Effects of the Addition of Microbial Agents After Dazomet Fumigation on the Microbial Community Structure in Soils with Continuous Cropping of Strawberry (Fragaria × Ananassa Duch.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Design
2.3. Experimental Methods
2.4. Statistical Analysis
3. Results
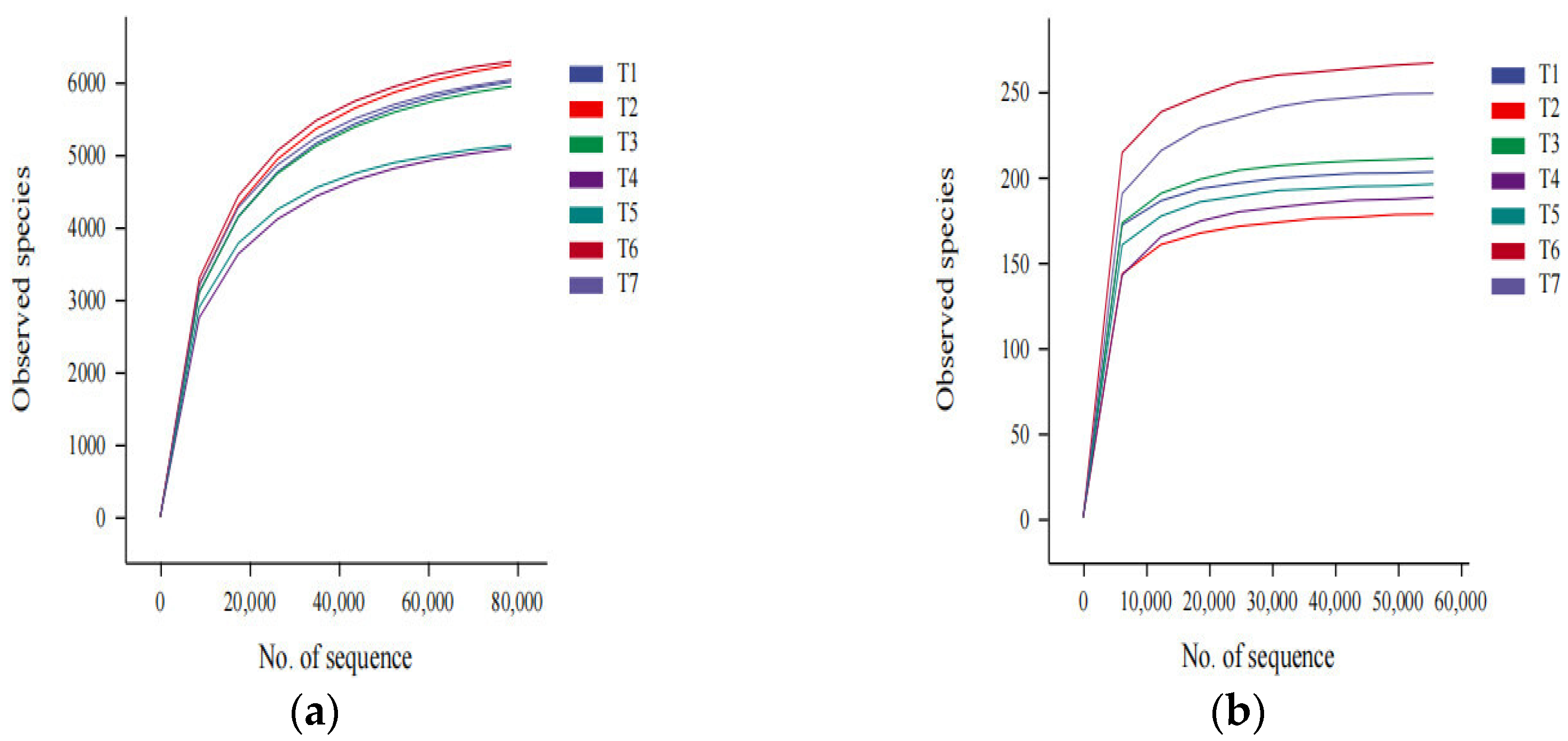
3.1. Evaluation of the Sequencing Depth of the Soil Samples
3.2. Soil Bacterial and Fungal Community Richness and Diversity Analysis
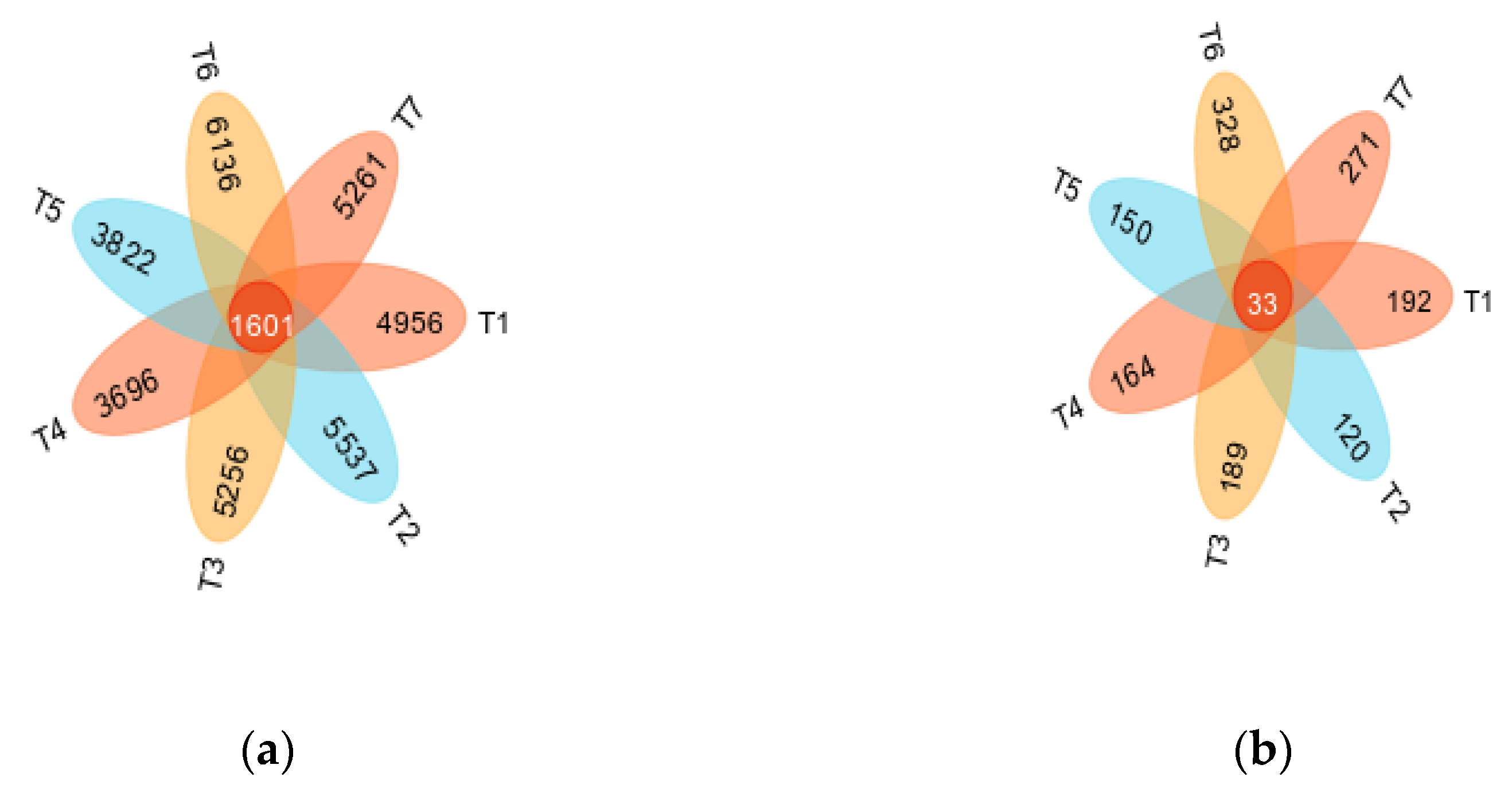
3.3. Analysis of the Soil Bacterial and Fungal Groups
3.4. Analysis of the Soil Bacterial and Fungal Community Compositions
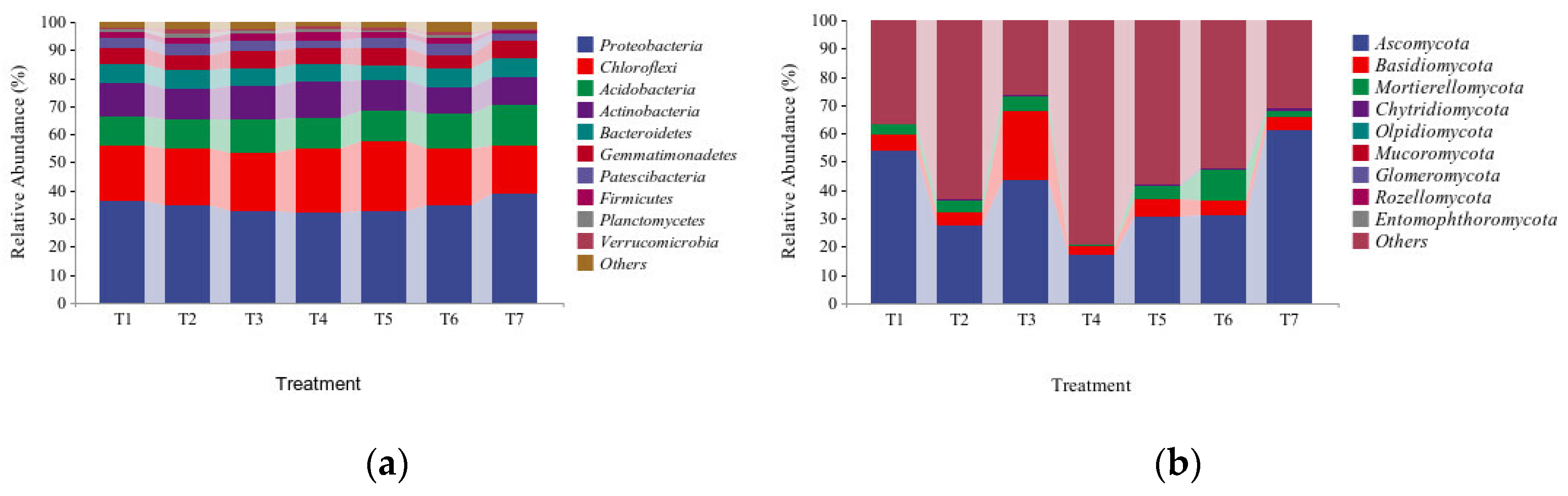
3.4.1. Analysis of the Soil Bacterial and Fungal Phylum-Level Community Compositions
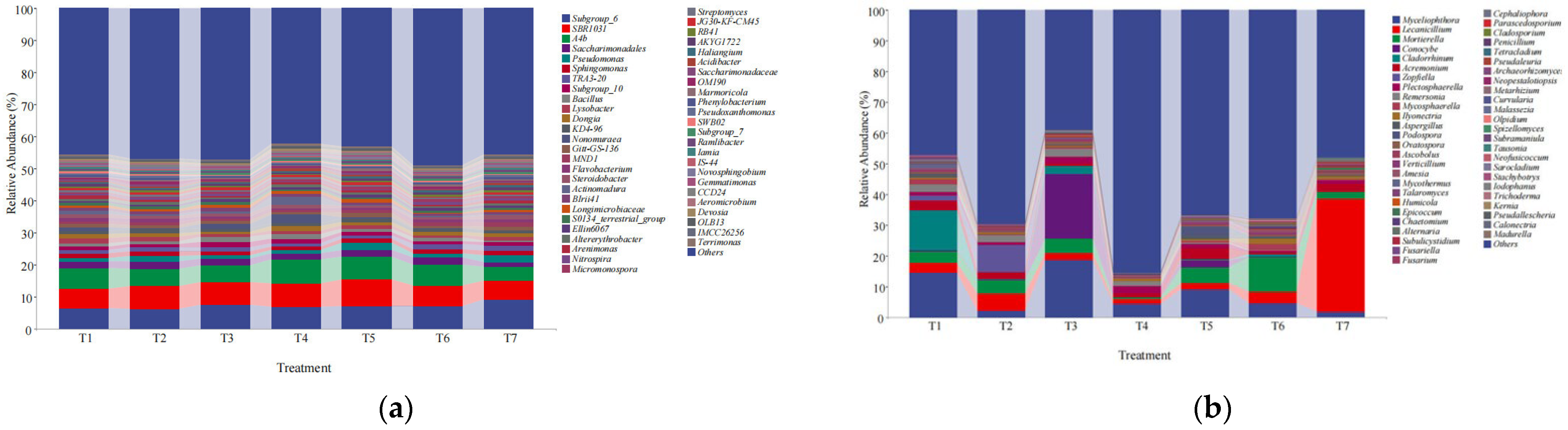
3.4.2. Analysis of the Soil Bacterial and Fungal Community Compositions at the Genus Level
3.4.3. Analysis of the Dominant Soil Bacterial and Fungal Communities at the Genus Level
3.5. Effects of Different Treatments on the Growth and Yield of Strawberry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Z.; Liang, T.; Kang, C. Molecular bases of strawberry fruit quality traits: Advances, challenges, and opportunities. Plant Physiol. 2023, 193, 900–914. [Google Scholar] [CrossRef]
- Cao, K.; Wang, S. Autotoxicity and soil sickness of strawberry (Fragaria×ananassa). Allelopath. J. 2007, 20, 103–114. [Google Scholar]
- Cota, L.V.; Maffia, L.A.; Mizubuti, E.S.; Macedo, P.E.; Antunes, R.F. Biological control of strawberry gray mold by Clonostachys rosea under field conditions. Biol. Control 2008, 46, 515–522. [Google Scholar] [CrossRef]
- Yao, H.; Jiao, X.; Wu, F. Effects of continuous cucumber cropping and alternative rotations under protected cultivation on soil microbial community diversity. Plant Soil 2006, 284, 195–203. [Google Scholar] [CrossRef]
