Synergistic Engineering of the Twin-Arginine Translocation (Tat) Pathway and Membrane Capacity Enhances Extracellular Production of Amylosucrase in Bacillus licheniformis
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains, Plasmids, and Culture Conditions
2.2. Genetic Manipulation
2.2.1. Standard Molecular Cloning Techniques
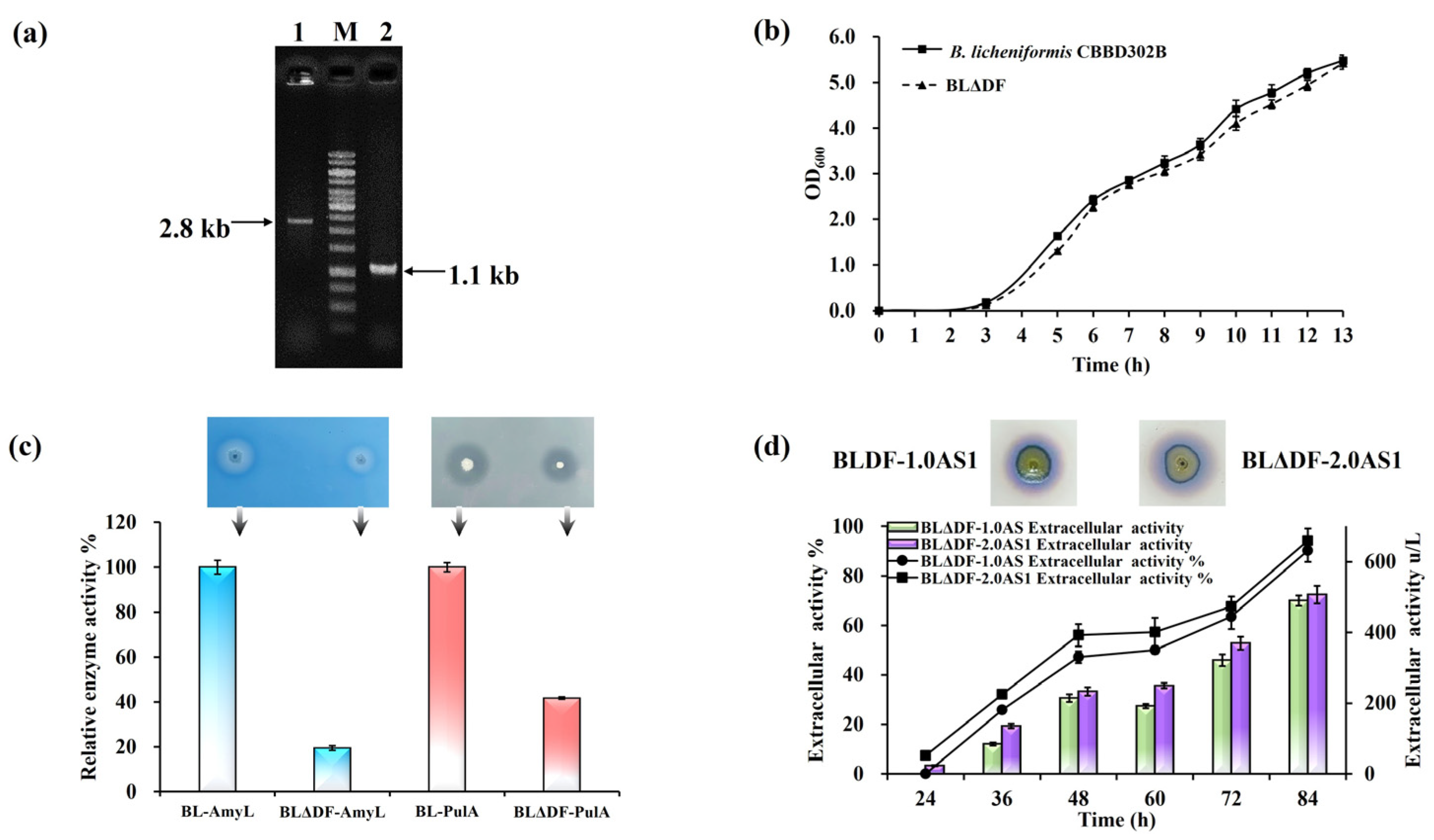
2.2.2. Gene Deletion in B. licheniformis
2.2.3. Prediction and Structure Analysis of NpAS Mutation Sites
2.2.4. Site-Directed Mutagenesis of TAT Translocases
2.3. Semi-Quantitative Enzyme Activity Analysis
2.4. Shaking Flask Fermentation
2.5. Enzyme Preparation and Activity Assays
2.5.1. Sample Preparation
2.5.2. Activity Assays
3. Results and Discussion
3.1. Impact of Hydrophobic Region Modifications on NpAS Secretion in B. licheniformis
3.2. Effect of TAT Pathway Modifications on NpAS Secretion Efficiency
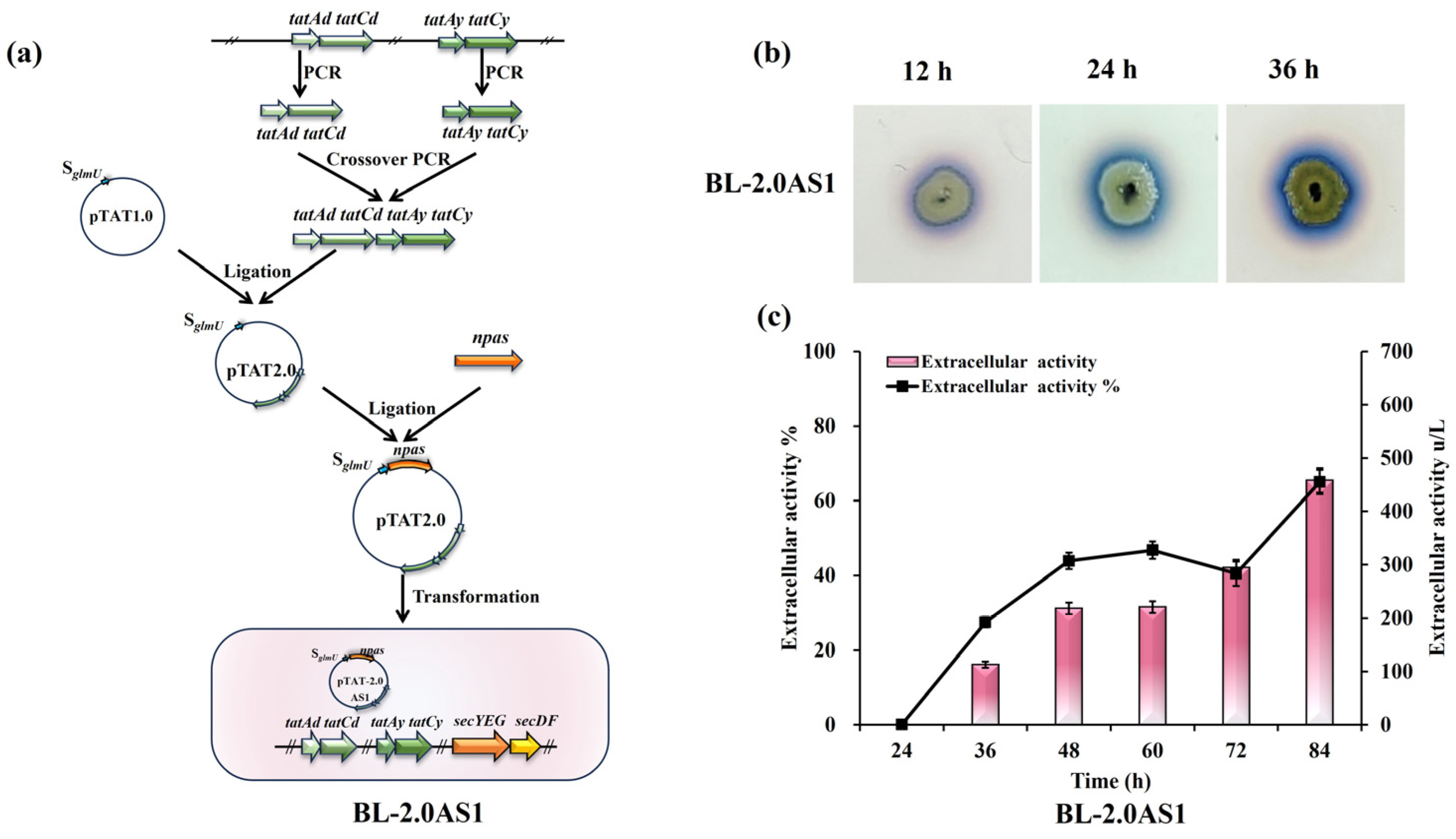
3.2.1. Influence of TAT Translocase Overexpression
3.2.2. Influence of TAT Translocase Mutations
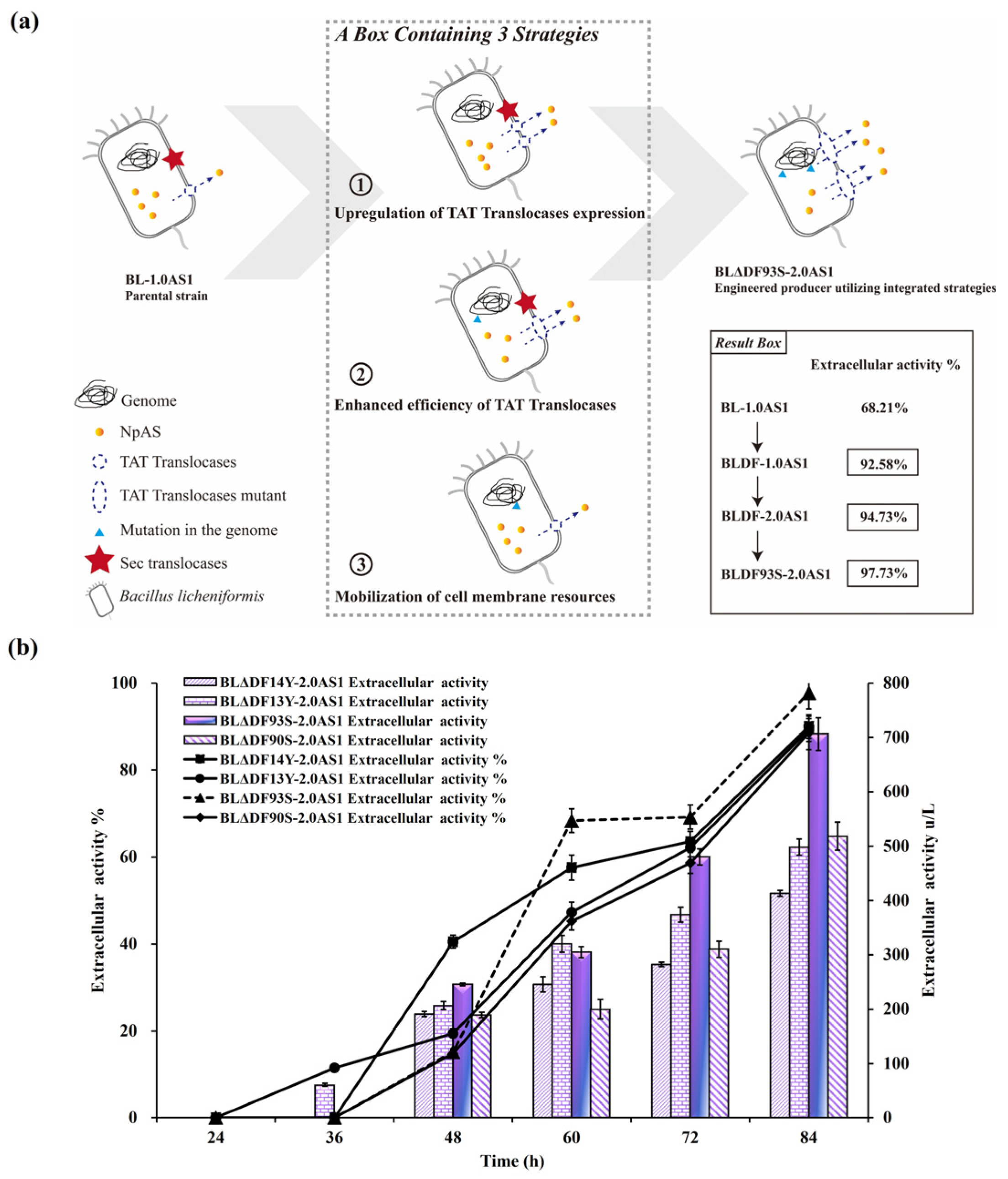
3.3. Enhancing TAT Pathway Efficiency Through Cell Membrane Resource Redistribution
3.4. Enhanced Secretion of NpAS Through Combined Regulation Strategies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AS | Amylosucrase |
| ADP | Adenosine diphosphate |
| UDP | Uridine diphosphate |
| NpAS | Neisseria polysaccharea amylosucrase |
| LB | Luria–Bertani medium |
| PCR | Polymerase chain reaction |
| DNS | Dinitrosalicylic acid |
References
- Seo, D.H.; Yoo, S.H.; Choi, S.J.; Kim, Y.R.; Park, C.S. Versatile biotechnological applications of amylosucrase, a novel glucosyltransferase. Food Sci. Biotechnol. 2020, 29, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; So, Y.S.; Baik, M.Y.; Kim, Y.R.; Yoo, S.H.; Seo, D.H.; Park, C.S. Enzymatic synthesis of α-glucan microparticles using amylosucrases from Bifidobacterium species and its physicochemical properties. Biomacromolecules 2024, 25, 2024–2032. [Google Scholar] [CrossRef]
