Identification and Feeding Characterization of Sterkiella histriomuscorum (Protozoa, Ciliophora, Hypotrichia) Isolated from Outdoor Mass Culture of Scenedesmus dimorphus
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Cultivation
2.2. Feeding Impact Analysis
2.3. Light Microscopy
2.4. Scanning and Transmission Electron Microscopy
2.5. Ability of the Ciliate to Feed on Other Microalgae
2.6. DNA Extraction, PCR, Sequencing, and Phylogenetic Analysis
2.7. Terminology
3. Results
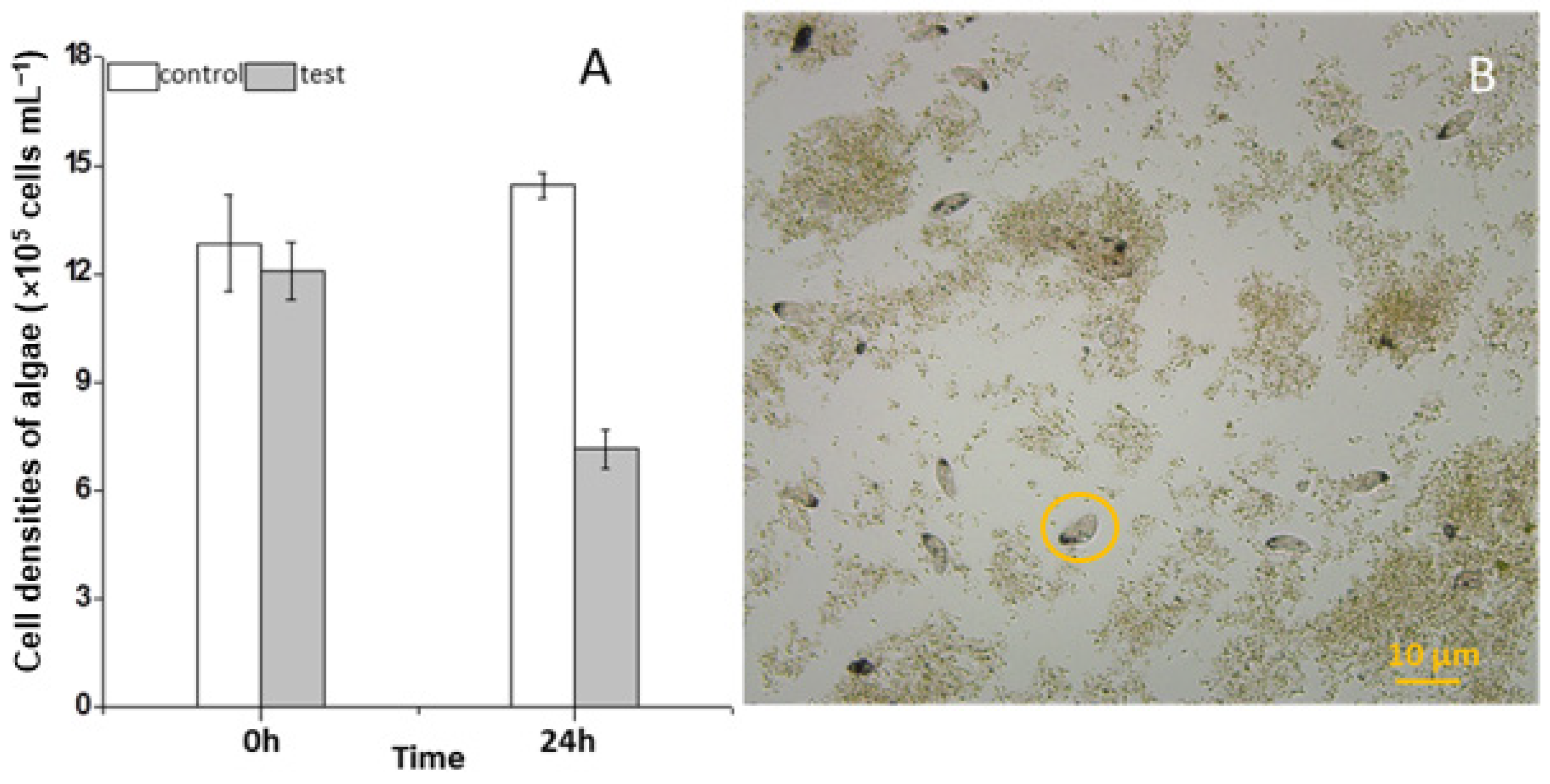
3.1. Feeding Impact of the Ciliate on the Growth of Scenedesmus
3.2. Morphological Observation
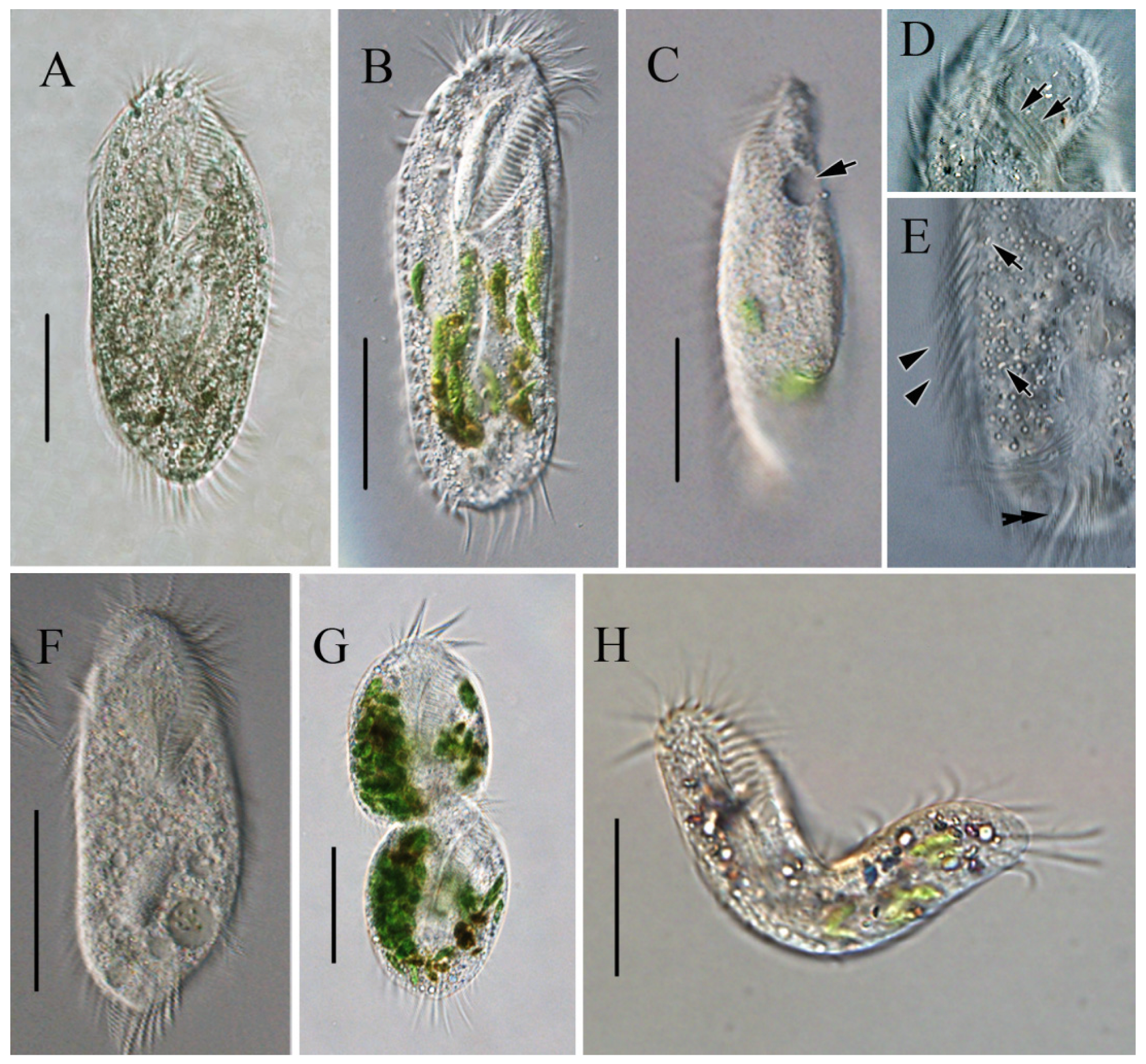
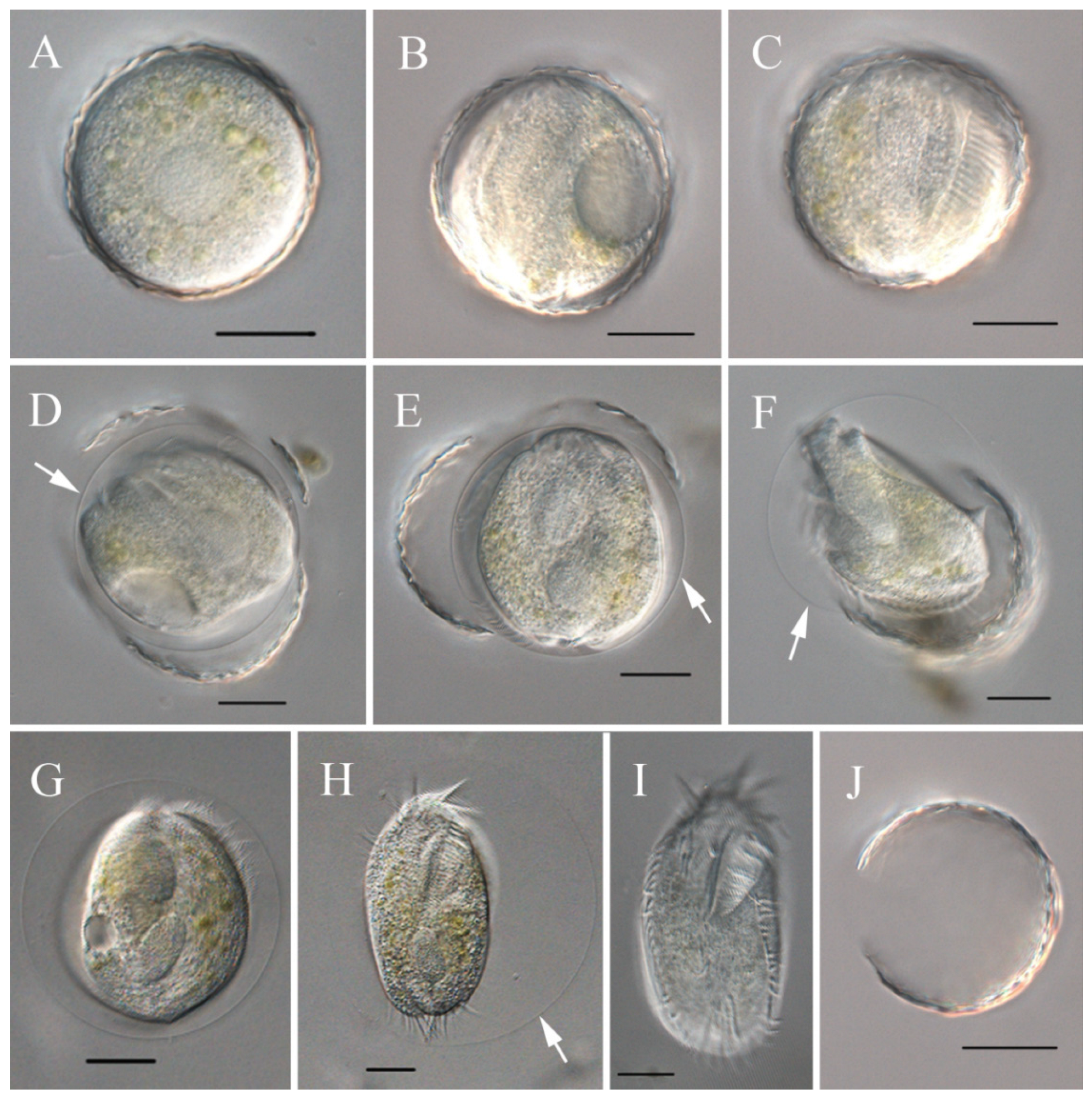
3.2.1. Light Microscopy of Sterkiella histriomuscorum
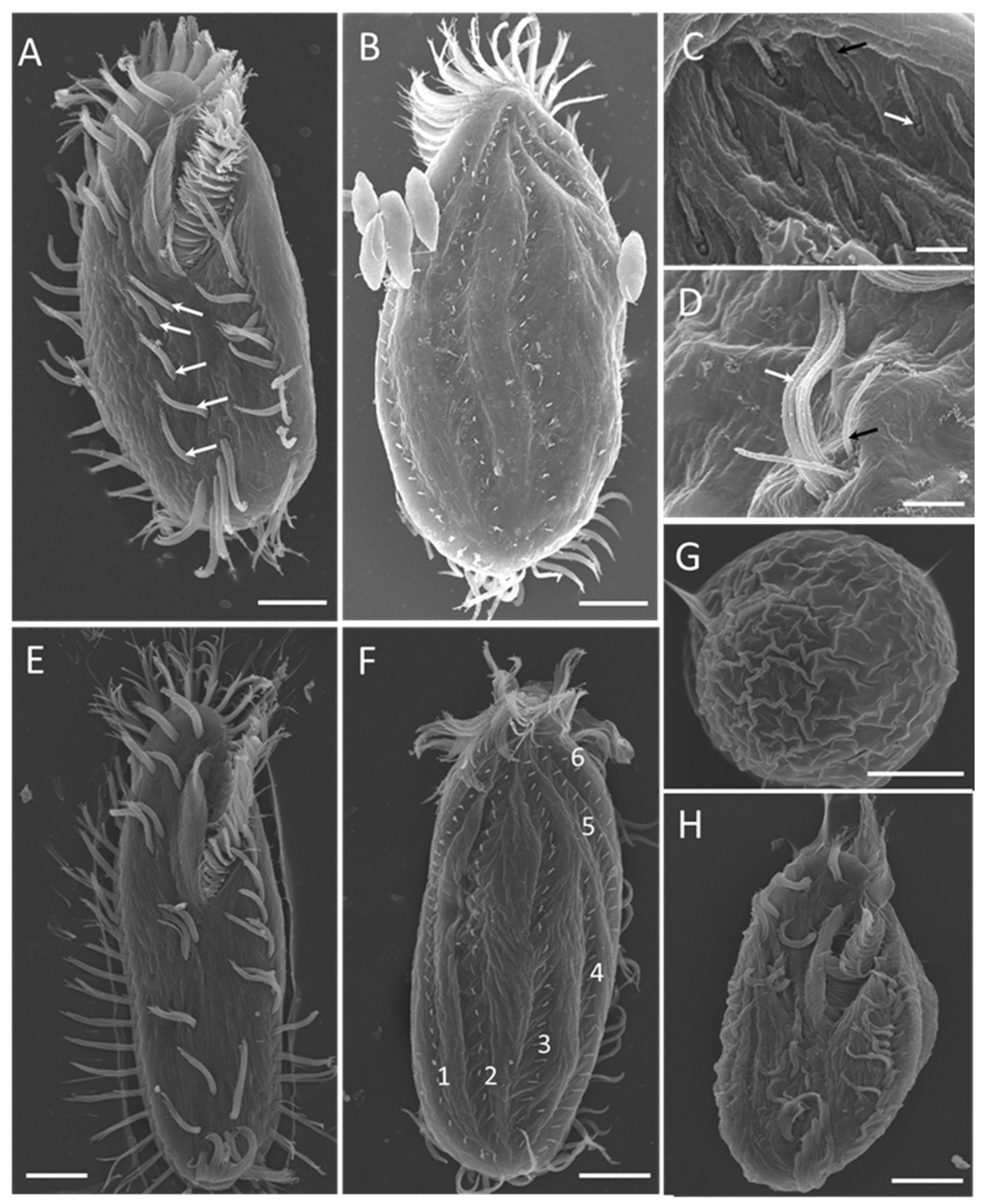
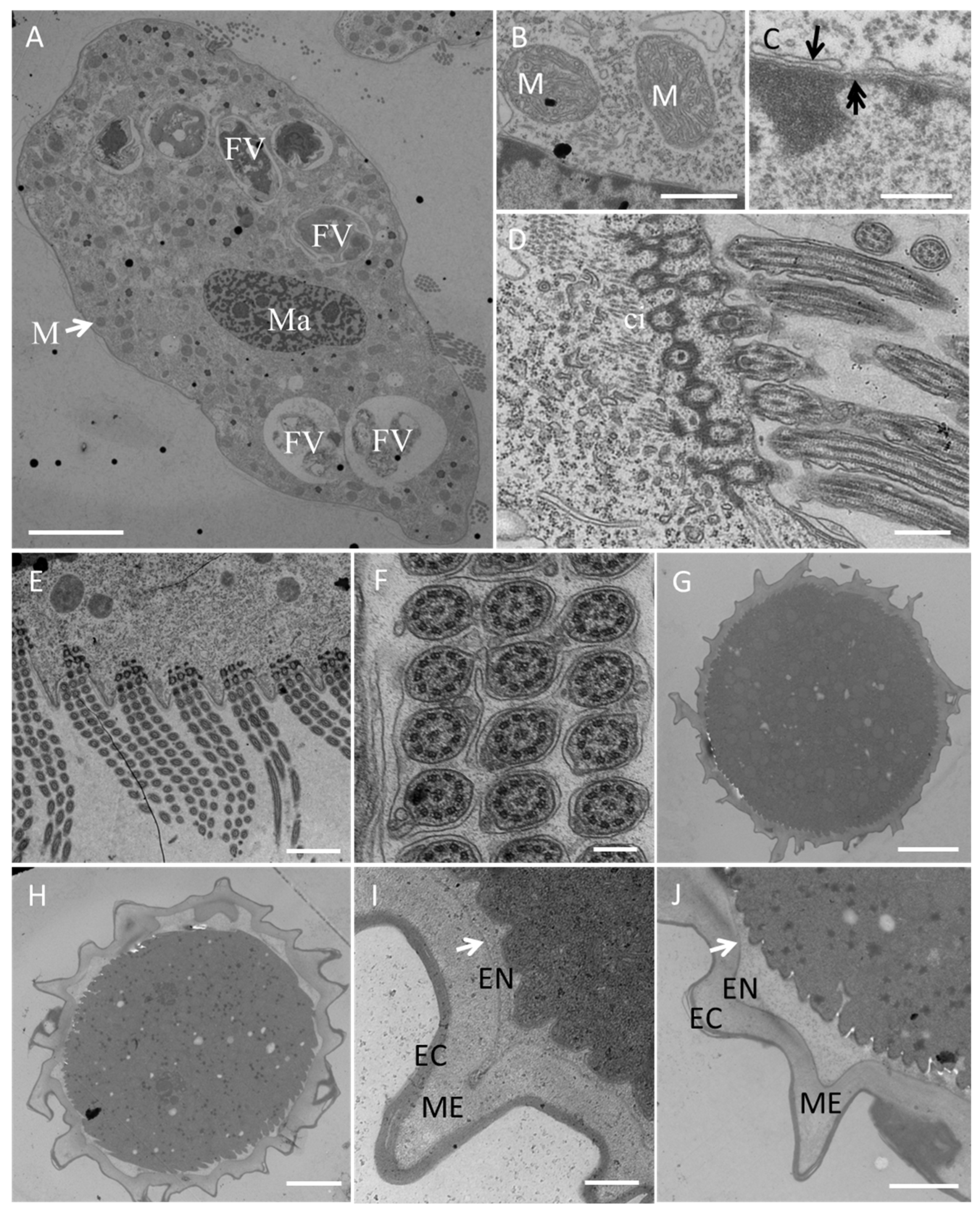
3.2.2. Electron Microscopy
3.3. Encystment and Excystment Processes
3.4. Feeding Selectivity
3.5. Molecular Phylogenetic Analysis
4. Discussion
4.1. Identification of S. histriomuscorum
4.2. Feeding Characteristics and Feeding Selectivity on the Microalgae
4.3. The Distribution of Sterkiella and the Function of the Cyst
4.4. The Improvement of SEM Observation on S. histriomuscorum
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tillmann, U. Interactions between planktonic microalgae and protozoan grazers. J. Eukaryot. Microbiol. 2004, 51, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Roberts, E.C.; Wootton, E.C.; Davidson, K.; Jeong, H.J.; Lowe, C.D.; Montagnes, D.J.S. Feeding in the dinolflagellate Oxyrrhis marina: Linking behaviour with mechanisms. J. Plankton Res. 2011, 33, 603–614. [Google Scholar] [CrossRef]
- Mandal, S.; Mallick, N. Microalga Scenedesmus obliquus as a potential source for biodiesel production. Appl. Microbiol. Biotechnol. 2009, 84, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Day, J.G.; Gong, Y.C.; Hu, Q. Microzooplanktonic grazers—A potentially devastating threat to the commercial success of microalgal mass culture. Algal Res. 2017, 27, 356–365. [Google Scholar] [CrossRef]
- Finlay, B.J.; Esteban, G.F. Planktonic ciliate species diversity as an integral component of ecosystem function in a freshwater pond. Protist 1998, 149, 155–165. [Google Scholar] [CrossRef]
- Beaver, J.R.; Crisman, T.L. The role of ciliated protozoa in pelagic freshwater ecosystems. Microbial Ecol. 1989, 17, 111–136. [Google Scholar] [CrossRef]
- Berger, H. Monograph of the Oxytrichidae (Ciliophora, Hypotrichia) (Monographiae Biologicae); Springer: Dordrecht, The Netherlands, 1999; Volume 78. [Google Scholar]
