Integrated Microbiota and Metabolomics Analysis of Candida utilis CU-3 Solid-State Fermentation Effects on Cottonseed Hull-Based Feed
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design and Sampling
2.3. Detection of Nutrient Composition
2.4. Free Gossypol Content Determination
2.5. ITS Sequencing
2.6. Untargeted Metabolomics
2.7. Statistical Analysis
3. Results
3.1. Analysis of Feed Nutritional Composition and Free Gossypol Content
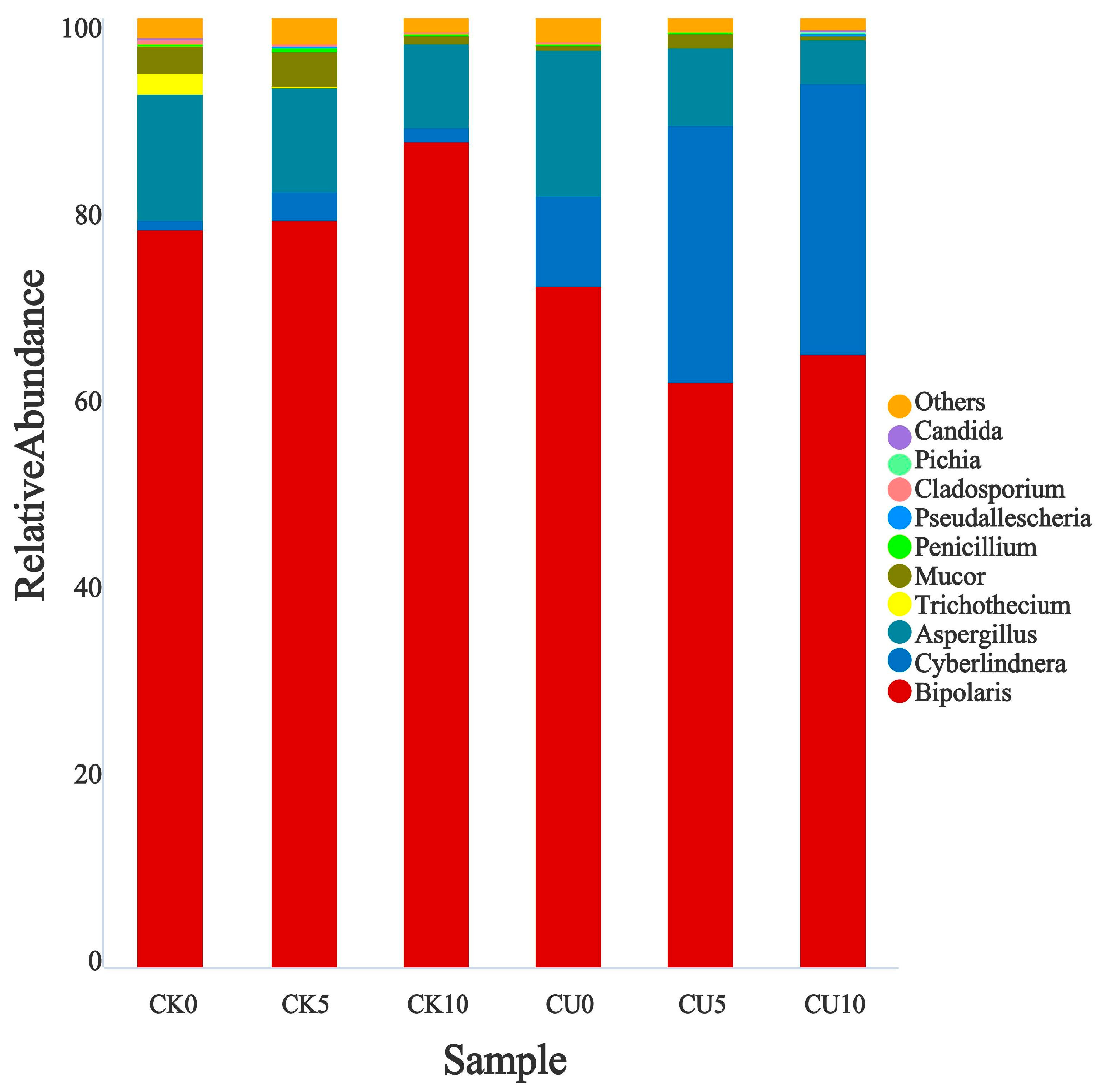
3.2. Microbial Diversity Analysis of Fermented Feed
3.3. Cluster Analysis of Metabolomics Between Experimental Group CU10 and Control Group CK10
3.4. Identification of Differential Metabolites Between Experimental Group CK10 and Control Group CU10
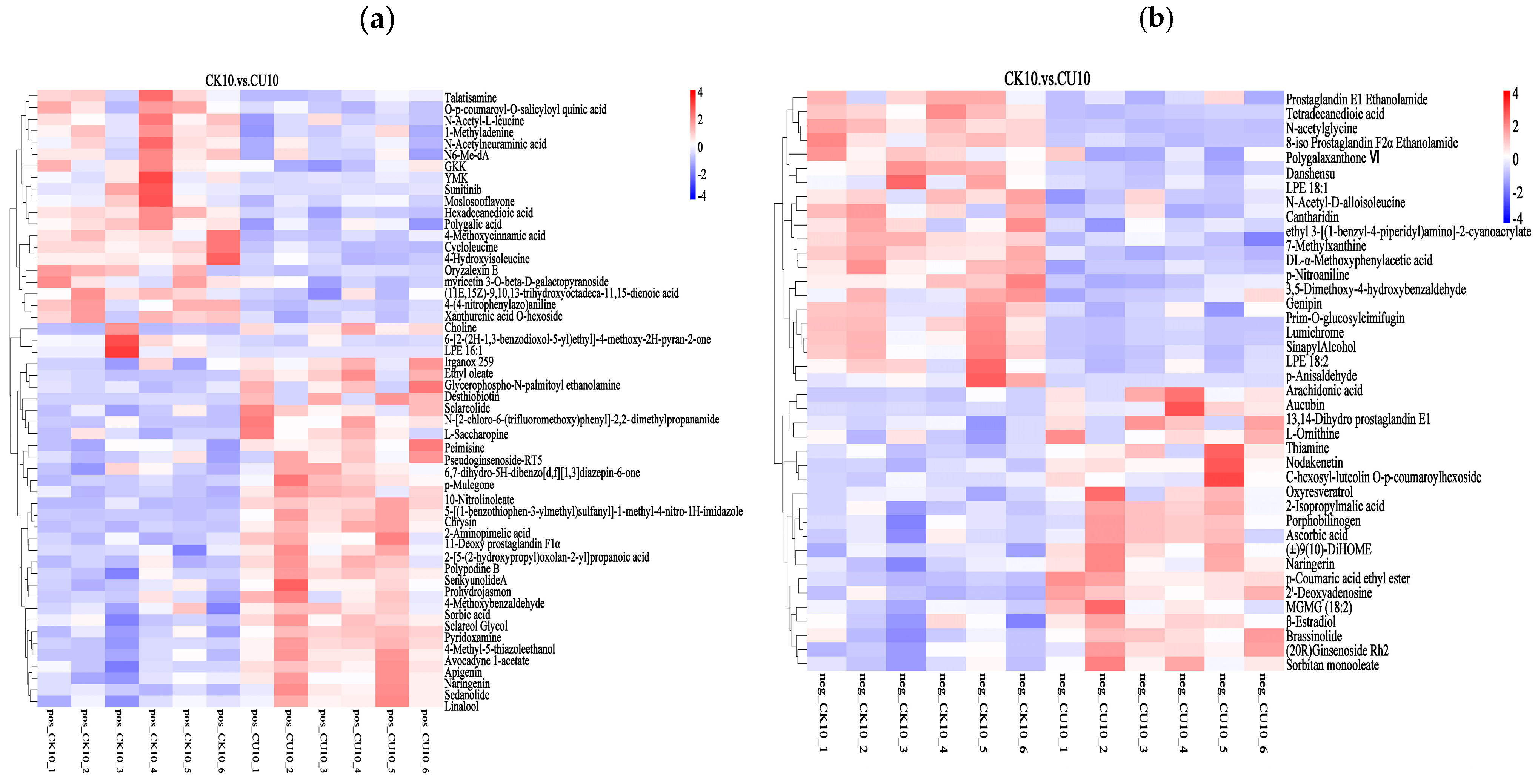
3.5. Heatmap Analysis of Differential Metabolites Between Experimental Group CK10 and Control Group CU10
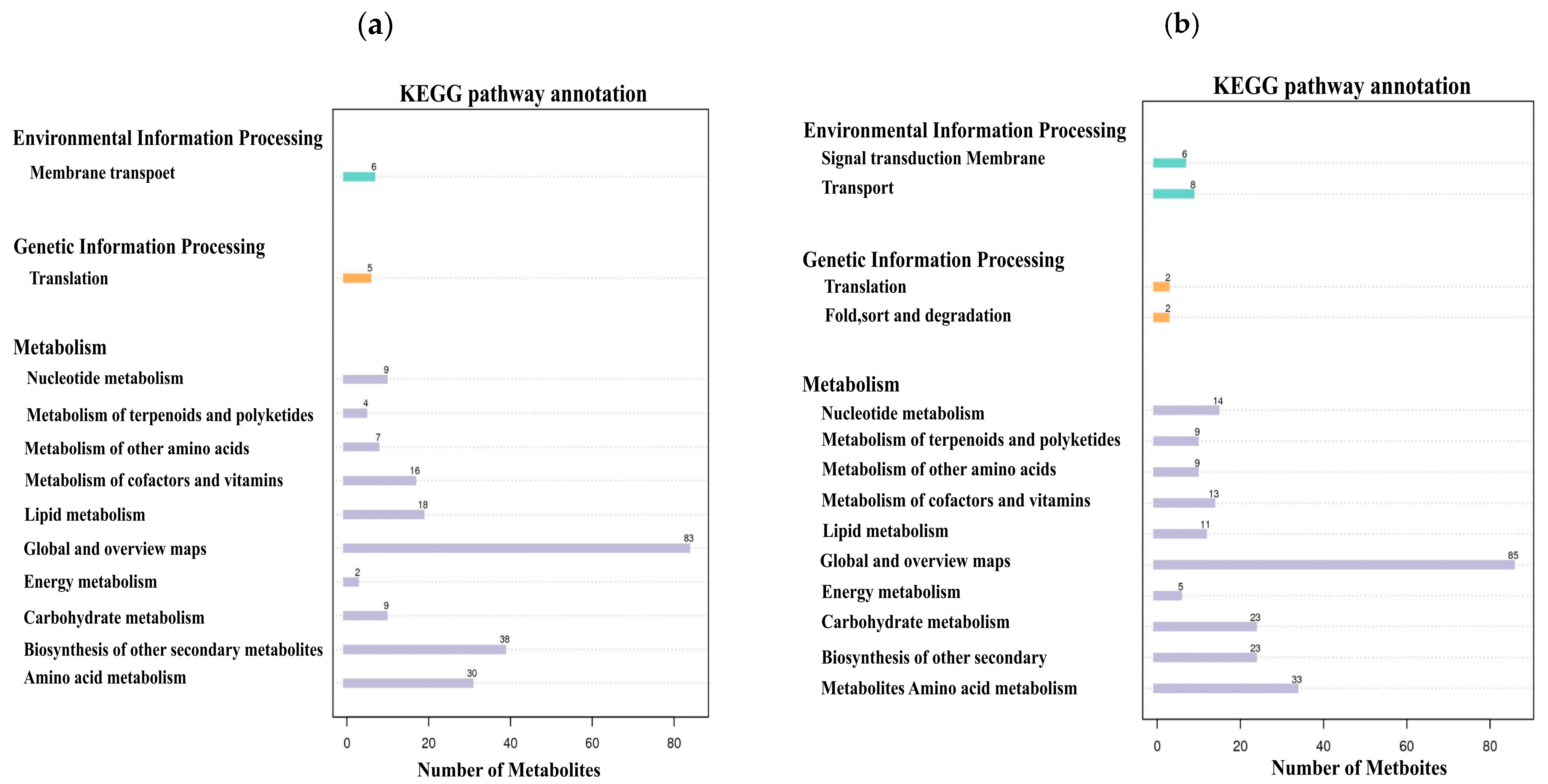
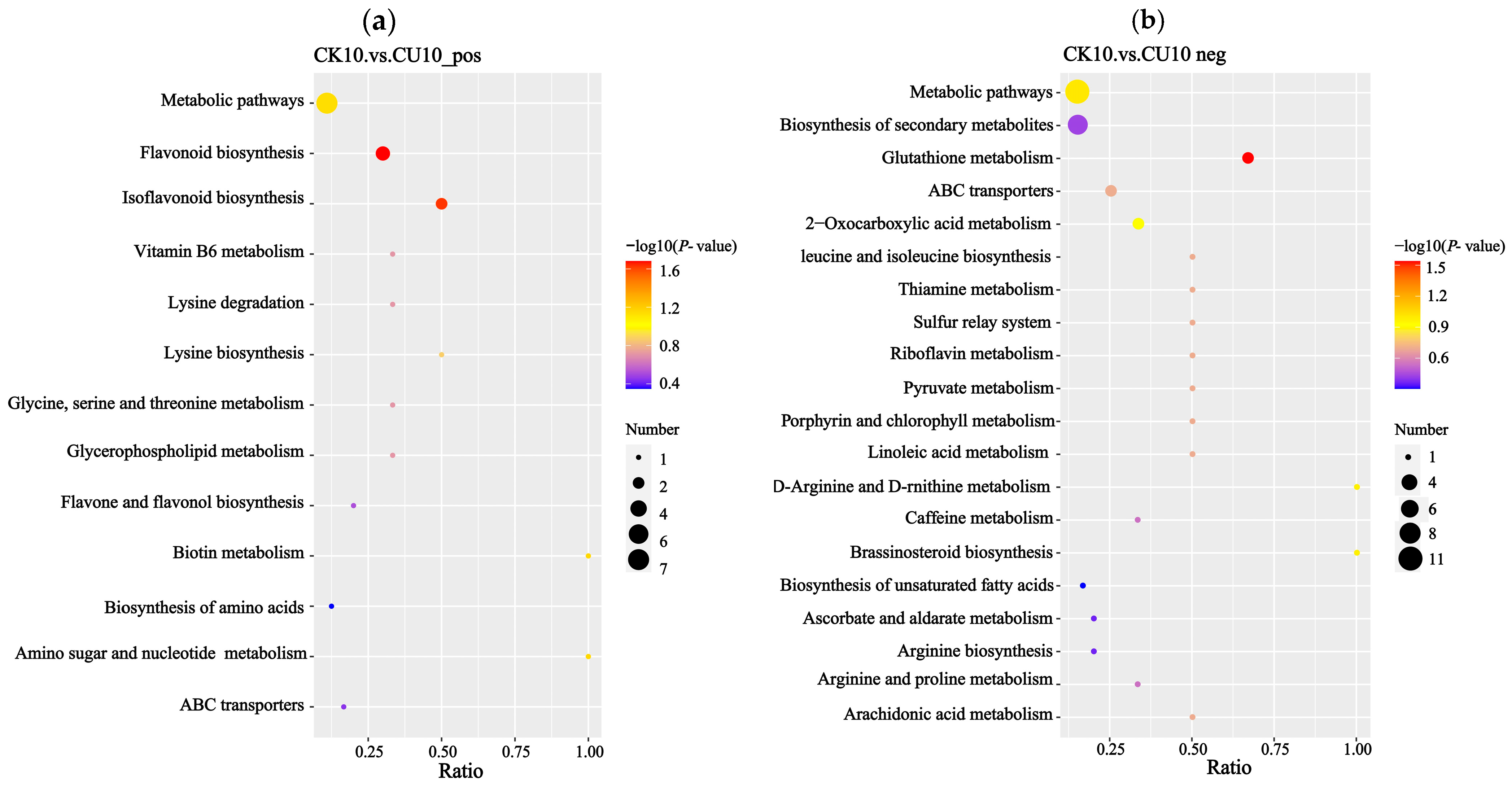
3.6. KEGG Pathway Analysis of Differential Metabolites Between Experimental Group CK10 and Control Group CU10
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, L.; Yang, X.; Nie, C.; Chen, C.; Zhang, W. Combined transcriptomics and cellular analyses reveal the molecular mechanism by which Candida tropicalis ZD-3 adapts to and degrades gossypol. Int. J. Biol. Macromol. 2024, 279, 135294. [Google Scholar] [CrossRef] [PubMed]
- Santosh, S.; Raghavendra, K.P.; Velmourougane, K.; Mageshwaran, V.; Blaise, D.; Waghmare, V.N. Microbial Detoxification of Gossypol in Cotton Seed Meal by Solid Substrate Fermentation. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 1654–1663. [Google Scholar] [CrossRef]
- Eliopoulos, C.; Langousi, I.; Kougia, E.; Saxami, G.; Markou, G.; Haroutounian, S.A.; Arapoglou, D. Solid-State Fermentation Initiated by Pleurotus ostreatus of a Cottonseed Cake and Lathyrus clymenum Pericarp Mixture: Impact on Nutritional Profile and Gossypol Content. Appl. Sci. 2024, 14, 5066. [Google Scholar] [CrossRef]
