Abstract
This study investigated the effects of salinity on the growth and cell morphotype of the coral-associated dinoflagellate Durusdinium glynnii under two acclimation strategies: abrupt saline shock (S5) and gradual reduction (S2). Results revealed optimal growth rates (µ = 0.22–0.35 day−1) at salinities of 20–30 g L−1, while extreme conditions (10 and 40 g L−1) significantly inhibited development. The S2 strategy enabled adaptation to salinities as low as 16 g L−1, maintaining higher cell densities compared to the S5 method. Gradual salinity reduction also influenced cellular morphology: below 12 g L−1, a predominant shift occurred from motile forms (mastigotes) to non-motile spherical structures (coccoid), suggesting an adaptive response to osmotic stress, gradually reducing the growth rate due to the lower reproductive rate of coccoid cells, as previously reported in studies. The findings conclude that D. glynnii is a euryhaline species, tolerant of moderate salinity variations (16–30 g L−1) but limited under extreme conditions. Its morphological plasticity and gradual acclimation capacity highlight its potential for cultivation in brackish environments and biomass production for biotechnological applications, such as antioxidants and antimicrobials. The data provide a foundation for future studies on molecular mechanisms of salinity tolerance, essential for coral conservation strategies and bioprospecting efforts.
1. Introduction
Microalgae are a diverse group of microorganisms that play a fundamental role in aquatic ecosystems and biogeochemical cycles. Among them, dinoflagellates constitute a lineage characterized by distinctive features, including the arrangement of their flagella, specific pigmentation patterns, specialized organelles, and unique nuclear attributes that differentiate them from other microalgal groups [1]. Dinoflagellates are among the most diverse unicellular microorganisms, playing a crucial role in primary production and global biogeochemical cycles. Within this group, Durusdinium glynnii, a member of the family Symbiodiniaceae—formerly classified as Symbiodinium clade D—stands out for its mutualistic association with marine invertebrates, particularly reef-building corals [2,3,4,5]. This symbiotic relationship is fundamental for the development and persistence of tropical and subtropical coral reef ecosystems, providing the host with essential nutrients through photosynthesis. Additionally, dinoflagellates contribute to coral health and resilience by enhancing their thermal tolerance and adaptive capacity under environmental stress, which is critical for mitigating the impacts of climate change on reef ecosystems [3,6].
Saline stress is a common factor in natural environments, however, microalgae develop survival strategies to maintain a balance between growth and stress responses, as eukaryotic algae exhibit high plasticity and adaptability to most abiotic stressors [7]. It is also known that the adaptability of microalgae to saline stress varies according to the species’ tolerance level [8]. Coastal environments, being highly dynamic, exhibit abrupt variations in the physicochemical properties of water, with salinity emerging as one of the main stressors for the biological community, influenced by rainfall, tidal cycles, and evaporation [9]. This suggests that D. glynnii is naturally subjected to salinity fluctuations in its environment, however, knowledge gaps remain regarding the ecophysiological impacts of this variable on the population of this dinoflagellate species.
Salinity is one of the factors that significantly influence the biology and physiology of dinoflagellates [10]. These organisms, in particular, are capable of crossing the marine/freshwater barrier more frequently than other microalgae, such as chlorophytes and rhodophytes, due to their ability to adapt to new conditions more rapidly and frequently than anticipated [11,12]. In this context, physiological studies emerge as important tools to determine the conditions under which algae thrive. It is known that changes in salinity can alter the dinoflagellate morphology [13,14], potentially slowing cell division, reducing size, disrupting motility, and inducing the formation of palmelloid structures in microalgae. However, some of these changes are species-specific [15], and studies investigating the effects of low salinity concentrations on marine microalgae remain limited in the scientific literature [16].
In summary, understanding how salinity influences the growth and cellular morphology of D. glynnii not only enhances our knowledge of its biology but also provides valuable insights for cultivation and biotechnological applications. Optimizing salinity conditions is crucial for laboratory and industrial-scale cultivation, where maintaining stable environments ensures the production of high-quality biomass. From a biotechnological perspective, the ability to cultivate marine algae in lower salinities (5–20 g L−1) opens new possibilities for utilizing brackish water—an underexploited resource unsuitable for human consumption or agriculture and costly to desalinate [17]. This approach aligns with several Sustainable Development Goals (SDGs), including SDG 1 (poverty eradication), SDG 8 (economic growth and employment), SDG 10 (reducing inequalities), and SDG 11 (sustainable cities and communities), by fostering economic opportunities in arid, often underdeveloped regions. Additionally, it contributes to SDG 6 (clean water and sanitation) and SDG 14 (life below water) by promoting innovative water use strategies. Moreover, previous studies from our research group have demonstrated that D. glynnii is a rich source of bioactive metabolites, including antioxidant [18] and antibacterial [19] compounds, making its biomass a promising candidate for high-value biotechnological applications. Given these prospects, the present study evaluated the impact of salinity on the growth and cellular morphology of D. glynnii, comparing two acclimation strategies: abrupt salinity shock and gradual salinity reduction.
