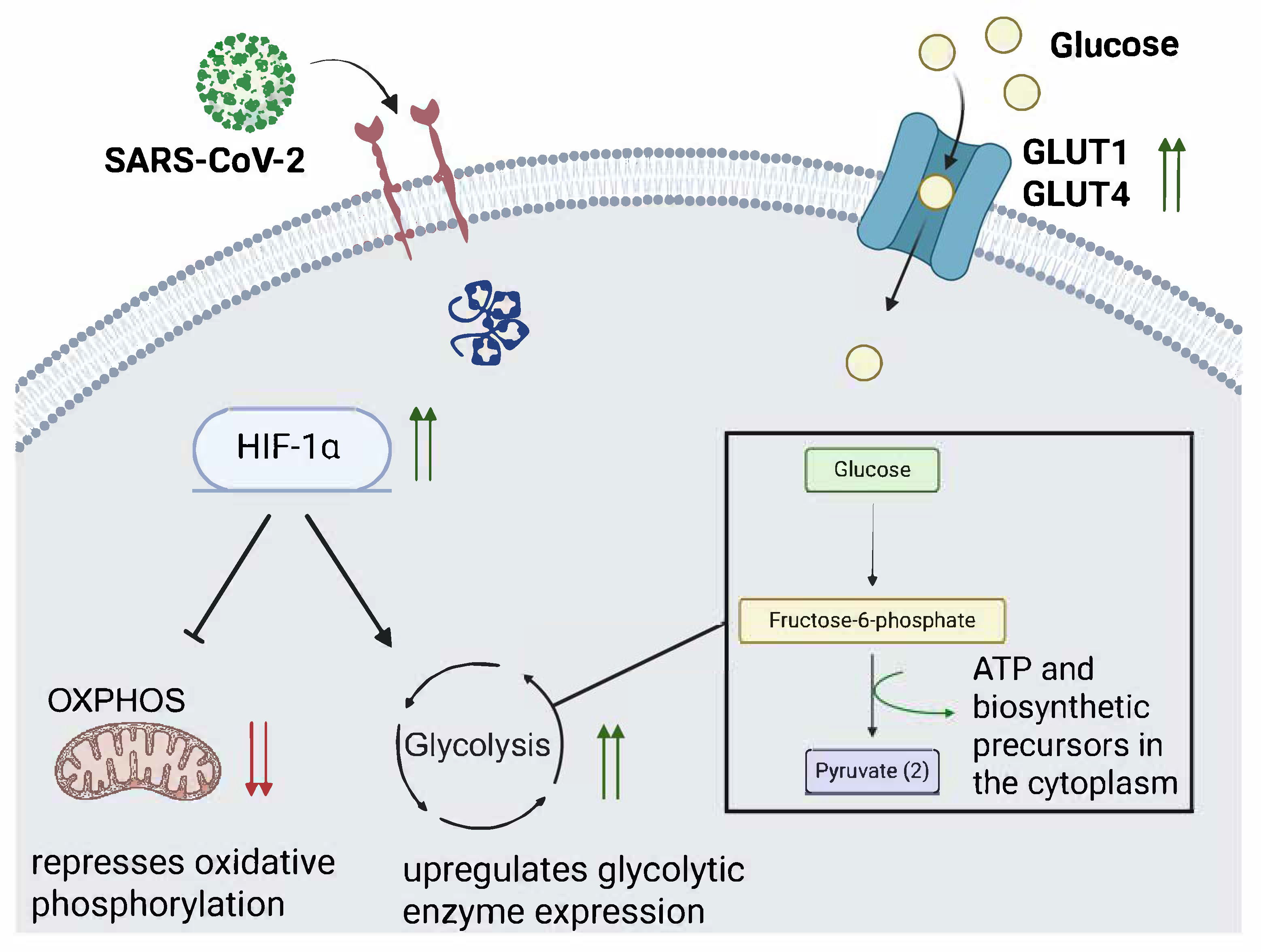
3.3. Disruption of Glucometabolic Control and Promotion of Glycolysis After SARS-CoV-2 Infection
3.4. COVID-19 Can Cause Direct and Indirect Damage to the Pancreas
3.5. COVID-19 Vaccination Has Been Speculated to Induce Latent Autoimmune Diabetes in Adults (LADA)
4. In Vivo Models Used to Study SARS-CoV-2 Infection in a Diabetic Host
4.1. Db/Db (Leptin Deficient) Mouse Model
4.2. HFD Mouse Model
4.3. Streptozotocin (STZ) Mouse Model
5. In Vitro Models of SARS-CoV-2 Infection in Diabetic Conditions
5.1. Continuous Cell Lines
5.2. Primary Kidney and Brain Cell Lines
5.3. Kidney Organoid
6. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Hui, D.S.; I Azhar, E.; Madani, T.A.; Ntoumi, F.; Kock, R.; Dar, O.; Ippolito, G.; McHugh, T.D.; Memish, Z.A.; Drosten, C.; et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health 2014; The latest 2019 novel coronavirus outbreak in Wuhan, China. Int. J. Infect. Dis. 2020, 91, 264–266. [Google Scholar] [CrossRef] [PubMed]
- de Wit, E.; van Doremalen, N.; Falzarano, D.; Munster, V.J. SARS and MERS: Recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 2016, 14, 523–534. [Google Scholar] [CrossRef]
- Lam, W.; Zhong, N.; Tan, W. Overview on SARS in Asia and the World. Respirology 2003, 8, S2–S5. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.Y.; Kang, J.-M.; Ha, Y.E.; Park, G.E.; Lee, J.Y.; Ko, J.-H.; Lee, J.Y.; Kim, J.M.; Kang, C.-I.; Jo, I.J.; et al. MERS-CoV outbreak following a single patient exposure in an emergency room in South Korea: An epidemiological outbreak study. Lancet 2016, 388, 994–1001. [Google Scholar] [CrossRef]
- Ramadan, N.; Shaib, H. Middle East respiratory syndrome coronavirus (MERS-CoV): A review. Germs 2019, 9, 35–42. [Google Scholar] [CrossRef]
- Li, W.; Moore, M.J.; Vasilieva, N.; Sui, J.; Wong, S.K.; Berne, M.A.; Somasundaran, M.; Sullivan, J.L.; Luzuriaga, K.; Greenough, T.C.; et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003, 426, 450–454. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Wu, L.; Niu, S.; Song, C.; Zhang, Z.; Lu, G.; Qiao, C.; Hu, Y.; Yuen, K.-Y.; et al. Structural and Functional Basis of SARS-CoV-2 Entry by Using Human ACE2. Cell 2020, 181, 894–904.e899. [Google Scholar] [CrossRef]
- Li, M.-Y.; Li, L.; Zhang, Y.; Wang, X.-S. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect. Dis. Poverty 2020, 9, 45. [Google Scholar] [CrossRef]
- Barreto, E.A.; Cruz, A.S.; Veras, F.P.; Martins, R.; Bernardelli, R.S.; Paiva, I.M.; Lima, T.M.; Singh, Y.; Guimarães, R.C.; Damasceno, S.; et al. COVID-19-related hyperglycemia is associated with infection of hepatocytes and stimulation of gluconeogenesis. Proc. Natl. Acad. Sci. USA 2023, 120, e2217119120. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Tao, W.; Flavell, R.A.; Zhu, S. Potential intestinal infection and faecal–oral transmission of SARS-CoV-2. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 269–283. [Google Scholar] [CrossRef]
- Stein, S.R.; Ramelli, S.C.; Grazioli, A.; Chung, J.-Y.; Singh, M.; Yinda, C.K.; Winkler, C.W.; Sun, J.; Dickey, J.M.; Ylaya, K.; et al. SARS-CoV-2 infection and persistence in the human body and brain at autopsy. Nature 2022, 612, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Qadir, M.M.F.; Bhondeley, M.; Beatty, W.; Gaupp, D.D.; Doyle-Meyers, L.A.; Fischer, T.; Bandyopadhyay, I.; Blair, R.V.; Bohm, R.; Rappaport, J.; et al. SARS-CoV-2 infection of the pancreas promotes thrombofibrosis and is associated with new-onset diabetes. JCI Insight 2021, 6, e151551. [Google Scholar] [CrossRef]