- Wang, W.-K.; Wang, Y.-L.; Li, W.-J.; Wu, Q.-C.; Yang, K.-L.; Li, S.-L.; Yang, H.-J. In situ rumen degradation characteristics and bacterial colonization of whole cottonseed, cottonseed hull and cottonseed meal with different gossypol content. AMB Express 2021, 11, 91. [Google Scholar] [CrossRef]
- Li, J.; Gao, T.; Hao, Z.; Guo, X.; Zhu, B. Anaerobic solid-state fermentation with Bacillus subtilis for digesting free gossypol and improving nutritional quality in cottonseed meal. Front. Nutr. 2022, 9, 1017637. [Google Scholar] [CrossRef]
- Niu, J.; Zhang, J.; Wei, L.; Ma, X.; Zhang, W.; Nie, C. Cottonseed meal fermented by Candida tropical reduces the fat deposition in white-feather broilers through cecum bacteria-host metabolic cross-talk. Appl. Microbiol. Biotechnol. 2020, 104, 4345–4357. [Google Scholar] [CrossRef]
- Yao, Y.; Li, H.; Li, J.; Zhu, B.; Gao, T. Anaerobic Solid-State Fermentation of Soybean Meal With Bacillus sp. to Improve Nutritional Quality. Front. Nutr. 2021, 8, 706977. [Google Scholar] [CrossRef]
- Agarussi, M.C.N.; Pereira, O.G.; Pimentel, F.E.; Azevedo, C.F.; da Silva, V.P.; e Silva, F.F. Microbiome of rehydrated corn and sorghum grain silages treated with microbial inoculants in different fermentation periods. Sci. Rep. 2022, 12, 16864. [Google Scholar] [CrossRef]
- Wang, L.; Chen, M.; Luo, X.; Fan, Y.; Zheng, Z.; He, Z.; Yin, R.; Meng, T.; Xu, S.; Pan, Y.; et al. Intramolecular Annulation of Gossypol by Laccase to Produce Safe Cottonseed Protein. Front. Chem. 2020, 8, 583176. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, H.; Han, B.; Li, H.Y.; Liu, X.L. Improvement of nutritional value, molecular weight patterns (soluble peptides), free amino acid patterns, total phenolics and antioxidant activity of fermented extrusion pretreatment rapeseed meal with Bacillus subtilis YY-1 and Saccharomyces cerevisiae YY-2. LWT 2022, 160, 113280. [Google Scholar]