- Foissner, W.; Berger, H.; Schaumburg, J. Identification and Ecology of Limnetic Plankton Ciliates; Informations berichte des Bayer; Landesamtes für Wasserwirtschaft: Ansbach, Germany, 1999; pp. 1–793. [Google Scholar]
- Augustin, H.; Foissner, W. Morphologie und Ökologie einiger Ciliaten (Protozoa: Ciliophora) aus dem Belebtschlamm. Arch. Fuer Protistenkd. 1992, 141, 243–283. [Google Scholar] [CrossRef]
- Petz, W.; Foissner, W. Morphology and infraciliature of some soil ciliates (Protozoa, Ciliophora) from continental Antarctica, with notes on the morphogenesis of Sterkiella histriomuscorum. Polar Rec. 1997, 33, 307–326. [Google Scholar] [CrossRef]
- Chen, X.M.; Gao, F.; Al-Farraj, S.A.; Al-Rasheid, K.A.S.; Xu, K.; Song, W.B. Morphology and morphogenesis of a novel mangrove ciliate, Sterkiella subtropica sp. nov. (Protozoa, Ciliophora, Hypotrichia), with phylogenetic analyses based on small-subunit rDNA sequence data. Int. J. Syst. Evol. Microbiol. 2015, 65, 2292–2303. [Google Scholar] [CrossRef]
- Kumar, S.; Kamra, K.; Bharti, D.; La Terza, A.; Sehgal, N.; Warren, A.; Sapra, G.R. Morphology, morphogenesis, and molecular phylogeny of Sterkiella tetracirrata n. sp. (Ciliophora, Oxytrichidae), from the Silent Valley National Park, India. Eur. J. Protistol. 2015, 51, 86–97. [Google Scholar] [CrossRef]
- Li, F.; Li, Y.; Luo, D.; Miao, M.; Shao, C. Morphology, morphogenesis, and molecular phylogeny of a new soil ciliate, Sterkiella multicirrata sp. nov. (Ciliophora, Hypotrichia) from China. J. Eukaryot. Microbiol. 2018, 65, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhao, X.; Shao, C.; Miao, M.; Clamp, J.C. Morphology and phylogeny of two new ciliates, Sterkiella sinica sp. nov. and Rubrioxytricha tsinlingensis sp. nov. (Protozoa, Ciliophora, Hypotrichia) from Northwest China. Syst. Biodivers. 2017, 15, 131–142. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, L.; Shao, C. Morphology, morphogenesis and molecular phylogeny of the new soil ciliate Sterkiella paratricirrata n. sp. (Ciliophora, Hypotrichia, Oxytrichidae). J. Nat. Hist. 2021, 54, 2471–2488. [Google Scholar] [CrossRef]
- Wang, M.; Hu, T.; Wang, S.; Tong, Z.; Wei, Q.; Fan, X. Morphology and molecular phylogeny of a new hypotrichous ciliate, Sterkiella zhangi n. sp. (Ciliophora, Oxytrichidae). Eur. J. Protistol. 2025, 98, 126141. [Google Scholar] [CrossRef]
- Grisvard, J.; Lemullois, M.; Morin, L.; Baroin-Tourancheau, A. Differentially expressed genes during the encystment—Excystment cycle of the ciliate Sterkiella histriomuscorum. Eur. J. Protistol. 2008, 44, 278–286. [Google Scholar] [CrossRef]
- Adl, S.M.; Berger, J.D. Timing of life cycle morphogenesis in synchronous samples of Sterkiella histriomuscorum. The vegetative cell cycle. Eur. J. Protistol. 1997, 33, 99–109. [Google Scholar] [CrossRef]