- Poloni, T.E.; Moretti, M.; Medici, V.; Turturici, E.; Belli, G.; Cavriani, E.; Visonà, S.D.; Rossi, M.; Fantini, V.; Ferrari, R.R.; et al. COVID-19 Pathology in the Lung, Kidney, Heart and Brain: The Different Roles of T-Cells, Macrophages, and Microthrombosis. Cells 2022, 11, 3124. [Google Scholar] [CrossRef]
- Menter, T.; Haslbauer, J.D.; Nienhold, R.; Savic, S.; Hopfer, H.; Deigendesch, N.; Frank, S.; Turek, D.; Willi, N.; Pargger, H.; et al. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology 2020, 77, 198–209. [Google Scholar] [CrossRef]
- Baj, J.; Karakuła-Juchnowicz, H.; Teresiński, G.; Buszewicz, G.; Ciesielka, M.; Sitarz, R.; Forma, A.; Karakuła, K.; Flieger, W.; Portincasa, P.; et al. COVID-19: Specific and Non-Specific Clinical Manifestations and Symptoms: The Current State of Knowledge. J. Clin. Med. 2020, 9, 1753. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef]
- Sutanto, H.; Soegiarto, G. Risk of Thrombosis during and after a SARS-CoV-2 Infection: Pathogenesis, Diagnostic Approach, and Management. Hematol. Rep. 2023, 15, 225–243. [Google Scholar] [CrossRef]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef]
- Solomon, T. Neurological infection with SARS-CoV-2—The story so far. Nat. Rev. Neurol. 2021, 17, 65–66. [Google Scholar] [CrossRef] [PubMed]
- Yong, S.J. Persistent Brainstem Dysfunction in Long-COVID: A Hypothesis. ACS Chem. Neurosci. 2021, 12, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Li, M.; Dong, Y.; Zhou, H.; Zhang, Z.; Tian, C.; Qin, R.; Wang, H.; Shen, Y.; Du, K.; et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes/Metab. Res. Rev. 2020, 36, e3319. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Gupta, R.; Ghosh, A.; Misra, A. Diabetes in COVID-19: Prevalence, pathophysiology, prognosis and practical considerations. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 303–310. [Google Scholar] [CrossRef]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; The Northwell COVID-19 Research Consortium. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef]
- Barron, E.; Bakhai, C.; Kar, P.; Weaver, A.; Bradley, D.; Ismail, H.; Knighton, P.; Holman, N.; Khunti, K.; Sattar, N.; et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: A whole-population study. Lancet Diabetes Endocrinol. 2020, 8, 813–822. [Google Scholar] [CrossRef]
- van den Berg, J.M.; Remmelzwaal, S.; Blom, M.T.; van Hoek, B.A.C.E.; Swart, K.M.A.; Overbeek, J.A.; Burchell, G.L.; Herings, R.M.C.; Elders, P.J.M. Effectiveness of COVID-19 Vaccines in Adults with Diabetes Mellitus: A Systematic Review. Vaccines 2023, 11, 24. [Google Scholar] [CrossRef]
- Sourij, C.; Tripolt, N.J.; Aziz, F.; Aberer, F.; Forstner, P.; Obermayer, A.M.; Kojzar, H.; Kleinhappl, B.; Pferschy, P.N.; Mader, J.K.; et al. Humoral immune response to COVID-19 vaccination in diabetes is age-dependent but independent of type of diabetes and glycaemic control: The prospective COVAC-DM cohort study. Diabetes Obes. Metab. 2022, 24, 849–858. [Google Scholar] [CrossRef]
- Sardu, C.; Gargiulo, G.; Esposito, G.; Paolisso, G.; Marfella, R. Impact of diabetes mellitus on clinical outcomes in patients affected by COVID-19. Cardiovasc. Diabetol. 2020, 19, 76. [Google Scholar] [CrossRef]
- Rawshani, A.; Kjölhede, E.A.; Rawshani, A.; Sattar, N.; Eeg-Olofsson, K.; Adiels, M.; Ludvigsson, J.; Lindh, M.; Gisslén, M.; Hagberg, E.; et al. Severe COVID-19 in people with type 1 and type 2 diabetes in Sweden: A nationwide retrospective cohort study. Lancet Reg. Health Eur. 2021, 4, 100105. [Google Scholar] [CrossRef]