- Meszka, B.; Malusà, E. Effects of soil disinfection on health status, growth and yield of strawberry stock plants. Crop Prot. 2014, 63, 113–119. [Google Scholar] [CrossRef]
- Chen, R.; Jiang, W.; Xu, S.; Fan, H.; Chen, X.; Shen, X.; Yin, C.; Mao, Z. An emerging chemical fumigant: Two-sided effects of dazomet on soil microbial environment and plant response. Environ. Sci. Pollut. Res. 2022, 29, 3022–3036. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, D.; Cheng, H.; Song, Z.; Ren, L.; Hao, B.; Zhu, J.; Fang, W.; Yan, D.; Li, Y. Chloropicrin alternated with dazomet improved the soil’s physicochemical properties, changed microbial communities and increased strawberry yield. Ecotoxicol. Environ. Saf. 2021, 220, 112362. [Google Scholar] [CrossRef]
- Mao, L.; Liu, X.; Sial, M.U.; Zhang, L.; Zhu, L.; Wu, C.; Cao, A. Soil application of dazomet combined with 1, 3-dichloropropene against soilborne pests for tomato production. Sci. Rep. 2024, 14, 31439. [Google Scholar] [CrossRef]
- Mao, L.G.; Wang, Q.X.; Yan, D.D.; Xie, H.W.; Li, Y.; Guo, M.X.; Cao, A. Evaluation of the combination of 1, 3-dichloropropene and dazomet as an efficient alternative to methyl bromide for cucumber production in China. Pest Manag. Sci. 2012, 68, 602–609. [Google Scholar] [CrossRef]
- Fang, W.; Yan, D.; Huang, B.; Ren, Z.; Wang, X.; Liu, X.; Li, Y.; Ouyang, C.; Migheli, Q.; Cao, A.; et al. Biochemical pathways used by microorganisms to produce nitrous oxide emissions from soils fumigated with dimethyl disulfide or allyl isothiocyanate. Soil Biol. Biochem. 2019, 132, 1–13. [Google Scholar] [CrossRef]
- Parthipan, B.; Mahadevan, A. Effects of methylisocyanate on soil microflora and the biochemical activity of soils. Environ. Pollurion 1995, 87, 283–287. [Google Scholar] [CrossRef]