- Bharti, D.; Kumar, S.; Varatharajan, G.R.; Kamra, K.; La Terza, A. Shedding light on the polyphyletic behavior of the genus Sterkiella: The importance of ontogenetic and molecular phylogenetic approaches. PLoS ONE 2018, 13, e0207688. [Google Scholar] [CrossRef]
- Boenigk, J.; Matz, C.; Jurgens, K.; Arndt, H. The influence of preculture conditions and food quality on the ingestion and digestion process of three species of heterotrophic nanoflagellates. Microb. Ecol. 2001, 42, 168–176. [Google Scholar] [CrossRef]
- Boenigk, J.; Arndt, H. Bacterivory by heterotrophic flagellates: Community structure and feeding strategies. Antonie Van. Leeuwenhoek 2002, 81, 465–480. [Google Scholar] [CrossRef]
- Bermúdez, J.R.; Metian, M.; Oberhänsli, F.; Taylor, A.; Swarzenski, P.W. Preferential grazing and repackaging of small polyethylene microplastic particles (≤5 μm) by the ciliate Sterkiella sp. Mar. Environ. Res. 2021, 166, 105260. [Google Scholar] [CrossRef]
- Montagnes, D.J.S.; Barbosa, A.B.; Boenigk, J.; Davidson, K.; Jurgens, K.; Macek, M.; Parry, J.D.; Roberts, E.C.; Simek, K. Selective feeding behaviour of key free-living protists: Avenues for continued study. Aquat. Microb. Ecol. 2008, 53, 83–98. [Google Scholar] [CrossRef]
- Allen, M.; Stanier, R.Y. Growth and division of some unicellular blue-green algae. J. Gen. Microbiol. 1968, 51, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M. Freshwater culture media. In Algal Culturing Techniques; Anderson, R.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 13–20. [Google Scholar]
- Schulze Dieckhoff, H.; Freiburg, M.; Heckmann, K. The isolation of gamones 3 and 4 of Euplotes octocarinatus. Eur. J. Biochem. 1987, 168, 89–94. [Google Scholar] [CrossRef]
- Gong, Y.C.; Patterson, D.J.; Li, Y.G.; Hu, Z.X.; Sommerfeld, M.; Chen, Y.S.; Hu, Q. Vernalophrys algivore gen. nov., sp. nov. (Rhizaria: Cercozoa: Vampyrellida), a new algal predator isolated from outdoor mass culture of Scenedesmus dimorphus. Appl. Environ. Microbiol. 2015, 81, 3900–3913. [Google Scholar] [CrossRef]
- He, Y.; Wei, W.; Wang, M.Y.; Wang, H.X.; Jia, J.; Gong, Y.C.; Hu, Q. Systematic study of microzooplankton in mass culture of the green microalga Scenedesmus acuminatus and quantitative assessment of its impact on biomass productivity throughout a year. Bioresour. Technol. 2024, 408, 131149. [Google Scholar] [CrossRef]
- Wilbert, N. Eine verbesserte Technik der Protargol imprägnation für Ciliaten. Mikrokosmos 1975, 64, 171–179. [Google Scholar]
- Gong, Y.C.; Gu, X.W.; Yu, Y.H.; Shen, Y.F.; Gu, F.K.; Ni, B. Preparation of the flagellate specimens for surface scanning electron microscopy. Period. Ocean Univ. China 2005, 35, 496–498. [Google Scholar]
