Synergistic Effect of Essential Oils and Rhamnolipid on Xanthomonas citri Subsp. citri
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
2.2. Essential Oils and Rhamnolipid
2.3. GC-MS Analysis
2.4. Bacterial Growth Inhibition
2.5. Synergism
- * IC 90 A combined: Refers to the concentration of compound A in combination with compound B required to inhibit 90% of bacterial growth.
- * IC 90 B combined: Refers to the concentration of compound B combined with compound A required to inhibit 90% of bacterial growth.
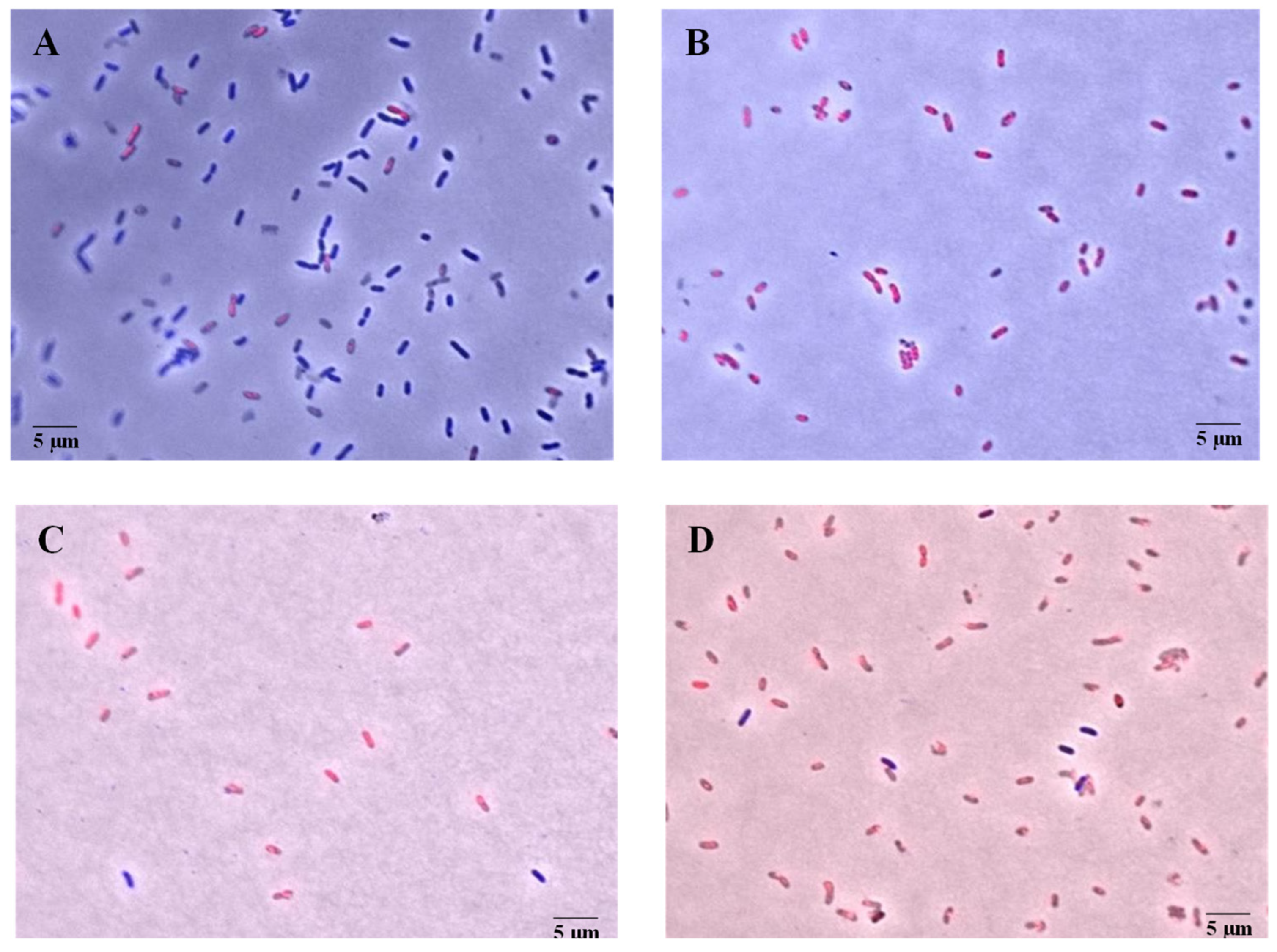
2.6. Cell Membrane Permeabilization
2.7. Effect of Bioactive Combinations on Biofilm Production by X. citri Subsp. citri
2.8. Time-Kill Assay
2.9. Statistical Analysis
3. Results
3.1. Bacterial Growth Inhibition
3.2. GC-MS Analysis
3.3. Synergism
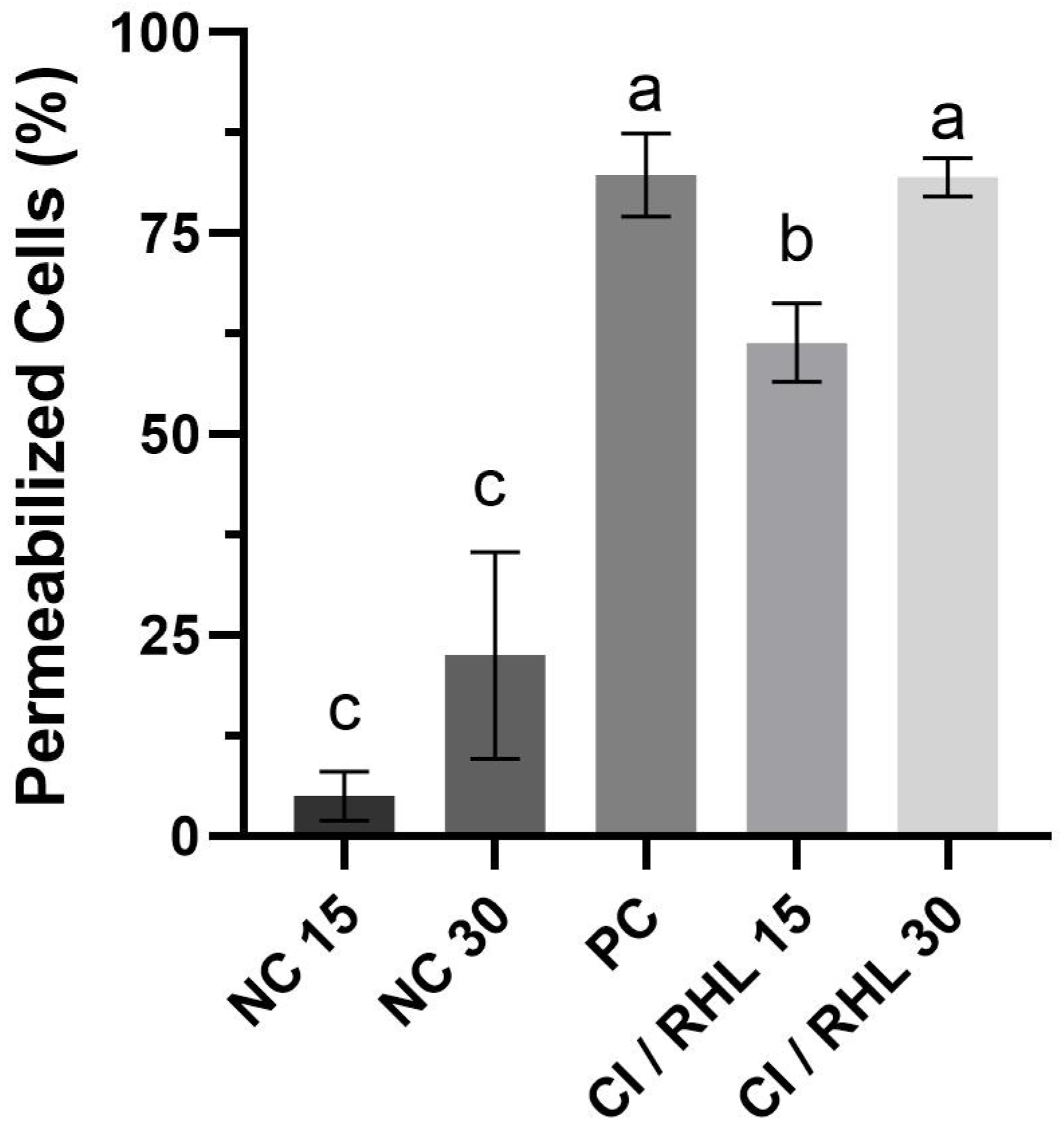
3.4. Cell Membrane Permeabilization
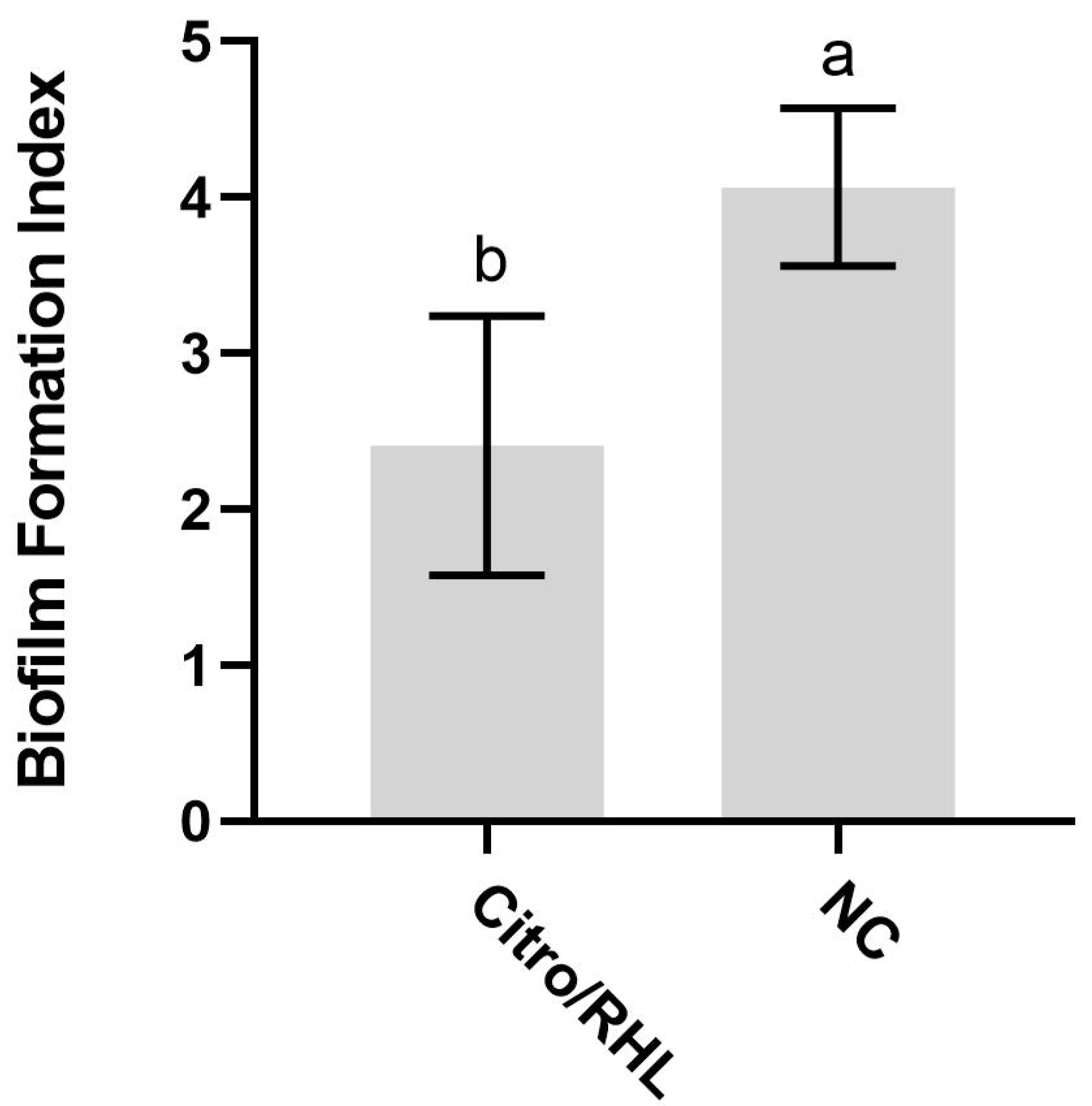
3.5. Effect of Synergic Combination on Biofilm Production
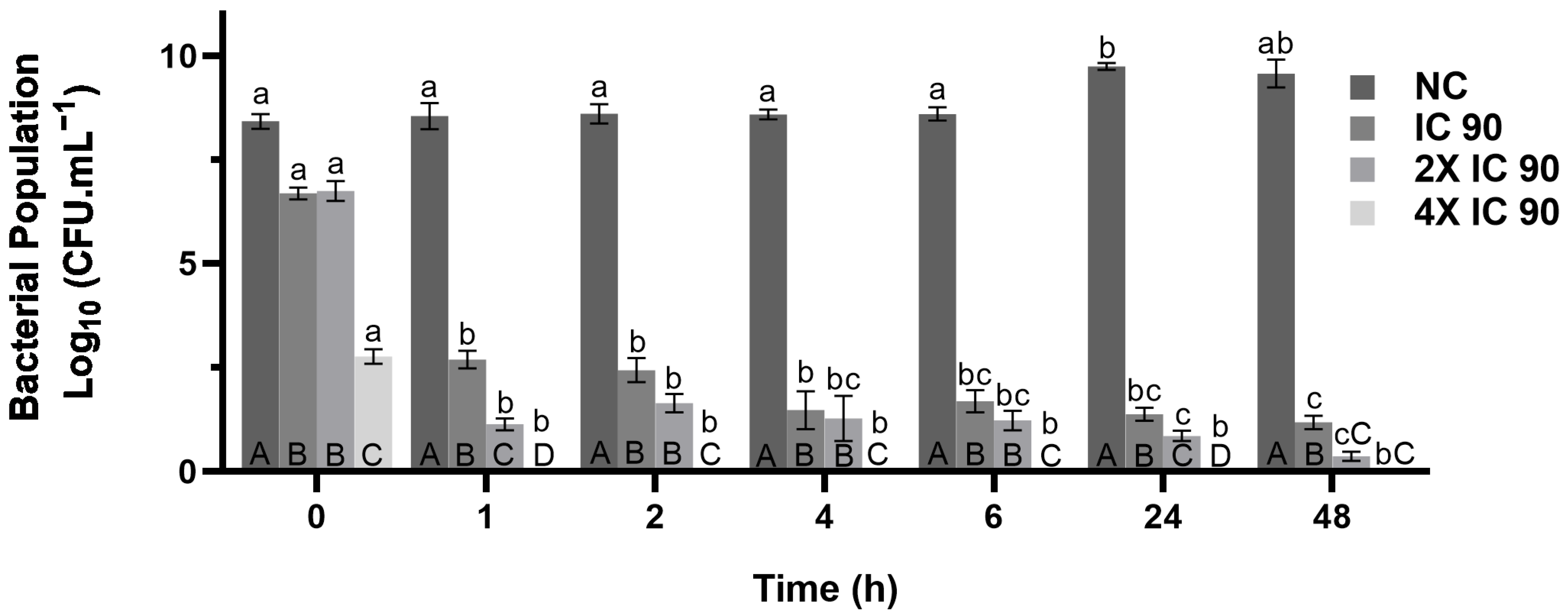
3.6. Time-Kill Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Naqvi, S.A.H.; Wang, J.; Malik, M.T.; Umar, U.U.D.; Ateeq-Ur-Rehman; Hasnain, A.; Sohail, M.A.; Shakeel, M.T.; Nauman, M.; Hafeez-ur-Rehman; et al. Citrus Canker-Distribution, Taxonomy, Epidemiology, Disease Cycle, Pathogen Biology, Detection, and Management: A Critical Review and Future Research Agenda. Agro 2022, 12, 1075. [Google Scholar] [CrossRef]
- Ference, C.M.; Gochez, A.M.; Behlau, F.; Wang, N.; Graham, J.H.; Jones, J.B. Recent Advances in the Understanding of Xanthomonas citri subsp. citri Pathogenesis and Citrus Canker Disease Management. Mol. Plant Pathol. 2018, 19, 1307–1325. [Google Scholar] [CrossRef]
- Ali, S.; Hameed, A.; Muhae-Ud-Din, G.; Ikhlaq, M.; Ashfaq, M.; Atiq, M.; Ali, F.; Zia, Z.U.; Naqvi, S.A.H.; Wang, Y. Citrus Canker: A Persistent Threat to the Worldwide Citrus Industry—An Analysis. Agronomy 2023, 13, 1112. [Google Scholar] [CrossRef]