- Wong, R.; Hall, M.; Vaddavalli, R.; Anand, A.; Arora, N.; Bramante, C.T.; Garcia, V.; Johnson, S.; Saltz, M.; Tronieri, J.S.; et al. Glycemic Control and Clinical Outcomes in U.S. Patients With COVID-19: Data From the National COVID Cohort Collaborative (N3C) Database. Diabetes Care 2022, 45, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, T.; Chubachi, S.; Namkoong, H.; Asakura, T.; Tanaka, H.; Lee, H.; Azekawa, S.; Okada, Y.; Koike, R.; Kimura, A.; et al. Clinical significance of prediabetes, undiagnosed diabetes and diagnosed diabetes on critical outcomes in COVID-19: Integrative analysis from the Japan COVID-19 task force. Diabetes Obes. Metab. 2023, 25, 144–155. [Google Scholar] [CrossRef]
- D’Onofrio, N.; Scisciola, L.; Sardu, C.; Trotta, M.C.; De Feo, M.; Maiello, C.; Mascolo, P.; De Micco, F.; Turriziani, F.; Municinò, E.; et al. Glycated ACE2 receptor in diabetes: Open door for SARS-COV-2 entry in cardiomyocyte. Cardiovasc. Diabetol. 2021, 20, 99. [Google Scholar] [CrossRef]
- Zhang, T.; Mei, Q.; Zhang, Z.; Walline, J.H.; Liu, Y.; Zhu, H.; Zhang, S. Risk for newly diagnosed diabetes after COVID-19: A systematic review and meta-analysis. BMC Med. 2022, 20, 444. [Google Scholar] [CrossRef] [PubMed]
- Kwan, A.C.; Ebinger, J.E.; Botting, P.; Navarrette, J.; Claggett, B.; Cheng, S. Association of COVID-19 Vaccination With Risk for Incident Diabetes After COVID-19 Infection. JAMA Netw. Open 2023, 6, e2255965. [Google Scholar] [CrossRef]
- Montefusco, L.; Ben Nasr, M.; D’Addio, F.; Loretelli, C.; Rossi, A.; Pastore, I.; Daniele, G.; Abdelsalam, A.; Maestroni, A.; Dell’Acqua, M.; et al. Acute and long-term disruption of glycometabolic control after SARS-CoV-2 infection. Nat. Metab. 2021, 3, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.G.; Terebuh, P.; Kaelber, D.C.; Xu, R.; Davis, P.B. SARS-CoV-2 Infection and New-Onset Type 2 Diabetes Among Pediatric Patients, 2020 to 2022. JAMA Netw. Open 2024, 7, e2439444. [Google Scholar] [CrossRef]
- Bally, K.; Ji, B.; Soni, L. COVID-19 Vaccine-Induced Latent Autoimmune Diabetes in Adults. Cureus 2023, 15, e33762. [Google Scholar] [CrossRef]
- Unnikrishnan, R.; Pradeepa, R.; Joshi, S.R.; Mohan, V. Type 2 Diabetes: Demystifying the Global Epidemic. Diabetes 2017, 66, 1432–1442. [Google Scholar] [CrossRef]
- Abel, E.D.; Gloyn, A.L.; Evans-Molina, C.; Joseph, J.J.; Misra, S.; Pajvani, U.B.; Simcox, J.; Susztak, K.; Drucker, D.J. Diabetes mellitus2014;Progress and opportunities in the evolving epidemic. Cell 2024, 187, 3789–3820. [Google Scholar] [CrossRef]
- Murphy, S.L.K.; Kenneth, D.; Xu, J.; Arias, E. Mortality in the United States, 2023; National Center Health Statistics: Hyattsville, MA, USA, 2024; pp. 1–13. [Google Scholar]
- Gwira, J.A.; Fryar, C.D.; Gu, Q. Prevalence of Total, Diagnosed, and Undiagnosed Diabetes in Adults: United States, August 2021–August 2023; NCHS Data Brief: Hyattsville, MD, USA, 2024. [Google Scholar]
- The Diabetes Control and Complications Trial Research Group. The Effect of Intensive Treatment of Diabetes on the Development and Progression of Long-Term Complications in Insulin-Dependent Diabetes Mellitus. N. Engl. J. Med. 1993, 329, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Laakso, M. Hyperglycemia and cardiovascular disease in type 2 diabetes. Diabetes 1999, 48, 937–942. [Google Scholar] [CrossRef]
- Giri, B.; Dey, S.; Das, T.; Sarkar, M.; Banerjee, J.; Dash, S.K. Chronic hyperglycemia mediated physiological alteration and metabolic distortion leads to organ dysfunction, infection, cancer progression and other pathophysiological consequences: An update on glucose toxicity. Biomed. Pharmacother. 2018, 107, 306–328. [Google Scholar] [CrossRef] [PubMed]