- Trabelsi, D.; Mhamdi, R. Microbial inoculants and their impact on soil microbial communities: A review. BioMed Res. Int. 2013, 2013, 863240. [Google Scholar] [CrossRef]
- Alori, E.T.; Dare, M.O.; Babalola, O.O. Microbial inoculants for soil quality and plant health. Sustain. Agric. Rev. 2017, 22, 281–307. [Google Scholar]
- Vestberg, M.; Kukkonen, S.; Saari, K.; Parikka, P.; Huttunen, J.; Tainio, L.; Devos, N.; Weekers, F.; Kevers, C.; Thonart, P.; et al. Microbial inoculation for improving the growth and health of micropropagated strawberry. Appl. Soil Ecol. 2004, 27, 243–258. [Google Scholar] [CrossRef]
- de Andrade, F.M.; de Assis Pereira, T.; Souza, T.P.; Guimarães, P.H.S.; Martins, A.D.; Schwan, R.F.; Pasqual, M.; Dória, J. Beneficial effects of inoculation of growth-promoting bacteria in strawberry. Microbiol. Res. 2019, 223, 120–128. [Google Scholar] [CrossRef]
- Mahajan, G.; Rattan, P.; Sharma, A. Effect of microbial inoculants on growth, yield and quality attributes of tomato (Solanum lycopersicum). Pharma Innov. J. 2023, 11, 1503–1511. [Google Scholar]
- Wu, J.J.; Zhu, J.H.; Zhang, D.Q.; Cheng, H.Y.; Hao, B.Q.; Cao, A.C.; Yan, D.D.; Wang, Q.X.; Li, Y. Beneficial effect on the soil microenvironment of Trichoderma applied after fumigation for cucumber production. PLoS ONE 2022, 17, e0266347. [Google Scholar] [CrossRef]
- Cheng, H.; Zhang, D.; Ren, L.; Song, Z.; Li, Q.; Wu, J.; Fang, W.; Huang, B.; Yan, D.; Li, Y. Bioactivation of soil with beneficial microbes after soil fumigation reduces soil-borne pathogens and increases tomato yield. Environ. Pollut. 2021, 283, 117160. [Google Scholar] [CrossRef]