- Kang, J.U.; So, Y.S.; Kim, G.; Lee, W.; Seo, D.H.; Shin, H.; Yoo, S.H. Efficient biosynthesis of theanderose, a potent prebiotic, using amylosucrase from Deinococcus deserti. J. Agric. Food Chem. 2024, 72, 25197–25209. [Google Scholar] [CrossRef] [PubMed]
- Im, J.K.; Seo, D.H.; Yu, J.S.; Yoo, S.H. Efficient and novel biosynthesis of myricetin α-triglucoside with improved solubility using amylosucrase from Deinococcus deserti. Int. J. Biol. Macromol. 2024, 273, 133205. [Google Scholar] [CrossRef]
- Zhan, Y.F.; Meng, Z.H.; Yan, C.H.; Tan, M.; Khurshid, M.; Li, Y.J.; Zheng, S.J.; Wang, J. A novel cascade catalysis for one-pot enzymatically modified isoquercitrin (EMIQ) conversion from rutin and sucrose using rationally designed gradient temperature control. Food Chem. 2024, 457, 140163. [Google Scholar] [CrossRef] [PubMed]
- Rha, C.S.; Kim, H.G.; Baek, N.I.; Kim, D.O.; Park, C.S. Using amylosucrase for the controlled synthesis of novel isoquercitrin glycosides with different glycosidic linkages. J. Agric. Food Chem. 2020, 68, 13798–13805. [Google Scholar] [CrossRef]
- Jung, Y.S.; Kim, H.G.; Oh, S.M.; Lee, D.Y.; Park, C.S.; Kim, D.O.; Baek, N.I. Synthesis of alpha-linked glucosides from soybean isoflavone aglycones using amylosucrase from Deinococcus geothermalis. J. Agric. Food Chem. 2023, 71, 2430–2437. [Google Scholar] [CrossRef]
- Tian, Y.; Xu, W.; Zhang, W.; Zhang, T.; Guang, C.; Mu, W. Amylosucrase as a transglucosylation tool: From molecular features to bioengineering applications. Biotechnol. Adv. 2018, 36, 1540–1552. [Google Scholar] [CrossRef]
- Bae, J.; Jun, S.J.; Chang, P.S.; Yoo, S.H. A unique biochemical reaction pathway towards trehalulose synthesis by an amylosucrase isolated from Deinococcus deserti. Nat. Biotechnol. 2022, 70, 1–8. [Google Scholar] [CrossRef]