- Hanaichi, T.; Sato, T.; Iwamoto, T.; Malavasi-Yamashiro, J.; Hoshino, M.; Mizuno, N. A stable lead by modification of Sato’s method. J. Electron. Microsc. 1986, 35, 304–306. [Google Scholar]
- Medlin, L.; Elwood, H.J.; Stickel, S.; Sogin, M.L. The characterization of enzymatically amplified eukaryotic 16S7 like rRNA7 coding regions. Gene 1998, 71, 491–499. [Google Scholar] [CrossRef]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The Clustal X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef]
- Castresana, J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 2000, 17, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Posada, D. jModelTest: Phylogenetic model averaging. Mol. Biol. Evol. 2008, 25, 1253–1256. [Google Scholar] [CrossRef] [PubMed]
- Guindon, S.; Guindon, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 307–321. [Google Scholar] [CrossRef]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef]
- Shimodaira, H. An approximately unbiased test of phylogenetic tree selection. Syst. Biol. 2002, 51, 492–508. [Google Scholar] [CrossRef]
- Zhang, Q.; Yi, Z.; Fan, X.; Warren, A.; Gong, J.; Song, W. Further insights into the phylogeny of two ciliate classes Nassophorea and Prostomatea (Protista, Ciliophora). Mol. Phylogenet Evol. 2014, 70, 162–170. [Google Scholar] [CrossRef]
- Lynn, D.H. The Ciliated Protozoa: Characterization, Classification and Guide to the Literature, 3rd ed.; Springer: Dordrecht, The Netherlands, 2008. [Google Scholar]
- Berger, H. Monograph of the Gonostomatidae and Kahliellidae (Ciliophora, Hypotricha) (Monographiae Biologicae); Springer: Dordrecht, The Netherlands, 2011; Volume 90. [Google Scholar]
- Foissner, W.; Al-Rasheid, K. A unified organization of the stichotrichine oral apparatus, including a description of the buccal seal (Ciliophora: Spirotrichea). Acta Protozool. 2006, 45, 1–16. [Google Scholar]
- Foissner, W.; Blatterer, H.; Berger, H.; Kohmann, F. Taxonomische und Okologische Revision der Ciliaten des Saprobien-Systems—Band I: Cyrtophorida, Oligotrichida, Hypotrichia, Colpodea. Informationsberichte des Bayer; Landesamtes für Wasserwirtschaft: Ansbach, Germany, 1991; Volume 91, pp. 1–478. [Google Scholar]
- Foissner, W. Taxonomische Studien über die Ciliaten des Grossglocknergebietes (Hohe Tauern, österreich). IX. Ordnungen Heterotrichida und Hypotrichida. Ber. Nat. Med. Ver. Salzbg. 1980, 5, 71–117. [Google Scholar]
- Foissner, W.; Berger, H. Identification and ontogenesis of the nomen nudum hypotrichs (Protozoa: Ciliophora) Oxytricha nova (=Sterkiella nova sp. n.) and O. trifallax (=S. histriomuscorum). Acta Protozool. 1999, 38, 215–248. [Google Scholar]