- Rigano, L.; Siciliano, F.; Enrique, R.; Sendín, L.; Filippone, P.; Torres, P.; Qüesta, J.; Dow, M.; Castagnaro, A.; Vojnov, A.; et al. Biofilm formation, epiphytic fitness, and canker development in Xanthomonas axonopodis pv. citri. Mol. Plant Microbe Interact. 2007, 20, 1222–1230. [Google Scholar] [CrossRef]
- Ficarra, F.; Grandellis, C.; Galván, E.; Ielpi, L.; Feil, R.; Lunn, J.; Gottig, N.; Ottado, J. Xanthomonas citri ssp. citri requires the outer membrane porin OprB for maximal virulence and biofilm formation. Mol. Plant Pathol. 2017, 18, 720–733. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, N. The wxacO gene of Xanthomonas citri ssp. citri encodes a protein with a role in lipopolysaccharide biosynthesis, biofilm formation, stress tolerance and virulence. Mol. Plant Pathol. 2011, 12, 381–396. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, N. Foliar application of biofilm formation-inhibiting compounds enhances control of citrus canker caused by Xanthomonas citri subsp. citri. Phytopathology 2014, 104, 134–142. [Google Scholar] [CrossRef]
- Behlau, F. An overview of citrus canker in Brazil. Trop. Plant Pathol. 2020, 46, 441–452. [Google Scholar] [CrossRef]
- Fan, X.; Saleem, T.; Zou, H. Copper resistance mechanisms in plant pathogenic bacteria. Phytopathol. Mediterr. 2022, 61, 129–138. [Google Scholar] [CrossRef]
- Dewdney, M.M.; Graham, J.H. Florida citrus pest management guide: Citrus canker. Univ. Fla. IFAS Ext. 2019, PP-182/CG040. [Google Scholar] [CrossRef]
- Behlau, F.; Belasque, J.; Graham, J.H.; Leite, R.P. Effect of Frequency of Copper Applications on Control of Citrus Canker and the Yield of Young Bearing Sweet Orange Trees. Crop Prot. 2010, 29, 300–305. [Google Scholar] [CrossRef]
- Areas, M.S.; Gonçalves, R.M.; Soman, J.M.; Souza Filho, R.C.; Gioria, R.; Da Silva Junior, T.A.F.; Maringoni, A.C. Resistance of Xanthomonas euvesicatoria Strains from Brazilian Pepper to Copper and Zinc Sulfates. An. Acad. Bras. Ciênc. 2018, 90, 2375–2380. [Google Scholar] [CrossRef] [PubMed]
- Lopes, S.A.; Quezado-Soares, A.M. Bacterial diseases of tomato. In Disease Control in Vegetable Crops; Zambolim, L., Vale, F.X.R., Costa, H., Eds.; UFV Press: Chilliwack, BC, Canada, 2000; pp. 754–784. [Google Scholar]
