Programming Effects of Maternal Nutrition on Intestinal Development and Microorganisms of Offspring: A Review on Pigs
Abstract
1. Introduction
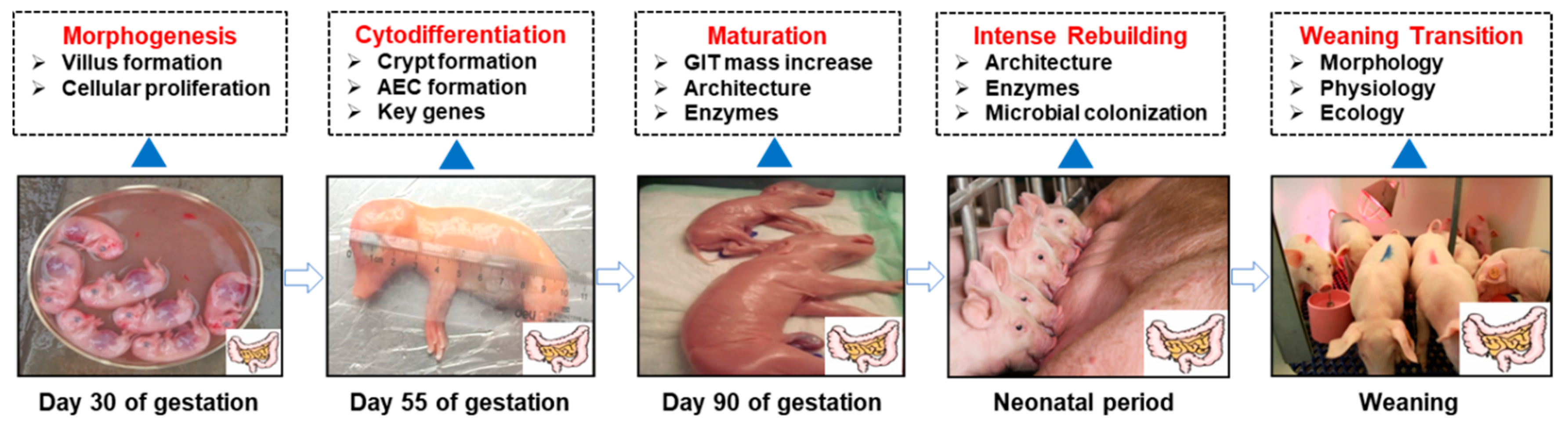
2. Intestinal Development (From Fetus to Neonates)
3. Immune System and Microorganism Colonization (From Fetus to Neonates)
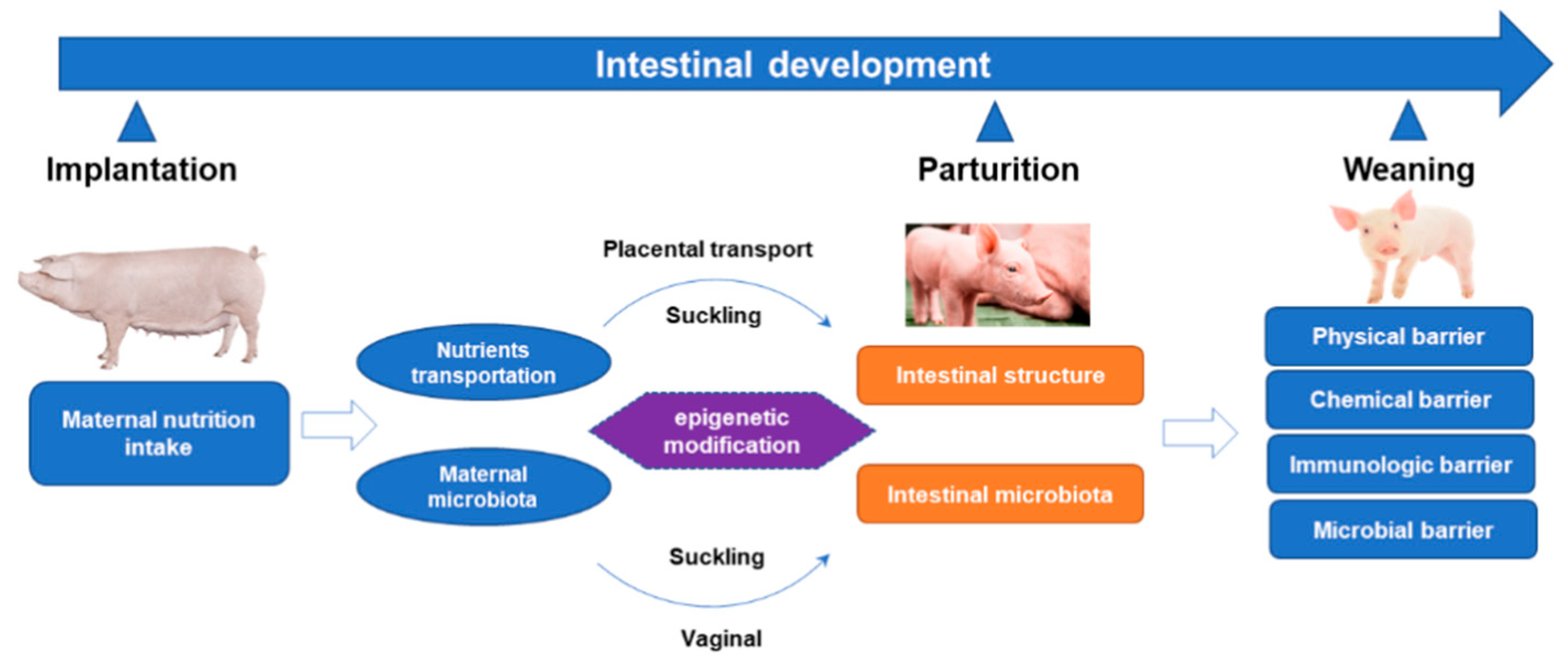
4. The Role of Maternal Nutrition in Intestinal Development and Microorganisms
4.1. Protein and Amino Acids
| Sources | Experimental Groups | Stage | Findings | Study |
|---|---|---|---|---|
| Pro | (1) CON (2) 1% Pro (3) 1% Pro + 0.0167% adifluoromethylornithine | From day 15 to 70 of gestation | Fetal weight (+) Protein and DNA concentrations of the fetal small intestine (+) mRNA levels for potassium voltage-gated channel, shaker-related subfamily, and member 1 (Kv1.1) in the fetal small and large intestines (+) | [46] |
| Arg | (1) CON (2) CON+ 0.28% Arg (3) CON+ 0.79% Arg | Day 70 of gestation to farrowing | Live litter weight (+) Piglet weight gain and litter weight gain (+) | [47] |
| Met and Cys | Met/Cys: 46% Met, 51% Met, 56% Met, and 62% Met | Day 90 of gestation to lactation | 46% Met: hippuric acid, retinoic acid, riboflavin, and δ-tocopherol (−) 51% Met: Firmicutes (+) (the optimum Met/Cys ratio) 62% Met: Proteobacteria (+) | [44] |
| Protein | (1) Normal protein: 14% CP (2) Low protein: 12% CP (3) Very low protein: 10% | During gestation | Low protein: the abundance and diversities of the jejunum microbiome (+); biological functions of the jejunum microbiome (+) | [39] |
| Glutamine | (1) CON (2) CON + 1% Gln | Day 85 of gestation to farrowing | Average birth weight (+) Gln concentration (+) Intestinal weight and morphologies (+) Extracellular matrix, tight junction protein (+) | [49] |
| Met | (1) CON (2) CON + 0.12% Met (3) CON+ 0.24% Met | Day 90 of gestation to day 21 of lactation | Malondialdehyde (−) glutathione peroxidase (+) Phascolarctobacterium and Bacteroidetes (+) | [45] |
| Met | (1) Met (2) HMTBA | During lactation | Reduced glutathione (+) Oxidized glutathione (GSSG)/GSH ratio (−) Glutathione peroxidase (+) Villus height and goblet cell numbers (+) | [50] |
| Protein | (1) Adequate protein (AP, 12.1%) (2) Low protein (LP, 6.5%) (3) High protein (HP, 30%) | During gestation | LP: Mortality (+) Cortisol (+) IL-6 (+) HP: CD4+ cell percentage (+) CD4+/CD8+ ratio (+) IL-6 (+) | [35] |
| Protein | (1) Adequate protein (AP, 12.1%) (2) Low protein (LP, 6.5%) (3) High protein (HP, 30%) | During gestation | HP and LP: body weight and catch-up growth (−) Ki67 and active caspase 3 (−) IUGR: brush border enzyme activities (−) Vacuolated enterocytes disappearance (−) | [34] |
| Protein | (1) CON (2) CON + 1% SDP | Day 30 before farrowing to weaning | Phylum Bacteroidota and genus Lactobacillus and Ruminococcus (+) Phylum Bacillota and genus Bacteroides, Escherichia/Shigella, and Clostridium (−) | [40] |
4.2. Fatty Acids
| Source | Experimental Groups | Stage | Key Findings | Study |
|---|---|---|---|---|
| Fat sources | (1) Lard (2) Linseed oil | During gestation and lactation | 18:3(n-3) and 20:5(n-3) in maternal RBC and piglet ileum (+) 22:6(n-3) and 20:4(n-6) in maternal RBC and piglet ileum (−) 18:3(n-3) in milk and piglet ileum (+) | [60] |
| Energy level | (1) NRC, 2012 (3.40 MCal DE) (2) Low-energy diet (3.00 MCal DE) | Day 1 of gestation to farrowing | Small intestinal weight (−) The ratio of villus height to crypt depth (−) Lactase and sucrase (−) IL-6, TNF-a (+) TLR-4, IL-1b and NF-kB (+) ZO-1 (−) | [54] |
| Energy level | (1) NRC, 2012 (2) High-energy diet (add 4.6% soybean oil) | Day 1 of gestation to farrowing | Small intestinal weight (+) Villus height (+) Lactase, sucrase (+) Insulin-like growth factor 1 receptor (+) | [53] |
| Fat sources | (1) 18:3n-3 (2) 18:2n-6 | Throughout gestation and lactation | Mesenteric lymph nodes (+) MHC class II+ antigen-presenting cells (+) | [59] |
| Nutrition level | (1) 75% of NRC (2) NRC (3) 150% of NRC | Day 1 of gestation to farrowing | Small intestine weight (+) Brush-border lactase (+) SGLT1, GLUT2, PEPT1, and GLP2R (+) | [52] |
| Fat sources | (1) Lard (2) Linseed oil | During gestation and lactation | Permeability (+) Paracellular permeability (−) Choline acetyltransferase (ChAT)-immunoreactive (IR) neurons (+) | [58] |
| Fat sources | (1) CON (2) CON + fish oil (3) CON + gold fat (4) CON + coconut fat | Entire gestation and lactation periods | (2) (3): Glucose transporter 2 (+) Sodium glucose transporter 1 protein (+) AMP-activated protein kinase activity (+) | [62] |
| Fish oil | (1) CON (2) CON + 30 g/d fish oil (3) CON + 60 g/d fish oil | Day 90 of gestation to weaning at day 21 of lactation | Plasma IgG, IgM and IgA (+) Cortisol (−) α-diversity of fecal microbiota, Lactobacillus genus (+) | [61] |
4.3. Carbohydrate
| Source | Experimental Groups | Stage | Key Findings | Study |
|---|---|---|---|---|
| Soluble fiber | (1) CON (2) CON + 2.0% pregelatinized waxy maize starch plus guar gum (SF) | During gestation | Growth rate (+) The incidence of diarrhea (−) The fecal and plasma levels of acetate and butyrate (+) Plasma zonulin and fecal lipocalin-2 (−) Plasma concentrations of interleukin 10 (IL-10) and Transforming growth factor (TGF-β) (+) Lactobacillus spp. (+) Bilophila spp. (−) | [10] |
| RS | (1) Containing 33% of digestible starch (DS diet) (2) Containing 33% of pea starch (RS) diet | Gestation and lactation | Firmicutes/Bacteroidetes ratio (+) Bifidobacterium (+) Milk protein concentration (−) Lactose concentration (+) Zonula occludens 1 (ZO-1) (+) | [70] |
| Fiber | Insoluble/soluble fiber ratio of 3.89 (R1), 5.59 (R2), 9.12 (R3), and 12.81 (R4) | During the entire gestation | Duodenal weight, jejunal villus height, and villus height/crypt depth (−) Lactase, sucrase, and maltase (−) Antioxidant capacity (−) Inflammatory response (+) | [71,72] |