- Foissner, W. Ontogenesis in ciliated protozoa, with emphasis on stomatogenesis. In Ciliates: Cells as Organisms; Hausmann, K., Bradbury, P.C., Eds.; Gustav Fischer: Jena, Germany, 1996; pp. 95–177. [Google Scholar]
- Zoller, S.D.; Hammersmith, R.L.; Swart, E.C.; Higgins, B.P.; Doak, T.G.; Herrick, G.; Landweber, L.F. Characterization and taxonomic validity of the ciliate Oxytricha trifallax (class Spirotrichea) based on multiple gene sequences: Limitations in identifying genera solely by morphology. Protist 2012, 163, 643–657. [Google Scholar] [CrossRef] [PubMed]
- Küppers, G.C.; Paiva, T.S.; Borges, B.N.; Harada, M.L.; Garraza, G.G.; Mataloni, G. An Antarctic hypotrichous ciliate, Parasterkiella thompsoni (Foissner) nov. gen., nov. comb., recorded in Argentinean peat-bogs: Morphology, morphogenesis, and molecular phylogeny. Eur. J. Protistol. 2011, 47, 103–123. [Google Scholar] [CrossRef]
- Zingel, P.; Agasild, H.; Nõges, T.; Kisand, V. Ciliates are the dominant grazers on pico- and nanoplankton in a shallow, naturally highly eutrophic lake. Microb. Ecol. 2007, 53, 134–142. [Google Scholar] [CrossRef]
- Ma, M.Y.; Gong, Y.C.; Hu, Q. Identification and feeding characteristics of the mixotrophic flagellate Poterioochromonas malhamensis, a microalgal predator isolated from outdoor massive Chlorella culture. Algal Res. 2018, 29, 142–153. [Google Scholar] [CrossRef]
- Zhang, H.W.; Patterson, D.J.; He, Y.; Wang, H.X.; Yuan, D.N.; Hu, Q.; Gong, Y.C. Kinopus chlorellivorus gen. nov., sp. nov. (Vampyrellida, Rhizaria), a new algivorous protist predator isolated from large-scale outdoor cultures of Chlorella sorokiniana. Appl. Environ. Microb. 2022, 88, e01215-22. [Google Scholar] [CrossRef]
- Zhang, H.W.; He, Q.; Jiang, X.Y.; Wang, H.X.; Wang, Y.L.; Ma, M.Y.; Hu, Q.; Gong, Y.C. A new algivorous heterolobosean amoeba, Euplaesiobystra perlucida sp. nov. (Tetramitia, Discoba), isolated from pilot-scale cultures of Phaeodactylum tricornutum. Microbiol. Spectr. 2023, 11, e0081723. [Google Scholar] [CrossRef]
- Goulder, R. Grazing by the ciliated protozoon Loxodes magnus on the alga Scenedesmus in a eutrophic pond. Oikos 1972, 23, 109–115. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, J.; Lu, Y.; Chen, Q.; Yang, Z. Grazer-induced morphological defense in Scenedesmus obliquus is affected by competition against Microcystis aeruginosa. Sci. Rep. 2015, 5, 12743. [Google Scholar] [CrossRef]
- Fyda, J.; Nosek, J.; Wiackowski, K.; Pajdak-Stós, A.; Fiałkowska, E. Effects of grazers’ species identity on cyanobacteria in bitrophic and tritrophic food webs. FEMS Microbiol. Ecol. 2009, 68, 329–339. [Google Scholar] [CrossRef]