2. Materials and Methods
2.1. Biological Material
The Durusdinium glynnii strain (BMK 211) was obtained from the Live Feed Production Laboratory at the Federal Rural University of Pernambuco, where it is maintained in filtered and autoclaved seawater with a salinity of 30 g L−1, enriched with f/2 culture medium. The cultures were acclimated to a controlled temperature of 22 ± 1 °C, an irradiance of approximately 150 µmol photons m−2 s−1, and a 12:12 h light:dark photoperiod.
2.2. Experimental Design and Cultivation Conditions
The dinoflagellate pre-culture, initially cultivated in f/2 medium, was transferred to modified Conway medium [20] to avoid nitrogen and phosphorus limitation. Following the transfer, the strain underwent an adaptation period to adjust to the new nutritional conditions, ensuring proper cell development under optimal conditions for the subsequent experiments.
Experiment 1: This study aimed to evaluate the effects of salinity shock stress, exposing the dinoflagellate to salinity levels of 10, 20, 30, and 40 g L−1 using sterile seawater (42 g L−1, autoclaved at 121 °C for 20 min) and distilled water. In this experiment, the 30 g L−1 salinity served as the control treatment. The experimental design was completely randomized, with three replicates per treatment, totaling 12 experimental units over a duration of 18 days. The assay was conducted in 50 mL screw-cap test tubes, which were manually agitated daily to maintain the cells in suspension. The lowest salinity level that maintained satisfactory growth performance was used as the baseline condition for the second phase of the study.
Experiment 2: The objective was to examine the growth capacity of D. glynnii under a long-term acclimation strategy. Two strategies for gradual salinity reduction were applied, with intervals of 2 g L−1 (S2) and 5 g L−1 (S5). The experimental design was also completely randomized, with four replicates per treatment, totaling eight experimental units. The cultures were maintained in borosilicate bottles with a total volume of 1 L and an initial culture volume of 250 mL, under constant aeration (at a rate of 0.05 vvm). Dilutions were performed weekly, whenever the microalgae reached the exponential growth phase, until either a salinity of 0 g L−1 was reached or total cell mortality occurred.
Both experiments were conducted under a semi-continuous system with controlled temperature (22 ± 1 °C), irradiance of approximately 150 µmol photons m−2 s−1, a 12:12 h light:dark photoperiod, and an initial inoculum density of 5 × 104 cells mL−1.
2.3. Growth Parameters
To evaluate microalgal growth in the conducted studies, 1.5 mL samples were periodically collected from the experimental units and fixed with formaldehyde (4%) for cell quantification using a hemocytometer under a binocular optical microscope. Based on the average density values, logistic growth curves were plotted for each treatment and adjusted according to Pindyck and Rubinfeld [21]. These values were also used to calculate the growth rate (K, divisions day−1), doubling time (DT, days division−1), and specific growth rate (µ, day−1) [22]. For the maximum cell density (MCD, ×104 cells mL−1), the highest average cell concentration observed during cultivation was considered, and the day of maximum cell density (dMCD, day) corresponded to the cultivation day on which the MCD was observed.
2.4. Cell Morphotype
Weekly, prior to each dilution, 1.5 mL samples were collected from each experimental unit to estimate the number of cells in the motile stage (mastigote) and symbiotic stage (coccoid). For this purpose, two types of sample preparations were performed: unfixed samples, which allowed the counting of coccoid cells present within the hemocytometer grid, and fixed samples, which were used to quantify the total average number of cells. The difference between the quantifications of fixed and unfixed samples corresponded to the density of mastigote cells (Equation (1)).
where: Dmast. represents the density of cells in the mastigote stage, Dtotal is the total density of coccoid and mastigote cells, and Dcoc. represents the density of coccoid cells only.
Dmast. = Dtotal − Dcoc.
2.5. Statistical Analyses
The results were expressed as mean ± standard deviation. Data were tested for normality and homogeneity using the Shapiro–Wilk and Cochran tests, respectively. Since the data met the assumptions of a normal distribution, they were subjected to analysis of variance (ANOVA), followed by Tukey’s test for mean comparisons. To assess the effect of gradual salinity reduction on the dinoflagellate’s cell density, a quadratic regression model was chosen as it provided the best fit to the data. A significance level of p < 0.05 was considered for all analyses.