| Fiber | (1) Control diet (CD, 16.15% dietary fiber) (2) High-dietary-fiber diet (HFD, 30.14% dietary fiber) | Day 90 of gestation to farrowing | α-diversity indices (+) Acidobacteria and Bacteroidetes at phylum level (+) Bradyrhizobium and Phyllobacterium at genus level (+) The abundances of proteins associated with oxidative status, energy metabolism, and immune and inflammatory responses (+) | [66] |
| Fiber | (1) CON (2) CON + sugar beet pulp (SBP) (3) CON + wheat bran (WB) | Day 85 of gestation to weaning | SBP: sow ADFI, litter. and piglet weaning weight, piglet ADG, immunoglobulin A (IgA), and interleukin-10 (IL-10) levels in the colostrum and IgA levels in the milk (+) Christensenellaceae and butyrate levels in the colon (+) WB: IL-10 levels in the milk (+) Lactobacillaceae in the colon (+) | [73] |
| RS | Maternal diets: digestible starch (DS) or RS diet Piglet treatment: control diet or high fat diet | Late gestation and lactation | RNA sequencing on liver and colon scrapings revealed minor differences | [74] |
| Inulin | (1) CON (2) CON + 3% inulin | Gestation and lactation | The cell numbers of enterococci (+) Cell numbers of eubacteria (stomach) and C. leptum (caecum) (+) Cell numbers of enterobacteria and L. amylovorus (stomach) (−) | [68] |
| Fiber source | (1) CON (2) Alfalfa meal (3) Beet pulp (4) Soybean skin | Day 60 of gestation to farrowing | Alfalfa meal improved sow and piglet performance and relieved gut and systemic inflammation | [69] |
4.4. Minerals and Vitamins
| Source | Experimental groups | Stage | Key Findings | Study |
|---|---|---|---|---|
| Mineral | (1) CON (contains 100 ppm Zn from ZnSO4) (2) CON + 100 ppm Zn from ZnSO4 (3) CON + 100 ppm additional Zn from ZnAA | Day 15 of gestation and continuing through lactation | ZnSO4: duodenal villus width (+) ZnAA: ileal villus width (−) | [75] |
| Mineral | (1) Na2SeO3 (2) Selenium-enriched yeast | Day 85 of gestation and continuing through lactation | Se content in the plasma and milk (+) T-AOC and GSH-Px in the colostrum (+) Protein abundances of MUC1, E-cadherin, ZO-1, occludin, and claudin (+) SCFA-producing microbiota (+) | [77] |
| Mineral | (1) Na2SeO3 (2) HMSeBA | During gestation | Ileal GPX2 and SePP1 (+) IL-1β, IL-6 and NF-κB genes (−) p-NF-κB, Beclin-1 and p-ERK proteins (−) | [82] |
| Vitamin | (1) 2000 IU/kg vitamin D3 (2) 50 μg/kg 25-OH-D3 | Day 107 of gestation to day 21 of lactation | Milk n-6/n-3 PUFA ratio (+) Bone-specific alkaline phosphatase (+) Calcium absorption rate (+) Milk fat content and immunoglobulin G level (+) Concentration of butyrate (+) | [79,80] |
| Vitamin | (1) Mock (2) Mock + Vitamin A (VA) (3) PEDV (4) PEDV + VA | Day 76 of gestation throughout lactation | Maternal IgA (+) Lactogenic immune protection in nursing piglets (+) | [81] |
4.5. Probiotics and Prebiotics
| Source | Experimental Groups | Stage | Key Findings | Study |
|---|---|---|---|---|
| Probiotic mixture | (1) CON (2) CON+ probiotic mixture | Day 0 of gestation until 20 days before delivery | Litter size and litter weigh at birth (+) Diarrhea incidence (−) Antioxidant capabilities Systemic immune status | [95] |
| Seaweed extracts (SWE) | 2 × 2 factorial design (1) CON (2) CON + SWE 10.0 g/d LPS challenge | From day 107 of gestation until weaning (d 26) | Colostrum IgA, IgG, and serum IgG (+) Colonic E. coli population (−) TNF-α mRNA expression (+) | [99] |
| Seaweed extracts (SWE) | 2 × 2 factorial design - SWE vs. +SWE - ETEC vs. +ETEC | Day 83 of gestation until weaning (day 28) | Heat-labile enterotoxin gene (−) Villus height in the ileum (+) | [100] |
| Methyl donor (MET) | (1) CON (2) BPA (50 mg/kg) (3) MET (3 g/kg betaine, 400 mg/kg choline, 150 µg/kg vitamin B12, and 15 mg/kg folic acid) (4) BPA + MET | Throughout gestation | The ratio of villus height to crypt depth (+) Lactase activity (+) Pept1, DNMT1, DNMT3a, and MTHFR (+) DNA methylation level of jejunum Pept1 (+) | [112] |
| Prebiotics (scFOS) | (1) CON (2) Mycotoxin deoxynivalenol (3) scFOS | During the last 4 weeks of gestation | T regulatory response (+) | [113] |
| scFOS | (1) CON (2) CON + scFOS | During the last third of gestation and throughout lactation | Ileal cytokine secretions (IFN)-γ (+) Cecal goblet cell number (+) IgA vaccine response (+) Bacterial fermentative activity (+) Colonic butyrate (+) | [102] |
| Prebiotics | Received daily 45 mL lactulose | 10 days before until 10 days after parturition | Daily weight gains (+) Total aerobic bacterial counts and C. perfringens counts (+) IgG antibody levels (+) | [114] |
| Resveratrol | (1) CON (2) CON + 300 mg/kg resveratrol | 20 days after breeding through gestation and lactation | Butyrate-producing bacteria (+) Diarrhea and intestinal inflammation (−) Intestinal morphology (+) T-cell receptor, MAPK, and Ras signaling (−) | [108] |
| Mannan oligosaccharide (MOS) | 2 × 2 factorial design Sow: (1) CON (2) CON + 400 mg/kg MOS Piglets: (1) CON (2) +800 mg/kg MOS | Day 86 of gestation until weaning | Lactobacillus (+) Escherichia coli (−) sIgA content (+) Toll-like receptor 2 (TLR2), toll-like receptor 4 (TLR4), and interleukin 8 (IL-8) (−) Cytokines IL-2 and IL-4 (−) | [105,115] |
| L-carnitine | 2 × 2 factorial design (soyabean meal vs. DDGS) and two L-carnitine levels | Gestation and lactation | Total superoxide dismutase activity (+) Malondialdehyde (−) IL-1β, IL-12, IL-6, and TNF-α (−) Lactobacillus spp. and bifidobacteria spp. (+) Tight junction proteins (+) | [107] |
| Yeast mannan-rich fraction (MRF) | (1) CON (2) CON + MRF (900 mg/kg) | Gestation and lactation | Protein and immunoglobulin G (IgG) in milk (+) Genes related to tissue development, functioning. and immunity, as well as greater cell proliferation and less migration of cells (+) | [109] |