- Yusuf, H.A.; Piao, M.; Ma, T.; Huo, R.; Tu, Y. Effect of lactic acid bacteria and yeast supplementation on anti-nutritional factors and chemical composition of fermented total mixed ration containing cottonseed meal or rapeseed meal. Anim. Biosci. 2022, 35, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.W.; Sun, H.; Yao, X.H.; Wu, Y.F.; Wang, X.; Feng, J. Effects of Replacement of Soybean Meal by Fermented Cottonseed Meal on Growth Performance, Serum Biochemical Parameters and Immune Function of Yellow-feathered Broilers. Asian-Australas. J. Anim. Sci. 2012, 25, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, F.; Avesani, M.; Lorenzini, M.; Zapparoli, G.; Simonato, B. Fermentation Performances and Aroma Contributions of Selected Non-Saccharomyces Yeasts for Cherry Wine Production. Foods 2024, 13, 2455. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Lin, X.; Liu, M.; Yan, X.; Liang, H.; Ji, C.; Li, S.; Zhang, S.; Chen, Y.; Zhu, B. Effect of Saccharomyces cerevisiae LXPSC1 on microorganisms and metabolites of sour meat during the fermentation. Food Chem. 2023, 402, 134213. [Google Scholar] [CrossRef]
- Vlassa, M.; Filip, M.; Țăranu, I.; Marin, D.; Untea, A.E.; Ropotă, M.; Dragomir, C.; Sărăcilă, M. The Yeast Fermentation Effect on Content of Bioactive, Nutritional and Anti-Nutritional Factors in Rapeseed Meal. Foods 2022, 11, 2972. [Google Scholar] [CrossRef]
- Wang, C.; Wei, S.; Jin, M.; Liu, B.; Yue, M.; Wang, Y. Integrated Microbiomic and Metabolomic Dynamics of Fermented Corn and Soybean By-Product Mixed Substrate. Front. Nutr. 2022, 9, 831243. [Google Scholar] [CrossRef]
- Huang, J.; Xu, J.; Wu, R.; Wang, J.; Yang, J.; Li, Y.; Wang, B.; Xiong, W.; Guo, Y. Influence of Cuticular Waxes from Triticale on Rumen Fermentation: A Metabolomic and Microbiome Profiling Study. J. Agric. Food Chem. 2024, 72, 1592–1606. [Google Scholar] [CrossRef]