3. Results
3.1. Growth Kinetics of Durusdinium glynnii Under Different Salinities
The results of the salinity shock study demonstrate that this variable influences the biological parameters related to the cell production of Durusdinium glynnii. At the maximum salinity level tested, the dinoflagellate exhibited the highest division rate (0.42 ± 0.18 div. day−1) in the shortest time (2.70 ± 0.96 days div.−1), reaching an average density of 30.44 ± 6.34 × 104 cells mL−1 at a specific growth rate of 0.62 ± 0.11 day−1 on the sixth day of cultivation. Conversely, at the lowest salinity, the dinoflagellate showed a low cell division rate (0.27 ± 0.05 div. day−1) and a high doubling time (3.89 ± 0.81 days div.−1), achieving a cell density of 27.25 ± 9.28 × 104 cells mL−1 at 0.19 ± 0.06 day−1 on the eighteenth day of cultivation. The salinity of 20 g L−1 did not differ statistically from the control (30 g L−1) in any growth parameter. Despite the lower division rate and longer doubling time, both treatments numerically exhibited the highest cell densities, ranging from 35.38 ± 12.79 to 34.38 ± 13.42 × 104 cells mL−1 for salinities of 20 and 30 g L−1, respectively (Table 1). The trends observed in the growth curves indicate that, at a salinity of 40 g L−1, the system reached the stationary phase starting from the 6th day, showing growth inhibition in comparison to the other treatments. The salinity of 10 g L−1, in turn, exhibited slower growth, not reaching the stationary phase by the end of the cultivation period. However, it demonstrated a greater growth potential compared to the salinity of 40 g L−1, achieving higher cell densities, albeit with a longer growth period. The curve corresponding to the salinity of 20 g L−1 was the one that most closely resembled the control treatment. The results indicate that the dinoflagellate D. glynnii is capable of adapting to a direct shock with a salinity of 20 g L−1 without impairing its growth.

Table 1.
Growth parameters of the dinoflagellate Durusdinium glynnii subjected to stress from different salinities.
3.2. Growth of D. glynnii Under Strategies of Gradual Salinity Reduction
Given that D. glynnii maintains its growth pattern at a salinity of 20 g L−1, the second experiment was initiated under this condition. Strategy S2 exhibited an initial growth trend during the early stages of the experiment, continuing to increase as salinity was gradually reduced to 18 g L−1. Upon reduction to a salinity of 16 g L−1, a slight decrease in cell density was observed, which remained stable over time, culminating in a peak in density at the end of this period, with an average value of 109.63 × 104 cells mL−1. When the salinity was reduced to 14 g L−1, a marked decrease in cell density occurred, which later stabilized (Figure 1A).
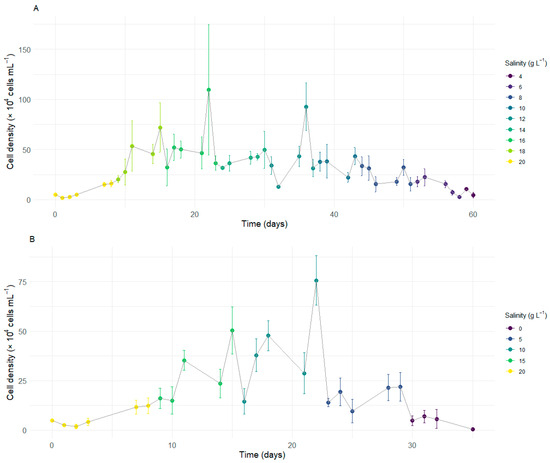
Figure 1.
Cell concentration of Durusdinium glynnii subjected to two strategies of gradual salinity reduction. Reduction in intervals of S2 (A) and S5 (B).
At a salinity of 12 g L−1, a less pronounced initial reduction in density was observed, followed by a significant increase over time, resulting in a second peak at the end of this stage, with an average of 92.75 × 104 cells mL−1. Upon reduction to 10 g L−1, another sharp decline in cell density was observed, which remained stable until the transition to a salinity of 6 g L−1. From this point onward, density began to gradually decrease until reaching a salinity of 2 g L−1, where no cells were observed in suspension at the end of the experiment on day 60 (Figure 1A).
Strategy S5 exhibited an initial growth trend during the early stages of cultivation, continuing to increase after the salinity was reduced to 15 g L−1. At this stage, the population density rapidly recovered over time, reaching a maximum average value of 50.42 × 104 cells mL−1. Upon reducing salinity to 10 g L−1, a sharp decline in density was observed. Despite this, D. glynnii gradually recovered, eventually reaching the highest average density observed throughout the cultivation for this strategy (75.63 × 104 cells mL−1). On the other hand, when salinity was reduced to 5 g L−1, a further significant decrease in density occurred, which, in this case, failed to recover over time. Finally, upon reduction to a salinity of 0 g L−1, there was a further pronounced decrease in density, culminating in the absence of cells in suspension by the end of the experiment on day 35 (Figure 1B).
The analysis of the density curve as a function of salinity revealed that strategy S2 achieves higher densities in a shorter time compared to strategy S5. However, both curves exhibit an intersection point at a salinity of 12 g L−1. Beyond this point, the density in strategy S2 gradually decreases, falling below the values observed for S5. On the other hand, strategy S5 reaches its highest density at a salinity of 10 g L−1, before the cell density begins to decrease (Figure 2). These results are consistent with the quadratic regression performed between density and salinity, where the vertex of the parabola passes through the salinity of 12 g L−1. Similar to what was observed for cell density, μmax values also varied according to salinity under both evaluated strategies. In strategy S2, the highest values were recorded at higher salinities (18 g L−1; μmax = 0.627 ± 0.13 day−1), with a sharp decline as salinity decreased. In contrast, strategy S5 showed its peak μmax at 10 g L−1 (μmax = 0.685 ± 0.02 day−1). Both strategies fitted significantly to quadratic models, with coefficients of determination (R2) of 0.7883 and 0.9161 for S2 and S5, respectively (Figure 3).