- Tian, Y.; Xu, W.; Guang, C.; Zhang, W.; Mu, W. Thermostable amylosucrase from Calidithermus timidus DSM 17022: Insight into its characteristics and tetrameric conformation. J. Agric. Food Chem. 2019, 67, 9868–9876. [Google Scholar] [CrossRef]
- Ding, H.Y.; Wang, T.Y.; Wu, J.Y.; Tsai, Y.L.; Chang, T.S. Enzymatic synthesis of novel and highly soluble puerarin glucoside by Deinococcus geothermalis amylosucrase. Molecules 2022, 27, 4074. [Google Scholar] [CrossRef]
- Zhu, X.; Tian, Y.; Xu, W.; Bai, Y.; Zhang, T.; Mu, W. Biochemical characterization of a highly thermostable amylosucrase from Truepera radiovictrix DSM 17093. Int. J. Biol. Macromol. 2018, 116, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Seo, D.H.; Kim, S.H.; Hong, Y.S.; Lee, J.H.; Kim, Y.J.; Jung, D.H.; Yoo, S.H.; Park, C.S. Comparative study on four amylosucrases from Bifidobacterium species. Int. J. Biol. Macromol. 2020, 155, 535–542. [Google Scholar] [CrossRef]
- Rha, C.S.; Kim, E.R.; Kim, Y.J.; Jung, Y.S.; Kim, D.O.; Park, C.S. Simple and efficient production of highly soluble daidzin glycosides by amylosucrase from Deinococcus geothermalis. J. Agric. Food Chem. 2019, 67, 12824–12832. [Google Scholar] [CrossRef]
- Jun, S.J.; Lee, J.A.; Kim, Y.W.; Yoo, S.H. Site-directed mutagenic engineering of a Bifidobacterium amylosucrase toward greater efficiency of turanose synthesis. J. Agric. Food Chem. 2022, 70, 1579–1588. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, W.; Bai, Y.; Zhang, T.; Jiang, B.; Mu, W. Identification of an alpha-(1,4)-glucan-synthesizing amylosucrase from Cellulomonas carboniz T26. J. Agric. Food Chem. 2017, 65, 2110–2119. [Google Scholar] [CrossRef]
- Schneider, J.; Fricke, C.; Overwin, H.; Hofer, B. High level expression of a recombinant amylosucrase gene and selected properties of the enzyme. Appl. Microbiol. Biotechnol. 2011, 89, 1821–1829. [Google Scholar] [CrossRef] [PubMed]