- Damiani, C.; Gaglio, D.; Sacco, E.; Alberghina, L.; Vanoni, M. Systems metabolomics: From metabolomic snapshots to design principles. Curr. Opin. Biotechnol. 2020, 63, 190–199. [Google Scholar] [CrossRef]
- Deng, X.; Jia, Y.; Ge, G.; Wang, Z.; Liu, M.; Bao, J.; Zhao, M.; Si, Q.; Liu, Y.; Zhao, W. Microbiomics and volatile metabolomics-based investigation of changes in quality and flavor of oat (Avena sativa L.) silage at different stages. Front. Plant Sci. 2023, 14, 1278715. [Google Scholar] [CrossRef]
- Kharnaior, P.; Tamang, J.P. Metagenomic-Metabolomic Mining of Kinema, a Naturally Fermented Soybean Food of the Eastern Himalayas. Front. Microbiol. 2022, 13, 868383. [Google Scholar] [CrossRef]
- Thiex, N. Evaluation of analytical methods for the determination of moisture, crude protein, crude fat, and crude fiber in distillers dried grains with solubles. J. AOAC Int. 2009, 92, 61–73. [Google Scholar] [CrossRef] [PubMed]
- ISO 6866-1985; Animal Feeding Stuffs-Determination of Free and Total Gossypol. Internal Organization for Standardization: Geneva, Switzerland, 1985.
- Wang, W.; Wu, Q.; Li, W.; Wang, Y.; Zhang, F.; Lv, L.; Li, S.; Yang, H. High-Gossypol Whole Cottonseed Exhibited Mediocre Rumen Degradability and Less Microbial Fermentation Efficiency than Cottonseed Hull and Cottonseed Meal with an In Vitro Gas Production Technique. Fermentation 2022, 8, 103. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, J.; Wang, G.; Wei, T.; Hu, K.; Yi, W.; Zeng, P.; Li, H.; Wu, Y.; He, Q. Synthesis and characterization of zeolitic imidazolate frameworks nanocrystals and their application in adsorption and detoxification of gossypol in cottonseed oil. Food Chem. 2023, 418, 135905. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zheng, H.; Zhang, X.; Chen, X.; Liu, Y.; Tang, Y.; Zhang, W.; Wang, Z.; Zhao, L.; Guo, Y. Effective Degradation of Free Gossypol in Defatted Cottonseed Meal by Bacterial Laccases: Performance and Toxicity Analysis. Foods 2024, 13, 566. [Google Scholar] [CrossRef]