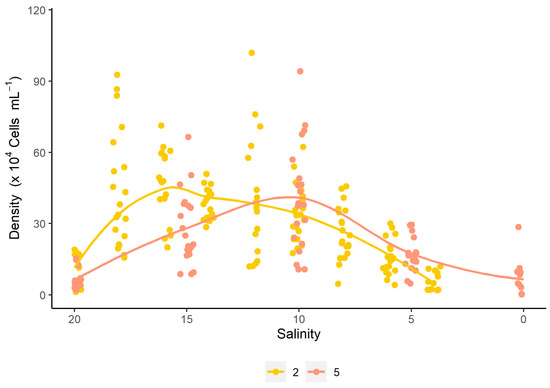
Figure 2.
Correlation between cell density and salinity for the different salinity reduction strategies (S2 and S5) for the dinoflagellate Durusdinium glynnii.
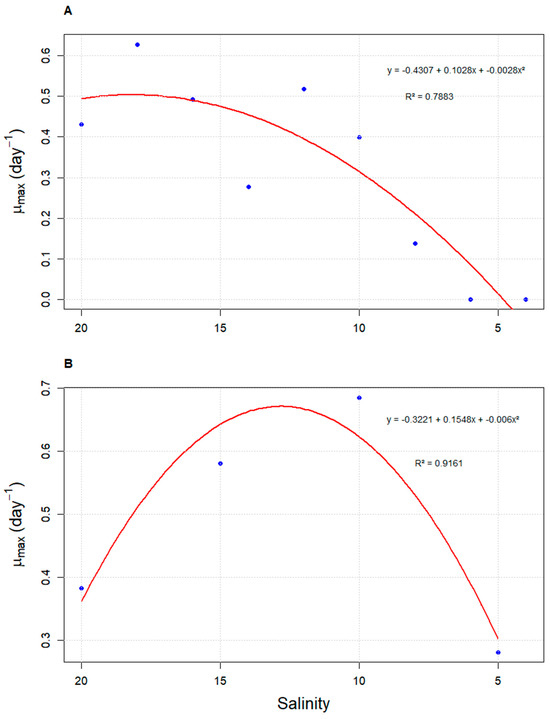
Figure 3.
Quadratic regression analysis of the maximum specific growth rate (μmax) of Durusdinium glynnii cultures as a function of salinity, under two different acclimation strategies to low salinity: S2 (A) and S5 (B).
3.3. Effects of Salinite on the Cell Morphotype
In the classification of cell morphotypes of D. glynnii, strategy S2 exhibited both coccoid cells and mastigotes at salinities ranging from 20 to 12 g L−1. Initially, the cells were predominantly mastigotes (approximately 70% of the total population), and as the dilutions progressed, coccoid cells became more prominent. At a salinity of 10 g L−1, mastigotes were no longer present, and the population consisted exclusively of coccoid cells, reaching 100% of this morphotype at salinities equal to or below 10 g L−1. In strategy S2, all salinities showed statistically significant differences between coccoid and mastigote cell morphotypes, with the exception of salinities 16 and 12 g L−1, where no significant differences between these two morphotypes were observed (Figure 4A).
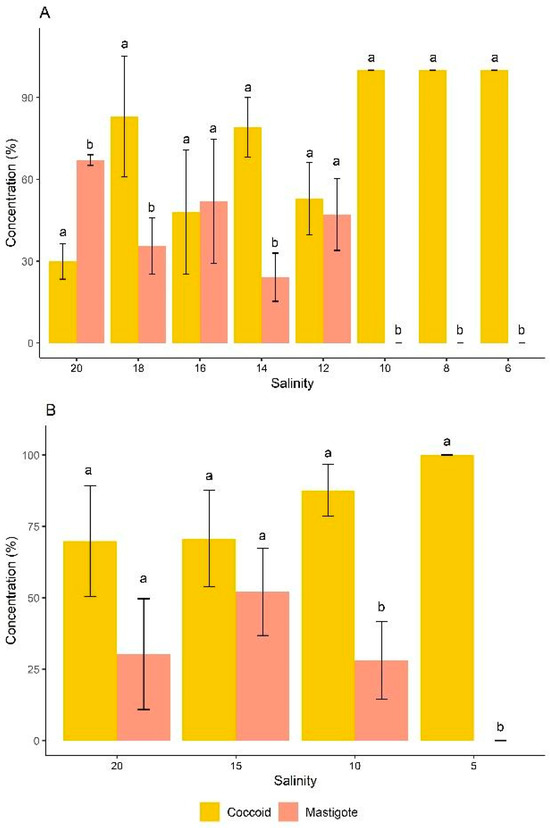
Figure 4.
Distribution of cell morphotypes of D. glynnii in strategies S2 (A) and S5 (B) at different salinities. Different letters indicate significant differences according to an ANOVA followed by Tukey’s test (p < 0.05).