- Berbudi, A.; Rahmadika, N.; Tjahjadi, A.I.; Ruslami, R. Type 2 Diabetes and its Impact on the Immune System. Curr. Diabetes Rev. 2020, 16, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Kulcsar, K.A.; Coleman, C.M.; Beck, S.E.; Frieman, M.B. Comorbid diabetes results in immune dysregulation and enhanced disease severity following MERS-CoV infection. JCI Insight 2019, 4, e131774. [Google Scholar] [CrossRef]
- Chan, J.W.M.; Ng, C.K.; Chan, Y.H.; Mok, T.Y.W.; Lee, S.; Chu, S.Y.Y.; Law, W.L.; Lee, M.P.; Li, P.C.K. Short term outcome and risk factors for adverse clinical outcomes in adults with severe acute respiratory syndrome (SARS). Thorax 2003, 58, 686–689. [Google Scholar] [CrossRef]
- Yang, J.K.; Feng, Y.; Yuan, M.Y.; Yuan, S.Y.; Fu, H.J.; Wu, B.Y.; Sun, G.Z.; Yang, G.R.; Zhang, X.L.; Wang, L.; et al. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet. Med. 2006, 23, 623–628. [Google Scholar] [CrossRef]
- Yang, J.K.; Lin, S.S.; Ji, X.J.; Guo, L.M. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010, 47, 193–199. [Google Scholar] [CrossRef]
- Alraddadi, B.M.; Watson, J.T.; Almarashi, A.; Abedi, G.R.; Turkistani, A.; Sadran, M.; Housa, A.; Almazroa, M.A.; Alraihan, N.; Banjar, A.; et al. Risk Factors for Primary Middle East Respiratory Syndrome Coronavirus Illness in Humans, Saudi Arabia, 2014. Emerg. Infect. Dis. 2016, 22, 49–55. [Google Scholar] [CrossRef]
- Alqahtani, F.Y.; Aleanizy, F.S.; Ali El Hadi Mohamed, R.; Alanazi, M.S.; Mohamed, N.; Alrasheed, M.M.; Abanmy, N.; Alhawassi, T. Prevalence of comorbidities in cases of Middle East respiratory syndrome coronavirus: A retrospective study. Epidemiol. Infect. 2018, 147, e35. [Google Scholar] [CrossRef]
- O’Meara, T.R.; Nanishi, E.; McGrath, M.E.; Barman, S.; Dong, D.; Dillen, C.; Menon, M.; Seo, H.-S.; Dhe-Paganon, S.; Ernst, R.K.; et al. Reduced SARS-CoV-2 mRNA vaccine immunogenicity and protection in mice with diet-induced obesity and insulin resistance. J. Allergy Clin. Immunol. 2023, 152, 1107–1120.e1106. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Berg, A.H.; Iyengar, P.; Lam, T.K.T.; Giacca, A.; Combs, T.P.; Rajala, M.W.; Du, X.; Rollman, B.; Li, W.; et al. The Hyperglycemia-induced Inflammatory Response in Adipocytes: The role of reactive oxyegen species. J. Biol. Chem. 2005, 280, 4617–4626. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.B. The complex role of adipokines in obesity, inflammation, and autoimmunity. Clin. Sci. 2021, 135, 731–752. [Google Scholar] [CrossRef]
- Leal, V.d.O.; Mafra, D. Adipokines in obesity. Clin. Chim. Acta 2013, 419, 87–94. [Google Scholar] [CrossRef]
- Zanza, C.; Romenskaya, T.; Manetti, A.C.; Franceschi, F.; La Russa, R.; Bertozzi, G.; Maiese, A.; Savioli, G.; Volonnino, G.; Longhitano, Y. Cytokine Storm in COVID-19: Immunopathogenesis and Therapy. Medicina 2022, 58, 144. [Google Scholar] [CrossRef]
- Angriman, F.; Ferreyro, B.L.; Burry, L.; Fan, E.; Ferguson, N.D.; Husain, S.; Keshavjee, S.H.; Lupia, E.; Munshi, L.; Renzi, S.; et al. Interleukin-6 receptor blockade in patients with COVID-19: Placing clinical trials into context. Lancet Respir. Med. 2021, 9, 655–664. [Google Scholar] [CrossRef]
- Rask-Madsen, C.; King, G.L. Vascular complications of diabetes: Mechanisms of injury and protective factors. Cell. Metab. 2013, 17, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Y.; Liu, S.; Gao, M.; Wang, W.; Chen, K.; Huang, L.; Liu, Y. Diabetic vascular diseases: Molecular mechanisms and therapeutic strategies. Signal Transduct. Target. Ther. 2023, 8, 152. [Google Scholar] [CrossRef]
- Mameli, C.; Ghezzi, M.; Mari, A.; Cammi, G.; Macedoni, M.; Redaelli, F.C.; Calcaterra, V.; Zuccotti, G.; D’Auria, E. The Diabetic Lung: Insights into Pulmonary Changes in Children and Adolescents with Type 1 Diabetes. Metabolites 2021, 11, 69. [Google Scholar] [CrossRef]