- Liu, B.; Liu, H.; Liu, D.; Zhou, M.; Jiang, Q.; Ma, X.; Wang, J.; Tan, B.e.; Zhang, C. Free Gossypol Removal and Nutritional Value Enhancement of Cottonseed Meal via Solid-State Fermentation with Rhodotorula mucilaginosa TG529. Agriculture 2024, 14, 1463. [Google Scholar] [CrossRef]
- Ni, S.; Zhao, D.; Li, K.; Wu, Y.; Yang, S.; Chen, X.; Cui, Z.; Yan, X.; Liu, G. Cofermentation of Cottonseed Meal by the Synergistic Action of Microbial Flora with Protease and Its Metabolic Kinetic. ACS Food Sci. Technol. 2024, 4, 773–785. [Google Scholar] [CrossRef]
- Niu, J.L.; Wei, L.Q.; Luo, Y.Q.; Yang, W.T.; Lu, Q.C.; Zheng, X.X.; Niu, Y.J.; Sheng, W.; Cheng, H.; Zhang, W.J.; et al. Fermented cottonseed meal improves production performance and reduces fat deposition in broiler chickens. Anim. Biosci. 2021, 34, 680–691. [Google Scholar] [CrossRef]
- Zhang, H.; Du, H.; Xu, Y.; Zhou, N.-Y. Volatile Organic Compound-Mediated Antifungal Activity of Pichia spp. and Its Effect on the Metabolic Profiles of Fermentation Communities. Appl. Environ. Microbiol. 2021, 87, e02992-20. [Google Scholar] [CrossRef]
- Meng, H.; Jiang, Y.; Wang, L.; Li, Y.; Wang, S.; Tong, X.; Wang, S. Dynamic Analysis of Fermentation Quality, Microbial Community, and Metabolome in the Whole Plant Soybean Silage. Fermentation 2024, 10, 535. [Google Scholar] [CrossRef]
- Semwal, D.; Semwal, R.; Combrinck, S.; Viljoen, A. Myricetin: A Dietary Molecule with Diverse Biological Activities. Nutrients 2016, 8, 90. [Google Scholar] [CrossRef]