In strategy S5, an inverse pattern was observed, with coccoid cells being dominant throughout the cultivation period. Statistically significant differences between coccoid and mastigote cell morphotypes were observed at salinities 10 and 5 g L−1. At salinities 20 and 15 g L−1, no significant differences between these two morphotypes were found. Furthermore, at a salinity of 5 g L−1, mastigote cells were no longer detected, with 100% of the cell population consisting of coccoid cells (Figure 4B).
4. Discussion
Currently, there is limited literature available on isolated cultures of Durusdinium glynnii, and our study evaluated, for the first time, the long-term responses of this dinoflagellate to stress caused by salinity variations, expressing its tolerance and survival at low salinity. We observed that, even under stress induced by salinity shock, D. glynnii was able to grow in salinities between 10 and 40 g L−1, with faster growth rate at 40 g L−1 and a longer exponential phase at 10 g L−1. A similar behavior was observed for the species Akashiwo sanguinea, which exhibited growth within the same salinity range [23]. On the other hand, the species Alexandrium minutum exhibited growth between 12 and 37 g L−1, with its optimal growth range between 20 and 30 g L−1, classifying it as euryhaline [24,25]. Strains of Ostreopsis spp. grew within a salinity range of 20 to 40 g L−1 [13]. D. glynnii exhibited an optimal growth range between salinities of 20 and 30 g L−1, resembling A. minutum. This plasticity suggests that the dinoflagellate we studied may also be euryhaline, which enables it to adapt to varying salinity conditions.
Despite the reported results, D. glynnii showed a clear preference for higher salinity conditions, as evidenced by the reduction in growth rate at lower salinities. At high salinities, it is known that enzymes can be deactivated, leading to a reduction in the photosynthetic rate and consequent loss of cellular water, factors that contribute to decreased cell density [26]. This mechanism explains the short exponential phase observed at a salinity of 40 g L−1, with growth inhibition from the sixth day of cultivation, even with a relatively high specific growth rate (0.62 ± 0.11 day−1). On the other hand, exposure to low salinities is often associated with oxidative stress in dinoflagellates [27], which may have contributed to the slower adaptation and reduced densities observed in our study at a salinity of 10 g L−1 (0.27 ± 0.05 div. day−1). A. minutum exhibited similar behavior to D. glynnii when cultivated at 10 g L−1, with very slow growth (~0.15 divisions day−1) and higher growth rates at elevated salinities [24].
Quantifying the growth rate provides valuable information regarding overall health and response to specific conditions, as it allows for the assessment of the organism is ability to adapt to different environments [28]. Within the optimal growth range (20 and 30 g L−1), the specific growth rate (µ) of D. glynnii ranged from 0.22 to 0.35 day−1. The dinoflagellate Alexandrium tamiyavanichii, which also grew well between 20 and 30 g L−1 (µ = 0.25 to 0.35 day−1) [25], exhibited similar results. For Vulcanodinium rugosum, the salinities that favored these results were 25 and 40 g L−1 (µ = 0.21–0.39 day−1) [10]. Prorocentrum micans showed better growth at a salinity of 30 g L−1 (µ = 0.38 day−1) and low growth at 25 g L−1 (µ = 0.15 day−1), while P. obtusum exhibited similar growth [27]. This comparison highlights that, although these dinoflagellate species share similar specific growth rates, their salinity preferences vary, reflecting species-specific adaptations.
During the salinity shock experiment, D. glynnii reached a maximum cell density of 35.38 ± 12.79 × 104 cells mL−1. Previous studies reported that this same species reached a maximum density of 70.25 ± 2 × 104 and 91.83 ± 16.83 × 104 cells mL−1 [28,29]. A reasonable explanation for the lower density observed in the present study is that the salinity shock experiment was conducted in test tubes without aeration, reducing the oxygen concentration in the culture medium, which likely favored slower growth or lower cell density. Regarding gradual acclimation, the highest cell density in strategy S2 was observed at a salinity of 16 g L−1, while in strategy S5, it occurred at a salinity of 10 g L−1. Although these salinities are similar, S2 achieved approximately 32% higher cell density compared to S5, suggesting that more gradual salinity reductions are more effective in promoting biomass production.
During the gradual acclimation process, it was observed that, starting at a salinity of 6 in strategy S2 and at a salinity of 5 g L−1 in strategy S5, there was a more pronounced decline in the growth curve. Under these conditions, the species was no longer able to adapt, indicating that this may be the lethal salinity threshold for D. glynnii. This behavior may be related to the osmoregulation process, which requires high energy expenditure from the cells, compromising growth rates [29,30]. Another important factor to consider is the age of the cultures, as the gradual salinity reduction test in the present study lasted between 35 and 60 days. Aged cultures of dinoflagellates show a decrease in the production of genes related to stress/stimulus responses when exposed to abiotic stress conditions, resulting in increased cell mortality in response to external stress in marine microalgal populations with the advancing chronological age of the culture [31,32,33]. Based on this, we hypothesize that the aging of D. glynnii cultures, combined with extreme salinity reduction, reduces cell motility, compromises stress responses, and increases cell mortality. This suggests that cells progressively become less adaptable and more vulnerable as they age, which may affect the survival of the dinoflagellate.