- Kazeminezhad, I.; Mosivand, S. Elimination of copper and nickel from wastewater by electrooxidation method. J. Magn. Magn. Mater. 2017, 422, 84–92. [Google Scholar] [CrossRef]
- Macomber, L.; Imlay, J.A. The Iron-Sulfur Clusters of Dehydratases Are Primary Intracellular Targets of Copper Toxicity. Proc. Natl. Acad. Sci. USA 2009, 106, 8344–8349. [Google Scholar] [CrossRef]
- Chillappagari, S.; Seubert, A.; Trip, H.; Kuipers, O.P.; Marahiel, M.A.; Miethke, M. Copper Stress Affects Iron Homeostasis by Destabilizing Iron-Sulfur Cluster Formation in Bacillus subtilis. J. Bacteriol. 2010, 192, 2512–2524. [Google Scholar] [CrossRef]
- Martinez, J.G.; Paran, G.P.; Rizon, R.; De Meester, N.; Moens, T. Copper effects on soil nematodes and their possible impact on leaf litter decomposition: A microcosm approach. Eur. J. Soil Biol. 2016, 73, 1–7. [Google Scholar] [CrossRef]
- Isman, M.B.; Miresmailli, S.; Machial, C. Commercial Opportunities for Pesticides Based on Plant Essential Oils in Agriculture, Industry, and Consumer Products. Phytochem. Rev. 2011, 10, 197–204. [Google Scholar] [CrossRef]
- Xiao, S.; Cui, P.; Shi, W.; Zhang, Y. Identification of Essential Oils with Activity against Stationary Phase Staphylococcus aureus. BMC Complement. Med. Ther. 2020, 20, 99. [Google Scholar] [CrossRef] [PubMed]
- Marin, V.R.; Zamunér, C.F.C.; Santos, E.A.; Ferreira, H.; Sass, D.C. Essential Oils as Alternatives in the Control of Phytopathogens: A Systematic Review of the Last Five Years. J. Essent. Oil-Bear. Plants 2024, 27, 903–937. [Google Scholar] [CrossRef]
- Nagy, T.; Tóth, M.; Papp, G.; Horváth, B.; Kocsis, B.; Kustos, I. Antibacterial Efficacy of Essential Oils against Xanthomonas arboricola pv. pruni: A Comparative Study. Molecules 2023, 28, 2345. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential Oils in Food Preservation: Mode of Action, Synergies, and Interactions with Food Matrix Components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef]
- Rooney, A.P.; Price, N.P.J.; Ray, K.J.; Kuo, T.M. Isolation and Characterization of Rhamnolipid-Producing Bacterial Strains from a Biodiesel Facility. Microbiology 2009, 155, 1028–1038. [Google Scholar] [CrossRef][Green Version]