- Li, Q.; Andom, O.; Fang, W.; Yan, D.; Li, Y.; Wang, Q.; Jin, X.; Cao, A.J.A. Effects of soil amendments on soil properties, soil-borne pathogens, and strawberry growth after Dazomet fumigation. Agriculture 2023, 14, 9. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Chao, A. Nonparametric estimation of the number of classes in a population. Scand. J. Stat. 1984, 11, 265–270. [Google Scholar]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Xiong, W.; Zhao, Q.; Zhao, J.; Xun, W.; Li, R.; Zhang, R.; Wu, H.; Shen, Q. Different continuous cropping spans significantly affect microbial community membership and structure in a vanilla-grown soil as revealed by deep pyrosequencing. Microb. Ecol. 2015, 70, 209–218. [Google Scholar] [CrossRef]
- Yim, B.; Smalla, K.; Winkelmann, T. Evaluation of apple replant problems based on different soil disinfection treatments—Links to soil microbial community structure. Plant Soil 2013, 366, 617–631. [Google Scholar] [CrossRef]
- Li, J.-p.; Li, M.-q.; Hui, N.-n.; Wang, L.; Ma, Y.-q.; Qi, Y.-h. Population dynamics of main fungal pathogens in soil of continuously cropped potato. Acta Pratacult. Sin. 2013, 22, 147. [Google Scholar]
- De Cal, A.; Martinez-Treceno, A.; Salto, T.; López-Aranda, J.; Melgarejo, P. Effect of chemical fumigation on soil fungal communities in Spanish strawberry nurseries. Appl. Soil Ecol. 2005, 28, 47–56. [Google Scholar] [CrossRef]
- Huang, B.; Chen, Y.; Pei, Z.; Jiang, L.; Zhang, Y.; Wang, J.; Wang, J. Application of microbial organic fertilizers promotes the utilization of nutrients and restoration of microbial community structure and function in rhizosphere soils after dazomet fumigation. Front. Microbiol. 2023, 13, 1122611. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, S.H.; Jo, H.Y.; Finneran, K.T.; Kwon, M.J. Diversity and composition of soil Acidobacteria and Proteobacteria communities as a bacterial indicator of past land-use change from forest to farmland. Sci. Total Environ. 2021, 797, 148944. [Google Scholar] [CrossRef]