- Watthanasakphuban, N.; Ninchan, B.; Pinmanee, P.; Rattanaporn, K.; Keawsompong, S. In silico analysis and development of the secretory expression of D-psicose-3-epimerase in Escherichia coli. Microorganisms 2024, 12, 1574. [Google Scholar] [CrossRef] [PubMed]
- Khlebodarova, T.M.; Bogacheva, N.V.; Zadorozhny, A.V.; Bryanskaya, A.V.; Vasilieva, A.R.; Chesnokov, D.O.; Pavlova, E.I.; Peltek, S.E. Komagataella phaffii as a platform for heterologous expression of enzymes used for industry. Microorganisms 2024, 12, 346. [Google Scholar] [CrossRef]
- Kim, E.R.; Rha, C.S.; Jung, Y.S.; Choi, J.M.; Kim, G.T.; Jung, D.H.; Kim, T.J.; Seo, D.H.; Kim, D.O.; Park, C.S. Enzymatic modification of daidzin using heterologously expressed amylosucrase in Bacillus subtilis. Food Sci. Biotechnol. 2019, 28, 165–174. [Google Scholar] [CrossRef]
- Wang, C.; Niu, D.; McHunu, N.P.; Zhang, M.; Singh, S.; Wang, Z. Secretory expression of amylosucrase in Bacillus licheniformis through twin-arginine translocation pathway. J. Ind. Microbiol. Biotechnol. 2024, 51, kuae004. [Google Scholar] [CrossRef] [PubMed]
- Arauzo-Aguilera, K.; Saaranen, M.J.; Robinson, C.; Ruddock, L.W. Highly efficient export of a disulfide-bonded protein to the periplasm and medium by the TAT pathway using CyDisCo in Escherichia coli. Microbiologyopen 2023, 12, e1350. [Google Scholar] [CrossRef]
- Rocco, M.A.; Waraho-Zhmayev, D.; DeLisa, M.P. Twin-arginine translocase mutations that suppress folding quality control and permit export of misfolded substrate proteins. Proc. Natl. Acad. Sci. USA 2012, 109, 13392–13397. [Google Scholar] [CrossRef] [PubMed]
- Pourhassan, N.Z.; Smits, S.H.J.; Ahn, J.H.; Schmitt, L. Biotechnological applications of type 1 secretion systems. Biotechnol. Adv. 2021, 53, 107864. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Li, J.; Liang, Y.; Li, L.; Wang, Y.; Pi, L.; Xing, P.; Nomura, C.T.; Chen, S.; Zhu, C.; et al. Engineering the TAT-secretion pathway of Bacillus licheniformis for the secretion of cytoplasmic enzyme arginase. Appl. Microbiol. Biotechnol. 2024, 108, 89. [Google Scholar] [CrossRef]