| Short-chain fructooligosaccharide (scFOS) | (1) CON (2) CON + (10 g scFOS/d) | The last 4 weeks of gestation and the 4 weeks of lactation | IgA, scFOS. and TGFb1 concentrations (+) | [104] |
| Oregano essential oils (OEO) | (1) CON (2) CON + 250 mg/kg of OEO | Gestation and lactation | Fat percentage in milk (−) T lymphocytes (+) | [116] |
| Bioactive substances | (1) CON (2) CON + flax seed, rapeseed, linden inflorescence, taurine, L-carnitine and tocopherol acetate | Day 80 of gestation to lactation | Apoptotic index (−) p53 expression (−) | [111] |
| Folic acid (FA) | (1) CON (1.8 mg FA per kg) (2) FA-supplemented diet (30.3 mg FA per kg) | During gestation | DNMT-1 and Bcl-2 (+) p53, Bax, Mpg, and Apex-1 (−) | [112] |
5. The Underlying Mechanism of Maternal Nutrition and Offspring Development
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lalles, J.P. Long term effects of pre- and early postnatal nutrition and environment on the gut. J. Anim. Sci. 2012, 90, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Burkey, T.E.; Skjolaas, K.A.; Minton, J.E. Board-invited review: Porcine mucosal immunity of the gastrointestinal tract. J. Anim. Sci. 2009, 87, 1493–1501. [Google Scholar] [CrossRef][Green Version]
- Zabielski, R.; Godlewski, M.M.; Guilloteau, P. Control of development of gastrointestinal system in neonates. J. Physiol. Pharmacol. 2008, 59 (Suppl. S1), 35–54. [Google Scholar] [PubMed]
- Pluske, J.R. Feed- and feed additives-related aspects of gut health and development in weanling pigs. J. Anim. Sci. Biotechnol. 2013, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Montagne, L.; Boudry, G.; Favier, C.; Le Huerou-Luron, I.; Lalles, J.P.; Seve, B. Main intestinal markers associated with the changes in gut architecture and function in piglets after weaning. Br. J. Nutr. 2007, 97, 45–57. [Google Scholar] [CrossRef]
- Guilloteau, P.; Zabielski, R.; Hammon, H.M.; Metges, C.C. Nutritional programming of gastrointestinal tract development. Is the pig a good model for man? Nutr. Res. Rev. 2010, 23, 4–22. [Google Scholar] [CrossRef]
- Chu, D.M.; Meyer, K.M.; Prince, A.L.; Aagaard, K.M. Impact of maternal nutrition in pregnancy and lactation on offspring gut microbial composition and function. Gut Microbes 2016, 7, 459–470. [Google Scholar] [CrossRef]
- Zhang, S.; Heng, J.; Song, H.; Zhang, Y.; Lin, X.; Tian, M.; Chen, F.; Guan, W. Role of Maternal Dietary Protein and Amino Acids on Fetal Programming, Early Neonatal Development, and Lactation in Swine. Animals 2019, 9, 19. [Google Scholar] [CrossRef]
- Klurfeld, D.M. Nutritional regulation of gastrointestinal growth. Front. Biosci. A J. Virtual Libr. 1999, 4, D299–D302. [Google Scholar] [CrossRef]
- Cheng, C.; Wei, H.; Xu, C.; Xie, X.; Jiang, S.; Peng, J. Maternal Soluble Fiber Diet during Pregnancy Changes the Intestinal Microbiota, Improves Growth Performance, and Reduces Intestinal Permeability in Piglets. Appl. Environ. Microbiol. 2018, 84, e01047-18. [Google Scholar] [CrossRef]
- Nowland, T.L.; Kirkwood, R.N.; Pluske, J.R. Review: Can early-life establishment of the piglet intestinal microbiota influence production outcomes? Animal 2022, 16, 100368. [Google Scholar] [CrossRef] [PubMed]
- Thum, C.; Cookson, A.L.; Otter, D.E.; McNabb, W.C.; Hodgkinson, A.J.; Dyer, J.; Roy, N.C. Can nutritional modulation of maternal intestinal microbiota influence the development of the infant gastrointestinal tract? J. Nutr. 2012, 142, 1921–1928. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; Carnielli, V.P.; Ksiazyk, J.; Luna, M.S.; Migacheva, N.; Mosselmans, J.M.; Picaud, J.C.; Possner, M.; Singhal, A.; Wabitsch, M. Factors affecting early-life intestinal microbiota development. Nutrition 2020, 78, 110812. [Google Scholar] [CrossRef]
- Dekaney, C.M.; Wu, G.; Jaeger, L.A. Gene expression and activity of enzymes in the arginine biosynthetic pathway in porcine fetal small intestine. Pediatr. Res. 2003, 53, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Dekaney, C.M.; Bazer, F.W.; Jaeger, L.A. Mucosal morphogenesis and cytodifferentiation in fetal porcine small intestine. Anat. Rec. 1997, 249, 517–523. [Google Scholar] [CrossRef]
- Perozzi, G.; Barilà, D.; Murgia, C.; Kelly, D.; Begbie, R.; King, T.P. Expression of differentiated functions in the developing porcine small intestine. J. Nutr. Biochem. 1993, 4, 699–705. [Google Scholar] [CrossRef]
- McPherson, R.L.; Ji, F.; Wu, G.; Blanton, J.R., Jr.; Kim, S.W. Growth and compositional changes of fetal tissues in pigs. J. Anim. Sci. 2004, 82, 2534–2540. [Google Scholar] [CrossRef]
- Sangild, P.; Fowden, A.; Trahair, J. How does the foetal gastrointestinal tract develop in preparation for enteral nutrition after birth? Livest. Prod. Sci. 2000, 66, 141–150. [Google Scholar] [CrossRef]
- Buddington, R.K.; Sangild, P.T.; Hance, B.; Huang, E.Y.; Black, D.D. Prenatal gastrointestinal development in the pig and responses after preterm birth. J. Anim. Sci. 2012, 90 (Suppl. S4), 290–298. [Google Scholar] [CrossRef]
- Kelly, D.; Coutts, A. Development of digestive and immunological function in neonates: Role of early nutrition. Livest. Prod. Sci. 2000, 66, 161–167. [Google Scholar] [CrossRef]
- Zhang, H.; Malo, C.; Buddington, R.K. Suckling induces rapid intestinal growth and changes in brush border digestive functions of newborn pigs. J. Nutr. 1997, 127, 418–426. [Google Scholar] [CrossRef]
- Fan, M.; Stoll, B.; Jiang, R.; Burrin, D. Enterocyte digestive enzyme activity along the crypt-villus and longitudinal axes in the neonatal pig small intestine. J. Anim. Sci. 2001, 79, 371–381. [Google Scholar] [CrossRef]