Most symbiotic species have the potential to transition between a free-living motile stage (mastigote) and a spherical symbiotic stage (coccoid) [34]. Microalgae respond to abiotic stress through various mechanisms, with morphological changes such as cell size alterations, flagellar loss, and reduction of motile cells as culture age increases [35,36]. This pattern was corroborated by the present study, which observed both cell morphotypes, with coccoid cells being predominant. This predominance progressively increased, reaching 100% coccoid cells as the stress caused by decreasing salinity intensified, demonstrating a clear transition from mastigote to coccoid cells in both strategies S2 and S5. A previous study indicated that under optimal growth conditions, D. glynnii was composed predominantly of mastigote cells [30]. The same authors further reported that thermal stress reduced this percentage to below 90%, except when there was a high urea concentration, which favored mastigotes (>95%). Conversely, low nitrate concentrations induced a predominance of coccoid cells during thermal stress. Despite the application of different types of stress, both studies indicate a transition between morphotypes, with environmental stress favoring the formation of coccoid cells, but suggesting that the factors inducing this transition vary depending on the type of stress applied.
5. Conclusions
Salinity has been shown to be a key variable for the growth of the dinoflagellate Durusdinium glynnii, with an optimal range between 20 and 40 g L−1. The experimental results suggest that this species can survive and maintain competitiveness in brackish water habitats, with the potential for adaptation to salinities up to 16 g L−1 through strategy S2, without significantly compromising cellular growth. Although it does not guarantee maximum performance under low salinity conditions, cultivation under these conditions holds potential for optimizing the production of biocompounds of biotechnological interest. These findings provide fundamental insights into the ecophysiological behavior of D. glynnii in response to salinity variations, an area still underexplored in the scientific literature. This preliminary study establishes basic parameters for the growth and adaptation of the species, which may serve as a foundation for future, more in-depth investigations. Subsequent research could explore the molecular, biochemical, and metabolic mechanisms underlying the observed plasticity, thus broadening the potential applications of this dinoflagellate in biotechnology and ecological applications.
Author Contributions
Conceptualization, B.d.C.S.B.; methodology, B.d.C.S.B., J.L.d.A., I.M.T.d.A., D.W.S.O. and P.R.d.S.; validation, B.d.C.S.B. and I.M.T.d.A.; formal analysis, C.Y.B.O. and A.O.G.; investigation, B.d.C.S.B., J.L.d.A. and P.R.d.S.; resources, A.O.G. and C.Y.B.O.; data curation, B.d.C.S.B. and C.V.F.d.S.C.; writing—original draft preparation, B.d.C.S.B.; writing—review and editing, J.L.d.A., C.V.F.d.S.C., C.Y.B.O. and A.O.G.; supervision, A.O.G. and J.L.d.A.; project administration, B.d.C.S.B. and A.O.G.; funding acquisition, A.O.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded in part by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)—finance code 001, by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq)—finance code: 409352/2023-3, and by Fundação de Amparo à Ciência e Tecnologia de Pernambuco (FACEPE)—finance code: APQ 1387-3.03/22. Moreover, A.O.G. are thankful to the CNPq for research fellowships PQ 310898/2023-4. Finally, B.d.C.S.B. and J.L.d.A. are thankful to the FACEPE for the research fellowship: IBPG-0098-5.06/21 and BFP-0131-5.06/22, respectively.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Carty, S.; Parrow, M.W. Dinoflagellates. In Freshwater Algae of North America, 2nd ed.; Wehr, J.D., Sheath, R.G., Kociolek, J.P., Eds.; Elsevier: Ann Arbor, MI, USA, 2015; pp. 773–807. [Google Scholar]
- Oliveira, C.Y.B.; Oliveira, C.D.L.; Müller, M.N.; Santos, E.P.; Dantas, D.M.; Gálvez, A.O. A scientometric overview of global dinoflagellate research. Publications 2020, 8, 50. [Google Scholar] [CrossRef]