- Yelle, D.J.; Ralph, J.; Lu, F.; Hammel, K.E. Evidence for cleavage of lignin by a brown rot basidiomycete. Environ. Microbiol. 2008, 10, 1844–1849. [Google Scholar] [CrossRef]
- Schneider, T.; Keiblinger, K.M.; Schmid, E.; Sterflinger-Gleixner, K.; Ellersdorfer, G.; Roschitzki, B.; Richter, A.; Eberl, L.; Zechmeister-Boltenstern, S.; Riedel, K. Who is who in litter decomposition? Metaproteomics reveals major microbial players and their biogeochemical functions. ISME J. 2012, 6, 1749–1762. [Google Scholar] [CrossRef]
- Xie, M.; Zhang, Y.-J.; Peng, D.-L.; Zhou, J.; Zhang, X.-L.; Zhang, Z.-R.; Zhao, J.-J.; Wu, Y.-H.J.P.O. Persistence and viability of Lecanicillium lecanii in Chinese agricultural soil. PLoS ONE 2015, 10, e0138337. [Google Scholar] [CrossRef] [PubMed]
- Barrera, V.A.; Martin, M.E.; Aulicino, M.; Martínez, S.; Chiessa, G.; Saparrat, M.C.; Gasoni, A.L.J.R.a.d.m. Carbon-substrate utilization profiles by Cladorrhinum (Ascomycota). Rev. Argent. De Microbiol. 2019, 51, 302–306. [Google Scholar] [CrossRef]
- Jia, H.-t.; Khashi-ur-Rahman, M.; Wu, F.-z.; Zhou, X.-a. Effects of rotation of Indian mustard on cucumber seedling rhizosphere fungal community composition. Int. J. Agric. Biol. 2020, 23, 757–762. [Google Scholar]
- Nayak, S.; Samanta, S.; Mukherjee, A.K. Beneficial role of Aspergillus sp. in agricultural soil and environment. In Frontiers in Soil and Environmental Microbiology; CRC Press: Boca Raton, FL, USA, 2020; pp. 17–36. [Google Scholar]

| Alkali-Hydrolyzed Nitrogen (mg·kg−1) | Available Phosphorus (mg·kg−1) | Available Potassium (mg·kg−1) | Organic Matter (g·kg−1) | pH | Conductivity (μs/cm) |
|---|---|---|---|---|---|
| 133.47 | 212.27 | 386.33 | 22.9 | 7.0 | 302.5 |
| Kingdom | Treatment | ASVs | Community Abundance Index | Community Diversity Index | ||
|---|---|---|---|---|---|---|
| Chao1 Index | Observed Species | Shannon | Simpson | |||
| p < 0.05 | ||||||