- Ulfig, A.; Freudl, R. The early mature part of bacterial twin-arginine translocation (Tat) precursor proteins contributes to TatBC receptor binding. J. Biol. Chem. 2018, 293, 7281–7299. [Google Scholar] [CrossRef]
- Taw, M.N.; Li, M.; Kim, D.; Rocco, M.A.; Waraho-Zhmayev, D.; DeLisa, M.P. Engineering a supersecreting strain of Escherichia coli by directed coevolution of the multiprotein TAT translocation machinery. ACS Synth. Biol. 2021, 10, 2947–2958. [Google Scholar] [CrossRef]
- Shen, P.; Niu, D.; Liu, X.; Tian, K.; Permaul, K.; Singh, S.; McHunu, N.P.; Wang, Z. High-efficiency chromosomal integrative amplification strategy for overexpressing alpha-amylase in Bacillus licheniformis. J. Ind. Microbiol. Biotechnol. 2022, 49, kuac009. [Google Scholar] [CrossRef]
- Niu, D.; Li, C.; Wang, P.; Huang, L.; McHunu, N.P.; Singh, S.; Prior, B.A.; Ye, X. Twin-arginine signal peptide of Bacillus licheniformis GlmU efficiently mediated secretory expression of protein glutaminase. Electron. J. Biotechnol 2019, 42, 49–55. [Google Scholar] [CrossRef]
- Niu, D.; Cong, H.; Zhang, Y.; McHunu, N.P.; Wang, Z.X. Pullulanase with high temperature and low pH optima improved starch saccharification efficiency. Sci. Rep. 2022, 12, 21942. [Google Scholar] [CrossRef]
- Niu, D.; Zuo, Z.; Shi, G.Y.; Wang, Z.X. High yield recombinant thermostable alpha-amylase production using an improved Bacillus licheniformis system. Microb. Cell. Fact. 2009, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J.; Russell, D.W. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Press: Cold Spring Harbor, NY, USA, 2001. [Google Scholar]
- Skov, L.K.; Mirza, O.; Henriksen, A.; De Montalk, G.P.; Remaud-Simeon, M.; Sarcabal, P.; Willemot, R.M.; Monsan, P.; Gajhede, M. Amylosucrase, a glucan-synthesizing enzyme from the alpha-amylase family. J. Biol. Chem. 2001, 276, 25273–25278. [Google Scholar] [CrossRef] [PubMed]