- LaJeunesse, T.C.; Parkinson, J.E.; Gabrielson, P.W.; Jeong, H.J.; Reimer, J.D.; Voolstra, C.R.; Santos, S.R. Systematic Revision of Symbiodiniaceae Highlights the Antiquity and Diversity of Coral Endosymbionts. Curr. Biol. 2018, 28, 2570–2580.e6. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.-H.; Jang, J.Y.; Kim, J.; Kim, S.; Keshavmurthy, S.; Agostini, S.; Reimer, J.D.; Chen, C.A.; Choi, K.-S.; Park, S.R.; et al. The Origin of the Subtropical Coral Alveopora japonica (Scleractinia: Acroporidae) in High-Latitude Environments. Front. Ecol. Evol. 2020, 8, 12. [Google Scholar] [CrossRef]
- Kannan, M.R.; Balakrishnan, R.; Thillaichidambaram, M.; Natesan, S.; Paramasamy, G.; Prakash, S.; Muthiah, R.C. Probing the Thermo-Tolerant Endosymbiont Genus Durusdinium (Clade D) in the Scleractinian Corals of Palk Bay, Southeast Coast of India. Biologia 2023, 78, 255–264. [Google Scholar] [CrossRef]
- Gordon, B.R.; Leggat, W. Symbiodinium–Invertebrate Symbioses and the Role of Metabolomics. Mar. Drugs 2010, 8, 2546–2568. [Google Scholar] [CrossRef]
- Ren, Y.; Sun, H.; Deng, J.; Huang, J.; Chen, F. Carotenoid Production from Microalgae: Biosynthesis, Salinity Responses and Novel Biotechnologies. Mar. Drugs 2021, 19, 713. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; Galal, H.R.; Mousa, A.S.H.; Farghl, A.A.M. Impact of Macronutrients and Salinity Stress on Biomass and Biochemical Constituents in Monoraphidium braunii to Enhance Biodiesel Production. Sci. Rep. 2024, 14, 2725. [Google Scholar] [CrossRef]
- Röthig, T.; Trevathan-Tackett, S.M.; Voolstra, C.R.; Ross, C.; Chaffron, S.; Durack, P.J.; Warmuth, L.M.; Sweet, M. Human-induced salinity changes impact marine organisms and ecosystems. Glob. Chang. Biol. 2023, 29, 4731–4749. [Google Scholar] [CrossRef]
- Abadie, E.; Muguet, A.; Berteaux, T.; Chomérat, N.; Hess, P.; D’OrbCastel, E.R.; Masseret, E.; Laabir, M. Toxin and Growth Responses of the Neurotoxic Dinoflagellate Vulcanodinium rugosum to Varying Temperature and Salinity. Toxins 2016, 8, 136. [Google Scholar] [CrossRef]
- Čalasan, A.Ž.; Kretschmann, J.; Gottschling, M. They Are Young, and They Are Many: Dating Freshwater Lineages in Unicellular Dinophytes. Environ. Microbiol. 2019, 21, 4125–4135. [Google Scholar] [CrossRef]
- Annenkova, N.V.; Hansen, G.; Rengefors, K. Closely Related Dinoflagellate Species in Vastly Different Habitats—An Example of a Marine–Freshwater Transition. Eur. J. Phycol. 2020, 55, 478–489. [Google Scholar] [CrossRef]
- Tawong, W.; Yoshimatsu, T.; Yamaguchi, H.; Adachi, M. Effects of Temperature, Salinity and Their Interaction on Growth of Benthic Dinoflagellates Ostreopsis spp. from Thailand. Harmful Algae 2015, 44, 37–45. [Google Scholar] [CrossRef]
- Heyerhoff, B.; Nguyen, T.; Hillebrand, H.; Rhiel, E. On the Morphology and Predatory Behavior of the Dinoflagellate Oxyrrhis marina Exposed to Reduced Salinity. Eur. J. Protistol. 2019, 68, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Kholssi, R.; Lougraimzi, H.; Moreno-Garrido, I. Effects of Global Environmental Change on Microalgal Photosynthesis, Growth and Their Distribution. Mar. Environ. Res. 2023, 184, 105877. [Google Scholar] [CrossRef]
- Chaloub, R.M.; Monteiro, V.H.S.; Giordano, M. Physiological Responses of the Marine Microalga Isochrysis galbana (Prymnesiophyceae) to Hyposalinity. J. Appl. Phycol. 2023, 35, 2789–2800. [Google Scholar] [CrossRef]
- Honarparvar, S.; Zhang, X.; Chen, T.; Na, C.; Reible, D. Modeling technologies for desalination of brackish water—Toward a sustainable water supply. Curr. Opin. Chem. Eng. 2019, 26, 104–111. [Google Scholar] [CrossRef]
- Oliveira, C.Y.B.; Abreu, J.L.; Santos, E.P.; Matos, Â.P.; Tribuzi, G.; Oliveira, C.D.L.; Veras, B.O.; Bezerra, R.S.; Müller, M.N.; Gálvez, A.O. Light induces peridinin and docosahexaenoic acid accumulation in the dinoflagellate Durusdinium glynnii. Appl. Microbiol. Biotechnol. 2022, 106, 6263–6276. [Google Scholar] [CrossRef]
- Oliveira, C.Y.B.; Abreu, J.L.; Brandão, B.C.; Oliveira, D.W.S.; de Sena, P.R.; da Silva, W.A.; Araújo, E.S.; Rörig, L.R.; Costa, G.K.A.; Silva, S.M.B.C.; et al. A Holistic Approach to Producing Anti-Vibrio Metabolites by an Endosymbiotic Dinoflagellate Using Wastewater from Shrimp Rearing. Microorganisms 2024, 12, 1598. [Google Scholar] [CrossRef]