- Lovaglio, R.B.; Dos Santos, F.J.; Jafelicci, M., Jr.; Contiero, J. Rhamnolipid Emulsifying Activity and Emulsion Stability: pH Rules. Colloids Surf. B Biointerfaces 2011, 85, 301–305. [Google Scholar] [CrossRef]
- Yochikawa, J.T.H. Evaluation of Anti-Xanthomonas Citri Activity of Rhamnolipids Produced by Pseudomonas Aeruginosa. Bachelor’s Thesis, São Paulo State University, Institute of Biosciences of Rio Claro, Rio Claro, Brazil, 2016. [Google Scholar]
- Vatsa, P.; Sanchez, L.; Clement, C.; Baillieul, F.; Dorey, S. Rhamnolipid Biosurfactants as New Players in Animal and Plant Defense against Microbes. Int. J. Mol. Sci. 2010, 11, 5095. [Google Scholar] [CrossRef]
- Abalos, A.; Pinazo, A.; Infante, M.R.; Casals, M.; García, F.; Manresa, A. Physicochemical and Antimicrobial Properties of New Rhamnolipids Produced by Pseudomonas aeruginosa AT10 from Soybean Oil Refinery Wastes. Langmuir 2001, 17, 1367–1371. [Google Scholar] [CrossRef]
- Haba, E.; Bouhdid, S.; Torrego-Solana, N.; Marqués, A.; Espuny, M.; García-Celma, M.; Manresa, Á. Rhamnolipids as Emulsifying Agents for Essential Oil Formulations: Antimicrobial Effect against Candida albicans and Methicillin-Resistant Staphylococcus aureus. Int. J. Pharm. 2014, 476, 134–141. [Google Scholar] [CrossRef]
- Salazar-Bryam, A.M.; Silva, V.L.; De Abreu, M.R.; Matos, R.S.; Da Rocha, M.A.G.; Neves, R.C.; Camargo-Mathias, M.I.; Von Zuben, C.J.; Lovaglio, R.B.; Contiero, J. Rhamnolipids and Essential Oils in the Control of Mosquito-Borne Tropical Diseases. Appl. Microbiol. Biotechnol. 2021, 105, 7505–7515. [Google Scholar] [CrossRef]
- Marin, V.R.; Zamuner, C.F.C.; Hypolito, G.B.; Ferrarezi, J.H.; Alleoni, N.; Caccalano, M.N.; Ferreira, H.; Sass, D.C. Antibacterial activity of Cymbopogon species essential oils against Xanthomonas citri and their use in post-harvest treatment for citrus canker management. Lett. Appl. Microbiol. 2024, 77, ovae041. [Google Scholar] [CrossRef]