- Xu, W.; Liu, M.; Li, H.; Chen, J.; Zhou, J. De Novo Synthesis of Chrysin in Saccharomyces cerevisiae. J. Agric. Food Chem. 2024, 72, 6481–6490. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Ma, X.; Yu, F.; Xu, L.; Lang, L. Dihydromyricetin Alleviates Non-Alcoholic Fatty Liver Disease by Modulating Gut Microbiota and Inflammatory Signaling Pathways. J. Microbiol. Biotechnol. 2024, 34, 2637–2647. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Xia, X.; Chen, D.; Ren, Y.; Liu, Y.; Xiang, H.; Li, X.; Zhi, Y.; Mo, Y. Antiviral activity of chrysin and naringenin against porcine epidemic diarrhea virus infection. Front. Vet. Sci. 2023, 10, 1278997. [Google Scholar] [CrossRef] [PubMed]
- Hamed, A.M.; Ali, F.A.Z.; Mohammed, A.e.M.E.; Alrasheedi, M.; Ragab, I.; Aldoghaim, M.; Soliman, S.S. The ameliorative effect of chrysin on ovarian toxicity caused by methidathion in female rats. Front. Mol. Biosci. 2024, 11, 1470711. [Google Scholar] [CrossRef]
- Sivashanmugam, M.; Jaidev, J.; Umashankar, V.; Sulochana, K.N. Ornithine and its role in metabolic diseases: An appraisal. Biomed. Pharmacother. 2017, 86, 185–194. [Google Scholar] [CrossRef]
- Paßlack, N.; Zentek, J. Effects of Dietary Arginine, Ornithine, and Zeolite Supplementation on Uremic Toxins in Cats. Toxins 2018, 10, 206. [Google Scholar] [CrossRef]
- Zhou, Z.; Sarwar, A.; Hu, G.; Zhang, J.; Hu, H.; Aziz, T.; Wu, J.; Yang, Z.; Yang, Z. Identification of potential key metabolites in synbiotic yoghurt made with probiotic Saccharomyces cerevisiae var. boulardii CNCM I-745 and prebiotic inulin by non-targeted metabolomics analysis. Food Chem. 2025, 464, 141923. [Google Scholar] [CrossRef]
- Kinyua, A.W.; Ko, C.M.; Doan, K.V.; Yang, D.J.; Huynh, M.K.Q.; Moh, S.H.; Choi, Y.-H.; Kim, K.W. 4-hydroxy-3-methoxycinnamic acid regulates orexigenic peptides and hepatic glucose homeostasis through phosphorylation of FoxO1. Exp. Mol. Med. 2018, 50, e437. [Google Scholar] [CrossRef]