- Sanchez, E.L.; Lagunoff, M. Viral activation of cellular metabolism. Virology 2015, 479–480, 609–618. [Google Scholar] [CrossRef]
- Smallwood, H.S.; Duan, S.; Morfouace, M.; Rezinciuc, S.; Shulkin, B.L.; Shelat, A.; Zink, E.E.; Milasta, S.; Bajracharya, R.; Oluwaseum, A.J.; et al. Targeting Metabolic Reprogramming by Influenza Infection for Therapeutic Intervention. Cell Rep. 2017, 19, 1640–1653. [Google Scholar] [CrossRef] [PubMed]
- Kohio, H.P.; Adamson, A.L. Glycolytic control of vacuolar-type ATPase activity: A mechanism to regulate influenza viral infection. Virology 2013, 444, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Thaker, S.K.; Chapa, T.; Garcia, G., Jr.; Gong, D.; Schmid, E.W.; Arumugaswami, V.; Sun, R.; Christofk, H.R. Differential Metabolic Reprogramming by Zika Virus Promotes Cell Death in Human versus Mosquito Cells. Cell Metab. 2019, 29, 1206–1216.e1204. [Google Scholar] [CrossRef]
- Fontaine, K.A.; Sanchez, E.L.; Camarda, R.; Lagunoff, M. Dengue Virus Induces and Requires Glycolysis for Optimal Replication. J. Virol. 2015, 89, 2358–2366. [Google Scholar] [CrossRef] [PubMed]
- Munger, J.; Bennett, B.D.; Parikh, A.; Feng, X.-J.; McArdle, J.; Rabitz, H.A.; Shenk, T.; Rabinowitz, J.D. Systems-level metabolic flux profiling identifies fatty acid synthesis as a target for antiviral therapy. Nat. Biotechnol. 2008, 26, 1179–1186. [Google Scholar] [CrossRef]
- Pouysségur, J.; Marchiq, I.; Parks, S.K.; Durivault, J.; Ždralević, M.; Vucetic, M. ‘Warburg effect’ controls tumor growth, bacterial, viral infections and immunity—Genetic deconstruction and therapeutic perspectives. Semin. Cancer Biol. 2022, 86, 334–346. [Google Scholar] [CrossRef]
- Thaker, S.K.; Ch’ng, J.; Christofk, H.R. Viral hijacking of cellular metabolism. BMC Biol. 2019, 17, 59. [Google Scholar] [CrossRef]
- López-Ayllón, B.D.; Marin, S.; Fernández, M.F.; García-García, T.; Fernández-Rodríguez, R.; de Lucas-Rius, A.; Redondo, N.; Mendoza-García, L.; Foguet, C.; Grigas, J.; et al. Metabolic and mitochondria alterations induced by SARS-CoV-2 accessory proteins ORF3a, ORF9b, ORF9c and ORF10. J. Med. Virol. 2024, 96, e29752. [Google Scholar] [CrossRef]
- Miller, B.; Silverstein, A.; Flores, M.; Cao, K.; Kumagai, H.; Mehta, H.H.; Yen, K.; Kim, S.-J.; Cohen, P. Host mitochondrial transcriptome response to SARS-CoV-2 in multiple cell models and clinical samples. Sci. Rep. 2021, 11, 3. [Google Scholar] [CrossRef]
- Guarnieri, J.W.; Dybas, J.M.; Fazelinia, H.; Kim, M.S.; Frere, J.; Zhang, Y.; Soto Albrecht, Y.; Murdock, D.G.; Angelin, A.; Singh, L.N.; et al. Core mitochondrial genes are down-regulated during SARS-CoV-2 infection of rodent and human hosts. Sci. Transl. Med. 2023, 15, eabq1533. [Google Scholar] [CrossRef]
- Rochowski, M.T.; Jayathilake, K.; Balcerak, J.-M.; Selvan, M.T.; Gunasekara, S.; Miller, C.; Rudd, J.M.; Lacombe, V.A. Impact of Delta SARS-CoV-2 Infection on Glucose Metabolism: Insights on Host Metabolism and Virus Crosstalk in a Feline Model. Viruses 2024, 16, 295. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guo, R.; Kim, S.H.; Shah, H.; Zhang, S.; Liang, J.H.; Fang, Y.; Gentili, M.; Leary, C.N.O.; Elledge, S.J.; et al. SARS-CoV-2 hijacks folate and one-carbon metabolism for viral replication. Nat. Commun. 2021, 12, 1676. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Liu, W.; Li, X.; Zhao, P.; Shereen, M.A.; Zhu, C.; Huang, S.; Liu, S.; Yu, X.; Yue, M.; et al. HIF-1α promotes SARS-CoV-2 infection and aggravates inflammatory responses to COVID-19. Signal Transduct. Target. Ther. 2021, 6, 308. [Google Scholar] [CrossRef]