- Palomino, J.C.; Martin, A.; Camacho, M.; Guerra, H.; Swings, J.; Portaels, F. Resazurin Microtiter Assay Plate: Simple and Inexpensive Method for Detection of Drug Resistance in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2002, 46, 2720–2722. [Google Scholar] [CrossRef]
- Rodríguez-Melcón, C.; Alonso-Calleja, C.; García-Fernández, C.; Carballo, J.; Capita, R. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) for Twelve Antimicrobials (Biocides and Antibiotics) in Eight Strains of Listeria monocytogenes. Biology 2021, 11, 46. [Google Scholar] [CrossRef]
- Parvekar, P.; Palaskar, J.; Metgud, S.; Maria, R.; Dutta, S. The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of silver nanoparticles against Staphylococcus aureus. Biomater. Investig. Dent. 2020, 7, 105–109. [Google Scholar] [CrossRef]
- Liu, Y.; Moore, J.; Kolling, G.; McGrath, J.; Papin, J.; Swami, N. Minimum Bactericidal Concentration of Ciprofloxacin to Pseudomonas aeruginosa Determined Rapidly Based on Pyocyanin Secretion. Sens. Actuators B Chem. 2020, 312, 127936. [Google Scholar] [CrossRef]
- Bellio, P.; Fagnani, L.; Nazzicone, L.; Celenza, G. New and simplified method for drug combination studies by checkerboard assay. MethodsX 2021, 8, 101543. [Google Scholar] [CrossRef]
- Mirzaei-Najafgholi, H.; Tarighi, S.; Golmohammadi, M.; Taheri, P. The Effect of Citrus Essential Oils and Their Constituents on Growth of Xanthomonas citri subsp. citri. Molecules 2017, 22, 591. [Google Scholar] [CrossRef]
- Zamuner, C.F.C.; Marin, V.R.; Dilarri, G.; Hypolito, G.B.; Sass, D.C.; Ferreira, H. Oregano essential oil and its main components Thymol and Carvacrol as alternatives to control citrus canker. Front. Agron. 2023, 5, 1148969. [Google Scholar] [CrossRef]
- Malamud, F.; Torres, P.S.; Roeschlin, R.; Rigano, L.A.; Enrique, R.; Bonomi, H.R.; Castagnaro, A.P.; Marano, M.R.; Vojnov, A.A. The Xanthomonas axonopodis pv. citri flagellum is required for mature biofilm and canker development. Microbiology 2011, 157, 819–829. [Google Scholar] [CrossRef]
- JASP Team. JASP (Version 0.14.1) [Computer Software]; JASP: Amsterdam, The Netherlands, 2020; Available online: https://jasp-stats.org/ (accessed on 10 October 2023).