- Fiałkowska, E.; Pajdak-Stós, A. Chemical and mechanical signals in inducing Phormidium (Cyanobacteria) defence against their grazers. FEMS Microbiol. Ecol. 2014, 89, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Li, X.; Li, Y.; Sun, Y.; Zhang, L.; Huang, Y.; Yang, Z. Rising temperature more strongly promotes low-abundance Paramecium to remove Microcystis and degrade microcystins. Environ. Pollut. 2021, 291, 118143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, Z.; Wang, N.; Gu, L.; Sun, Y.; Huang, Y.; Chen, Y.; Yang, Z. Mixotrophic Ochromonas addition improves the harmful Microcystis-dominated phytoplankton community in in situ microcosms. Environ. Sci. Technol. 2020, 54, 4609–4620. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.Y.; Wang, F.C.; Wei, C.J.; Chen, J.P.; Jin, H.; Wang, H.X.; Song, L.R.; Hu, Q.; Gong, Y.C. Establishment of high-cell-density heterotrophic cultivation of Poterioochromonas malhamensis contributes to achieving biological control of Microcystis. J. Appl. Phycol. 2022, 34, 423–434. [Google Scholar] [CrossRef]
- Berger, H.; Foissner, W.; Adam, H. Morphological variation and comparative analysis of morphogenesis in Parakahliella macrostoma (Foissner, 1982) nov. gen. and Histriculus muscorum (Kahl, 1932), (Ciliophora, Hypotrichida). Protistologica 1985, 21, 295–311. [Google Scholar]
- Hewitt, E.A.; Müller, K.M.; Cannone, J.; Hogan, D.J.; Gutell, R.; Prescott, D.M. Phylogenetic relationships among 28 spirotrichous ciliates documented by rDNA. Mol. Phylogenet. Evol. 2003, 29, 258–267. [Google Scholar] [CrossRef]
- Li, L.; Khan, S.N.; Ji, D.; Shin, M.K.; Berger, H. Morphology and small subunit (SSU) rRNA gene sequence of the new brackish water ciliate Neobakuella flava n. g., n. sp. (Ciliophora, Spirotricha, Bakuellidae) and SSU rRNA gene sequences of six additional hypotrichs from Korea. J. Eukaryot. Microbiol. 2011, 58, 339–351. [Google Scholar] [CrossRef]
- Corliss, J.O.; Esser, S.C. Comments on the role of the cyst in the life cycle and survival of free-living protozoa. Trans. Am. Microsc. Soc. 1974, 93, 578–593. [Google Scholar] [CrossRef]
- Gutierrez, J.C.; Callejas, S.; Borniquel, S.; Benitez, L.; Martin-Gonzales, A. Ciliate cryptobiosis: A microbial strategy against environmental starvation. Int. Microbiol. 2001, 4, 151–157. [Google Scholar] [CrossRef]
- Wang, X.H.; Li, H.N.; Zhan, X.L.; Ma, M.Y.; Yuan, D.N.; Hu, Q.; Gong, Y.C. Development and application of quantitative real-time PCR based on the mitochondrial cytochrome oxidase subunit I gene for early detection of the grazer Poterioochromonas malhamensis contaminating Chlorella culture. Algal Res. 2021, 53, 102133. [Google Scholar] [CrossRef]