- Morris, D.R.; Qu, Y.; Agrawal, A.; Garofalo, R.P.; Casola, A. HIF-1α Modulates Core Metabolism and Virus Replication in Primary Airway Epithelial Cells Infected with Respiratory Syncytial Virus. Viruses 2020, 12, 1088. [Google Scholar] [CrossRef] [PubMed]
- Codo, A.C.; Davanzo, G.G.; Monteiro, L.d.B.; de Souza, G.F.; Muraro, S.P.; Virgilio-da-Silva, J.V.; Prodonoff, J.S.; Carregari, V.C.; de Biagi Junior, C.A.O.; Crunfli, F.; et al. Elevated Glucose Levels Favor SARS-CoV-2 Infection and Monocyte Response through a HIF-1α/Glycolysis-Dependent Axis. Cell Metab. 2020, 32, 437–446.e435. [Google Scholar] [CrossRef]
- Zhou, Y.; Fu, B.; Zheng, X.; Wang, D.; Zhao, C.; Qi, Y.; Sun, R.; Tian, Z.; Xu, X.; Wei, H. Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. Natl. Sci. Rev. 2020, 7, 998–1002. [Google Scholar] [CrossRef]
- Park, J.; Dean, L.S.; Jiyarom, B.; Gangcuangco, L.M.; Shah, P.; Awamura, T.; Ching, L.L.; Nerurkar, V.R.; Chow, D.C.; Igno, F.; et al. Elevated circulating monocytes and monocyte activation in COVID-19 convalescent individuals. Front. Immunol. 2023, 14, 1151780. [Google Scholar] [CrossRef]
- Duan, X.; Tang, X.; Nair, M.S.; Zhang, T.; Qiu, Y.; Zhang, W.; Wang, P.; Huang, Y.; Xiang, J.; Wang, H.; et al. An airway organoid-based screen identifies a role for the HIF1α-glycolysis axis in SARS-CoV-2 infection. Cell Rep. 2021, 37, 109920. [Google Scholar] [CrossRef]
- Deng, W.; Bao, L.; Song, Z.; Zhang, L.; Yu, P.; Xu, Y.; Wang, J.; Zhao, W.; Zhang, X.; Han, Y.; et al. Infection with SARS-CoV-2 can cause pancreatic impairment. Signal Transduct. Target. Ther. 2024, 9, 98. [Google Scholar] [CrossRef]
- Wu, C.-T.; Lidsky, P.V.; Xiao, Y.; Lee, I.T.; Cheng, R.; Nakayama, T.; Jiang, S.; Demeter, J.; Bevacqua, R.J.; Chang, C.A.; et al. SARS-CoV-2 infects human pancreatic β cells and elicits β cell impairment. Cell Metab. 2021, 33, 1565–1576.e1565. [Google Scholar] [CrossRef]
- Hollstein, T.; Schulte, D.M.; Schulz, J.; Glück, A.; Ziegler, A.G.; Bonifacio, E.; Wendorff, M.; Franke, A.; Schreiber, S.; Bornstein, S.R.; et al. Autoantibody-negative insulin-dependent diabetes mellitus after SARS-CoV-2 infection: A case report. Nat. Metab. 2020, 2, 1021–1024. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Calvo, T.; Sabouri, S.; Anquetil, F.; von Herrath, M.G. The viral paradigm in type 1 diabetes: Who are the main suspects? Autoimmun. Rev. 2016, 15, 964–969. [Google Scholar] [CrossRef]
- Huang, J.; Pearson, J.A.; Wong, F.S.; Wen, L.; Zhou, Z. Innate immunity in latent autoimmune diabetes in adults. Diabetes/Metab. Res. Rev. 2022, 38, e3480. [Google Scholar] [CrossRef] [PubMed]
- Lui, D.T.W.; Lee, K.K.; Lee, C.H.; Lee, A.C.H.; Hung, I.F.N.; Tan, K.C.B. Development of Graves’ Disease After SARS-CoV-2 mRNA Vaccination: A Case Report and Literature Review. Front. Public Health 2021, 9, 778964. [Google Scholar] [CrossRef]
- Chen, H.; Charlat, O.; Tartaglia, L.A.; Woolf, E.A.; Weng, X.; Ellis, S.J.; Lakey, N.D.; Culpepper, J.; Moore, K.J.; Breitbart, R.E.; et al. Evidence that the diabetes gene encodes the leptin receptor: Identification of a mutation in the leptin receptor gene in db/db mice. Cell 1996, 84, 491–495. [Google Scholar] [CrossRef]
- Huang, Q.; An, R.; Wang, H.; Yang, Y.; Tang, C.; Wang, J.; Yu, W.; Zhou, Y.; Zhang, Y.; Wu, D.; et al. Aggravated pneumonia and diabetes in SARS-CoV-2 infected diabetic mice. Emerg. Microbes Infect. 2023, 12, 2203782. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, N.; Lu, X.; Zhou, M.; Yan, X.; Gu, W.; Yang, J.; Zhang, Q.; Zhang, C.; Gong, Y.; et al. Anti-infection effects of heparin on SARS-CoV-2 in a diabetic mouse model. Zool. Res. 2023, 44, 1003–1014. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Zhao, M.-M.; Li, M.-J.; Li, X.-Y.; Jin, J.-M.; Feng, Y.-M.; Zhang, L.; Huang, W.J.; Yang, F.; Yang, J.-K. Hyperglycemia induced cathepsin L maturation linked to diabetic comorbidities and COVID-19 mortality. eLife 2024, 13, RP92826. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.M.; Ardanuy, J.; Hammond, H.; Logue, J.; Jackson, L.; Baracco, L.; McGrath, M.; Dillen, C.; Patel, N.; Smith, G.; et al. Diet-induced obesity and diabetes enhance mortality and reduce vaccine efficacy for SARS-CoV-2. J. Virol. 2023, 97, e0133623. [Google Scholar] [CrossRef]