- Goenka, A.; Kollmann, T.R. Development of immunity in early life. J. Infect. 2015, 71 (Suppl. S1), S112–S120. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.N.; Himes, J.E.; Martinez, D.R.; Permar, S.R. The Impact of the Gut Microbiota on Humoral Immunity to Pathogens and Vaccination in Early Infancy. PLoS Pathog. 2016, 12, e1005997. [Google Scholar] [CrossRef] [PubMed]
- Mackie, R.I.; Sghir, A.; Gaskins, H.R. Developmental microbial ecology of the neonatal gastrointestinal tract. Am. J. Clin. Nutr. 1999, 69, 1035S–1045S. [Google Scholar] [CrossRef] [PubMed]
- Nyangahu, D.D.; Lennard, K.S.; Brown, B.P.; Darby, M.G.; Wendoh, J.M.; Havyarimana, E.; Smith, P.; Butcher, J.; Stintzi, A.; Mulder, N.; et al. Disruption of maternal gut microbiota during gestation alters offspring microbiota and immunity. Microbiome 2018, 6, 124. [Google Scholar] [CrossRef]
- Macpherson, A.J.; de Aguero, M.G.; Ganal-Vonarburg, S.C. How nutrition and the maternal microbiota shape the neonatal immune system. Nat. Reviews. Immunol. 2017, 17, 508–517. [Google Scholar] [CrossRef]
- Chowdhury, S.R.; King, D.E.; Willing, B.P.; Band, M.R.; Beever, J.E.; Lane, A.B.; Loor, J.J.; Marini, J.C.; Rund, L.A.; Schook, L.B.; et al. Transcriptome profiling of the small intestinal epithelium in germfree versus conventional piglets. BMC Genom. 2007, 8, 215. [Google Scholar] [CrossRef]
- Hooper, L.V. Bacterial contributions to mammalian gut development. Trends Microbiol. 2004, 12, 129–134. [Google Scholar] [CrossRef]
- Sangild, P.T. Prenatal Development of Gastrointestinal Function in the Pig and the Effects of Fetal Esophageal Obstruction. Pediatr. Res. 2002, 52, 416–424. [Google Scholar] [CrossRef]
- Jacobi, S.K.; Odle, J. Nutritional factors influencing intestinal health of the neonate. Adv. Nutr. 2012, 3, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Terstappen, F.; Tol, A.J.C.; Gremmels, H.; Wever, K.E.; Paauw, N.D.; Joles, J.A.; Beek, E.M.V.; Lely, A.T. Prenatal Amino Acid Supplementation to Improve Fetal Growth: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 2535. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Zheng, J.; Wu, F.; Fang, Z.; Che, L.; Wu, D. Effects of energy and amino acid intake during gestation on reproductive performance, milk composition, antioxidant status and placental nutrient transport in high-parity sows. Front. Vet. Sci. 2025, 12, 1585925. [Google Scholar] [CrossRef]
- Mickiewicz, M.; Zabielski, R.; Grenier, B.; Le Normand, L.; Savary, G.; Holst, J.J.; Oswald, I.P.; Metges, C.C.; Guilloteau, P. Structural and functional development of small intestine in intrauterine growth retarded porcine offspring born to gilts fed diets with differing protein ratios throughout pregnancy. J. Physiol. Pharmacol. 2012, 63, 225–239. [Google Scholar] [PubMed]
- Tuchscherer, M.; Otten, W.; Kanitz, E.; Grabner, M.; Tuchscherer, A.; Bellmann, O.; Rehfeldt, C.; Metges, C.C. Effects of inadequate maternal dietary protein:carbohydrate ratios during pregnancy on offspring immunity in pigs. BMC Vet. Res. 2012, 8, 232. [Google Scholar] [CrossRef]
- Pinheiro, D.F.; Pinheiro, P.F.; Buratini, J., Jr.; Castilho, A.C.; Lima, P.F.; Trinca, L.A.; Vicentini-Paulino, M.d.L.M. Maternal protein restriction during pregnancy affects gene expression and immunolocalization of intestinal nutrient transporters in rats. Clin. Sci. 2013, 125, 281–289. [Google Scholar] [CrossRef]
- Aubert, P.; Oleynikova, E.; Rizvi, H.; Ndjim, M.; Le Berre-Scoul, C.; Grohard, P.A.; Chevalier, J.; Segain, J.P.; Le Drean, G.; Neunlist, M.; et al. Maternal protein restriction induces gastrointestinal dysfunction and enteric nervous system remodeling in rat offspring. FASEB J. 2019, 33, 770–781. [Google Scholar] [CrossRef]
- Warren, M.F.; Hallowell, H.A.; Higgins, K.V.; Liles, M.R.; Hood, W.R. Maternal Dietary Protein Intake Influences Milk and Offspring Gut Microbial Diversity in a Rat (Rattus norvegicus) Model. Nutrients 2019, 11, 2257. [Google Scholar] [CrossRef]
- Jin, J.; Zhang, L.; Jia, J.; Chen, Q.; Yuan, Z.; Zhang, X.; Sun, W.; Ma, C.; Xu, F.; Zhan, S.; et al. Effects of Maternal Low-Protein Diet on Microbiota Structure and Function in the Jejunum of Huzhu Bamei Suckling Piglets. Animals 2019, 9, 713. [Google Scholar] [CrossRef]
- Lee, J.J.; Kyoung, H.; Cho, J.H.; Park, K.I.; Kim, Y.; Ahn, J.; Choe, J.; Kim, Y.; Kim, H.B.; Song, M. Change in the Gut Microbiota of Lactating Sows and Their Piglets by Inclusion of Dietary Spray-Dried Plasma in Sow Diets. J. Microbiol. Biotechnol. 2023, 34, 516–524. [Google Scholar] [CrossRef]
- Wu, G. Amino acids: Metabolism, functions, and nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef]
- Fang, Z.; Yao, K.; Zhang, X.; Zhao, S.; Sun, Z.; Tian, G.; Yu, B.; Lin, Y.; Zhu, B.; Jia, G. Nutrition and health relevant regulation of intestinal sulfur amino acid metabolism. Amino Acids 2010, 39, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Lai, S.; Yuan, P.; Zhe, L.; Yang, L.; Mercier, Y.; Hu, L.; Zhang, X.; Hua, L.; Zhuo, Y.; et al. Increased maternal consumption of methionine as its hydroxyl analog improves placental angiogenesis and antioxidative capacity in sows. J. Anim. Sci. Biotechnol. 2025, 16, 39. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.A.K.; Liu, G.; Bin, P.; Ding, S.; Kong, X.; Guan, G.; Yin, Y. Sulfur-containing amino acid supplementation to gilts from late pregnancy to lactation altered offspring’s intestinal microbiota and plasma metabolites. Appl. Microbiol. Biotechnol. 2020, 104, 1227–1242. [Google Scholar] [CrossRef]
- Azad, M.A.K.; Bin, P.; Liu, G.; Fang, J.; Li, T.; Yin, Y. Effects of different methionine levels on offspring piglets during late gestation and lactation. Food Funct. 2018, 9, 5843–5854. [Google Scholar] [CrossRef]
- Wang, J.; Tan, B.; Li, J.; Kong, X.; Tan, M.; Wu, G. Regulatory role of l-proline in fetal pig growth and intestinal epithelial cell proliferation. Anim. Nutr. 2020, 6, 438–446. [Google Scholar] [CrossRef]