- Gerits, E.; Blommaert, E.; Lippell, A.; O’Neill, A.J.; Weytjens, B.; De Maeyer, D.; Fierro, A.C.; Marchal, K.; Marchand, A.; Chaltin, P. Elucidation of the mode of action of a new antibacterial compound active against Staphylococcus aureus and Pseudomonas aeruginosa. PLoS ONE 2016, 11, e0155139. [Google Scholar] [CrossRef]
- Rasmussen, T.B.; Skindersoe, M.E.; Bjarnsholt, T.; Phipps, R.K.; Bisgaard, K.; Jensen, P.O.; Andersen, J.B.; Koch, B.; Larsen, T.O.; Hentzer, M.; et al. Identity and Effects of Quorum-Sensing Inhibitors Produced by Penicillium Species. Microbiology 2005, 151, 1325–1340. [Google Scholar] [CrossRef]
- Liaqat, I.; Bachmann, R.T.; Edyvean, R.G.J. Type 2 Quorum Sensing Monitoring, Inhibition, and Biofilm Formation in Marine Microorganisms. Curr. Microbiol. 2014, 68, 342–351. [Google Scholar] [CrossRef]
- Santamarta, S.; Aldavero, A.C.; Rojo, M.A. Essential Oil of Cymbopogon martini, Source of Geraniol, as a Potential Antibacterial Agent against Bacillus subtilis, a Pathogen of the Bakery Industry. F1000Research 2021, 10, 1027. [Google Scholar] [CrossRef]
- Cerri, A.C.; Esmerino, L.A. Antimicrobial Activity and Antibiofilm Effect of Essential Oils: A Comparative Study. Braz. J. Dev. 2022, 8, 73850–73863. [Google Scholar] [CrossRef]
- Sharma, Y.; Khan, L.A.; Manzoor, N. Anti-Candida activity of geraniol involves disruption of cell membrane integrity and function. J. Mycol. Méd. 2016, 26, 244–254. [Google Scholar] [CrossRef]
- Zeferino, R.C.F. Synthesis of Geranyl Acetate and Neryl Acetate via Heterogeneous Catalysis with Ion Exchange Resin and Characterization of the Esters for Potential Applications as Antimicrobial Additives. Ph.D. Thesis, Federal University of Santa Catarina, Florianópolis, Brazil, 2021. Available online: https://repositorio.ufsc.br/handle/123456789/231154 (accessed on 10 October 2023).