| Bacteria | T1 | 13,027 | 6335.19 ± 250.41 a | 6008.70 ± 233.75 a | 11.26 ± 0.17 ab | 0.9988 ± 0.0005 a |
| T2 | 13,971 | 6551.68 ± 273.69 a | 6242.50 ± 239.18 a | 11.35 ± 0.28 ab | 0.9988 ± 0.0005 a | |
| T3 | 13,229 | 6236.40 ± 121.48 a | 5944.23 ± 69.11 a | 11.27 ± 0.60 ab | 0.9988 ± 0.0002 a | |
| T4 (CK) | 10,856 | 5332.01 ± 210.10 b | 5089.53 ± 156.99 b | 10.87 ± 0.01 b | 0.9981 ± 0.0001 b | |
| T5 | 11,238 | 5308.26 ± 289.95 b | 5129.43 ± 222.82 b | 11.12 ± 0.21 ab | 0.9987 ± 0.0002 a | |
| T6 | 14,267 | 6532.83 ± 504.53 a | 6288.47 ± 445.66 a | 11.48 ± 0.11 a | 0.9991 ± 0.0001 a | |
| T7 | 13,137 | 6332.29 ± 417.81 a | 6036.10 ± 206.82 a | 11.40 ± 0.14 ab | 0.9990 ± 0.0002 a | |
| Fungi | T1 | 432 | 203.90 ± 7.86 bc | 203.13 ± 8.33 bc | 5.28 ± 0.31 ab | 0.9298 ± 0.0238 ab |
| T2 | 356 | 179.95 ± 8.52 c | 178.50 ± 7.88 c | 3.51 ± 0.22 d | 0.7128 ± 0.0184 d | |
| T3 | 449 | 212.27 ± 9.38 b | 210.90 ± 15.68 b | 4.71 ± 0.16 bc | 0.8986 ± 0.0254 ab | |
| T4 (CK) | 391 | 189.84 ± 10.65 bc | 187.97 ± 15.22 bc | 2.57 ± 0.41 e | 0.5083 ± 0.0694 e | |
| T5 | 413 | 196.55 ± 22.28 bc | 195.77 ± 21.78 bc | 4.02 ± 0.32 cd | 0.7794 ± 0.0758 cd | |
| T6 | 616 | 268.21 ± 13.31 a | 266.60 ± 12.52 a | 5.86 ± 0.70 a | 0.9589 ± 0.0211 a | |
| T7 | 556 | 250.93 ± 20.05 a | 249.23 ± 20.58 a | 4.82 ± 0.90 bc | 0.8491 ± 0.0457 c | |
| Kingdom | Serial Number | Genus | Relative Abundance (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | T5 | T6 | T7 | |||
| 1 | Subgroup_6 | 6.48 | 6.25 | 7.48 | 6.96 | 7.10 | 7.07 | 9.15 | |
| 2 | SBR1031 | 5.98 | 7.15 | 7.01 | 7.29 | 8.31 | 6.49 | 5.93 | |
| 3 | A4b | 6.52 | 5.41 | 5.37 | 7.45 | 7.18 | 6.44 | 4.35 | |
| 4 | Saccharimonadales | 2.01 | 2.28 | 1.98 | 1.76 | 1.92 | 2.20 | 1.50 | |
| 5 | Pseudomonas | 1.19 | 1.55 | 1.05 | 1.02 | 2.37 | 1.44 | 2.04 | |