- Walne, P.R. Experiments in the Large-Scale Culture of the Larvae of Ostrea edulis. Fish Investig. 1966, 25, 1–53. [Google Scholar]
- Pindyck, R.S.; Rubinfeld, D.L. Econometric Models and Economic Forecasts; McGraw-Hill: New York, NY, USA, 1981; 630p. [Google Scholar]
- Stein, J.R. Handbook of Phycological Methods: Culture Methods and Growth Measurements; Cambridge University Press: London, UK, 1973; pp. 1–448. [Google Scholar]
- Matsubara, T.; Nagasoe, S.; Yamasaki, Y.; Shikata, T.; Shimasaki, Y.; Oshima, Y.; Honjo, T. Effects of temperature, salinity, and irradiance on the growth of the dinoflagellate Akashiwo sanguinea. J. Exp. Mar. Bio. Ecol. 2007, 342, 226–230. [Google Scholar] [CrossRef]
- Grzebyk, D.; Béchemin, C.; Ward, C.J.; Vérité, C.; Codd, G.A.; Maestrini, S.Y. Effects of salinity and two coastal waters on the growth and toxin content of the dinoflagellate Alexandrium minutum. J. Plankton Res. 2003, 25, 1185–1199. [Google Scholar] [CrossRef]
- Lim, P.-T.; Ogata, T. Salinity effect on growth and toxin production of four tropical Alexandrium species (Dinophyceae). Toxicon 2005, 45, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Zafar, A.M.; Javed, M.A.; Aly Hassan, A.; Mehmood, K.; Sahle-Demessie, E. Recent updates on ions and nutrients uptake by halotolerant freshwater and marine microalgae in conditions of high salinity. J. Water Process Eng. 2021, 44, 102382. [Google Scholar] [CrossRef]
- Bui, Q.T.N.; Kim, H.-S.; Ki, J.-S. Low salinity causes oxidative stress and modulates specific antioxidant gene expression in the toxic dinoflagellate Alexandrium pacificum. J. Appl. Phycol. 2022, 34, 2437–2447. [Google Scholar] [CrossRef]
- Klueter, A.; Trapani, J.; Archer, F.I.; McIlroy, S.E.; Coffroth, M.A. Comparative growth rates of cultured marine dinoflagellates in the genus Symbiodinium and the effects of temperature and light. PLoS ONE 2017, 12, e0187707. [Google Scholar] [CrossRef]
- Guimarães, S.C.P.; Rörig, L.R. Efeito da salinidade no crescimento dos dinoflagelados Prorocentrum micans Ehrenberg e Prorocentrum cf. obtusum ostenfeld isolados da costa catarinense—Brasil. Rev. Estud. Biologia 2004, 26, 29.36. [Google Scholar] [CrossRef]
- Oliveira, C.Y.B.; Brandão, B.C.S.; Jannuzzi, L.G.D.S.; Oliveira, D.W.S.; Yogui, G.T.; Müller, M.N.; Gálvez, A.O. New insights on the role of nitrogen in the resistance to environmental stress in an endosymbiotic dinoflagellate. Environ. Sci. Pollut. Res. 2023, 30, 82142–82151. [Google Scholar] [CrossRef]
- Martens, H.; Van de Waal, D.B.; Brandenburg, K.M.; Krock, B.; Tillmann, U. Salinity effects on growth and toxin production in an Alexandrium ostenfeldii (Dinophyceae) isolate from The Netherlands. J. Plankton Res. 2016, 38, 1302–1316. [Google Scholar] [CrossRef]
- Johnson, J.G.; Morey, J.S.; Neely, M.G.; Ryan, J.C.; Van Dolah, F.M. Transcriptome remodeling associated with chronological aging in the dinoflagellate, Karenia brevis. Mar. Genom. 2012, 5, 15–25. [Google Scholar] [CrossRef]
- Gao, Y.; Erdner, D.L. Insights into Stress-Induced Death Processes during Aging in the Marine Bloom-Forming Dinoflagellate Karenia brevis. J. Mar. Sci. Eng. 2022, 10, 1993. [Google Scholar] [CrossRef]
- González-Pech, R.A.; Stephens, T.G.; Chen, Y.; Mohamed, A.R.; Cheng, Y.; Shah, S.; Dougan, K.E.; Fortuin, M.D.A.; Lagorce, R.; Burt, D.W.; et al. Comparison of 15 dinoflagellate genomes reveals extensive sequence and structural divergence in family Symbiodiniaceae and genus Symbiodinium. BMC Biol. 2021, 19, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Novosel, N.; Mišić Radić, T.; Levak Zorinc, M.; Zemla, J.; Lekka, M.; Vrana, I.; Gašparović, B.; Horvat, L.; Kasum, D.; Legović, T.; et al. Salinity-induced chemical, mechanical, and behavioral changes in marine microalgae. J. Appl. Phycol. 2022, 34, 1293–1309. [Google Scholar] [CrossRef]
- Jeong, H.J.; Lee, S.Y.; Kang, N.S.; Du Yoo, Y.; Lim, A.S.; Lee, M.J.; Kim, H.S.; Yih, W.; Yamashita, H.; LaJeunesse, T.C. Genetics and Morphology Characterize the Dinoflagellate Symbiodinium voratum, n. sp., (Dinophyceae) as the Sole Representative of Symbiodinium Clade E. J. Eukaryot. Microbiol. 2013, 61, 75–94. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).