- Finlay, T.; Fenchel, T. Cosmopolitan metapopulations of free-living eukaryotes. Protist 2004, 155, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Weisse, T. Meseres corlissi: A rare oligotrich ciliate adapted to warm water and temporary habitats. Aquat. Microb. Ecol. 2004, 37, 75–83. [Google Scholar] [CrossRef]
- Müller, H.; Foissner, W.; Weisse, T. Role of soil in the life cycle of Meseres corlissi (Ciliophora, Oligotrichea): Experiments with two clonal strains from the type locality, an astatic meadow pond. Aquat. Microb. Ecol. 2006, 42, 199–208. [Google Scholar] [CrossRef]
- Gutierrez, J.C.; Diaz, S.; Ortega, R.; Martin-Gonzales, A. Ciliate resting cyst walls: A comparative review. Recent. Res. Dev. Microbiol. 2003, 7, 361–379. [Google Scholar]
- Gu, F.K.; Ni, B. The exploration of preparing protozoan specimens for scanning electron microscopy. J. Chin. Electron Microsc. Soc. 1993, 6, 525–529. [Google Scholar]
- Shin, M.K.; Kim, W. Morphology and biometry of two oxytrichid species of genus Histriculus Corliss, 1960 (Ciliophora, Hypotrichida, Oxytrichidae) from Seoul, Korea. Korean J. Zool. 1994, 37, 113–119. [Google Scholar]
- Jiang, J.M.; Ma, H.G.; Shao, C. Morphology and morphogenesis of Sterkiella histriomuscorum (Ciliophora, Hypotricha). Acta Hydrobiol. Sin. 2013, 37, 227–234. [Google Scholar]


| Food Group | Algal Species | Strain | Morphology | Cell Size (µm) | Algal Category |
|---|---|---|---|---|---|
| Chlorophyte | Chlorella vulgaris | FACHB-8 | unicellular | 2–3 | ++ |
| Chlorella pyrenoidosa | FACHB-9 | unicellular | 2–3 | ++ | |
| Chlorella sorokiniana | CGMCC11801 | unicellular | 3–4 | ++ | |
| Scenedesmus acuminatus | CMBB154 | unicellular | 2–3 × 8–10 | ++ | |
| Chlorogonium elongatum | CMBB132 | unicellular | 2–3 × 6–8 | ++ | |
| Chlamydomonas reinhardtii | CC124 | unicellular | 7–10 | ++ | |
| Chlorella sorokiniana | FACHB-275 | unicellular | 5–6 | + | |
| Chlorella sorokiniana | CMBB146 | unicellular | 3–8 | + | |
| Nannochloropsis oceanica | IMET1 | unicellular | 2–4 | - | |
| Ulothrix sp. | FACHB-1747 | filamentous | 1–2 × 10–15 | - | |
| Chrysophyte | Isochrysis zhanjiangensis | CMBB282 | unicellular | 5–6 × 6–7 | - |
| Isochrysis sp. | CMBB112 | unicellular | 3–4 × 4–5 | - | |
| Diatom | Phaeodactylum tricornutum | UTEX640 | unicellular | 3–4 × 10–15 | ++ |
| Cyanobacteria | Microcystis aeruginosa | FACHB-928 | unicellular | 2–3 | - |
| Microcystis aeruginosa | FACHB-905 | unicellular | 2–3 | - | |
| Microcystis flos-aquae | FACHB-1028 | unicellular | 2–3 | - | |
| Microcystis aeruginosa | FACHB-942 | unicellular | 2–3 | - |