| 6 | Sphingomonas | 1.46 | 1.42 | 1.34 | 1.09 | 1.22 | 1.15 | 1.34 | |
| 7 | TRA3-20 | / | 1.29 | 1.20 | 1.00 | 1.17 | 1.62 | 1.70 | |
| 8 | Subgroup_10 | 1.04 | 1.17 | 1.58 | 1.46 | / | / | 1.01 | |
| 9 | Bacillus | / | / | 1.66 | 1.62 | 1.14 | 1.22 | / | |
| 10 | Lysobacter | 1.65 | 1.46 | / | 1.01 | / | / | 1.19 | |
| 11 | Dongia | 1.23 | / | / | 1.41 | 1.02 | / | / | |
| The relative abundance > 1% of the genus number | 9 | 9 | 9 | 11 | 9 | 8 | 9 | ||
| Dominant genus proportion/% | 27.56 | 27.98 | 28.67 | 32.07 | 31.43 | 27.63 | 28.21 | ||
| 1 | Myceliophthora | 14.64 | 2.17 | 18.81 | 4.27 | 9.17 | 4.67 | 1.96 | |
| 2 | Lecanicillium | 3.04 | 5.48 | 1.97 | 1.50 | 2.13 | 3.61 | 36.55 | |
| 3 | Mortierella | 3.86 | 4.34 | 4.87 | / | 4.99 | 11.37 | 2.30 | |
| 4 | Conocybe | / | / | 20.98 | / | 2.20 | / | / | |
| 5 | Cladorrhinum | 12.81 | / | 2.61 | / | / | / | / | |
| 6 | Acremonium | 3.29 | 2.23 | / | 1.23 | 3.70 | / | 2.50 | |
| 7 | Zopfiella | 1.52 | 8.85 | / | / | / | / | / | |
| 8 | Plectosphaerella | 1.39 | / | 2.06 | 2.55 | 1.53 | / | 1.35 | |
| 9 | Remersonia | 2.13 | 2.04 | 2.83 | 1.34 | / | / | / | |
| 10 | Mycosphaerella | 2.04 | / | / | / | / | 2.14 | / | |
| 11 | Ilyonectria | / | / | / | / | / | 1.53 | / | |
| 12 | Aspergillus | 1.53 | / | / | / | 1.13 | / | / | |
| 13 | Podospora | / | / | / | / | 3.02 | / | / | |
| 14 | Mycothermus | 1.26 | / | / | / | / | / | / | |
| The relative abundance > 1% of the genus number | 11 | 6 | 7 | 5 | 8 | 5 | 5 | ||
| Dominant genus proportion/% | 47.51 | 25.11 | 54.13 | 10.89 | 27.87 | 23.32 | 44.66 | ||
| Treatment | Seedling Mortality (%) | Number of Surviving Plants (667 m−2) | Average Fruit per Plant (Units) | Average Single Fruit Weight (g−1) | Yield kg·667 m−2 |
|---|---|---|---|---|---|
| p < 0.05 | |||||
| T1 | 4.7 ± 2.52 b | 7626.7 ± 201.33 a | 7.3 ± 0.15 a | 24.3 ± 0.47 a | 1143.4 ± 15.8 abcd |