- Hong, J.; Fang, L.H.; Jeong, J.H.; Kim, Y.Y. Effects of L-Arginine Supplementation during Late Gestation on Reproductive Performance, Piglet Uniformity, Blood Profiles, and Milk Composition in High Prolific Sows. Animals 2020, 10, 1313. [Google Scholar] [CrossRef]
- Blachier, F.; Boutry, C.; Bos, C.; Tome, D. Metabolism and functions of L-glutamate in the epithelial cells of the small and large intestines. Am. J. Clin. Nutr. 2009, 90, 814S–821S. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, T.; Huang, S.; Wang, W.; Dai, Z.; Feng, C.; Wu, G.; Wang, J. Maternal L-glutamine supplementation during late gestation alleviates intrauterine growth restriction-induced intestinal dysfunction in piglets. Amino Acids 2018, 50, 1289–1299. [Google Scholar] [CrossRef]
- Kumar, A.; Vlasova, A.N.; Liu, Z.; Chattha, K.S.; Kandasamy, S.; Esseili, M.; Zhang, X.; Rajashekara, G.; Saif, L.J. In vivo gut transcriptome responses to Lactobacillus rhamnosus GG and Lactobacillus acidophilus in neonatal gnotobiotic piglets. Gut Microbes 2014, 5, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, K. Fatty acids as modulators of the immune response. Annu. Rev. Nutr. 2006, 26, 45–73. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Che, L.; Wang, J.; Yang, M.; Su, G.; Fang, Z.; Lin, Y.; Xu, S.; Wu, D. Effects of maternal over- and undernutrition on intestinal morphology, enzyme activity, and gene expression of nutrient transporters in newborn and weaned pigs. Nutrition 2014, 30, 1442–1447. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Che, L.; Yang, Z.; Feng, B.; Che, L.; Xu, S.; Lin, Y.; Fang, Z.; Li, J.; Wu, D. A Maternal High-Energy Diet Promotes Intestinal Development and Intrauterine Growth of Offspring. Nutrients 2016, 8, 258. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Mou, D.; Hu, L.; Zhen, J.; Che, L.; Fang, Z.; Xu, S.; Lin, Y.; Feng, B.; Li, J.; et al. Effects of Maternal Low-Energy Diet during Gestation on Intestinal Morphology, Disaccharidase Activity, and Immune Response to Lipopolysaccharide Challenge in Pig Offspring. Nutrients 2017, 9, 1115. [Google Scholar] [CrossRef]
- Ma, J.; Prince, A.L.; Bader, D.; Hu, M.; Ganu, R.; Baquero, K.; Blundell, P.; Alan Harris, R.; Frias, A.E.; Grove, K.L.; et al. High-fat maternal diet during pregnancy persistently alters the offspring microbiome in a primate model. Nat. Commun. 2014, 5, 3889. [Google Scholar] [CrossRef]
- Chu, D.M.; Antony, K.M.; Ma, J.; Prince, A.L.; Showalter, L.; Moller, M.; Aagaard, K.M. The early infant gut microbiome varies in association with a maternal high-fat diet. Genome Med. 2016, 8, 77. [Google Scholar] [CrossRef]
- Innis, S.M.; Dai, C.; Wu, X.; Buchan, A.M.; Jacobson, K. Perinatal lipid nutrition alters early intestinal development and programs the response to experimental colitis in young adult rats. Am. J. Physiology. Gastrointest. Liver Physiol. 2010, 299, G1376–G1385. [Google Scholar] [CrossRef]
- De Quelen, F.; Chevalier, J.; Rolli-Derkinderen, M.; Mourot, J.; Neunlist, M.; Boudry, G. n-3 polyunsaturated fatty acids in the maternal diet modify the postnatal development of nervous regulation of intestinal permeability in piglets. J. Physiol. 2011, 589, 4341–4352. [Google Scholar] [CrossRef]
- Desaldeleer, C.; Ferret-Bernard, S.; de Quelen, F.; Le Normand, L.; Perrier, C.; Savary, G.; Romé, V.; Michel, C.; Mourot, J.; Le Huërou-Luron, I.; et al. Maternal 18:3n-3 favors piglet intestinal passage of LPS and promotes intestinal anti-inflammatory response to this bacterial ligand. J. Nutr. Biochem. 2014, 25, 1090–1098. [Google Scholar] [CrossRef]
- Boudry, G.; Douard, V.; Mourot, J.; Lalles, J.P.; Le Huerou-Luron, I. Linseed oil in the maternal diet during gestation and lactation modifies fatty acid composition, mucosal architecture, and mast cell regulation of the ileal barrier in piglets. J. Nutr. 2009, 139, 1110–1117. [Google Scholar] [CrossRef][Green Version]
- Han, P.; Du, Z.; Liu, X.; You, J.; Shi, X.E.; Sun, S.; Yang, G.; Li, X. Effects of maternal supplementation of fish oil during late gestation and lactation on growth performance, fecal microbiota structure and post-weaning diarrhoea of offspring piglets. Br. J. Nutr. 2022, 130, 966–977. [Google Scholar] [CrossRef]
- Gabler, N.K.; Spencer, J.D.; Webel, D.M.; Spurlock, M.E. In utero and postnatal exposure to long chain (n-3) PUFA enhances intestinal glucose absorption and energy stores in weanling pigs. J. Nutr. 2007, 137, 2351–2358. [Google Scholar] [CrossRef] [PubMed]
- Aluthge, N.D.; Van Sambeek, D.M.; Carney-Hinkle, E.E.; Li, Y.S.; Fernando, S.C.; Burkey, T.E. BOARD INVITED REVIEW: The pig microbiota and the potential for harnessing the power of the microbiome to improve growth and health. J. Anim. Sci. 2019, 97, 3741–3757. [Google Scholar] [CrossRef]
- Metzler-Zebeli, B.U.; Hooda, S.; Pieper, R.; Zijlstra, R.T.; van Kessel, A.G.; Mosenthin, R.; Ganzle, M.G. Nonstarch polysaccharides modulate bacterial microbiota, pathways for butyrate production, and abundance of pathogenic Escherichia coli in the pig gastrointestinal tract. Appl. Env. Microbiol. 2010, 76, 3692–3701. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, J.; Ren, E.; Su, Y.; Zhu, W. Co-occurrence of early gut colonization in neonatal piglets with microbiota in the maternal and surrounding delivery environments. Anaerobe 2018, 49, 30–40. [Google Scholar] [CrossRef]
- He, Y.; Peng, X.; Liu, Y.; Wu, Q.; Zhou, Q.; Hu, L.; Fang, Z.; Lin, Y.; Xu, S.; Feng, B.; et al. Effects of Maternal Fiber Intake on Intestinal Morphology, Bacterial Profile and Proteome of Newborns Using Pig as Model. Nutrients 2020, 13, 42. [Google Scholar] [CrossRef]