| Characteristics | Min | Max | Mean | SD | CV (%) | n |
|---|---|---|---|---|---|---|
| Body length | 93 | 138 | 110 | 12.16 | 0.11 | 30 |
| Body width | 35 | 60 | 47 | 8.39 | 0.18 | 30 |
| Adoral zone, length | 34 | 52 | 42 | 5.20 | 0.13 | 30 |
| Adoral membranelles, No. | 28 | 38 | 32 | 2.76 | 0.09 | 30 |
| Buccal cirri, No. | 1 | 1 | 1 | 0 | 0 | 30 |
| Frontal cirri, No. | 3 | 3 | 3 | 0 | 0 | 30 |
| Frontoventral cirri, No. | 3 | 4 | 4 | 0.18 | 0.05 | 30 |
| Posterior ventral cirri, No. | 3 | 3 | 3 | 0 | 0 | 16 |
| Pretransverse ventral cirri, No. | 2 | 2 | 2 | 0 | 0 | 16 |
| Transverse cirri, No. | 4 | 5 | 5 | 0.41 | 0.08 | 30 |
| Cirri in left marginal row, No. | 18 | 25 | 21 | 2.16 | 0.10 | 30 |
| Cirri in right marginal row, No. | 22 | 27 | 25 | 1.27 | 0.05 | 30 |
| Caudal cirri, No. | 3 | 3 | 3 | 0 | 0 | 16 |
| Dorsal kineties, No. | 5 | 7 | 6 | 0.62 | 0.11 | 16 |
| Macronuclear nodules, No. | 2 | 2 | 2 | 0 | 0 | 30 |
| Macronuclear nodule, length | 25 | 42 | 32 | 4.24 | 0.13 | 50 |
| Macronuclear nodule, width | 18 | 30 | 23 | 3.17 | 0.14 | 50 |
| Micronuclear nodules, No. | 1 | 3 | 2 | 0.78 | 0.41 | 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Chen, P.; Wang, H.; Deng, Q.; Zhang, X.; Yuan, G.; Jiang, M.; Zheng, L.; Hu, Z.; Gu, Z.; et al. Identification and Feeding Characterization of Sterkiella histriomuscorum (Protozoa, Ciliophora, Hypotrichia) Isolated from Outdoor Mass Culture of Scenedesmus dimorphus. Microorganisms 2025, 13, 1016. https://doi.org/10.3390/microorganisms13051016
Wang M, Chen P, Wang H, Deng Q, Zhang X, Yuan G, Jiang M, Zheng L, Hu Z, Gu Z, et al. Identification and Feeding Characterization of Sterkiella histriomuscorum (Protozoa, Ciliophora, Hypotrichia) Isolated from Outdoor Mass Culture of Scenedesmus dimorphus. Microorganisms. 2025; 13(5):1016. https://doi.org/10.3390/microorganisms13051016
Chicago/Turabian StyleWang, Mengyun, Pei Chen, Hongxia Wang, Qiong Deng, Xiaonan Zhang, Guoqing Yuan, Mixue Jiang, Lingling Zheng, Zixuan Hu, Zemao Gu, and et al. 2025. "Identification and Feeding Characterization of Sterkiella histriomuscorum (Protozoa, Ciliophora, Hypotrichia) Isolated from Outdoor Mass Culture of Scenedesmus dimorphus" Microorganisms 13, no. 5: 1016. https://doi.org/10.3390/microorganisms13051016
APA StyleWang, M., Chen, P., Wang, H., Deng, Q., Zhang, X., Yuan, G., Jiang, M., Zheng, L., Hu, Z., Gu, Z., Tikhonenkov, D. V., & Gong, Y. (2025). Identification and Feeding Characterization of Sterkiella histriomuscorum (Protozoa, Ciliophora, Hypotrichia) Isolated from Outdoor Mass Culture of Scenedesmus dimorphus. Microorganisms, 13(5), 1016. https://doi.org/10.3390/microorganisms13051016