- Serero, A.; Bedrat, A.; Baulande, S.; Bastianelli, G.; Colavizza, D.; Desfougères, T.; Pignède, G.; Quipourt-Isnard, A.D.; Nicolas, A. Recombination in a sterile polyploid hybrid yeast upon meiotic Return-To-Growth. Microb. Res. 2021, 250, 126789. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological Effects of Essential Oils—A Review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Noorshilawati, A.A.; Nurul Asyiqin, A.H.B.; Nur Suraya, A.; Aiza, H. Evaluation of antibacterial activity of Piper betle leaf extracts against pathogen of soft rot disease. IOP Conf. Ser. Earth Environ. Sci. 2023, 1182, 012049. [Google Scholar] [CrossRef]
- Marchese, A.; Barbieri, R.; Coppo, E.; Orhan, I.E.; Daglia, M.; Nabavi, S.F.; Nabavi, S.M. Antimicrobial Activity of Eugenol and Essential Oils Containing Eugenol: A Mechanistic Viewpoint. Crit. Rev. Microbiol. 2017, 43, 668–689. [Google Scholar] [CrossRef]
- Abdel-Mawgoud, A.M.; Lépine, F.; Déziel, E. Rhamnolipids: Diversity of structures, microbial origins and roles. Appl. Microbiol. Biotechnol. 2010, 86, 1323–1336. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, J.Y.; Park, J.Y.; Kim, J.S.; Seo, M.K.; Shin, M.K.; Kim, J.H. Synergistic bactericidal effects of carvone and β-lactams against Xanthomonas campestris pv. vesicatoria. Appl. Biol. Chem. 2023, 66, 44. [Google Scholar] [CrossRef]
- Côté, I.M.; Darling, E.S.; Brown, C.J. Interactions among ecosystem stressors and their importance in conservation. Proc. R. Soc. B 2016, 283, 20152592. [Google Scholar] [CrossRef]
- Tang, C.; Chen, J.; Zhang, L.; Zhang, R.; Zhang, S.; Ye, S.; Zhao, Z.; Yang, D. Exploring the Antibacterial Mechanism of Essential Oils by Membrane Permeability, Apoptosis, and Biofilm Formation Combination with Proteomic Analysis against Methicillin-Resistant Staphylococcus aureus. Int. J. Med. Microbiol. 2020, 310, 151435. [Google Scholar] [CrossRef]
- Bharali, P.; Saikia, J.; Ray, A.; Konwar, B. Rhamnolipid (RL) from Pseudomonas aeruginosa OBP1: A Novel Chemotaxis and Antibacterial Agent. Colloids Surf. B Biointerfaces 2013, 103, 502–509. [Google Scholar] [CrossRef]
- Pontes, E.; Melo, H.; Nogueira, J.; Firmino, N.; De Carvalho, M.; Júnior, F.; Cavalcante, T. Antibiofilm Activity of the Essential Oil of Citronella (Cymbopogon nardus) and Its Major Component, Geraniol, on the Bacterial Biofilms of Staphylococcus aureus. Food Sci. Biotechnol. 2018, 28, 633–639. [Google Scholar] [CrossRef]
- Cunha, B.; Duque, C.; Caiaffa, K.; Massunari, L.; Catanoze, I.; Santos, D.; De Oliveira, S.; Guiotti, A. Cytotoxicity and Antimicrobial Effects of Citronella Oil (Cymbopogon nardus) and Commercial Mouthwashes on S. aureus and C. albicans Biofilms on Prosthetic Materials. Arch. Oral Biol. 2019, 109, 104577. [Google Scholar] [CrossRef]
- Kim, L.; Jung, Y.; Yu, H.; Chae, K.; Kim, I. Physicochemical Interactions between Rhamnolipids and Pseudomonas aeruginosa Biofilm Layers. Environ. Sci. Technol. 2015, 49, 3718–3726. [Google Scholar] [CrossRef]
- NCCLS. Methods for Determining Bactericidal Activity of Antimicrobial Agents: Approved Guideline M26-A; National Committee for Clinical Laboratory Standards: Wayne, PA, USA, 1999. [Google Scholar]
- Petersen, P.J.; Jones, C.H.; Bradford, P.A. In vitro Antibacterial Activities of Tigecycline and Comparative Agents by Time-Kill Kinetic Studies in Fresh Mueller-Hinton Broth. Diagn. Microbiol. Infect. Dis. 2007, 59, 347–349. [Google Scholar] [CrossRef]
- Silva, F.; Lourenço, O.; Queiroz, J.; Domingues, F.C. Bacteriostatic versus Bactericidal Activity of Ciprofloxacin in Escherichia coli Assessed by Flow Cytometry Using a Novel Far-Red Dye. J. Antibiot. 2011, 64, 321–325. [Google Scholar] [CrossRef]
- Pavela, R.; Benelli, G. Essential oils as ecofriendly biopesticides? Challenges and constraints. Trends Plant Sci. 2016, 21, 1000–1007. [Google Scholar] [CrossRef]
- Henkel, M.; Geissler, M.; Weggenmann, F.; Hausmann, R. Production of microbial biosurfactants: Status quo of rhamnolipid and surfactin towards large-scale production. Biotechnol. J. 2017, 12, 1600561. [Google Scholar] [CrossRef]
- Damalas, C.A.; Koutroubas, S.D. Current status and recent developments in biopesticide use. Agriculture 2018, 8, 13. [Google Scholar] [CrossRef]
| Samples | IC 90 | MBC |
|---|---|---|
| Palmarosa | 0.06 | 0.06 |
| Citronella | 0.15 | 0.25 |