- Burnett, F.N.; Coucha, M.; Bolduc, D.R.; Hermanns, V.C.; Heath, S.P.; Abdelghani, M.; Macias-Moriarity, L.Z.; Abdelsaid, M. SARS-CoV-2 Spike Protein Intensifies Cerebrovascular Complications in Diabetic hACE2 Mice through RAAS and TLR Signaling Activation. Int. J. Mol. Sci. 2023, 24, 6394. [Google Scholar] [CrossRef]
- Atef, Y.; Ito, T.; Masuda, A.; Kato, Y.; Nishimura, A.; Kanda, Y.; Kunisawa, J.; Kusakabe, T.; Nishida, M. Diabetic Mice Spleen Vulnerability Contributes to Decreased Persistence of Antibody Production after SARS-CoV-2 Vaccine. Int. J. Mol. Sci. 2024, 25, 10379. [Google Scholar] [CrossRef] [PubMed]
- Alkhawaldeh, O.; Jarrar, Y.; Gharaibeh, M.; Abudahab, S.; Abulebdah, D.; Jarrar, B. Alterations in the gene expression of SARS-COV-2 entry receptors and enzymes in lungs and hearts of controlled and uncontrolled diabetic mice. Fundam. Clin. Pharmacol. 2024, 38, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, Y.; Nakayama, S.; Yamamoto, A.; Kitazawa, T. High D-glucose levels induce ACE2 expression via GLUT1 in human airway epithelial cell line Calu-3. BMC Mol. Cell Biol. 2022, 23, 29. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Rodriguez, J.R.; Valdés Aguayo, J.J.; Garza-Veloz, I.; Martinez-Rendon, J.; Del Refugio Rocha Pizaña, M.; Cabral-Pacheco, G.A.; Juárez-Alcalá, V.; Martinez-Fierro, M.L. Sustained Hyperglycemia and Its Relationship with the Outcome of Hospitalized Patients with Severe COVID-19: Potential Role of ACE2 Upregulation. J. Pers. Med. 2022, 12, 805. [Google Scholar] [CrossRef]
- Baristaite, G.; Gurwitz, D. d-Galactose treatment increases and reduces mRNA expression in A549 human lung epithelial cells. Drug Dev. Res. 2022, 83, 622–627. [Google Scholar] [CrossRef]
- Garreta, E.; Prado, P.; Stanifer, M.L.; Monteil, V.; Marco, A.; Ullate-Agote, A.; Moya-Rull, D.; Vilas-Zornoza, A.; Tarantino, C.; Romero, J.P.; et al. A diabetic milieu increases ACE2 expression and cellular susceptibility to SARS-CoV-2 infections in human kidney organoids and patient cells. Cell Metab. 2022, 34, 857–873.e859. [Google Scholar] [CrossRef]
- Bülow, R.D.; Boor, P. Extracellular Matrix in Kidney Fibrosis: More Than Just a Scaffold. J. Histochem. Cytochem. 2019, 67, 643–661. [Google Scholar] [CrossRef]
- Yang, Y.-F.; Li, T.-C.; Li, C.-I.; Liu, C.-S.; Lin, W.-Y.; Yang, S.-Y.; Chiang, J.-H.; Huang, C.-C.; Sung, F.-C.; Lin, C.-C. Visit-to-Visit Glucose Variability Predicts the Development of End-Stage Renal Disease in Type 2 Diabetes: 10-Year Follow-Up of Taiwan Diabetes Study. Medicine 2015, 94, e1804. [Google Scholar] [CrossRef]
| Year | Country | Conclusions | Reference |
|---|---|---|---|
| 2020 | USA | COVID-19 patients with diabetes had significantly greater levels of inflammatory biomarkers and risk of severe pneumonia than COVID-19 patients who were not diabetic. | [23] |
| 2020 | China, Italy, USA | Meta-analyses of clinical cohort studies indicate that diabetes is associated with a greater incidence of COVID-19. Additionally, severity is increased in diabetic COVID-19 patients compared to non-diabetic patients. | [24] |
| 2020 | USA | In a cohort of 5700 hospitalized COVID-19 patients, diabetes was the third most common comorbidity (33.8%), behind obesity (41.7%) and hypertension (56.6%). | [25] |