| T2 | 10.7 ± 3.79 a | 7146.7 ± 302.88 b | 7.3 ± 0.25 a | 24.4 ± 0.70 a | 1088.2 ± 13.89 cd |
| T3 | 3.7 ± 1.53 b | 7706.7 ± 122.2 a | 7.4 ± 0.1 a | 24.7 ± 0.42 a | 1181.2 ± 14.73 ab |
| T4 | 12.3 ± 1.53 a | 7013.3 ± 122.2 b | 7.3 ± 0.46 a | 24.5 ± 0.66 a | 1065.5 ± 71.11 d |
| T5 | 6.3 ± 1.53 b | 7493.3 ± 122.2 a | 7.2 ± 0.36 a | 24.0 ± 0.42 a | 1102.5 ± 79.92 bcd |
| T6 | 2.3 ± 0.58 b | 7813.3 ± 46.19 a | 7.3 ± 0.21 a | 24.6 ± 1.22 a | 1195.5 ± 31.68 a |
| T7 | 4.3 ± 3.97 b | 7653.3 ± 122.2 a | 7.4 ± 0.12 a | 24.0 ± 0.61 a | 1152.0 ± 28.46 abc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, R.; Li, Y.; Meng, J.; Han, J. Effects of the Addition of Microbial Agents After Dazomet Fumigation on the Microbial Community Structure in Soils with Continuous Cropping of Strawberry (Fragaria × Ananassa Duch.). Microorganisms 2025, 13, 1178. https://doi.org/10.3390/microorganisms13061178
Wu R, Li Y, Meng J, Han J. Effects of the Addition of Microbial Agents After Dazomet Fumigation on the Microbial Community Structure in Soils with Continuous Cropping of Strawberry (Fragaria × Ananassa Duch.). Microorganisms. 2025; 13(6):1178. https://doi.org/10.3390/microorganisms13061178
Chicago/Turabian StyleWu, Ran, Yan Li, Jian Meng, and Jiangwei Han. 2025. "Effects of the Addition of Microbial Agents After Dazomet Fumigation on the Microbial Community Structure in Soils with Continuous Cropping of Strawberry (Fragaria × Ananassa Duch.)" Microorganisms 13, no. 6: 1178. https://doi.org/10.3390/microorganisms13061178
APA StyleWu, R., Li, Y., Meng, J., & Han, J. (2025). Effects of the Addition of Microbial Agents After Dazomet Fumigation on the Microbial Community Structure in Soils with Continuous Cropping of Strawberry (Fragaria × Ananassa Duch.). Microorganisms, 13(6), 1178. https://doi.org/10.3390/microorganisms13061178