| Geranium | 0.06 | 0.06 |
| Clove | 0.06 | 0.06 |
| Rhamnolipids | 0.3 | 0.5 |
| Compounds | Ret. Time | Height % | Area % |
|---|---|---|---|
| β-Myrcene | 4.282 | 1.65 | 1.08 |
| β-cis-Ocimene | 5.142 | 3.56 | 2.46 |
| Linalool | 6.057 | 4.75 | 3.02 |
| Geraniol | 9.258 | 61.09 | 71.2 |
| Citral | 9.611 | 1.68 | 1.16 |
| Neryl acetate (Z) | 11.999 | 24.84 | 19.17 |
| Caryophyllene | 13.063 | 2.43 | 1.91 |
| Total | 100 |
| Compounds | Ret. Time | Height % | Area % |
|---|---|---|---|
| Sulcatone | 4.211 | 2.00 | 0.99 |
| Myrcene | 4.284 | 1.94 | 0.59 |
| D-Limonene | 4.927 | 2.45 | 3.61 |
| Linalol | 6.057 | 2.04 | 1.79 |
| Citronellal | 7.099 | 2.32 | 7.78 |
| Cis-2-Decen-1-ol | 8.156 | 2.08 | 0.59 |
| Citronellol | 8.625 | 2.23 | 6.33 |
| Citral (Z) | 8.992 | 2.43 | 9.48 |
| Geraniol | 9.239 | 3.16 | 43.68 |
| Citral (E) | 9.614 | 2.46 | 13.53 |
| Citronellyl acetate | 11.315 | 2.30 | 1.75 |
| Neryl Acetate (Z) | 11.992 | 2.36 | 4.74 |
| Caryophyllene | 13.062 | 2.61 | 3.42 |
| Γ-Muurolene | 15.034 | 2.59 | 1.72 |
| Total | 100 |
| Compounds | Ret. Time | Height % | Area % |
|---|---|---|---|
| Linalool | 6.060 | 6.46 | 5.2 |
| Rose Oxide (E) | 6.661 | 0.66 | 0.54 |
| (+)-Isomenthone (Cis) | 7.253 | 4.75 | 4.19 |
| (+)-Isomenthone (Trans) | 7.481 | 4.65 | 4.28 |
| Citronellol | 8.643 | 31.52 | 34.63 |
| Citral | 8.994 | 0.6 | 0.51 |
| Geraniol (Z) | 9.218 | 16.48 | 15.73 |
| Citronellyl formate | 9.648 | 10.97 | 11.02 |
| Geranyl Bromide | 10.247 | 3.75 | 3.48 |
| 2,6-Octadiene, 2,6 dimethyl | 11.319 | 0.62 | 0.53 |
| α-Copaene | 12.056 | 0.7 | 0.61 |
| β-Bourbonene | 12.285 | 1.58 | 1.48 |
| Caryophyllene | 13.066 | 1.32 | 1.26 |
| Citronellyl propionate | 13.271 | 0.81 | 0.77 |
| β-Bisabolene | 13.949 | 0.93 | 0.95 |
| Citronellyl butyrate | 15.073 | 0.82 | 0.66 |
| γ-Muurolene | 15.190 | 1.53 | 1.87 |
| Geranyl isobutyrate | 15.748 | 1.01 | 0.9 |
| Phenylethyl tiglate | 16.420 | 1.32 | 1.34 |
| epi-γ-Eudesmol | 17.307 | 6.5 | 7.33 |
| Geranyl tiglate | 18.589 | 1.37 | 1.31 |
| Total | 100 |
| Compounds | Ret. Time | Height % | Area % |
|---|---|---|---|
| Chavibetol | 11.617 | 58.99 | 63.84 |
| Caryophyllene | 13.071 | 6.64 | 5.3 |
| Eugenol Acetate | 15.175 | 34.37 | 30.86 |
| Total | 100 |
| Essential Oils | RHL * | |||||||
|---|---|---|---|---|---|---|---|---|
| 0.002 | 0.004 | 0.009 | 0.018 | 0.030 | 0.075 | 0.15 | 0.3 | |
| Citronella | ||||||||
| 0.15 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 |
| 0.075 | 0.5 | 0.5 | 0.5 | 0.6 | 0.6 | 0.7 | 1 | 1.5 |
| 0.03 | 0.7 | 1.2 | ||||||
| 0.018 | 0.6 | 1.1 | ||||||
| Palmarosa | ||||||||
| 0.06 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 |
| 0.03 | 1 | 1.5 | ||||||
| 0.015 | 0.7 | 1.2 | ||||||
| 0.0075 | 0.6 | 1.1 | ||||||
| Geranium | ||||||||
| 0.06 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 |
| 0.03 | 1 | 1.5 | ||||||
| 0.015 | 1.2 | |||||||
| 0.0075 | 1.1 | |||||||
| Clove | ||||||||
| 0.06 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 |
| 0.03 | 1 | 1.5 | ||||||
| 0.015 | 1.2 | |||||||
| 0.0075 | 1.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sereia, M.O.P.; Santos, E.A.d.; Leite, L.P.; Neves, R.C.; Marin, V.R.; Ferreira, H.; Contiero, J.; Sass, D.C. Synergistic Effect of Essential Oils and Rhamnolipid on Xanthomonas citri Subsp. citri. Microorganisms 2025, 13, 1153. https://doi.org/10.3390/microorganisms13051153
Sereia MOP, Santos EAd, Leite LP, Neves RC, Marin VR, Ferreira H, Contiero J, Sass DC. Synergistic Effect of Essential Oils and Rhamnolipid on Xanthomonas citri Subsp. citri. Microorganisms. 2025; 13(5):1153. https://doi.org/10.3390/microorganisms13051153
Chicago/Turabian StyleSereia, Maria Olimpia Pereira, Eduarda Araujo dos Santos, Lucas Prado Leite, Raphael Culim Neves, Vítor Rodrigues Marin, Henrique Ferreira, Jonas Contiero, and Daiane Cristina Sass. 2025. "Synergistic Effect of Essential Oils and Rhamnolipid on Xanthomonas citri Subsp. citri" Microorganisms 13, no. 5: 1153. https://doi.org/10.3390/microorganisms13051153
APA StyleSereia, M. O. P., Santos, E. A. d., Leite, L. P., Neves, R. C., Marin, V. R., Ferreira, H., Contiero, J., & Sass, D. C. (2025). Synergistic Effect of Essential Oils and Rhamnolipid on Xanthomonas citri Subsp. citri. Microorganisms, 13(5), 1153. https://doi.org/10.3390/microorganisms13051153