- Ojo, O.A.; Ogunlakin, A.D.; Maimako, R.F.; Gyebi, G.A.; Olowosoke, C.B.; Taiwo, O.A.; Elebiyo, T.C.; Adeniyi, D.; David, B.; Iyobhebhe, M.; et al. Therapeutic Study of Cinnamic Acid Derivative for Oxidative Stress Ablation: The Computational and Experimental Answers. Molecules 2023, 28, 7425. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, W.; Zhang, H.; Cui, Y.; Wang, F.; Wu, J.; Chao, H.; Yan, D. The Complete Genome Sequence of a Gossypol-Degrading Bacterial Strain, Raoultella sp. YL01. Curr. Microbiol. 2023, 80, 163. [Google Scholar] [CrossRef]
- Li, Z.; Gao, H.; Mei, H.; Wu, G.; Soloshonok, V.A.; Han, J. Synthesis of Aminoalkyl Sclareolide Derivatives and Antifungal Activity Studies. Molecules 2023, 28, 4067. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Tang, K.; Guo, Y. Discovery of sclareol and sclareolide as filovirus entry inhibitors. J. Asian Nat. Prod. Res. 2020, 22, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhuge, A.; Chen, H.; Han, S.; Shen, J.; Wang, K.; Xia, J.; Xia, H.; Jiang, S.; Wu, Y.; et al. Sedanolide alleviates DSS-induced colitis by modulating the intestinal FXR-SMPD3 pathway in mice. J. Adv. Res. 2025, 69, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Tabei, Y.; Abe, H.; Suzuki, S.; Takeda, N.; Arai, J.-I.; Nakajima, Y. Sedanolide Activates KEAP1–NRF2 Pathway and Ameliorates Hydrogen Peroxide-Induced Apoptotic Cell Death. Int. J. Mol. Sci. 2023, 24, 16532. [Google Scholar] [CrossRef]
- Chen, L.-X.; Qi, Z.; Shao, Z.-J.; Li, S.-S.; Qi, Y.-L.; Gao, K.; Liu, S.-X.; Li, Z.; Sun, Y.-S.; Li, P.-Y. Study on Antidepressant Activity of Pseudo-Ginsenoside HQ on Depression-Like Behavior in Mice. Molecules 2019, 24, 870. [Google Scholar] [CrossRef]
- Wickramasinghe, P.C.K.; Munafo, J.P., Jr. Key Odorants from the Fermentation Broth of the Edible Mushroom Ischnoderma resinosum. J. Agric. Food Chem. 2019, 67, 2036–2042. [Google Scholar] [CrossRef]





| Items | Fermentation Time | Control Group | Treatment Group |
|---|---|---|---|
| Crude protein (CP, %) | 0d | 2.66 ± 0.10 Aa | 2.68 ± 0.14 Ab |