- Kryshtafovych, A.; Fidelis, K. Protein structure prediction and model quality assessment. Drug Discov. Today 2009, 14, 386–393. [Google Scholar] [CrossRef]
- Yuan, S.; Chan, H.C.S.; Filipek, S.; Vogel, H. PyMOL and inkscape bridge the data and the data visualization. Structure 2016, 24, 2041–2042. [Google Scholar] [CrossRef] [PubMed]
- Büttcher, V.; Welsh, T.; Willmitzer, L.; Kossmann, J. Cloning and characterization of the gene for amylosucrase from Neisseria polysaccharea: Production of a linear alpha-1,4-glucan. J. Bacteriol. 1997, 179, 3324–3330. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.h.; Li, Y.; Liu, X.g.; Lu, F.p. Extracellular expression of pullulanase from Bacillus naganoensis in Escherichia coli. Ann. Microbiol. 2012, 63, 289–294. [Google Scholar] [CrossRef]
- Zhu, G.J.; Wang, Z.X. A Lab Manual for Industrial Microbiology; China Light Industry Press: Beijing, China, 1994. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Frain, K.M.; Robinson, C.; van Dijl, J.M. Transport of folded proteins by the TAT system. Protein J. 2019, 38, 377–388. [Google Scholar] [CrossRef]
- Guigas, G.; Weiss, M. Effects of protein crowding on membrane systems. Biochim. Biophys. Acta 2016, 1858, 2441–2450. [Google Scholar] [CrossRef]
- Liese, S.; Carlson, A. Membrane shape remodeling by protein crowding. Biophys. J. 2021, 120, 2482–2489. [Google Scholar] [CrossRef]
- Palmer, T.; Berks, B.C. The twin-arginine translocation (TAT) protein export pathway. Nat. Rev. Microbiol. 2012, 10, 483–496. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Jiang, J.; Zhang, H.; Liu, J.; Ruan, H. Transcending membrane barriers: Advances in membrane engineering to enhance the production capacity of microbial cell factories. Microb. Cell. Fact. 2024, 23, 154. [Google Scholar] [CrossRef] [PubMed]