- Leblois, J.; Massart, S.; Li, B.; Wavreille, J.; Bindelle, J.; Everaert, N. Modulation of piglets’ microbiota: Differential effects by a high wheat bran maternal diet during gestation and lactation. Sci. Rep. 2017, 7, 7426. [Google Scholar] [CrossRef]
- Passlack, N.; Vahjen, W.; Zentek, J. Dietary inulin affects the intestinal microbiota in sows and their suckling piglets. BMC Vet. Res. 2015, 11, 51. [Google Scholar] [CrossRef]
- Liu, B.S.; Zhu, X.Y.; Cui, Y.L.; Wang, W.J.; Liu, H.; Li, Z.D.; Guo, Z.G.; Ma, S.; Li, D.F.; Wang, C.Z.; et al. Consumption of Dietary Fiber from Different Sources during Pregnancy Alters Sow Gut Microbiota and Improves Performance and Reduces Inflammation in Sows and Piglets. mSystems 2021, 6. [Google Scholar] [CrossRef] [PubMed]
- Leblois, J.; Massart, S.; Soyeurt, H.; Grelet, C.; Dehareng, F.; Schroyen, M.; Li, B.; Wavreille, J.; Bindelle, J.; Everaert, N. Feeding sows resistant starch during gestation and lactation impacts their faecal microbiota and milk composition but shows limited effects on their progeny. PLoS ONE 2018, 13, e0199568. [Google Scholar] [CrossRef]
- Li, Y.; Liu, H.; Zhang, L.; Yang, Y.; Lin, Y.; Zhuo, Y.; Fang, Z.; Che, L.; Feng, B.; Xu, S.; et al. Maternal Dietary Fiber Composition during Gestation Induces Changes in Offspring Antioxidative Capacity, Inflammatory Response, and Gut Microbiota in a Sow Model. Int. J. Mol. Sci. 2019, 21, 31. [Google Scholar] [CrossRef] [PubMed]
- Brink, L.R.; Matazel, K.; Piccolo, B.D.; Bowlin, A.K.; Chintapalli, S.V.; Shankar, K.; Yeruva, L. Neonatal Diet Impacts Bioregional Microbiota Composition in Piglets Fed Human Breast Milk or Infant Formula. J. Nutr. 2019, 149, 2236–2246. [Google Scholar] [CrossRef]
- Shang, Q.; Liu, H.; Liu, S.; He, T.; Piao, X. Effects of dietary fiber sources during late gestation and lactation on sow performance, milk quality, and intestinal health in piglets. J. Anim. Sci. 2019, 97, 4922–4933. [Google Scholar] [CrossRef]
- Schroyen, M.; Leblois, J.; Uerlings, J.; Li, B.; Sureda, E.A.; Massart, S.; Wavreille, J.; Bindelle, J.; Everaert, N. Maternal dietary resistant starch does not improve piglet’s gut and liver metabolism when challenged with a high fat diet. BMC Genom. 2020, 21, 439. [Google Scholar] [CrossRef]
- Payne, R.L.; Bidner, T.D.; Fakler, T.M.; Southern, L.L. Growth and intestinal morphology of pigs from sows fed two zinc sources during gestation and lactation. J. Anim. Sci. 2006, 84, 2141–2149. [Google Scholar] [CrossRef]
- Mou, D.; Ding, D.; Yan, H.; Qin, B.; Dong, Y.; Li, Z.; Che, L.; Fang, Z.; Xu, S.; Lin, Y.; et al. Maternal supplementation of organic selenium during gestation improves sows and offspring antioxidant capacity and inflammatory status and promotes embryo survival. Food Funct. 2020, 11, 7748–7761. [Google Scholar] [CrossRef]
- Xiong, L.; Lin, T.; Yue, X.; Zhang, S.; Liu, X.; Chen, F.; Zhang, S.; Guan, W. Maternal Selenium-Enriched Yeast Supplementation in Sows Enhances Offspring Growth and Antioxidant Status through the Nrf2/Keap1 Pathway. Antioxidants 2023, 12, 2064. [Google Scholar] [CrossRef]
- Yang, K.; Zhu, J.; Wu, J.; Zhong, Y.; Shen, X.; Petrov, B.; Cai, W. Maternal Vitamin D Deficiency Increases Intestinal Permeability and Programs Wnt/beta-Catenin Pathway in BALB/C Mice. JPEN J. Parenter. Enter. Nutr. 2020, 45, 102–114. [Google Scholar] [CrossRef]
- Zhang, L.; Li, M.; Shang, Q.; Hu, J.; Long, S.; Piao, X. Effects of maternal 25-hydroxycholecalciferol on nutrient digestibility, milk composition and fatty-acid profile of lactating sows and gut bacterial metabolites in the hindgut of suckling piglets. Arch. Anim. Nutr. 2019, 73, 271–286. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hu, J.; Li, M.; Shang, Q.; Liu, S.; Piao, X. Maternal 25-hydroxycholecalciferol during lactation improves intestinal calcium absorption and bone properties in sow-suckling piglet pairs. J. Bone Miner. Metab. 2019, 37, 1083–1094. [Google Scholar] [CrossRef] [PubMed]
- Langel, S.N.; Paim, F.C.; Alhamo, M.A.; Lager, K.M.; Vlasova, A.N.; Saif, L.J. Oral vitamin A supplementation of porcine epidemic diarrhea virus infected gilts enhances IgA and lactogenic immune protection of nursing piglets. Vet. Res. 2019, 50, 101. [Google Scholar] [CrossRef]
- Mou, D.; Ding, D.; Yang, M.; Jiang, X.; Zhao, L.; Che, L.; Fang, Z.; Xu, S.; Lin, Y.; Zhuo, Y.; et al. Maternal organic selenium supplementation during gestation improves the antioxidant capacity and reduces the inflammation level in the intestine of offspring through the NF-kappaB and ERK/Beclin-1 pathways. Food Funct. 2020, 12, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Sanz, Y. Gut microbiota and probiotics in maternal and infant health. Am. J. Clin. Nutr. 2011, 94, 2000S–2005S. [Google Scholar] [CrossRef] [PubMed]
- Taras, D.; Vahjen, W.; Macha, M.; Simon, O. Response of performance characteristics and fecal consistency to long-lasting dietary supplementation with the probiotic strain Bacillus cereus var. toyoi to sows and piglets. Arch. Anim. Nutr. 2005, 59, 405–417. [Google Scholar] [CrossRef]
- Thompson, C.L.; Wang, B.; Holmes, A.J. The immediate environment during postnatal development has long-term impact on gut community structure in pigs. ISME J. 2008, 2, 739–748. [Google Scholar] [CrossRef]
- Liao, S.F.; Nyachoti, M. Using probiotics to improve swine gut health and nutrient utilization. Anim. Nutr. 2017, 3, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Markowiak, P.; Slizewska, K. The role of probiotics, prebiotics and synbiotics in animal nutrition. Gut Pathog. 2018, 10, 21. [Google Scholar] [CrossRef]
- Menegat, M.B.; DeRouchey, J.M.; Woodworth, J.C.; Dritz, S.S.; Tokach, M.D.; Goodband, R.D. Effects of Bacillus subtilis C-3102 on sow and progeny performance, fecal consistency, and fecal microbes during gestation, lactation, and nursery periods. J. Anim. Sci. 2019, 97, 3920–3937. [Google Scholar] [CrossRef]