| 5d | 2.76 ± 0.13 Ba | 3.52 ± 0.28 Aa | |
| 10d | 2.88 ± 0.09 Ba | 3.68 ± 0.27 Aa | |
| Crude fat (EE, %) | 0d | 3.23 ± 0.15 Aa | 3.37 ± 0.15 Aa |
| 5d | 3.43 ± 0.32 Aa | 3.50 ± 0.20 Aa | |
| 10d | 3.60 ± 0.10 Aa | 3.30 ± 0.20 Aa | |
| Crude fiber (CF, %) | 0d | 38.70 ± 0.93 Aa | 37.97 ± 0.25 Aa |
| 5d | 38.24 ± 0.51 Aab | 36.30 ± 0.94 Ba | |
| 10d | 36.95 ± 0.45 Ab | 31.61 ± 1.66 Bb | |
| Free gossypol (FG, mg/kg) | 0d | 189.25 ± 2.31 Aa | 186.95 ± 4.32 Aa |
| 5d | 188.97 ± 1.66 Aa | 129.60 ± 8.59 Bb | |
| 10d | 170.08 ± 2.23 Ab | 64.80 ± 4.35 Bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, D.; Yan, Y.; Yang, F.; Yao, H.; Li, Y.; Huang, X.; Aihemaiti, M.; Zhan, F.; Hou, M.; Cui, W. Integrated Microbiota and Metabolomics Analysis of Candida utilis CU-3 Solid-State Fermentation Effects on Cottonseed Hull-Based Feed. Microorganisms 2025, 13, 1380. https://doi.org/10.3390/microorganisms13061380
Dong D, Yan Y, Yang F, Yao H, Li Y, Huang X, Aihemaiti M, Zhan F, Hou M, Cui W. Integrated Microbiota and Metabolomics Analysis of Candida utilis CU-3 Solid-State Fermentation Effects on Cottonseed Hull-Based Feed. Microorganisms. 2025; 13(6):1380. https://doi.org/10.3390/microorganisms13061380
Chicago/Turabian StyleDong, Deli, Yuanyuan Yan, Fan Yang, Huaibing Yao, Yang Li, Xin Huang, Maierhaba Aihemaiti, Faqiang Zhan, Min Hou, and Weidong Cui. 2025. "Integrated Microbiota and Metabolomics Analysis of Candida utilis CU-3 Solid-State Fermentation Effects on Cottonseed Hull-Based Feed" Microorganisms 13, no. 6: 1380. https://doi.org/10.3390/microorganisms13061380
APA StyleDong, D., Yan, Y., Yang, F., Yao, H., Li, Y., Huang, X., Aihemaiti, M., Zhan, F., Hou, M., & Cui, W. (2025). Integrated Microbiota and Metabolomics Analysis of Candida utilis CU-3 Solid-State Fermentation Effects on Cottonseed Hull-Based Feed. Microorganisms, 13(6), 1380. https://doi.org/10.3390/microorganisms13061380