- Elhadi, D.; Lv, L.; Jiang, X.R.; Wu, H.; Chen, G.Q. CRISPRi engineering E. coli for morphology diversification. Metab. Eng. 2016, 38, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Neef, J.; Bongiorni, C.; Schmidt, B.; Goosens, V.J.; van Dijl, J.M. Relative contributions of non-essential Sec pathway components and cell envelope-associated proteases to high-level enzyme secretion by Bacillus subtilis. Microb. Cell. Fact. 2020, 19, 52. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Niu, D.; Zhou, Y.; Liu, H.; Mchunu, N.P.; Zhang, M.; Singh, S.; Wang, Z. Synergistic Engineering of the Twin-Arginine Translocation (Tat) Pathway and Membrane Capacity Enhances Extracellular Production of Amylosucrase in Bacillus licheniformis. Microorganisms 2025, 13, 1179. https://doi.org/10.3390/microorganisms13061179
Wang C, Niu D, Zhou Y, Liu H, Mchunu NP, Zhang M, Singh S, Wang Z. Synergistic Engineering of the Twin-Arginine Translocation (Tat) Pathway and Membrane Capacity Enhances Extracellular Production of Amylosucrase in Bacillus licheniformis. Microorganisms. 2025; 13(6):1179. https://doi.org/10.3390/microorganisms13061179
Chicago/Turabian StyleWang, Caizhe, Dandan Niu, Yongqing Zhou, Hui Liu, Nokuthula Peace Mchunu, Meng Zhang, Suren Singh, and Zhengxiang Wang. 2025. "Synergistic Engineering of the Twin-Arginine Translocation (Tat) Pathway and Membrane Capacity Enhances Extracellular Production of Amylosucrase in Bacillus licheniformis" Microorganisms 13, no. 6: 1179. https://doi.org/10.3390/microorganisms13061179
APA StyleWang, C., Niu, D., Zhou, Y., Liu, H., Mchunu, N. P., Zhang, M., Singh, S., & Wang, Z. (2025). Synergistic Engineering of the Twin-Arginine Translocation (Tat) Pathway and Membrane Capacity Enhances Extracellular Production of Amylosucrase in Bacillus licheniformis. Microorganisms, 13(6), 1179. https://doi.org/10.3390/microorganisms13061179