- Veljovic, K.; Dinic, M.; Lukic, J.; Mihajlovic, S.; Tolinacki, M.; Zivkovic, M.; Begovic, J.; Mrvaljevic, I.; Golic, N.; Terzic-Vidojevic, A. Promotion of Early Gut Colonization by Probiotic Intervention on Microbiota Diversity in Pregnant Sows. Front. Microbiol. 2017, 8, 2028. [Google Scholar] [CrossRef]
- Wang, K.; Zhu, Q.; Kong, X.; Song, M.; Azad, M.A.K.; Xiong, L.; Zheng, Y.; He, Q. Dietary Probiotics or Synbiotics Supplementation During Gestation, Lactation, and Nursery Periods Modifies Colonic Microbiota, Antioxidant Capacity, and Immune Function in Weaned Piglets. Front. Vet. Sci. 2020, 7, 597832. [Google Scholar] [CrossRef]
- Cao, M.; Li, Y.; Wu, Q.J.J.; Zhang, P.; Li, W.T.T.; Mao, Z.Y.Y.; Wu, D.M.M.; Jiang, X.M.M.; Zhuo, Y.; Fang, Z.F.F.; et al. Effects of dietary Clostridium butyricum addition to sows in late gestation and lactation on reproductive performance and intestinal microbiota. J. Anim. Sci. 2019, 97, 3426–3439. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, J.; Cao, M.; Li, Y.; Zhuo, Y.; Fang, Z.; Che, L.; Xu, S.; Feng, B.; Lin, Y.; et al. Dietary supplementation of Bacillus subtilis PB6 improves sow reproductive performance and reduces piglet birth intervals. Anim. Nutr. 2020, 6, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.A.; Davis, E.; Spencer, J.D.; Moser, R.; Rehberger, T. The effect of a Bacillus-based direct-fed microbial supplemented to sows on the gastrointestinal microbiota of their neonatal piglets. J. Anim. Sci. 2013, 91, 3390–3399. [Google Scholar] [CrossRef]
- Hayakawa, T.; Masuda, T.; Kurosawa, D.; Tsukahara, T. Dietary administration of probiotics to sows and/or their neonates improves the reproductive performance, incidence of post-weaning diarrhea and histopathological parameters in the intestine of weaned piglets. Anim. Sci. J. Nihon Chikusan Gakkaiho 2016, 87, 1501–1510. [Google Scholar] [CrossRef]
- Hu, T.; Song, Z.; Yang, L.; Chen, K.; Wu, Y.; Xie, F.; Wang, J.; Yang, G.; Zhu, Y. Maternal probiotic mixture supplementation optimizes the gut microbiota structure of offspring piglets through the gut–breast axis. Anim. Nutr. 2024, 19, 386–400. [Google Scholar] [CrossRef]
- Additives, E.P.o.; Products or Substances used in Animal, F.; Rychen, G.; Aquilina, G.; Azimonti, G.; Bampidis, V.; Bastos, M.L.; Bories, G.; Chesson, A.; Cocconcelli, P.S.; et al. Safety and efficacy of Bacillus subtilis PB6 (Bacillus subtilis ATCC PTA-6737) as a feed additive for sows. EFSA J. Eur. Food Saf. Auth. 2017, 15, e04855. [Google Scholar] [CrossRef][Green Version]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef] [PubMed]
- Gaggia, F.; Mattarelli, P.; Biavati, B. Probiotics and prebiotics in animal feeding for safe food production. Int. J. Food Microbiol. 2010, 141 (Suppl. S1), S15–S28. [Google Scholar] [CrossRef]
- Leonard, S.G.; Sweeney, T.; Bahar, B.; O’Doherty, J.V. Effect of maternal seaweed extract supplementation on suckling piglet growth, humoral immunity, selected microflora, and immune response after an ex vivo lipopolysaccharide challenge. J. Anim. Sci. 2012, 90, 505–514. [Google Scholar] [CrossRef]
- Heim, G.; Sweeney, T.; O’Shea, C.J.; Doyle, D.N.; O’Doherty, J.V. Effect of maternal supplementation with seaweed extracts on growth performance and aspects of gastrointestinal health of newly weaned piglets after challenge with enterotoxigenic Escherichia coli K88. Br. J. Nutr. 2014, 112, 1955–1965. [Google Scholar] [CrossRef]
- McKay, J.A.; Mathers, J.C. Diet induced epigenetic changes and their implications for health. Acta Physiol. 2011, 202, 103–118. [Google Scholar] [CrossRef] [PubMed]
- Le Bourgot, C.; Le Normand, L.; Formal, M.; Respondek, F.; Blat, S.; Apper, E.; Ferret-Bernard, S.; Le Huerou-Luron, I. Maternal short-chain fructo-oligosaccharide supplementation increases intestinal cytokine secretion, goblet cell number, butyrate concentration and Lawsonia intracellularis humoral vaccine response in weaned pigs. Br. J. Nutr. 2017, 117, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Schaible, T.D.; Harris, R.A.; Dowd, S.E.; Smith, C.W.; Kellermayer, R. Maternal methyl-donor supplementation induces prolonged murine offspring colitis susceptibility in association with mucosal epigenetic and microbiomic changes. Hum. Mol. Genet. 2011, 20, 1687–1696. [Google Scholar] [CrossRef]
- Le Bourgot, C.; Ferret-Bernard, S.; Le Normand, L.; Savary, G.; Menendez-Aparicio, E.; Blat, S.; Appert-Bossard, E.; Respondek, F.; Le Huerou-Luron, I. Maternal short-chain fructooligosaccharide supplementation influences intestinal immune system maturation in piglets. PLoS ONE 2014, 9, e107508. [Google Scholar] [CrossRef]
- Duan, X.; Tian, G.; Chen, D.; Huang, L.; Zhang, D.; Zheng, P.; Mao, X.; Yu, J.; He, J.; Huang, Z.; et al. Mannan oligosaccharide supplementation in diets of sow and (or) their offspring improved immunity and regulated intestinal bacteria in piglet. J. Anim. Sci. 2019, 97, 4548–4556. [Google Scholar] [CrossRef]
- Shadid, R.; Haarman, M.; Knol, J.; Theis, W.; Beermann, C.; Rjosk-Dendorfer, D.; Schendel, D.J.; Koletzko, B.V.; Krauss-Etschmann, S. Effects of galactooligosaccharide and long-chain fructooligosaccharide supplementation during pregnancy on maternal and neonatal microbiota and immunity—A randomized, double-blind, placebo-controlled study. Am. J. Clin. Nutr. 2007, 86, 1426–1437. [Google Scholar] [CrossRef]
- Wei, B.; Nie, S.; Meng, Q.; Qu, Z.; Shan, A.; Chen, Z. Effects of l-carnitine and/or maize distillers dried grains with solubles in diets of gestating and lactating sows on the intestinal barrier functions of their offspring. Br. J. Nutr. 2016, 116, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Sun, S.; Luo, Z.; Shi, B.; Shan, A.; Cheng, B. Maternal dietary resveratrol alleviates weaning-associated diarrhea and intestinal inflammation in pig offspring by changing intestinal gene expression and microbiota. Food Funct. 2019, 10, 5626–5643. [Google Scholar] [CrossRef]