| 2020 | England | Type 1 and type 2 diabetes independently increased a COVID-19 patient’s odds of in-hospital death. | [26] |
| 2022 | International | Meta-analysis of 17 clinical studies conducted across the world revealed that SARS-CoV-2 vaccine effectiveness was diminished in diabetic cohorts compared to non-diabetics. | [27] |
| 2022 | Austria and Germany | Type 1 and type 2 diabetics who had been vaccinated had a similar humoral immune response to the SARS-CoV-2 receptor binding domain (RBD) compared to healthy individuals. | [28] |
| 2020 | International | A group in Italy conducted a meta-analysis which revealed that severe outcomes such as thromboembolism and reduced lung function were predominantly seen in type 2 diabetic COVID-19 patients. | [29] |
| 2021 | Sweden | There was observed a higher risk of severe COVID-19 outcomes associated with raised glycated hemoglobin (HbA1c) levels. Type 2 diabetes was associated with a greater risk of hospitalization, intensive care, and death. | [30] |
| 2022 | USA | The risk of hospitalization, invasive ventilation, and death from COVID-19 is increased with increasing HbA1c levels. | [31] |
| 2022 | Japan | Undiagnosed diabetes, prediabetes, and diagnosed diabetes were significant risk factors for severe COVID-19 outcomes. Additionally, increased HbA1c levels were associated with COVID-19 severity. | [32] |
| 2021 | Italy | Myocardial tissue from autopsy tissue of patients who died from COVID-19 had a higher percentage of positive staining for SARS-CoV-2. | [33] |
| 2022 | USA, England, Germany | Meta-analysis of patients after recovering from COVID-19 revealed an increased incidence and risk of developing new-onset diabetes. | [34] |
| 2023 | USA | Patients who had recovered from COVID-19 had an elevated risk of developing new-onset diabetes. The risk was higher in unvaccinated patients than in vaccinated patients. | [35] |
| 2021 | Italy | Nearly half of this Italian cohort of COVID-19 patients presented with hyperglycemia. Also, in their study, they reported insulin resistance and new-onset diabetes in recovered COVID-19 patients who did not have a history of diabetes. | [36] |
| 2023 | Brazil | Hyperglycemia was higher among COVID-19 patients than non-COVID-19 patients, regardless of diabetes status. | [11] |
| 2024 | International | Pediatric patients aged 10 to 19 years old who recovered from COVID-19 were significantly more likely to be diagnosed with new-onset diabetes within 6 months of recovery compared to non-COVID-19 respiratory infection control patients. | [37] |
| 2023 | USA | This case study describes a patient admitted to the hospital presenting diabetic ketoacidosis (DKA) one week after receiving the second dose of the Pfizer-BioNTech vaccine. The patient was soon diagnosed with latent autoimmune diabetes (LADA). | [38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nichols, J.H.; Smith, A.M.; Jonsson, C.B. The Intersection of SARS-CoV-2 and Diabetes. Microorganisms 2025, 13, 1390. https://doi.org/10.3390/microorganisms13061390
Nichols JH, Smith AM, Jonsson CB. The Intersection of SARS-CoV-2 and Diabetes. Microorganisms. 2025; 13(6):1390. https://doi.org/10.3390/microorganisms13061390
Chicago/Turabian StyleNichols, Jacob H., Amber M. Smith, and Colleen B. Jonsson. 2025. "The Intersection of SARS-CoV-2 and Diabetes" Microorganisms 13, no. 6: 1390. https://doi.org/10.3390/microorganisms13061390
APA StyleNichols, J. H., Smith, A. M., & Jonsson, C. B. (2025). The Intersection of SARS-CoV-2 and Diabetes. Microorganisms, 13(6), 1390. https://doi.org/10.3390/microorganisms13061390