- Graugnard, D.E.; Samuel, R.S.; Xiao, R.; Spangler, L.F.; Brennan, K.M. Intestinal gene expression profiles of piglets benefit from maternal supplementation with a yeast mannan-rich fraction during gestation and lactation. Animal 2015, 9, 622–628. [Google Scholar] [CrossRef]
- Liu, H.; Zeng, X.; Zhang, G.; Hou, C.; Li, N.; Yu, H.; Shang, L.; Zhang, X.; Trevisi, P.; Yang, F.; et al. Maternal milk and fecal microbes guide the spatiotemporal development of mucosa-associated microbiota and barrier function in the porcine neonatal gut. BMC Biol. 2019, 17, 106. [Google Scholar] [CrossRef]
- Strzałkowski, A.; Godlewski, M.M.; Hallay, N.; Kulasek, G.; Gajewski, Z.; Zabielski, R. The effect of supplementing sow with bioactive substances on neonatal small intestinal epithelium. J. Physiol. Pharmacol. 2007, 58, 115–122. [Google Scholar] [PubMed]
- Liu, H.; Wang, J.; Mou, D.; Che, L.; Fang, Z.; Feng, B.; Lin, Y.; Xu, S.; Li, J.; Wu, D. Maternal Methyl Donor Supplementation during Gestation Counteracts the Bisphenol A-Induced Impairment of Intestinal Morphology, Disaccharidase Activity, and Nutrient Transporters Gene Expression in Newborn and Weaning Pigs. Nutrients 2017, 9, 423. [Google Scholar] [CrossRef] [PubMed]
- Ferret-Bernard, S.; Le Normand, L.; Rome, V.; Le Bourgot, C.; Seeboth, J.; Savary, G.; Laurent, F.; Le Huerou-Luron, I.; Guzylack-Piriou, L. Maternal Supplementation of Food Ingredient (Prebiotic) or Food Contaminant (Mycotoxin) Influences Mucosal Immune System in Piglets. Nutrients 2020, 12, 2115. [Google Scholar] [CrossRef]
- Krueger, M.; Schroedl, W.; Isik, W.; Lange, W.; Hagemann, L. Effects of lactulose on the intestinal microflora of periparturient sows and their piglets. Eur. J. Nutr. 2002, 41 (Suppl. S1), I26–I31. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.D.; Chen, D.W.; Zheng, P.; Tian, G.; Wang, J.P.; Mao, X.B.; Yu, J.; He, J.; Li, B.; Huang, Z.Q.; et al. Effects of dietary mannan oligosaccharide supplementation on performance and immune response of sows and their offspring. Anim. Feed. Sci. Technol. 2016, 218, 17–25. [Google Scholar] [CrossRef]
- Ariza-Nieto, C.; Bandrick, M.; Baidoo, S.K.; Anil, L.; Molitor, T.W.; Hathaway, M.R. Effect of dietary supplementation of oregano essential oils to sows on colostrum and milk composition, growth pattern and immune status of suckling pigs. J. Anim. Sci. 2011, 89, 1079–1089. [Google Scholar] [CrossRef]
- Elolimy, A.; Vailati-Riboni, M.; Liang, Y.; Loor, J.J. Cellular Mechanisms and Epigenetic Changes: Role of Nutrition in Livestock. Vet. Clin. N. Am. Food Anim. Pract. 2019, 35, 249–263. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W.; Cudd, T.A.; Meininger, C.J.; Spencer, T.E. Maternal nutrition and fetal development. J. Nutr. 2004, 134, 2169–2172. [Google Scholar] [CrossRef]
- Hu, Y.; Hu, L.; Gong, D.; Lu, H.; Xuan, Y.; Wang, R.; Wu, D.; Chen, D.; Zhang, K.; Gao, F.; et al. Genome-wide DNA methylation analysis in jejunum of Sus scrofa with intrauterine growth restriction. Mol. Genet. Genom. MGG 2018, 293, 807–818. [Google Scholar] [CrossRef]
- Anderson, O.S.; Sant, K.E.; Dolinoy, D.C. Nutrition and epigenetics: An interplay of dietary methyl donors, one-carbon metabolism and DNA methylation. J. Nutr. Biochem. 2012, 23, 853–859. [Google Scholar] [CrossRef]
- Bai, G.; Yuan, H.; Cao, M.; Jiang, X.; Shi, B.; Bin, P. Glyphosate-Based Herbicide Stress During Pregnancy Impairs Intestinal Development in Newborn Piglets by Modifying DNA Methylation. J. Agric. Food Chem. 2025, 73, 2483–2498. [Google Scholar] [CrossRef]
- Murdoch, B.M.; Murdoch, G.K.; Greenwood, S.; McKay, S. Nutritional influence on epigenetic marks and effect on livestock production. Front. Genet. 2016, 7, 182. [Google Scholar] [CrossRef] [PubMed]
- Waterland, R.A.; Jirtle, R.L. Transposable elements: Targets for early nutritional effects on epigenetic gene regulation. Mol. Cell. Biol. 2003, 23, 5293–5300. [Google Scholar] [CrossRef] [PubMed]
- Jang, K.B.; Kim, J.H.; Purvis, J.M.; Chen, J.; Ren, P.; Vazquez-Anon, M.; Kim, S.W. Effects of mineral methionine hydroxy analog chelate in sow diets on epigenetic modification and growth of progeny. J. Anim. Sci. 2020, 98, skaa271. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Guo, S.; Gao, J.; Guo, Y.; Du, E.; Lv, Z.; Zhang, B. Maternal high-zinc diet attenuates intestinal inflammation by reducing DNA methylation and elevating H3K9 acetylation in the A20 promoter of offspring chicks. J. Nutr. Biochem. 2015, 26, 173–183. [Google Scholar] [CrossRef]
- Delage, B.; Dashwood, R.H. Dietary manipulation of histone structure and function. Annu. Rev. Nutr. 2008, 28, 347–366. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, L.; Wu, F.; Che, L. Programming Effects of Maternal Nutrition on Intestinal Development and Microorganisms of Offspring: A Review on Pigs. Microorganisms 2025, 13, 1151. https://doi.org/10.3390/microorganisms13051151
Hu L, Wu F, Che L. Programming Effects of Maternal Nutrition on Intestinal Development and Microorganisms of Offspring: A Review on Pigs. Microorganisms. 2025; 13(5):1151. https://doi.org/10.3390/microorganisms13051151
Chicago/Turabian StyleHu, Liang, Fali Wu, and Lianqiang Che. 2025. "Programming Effects of Maternal Nutrition on Intestinal Development and Microorganisms of Offspring: A Review on Pigs" Microorganisms 13, no. 5: 1151. https://doi.org/10.3390/microorganisms13051151
APA StyleHu, L., Wu, F., & Che, L. (2025). Programming Effects of Maternal Nutrition on Intestinal Development and Microorganisms of Offspring: A Review on Pigs. Microorganisms, 13(5), 1151. https://doi.org/10.3390/microorganisms13051151