Carbonate Buffer Mixture Alleviates Subacute Rumen Acidosis Induced by Long-Term High-Concentrate Feeding in Dairy Goats by Regulating Rumen Microbiota
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Animals and Experimental Protocol
2.3. Rumen pH and LPS Testing
2.4. Histopathologic Analysis
2.5. Comprehensive Metabolic Panel, Blood Biochemical Analysis, and LPS Tests of Serum
2.6. Analysis of Milk Composition
2.7. ELISA
2.8. Western Blot
2.9. Rumen Fluid DNA Extraction and Illumina MiSeq Sequencing
2.10. Statistical Analysis
3. Results
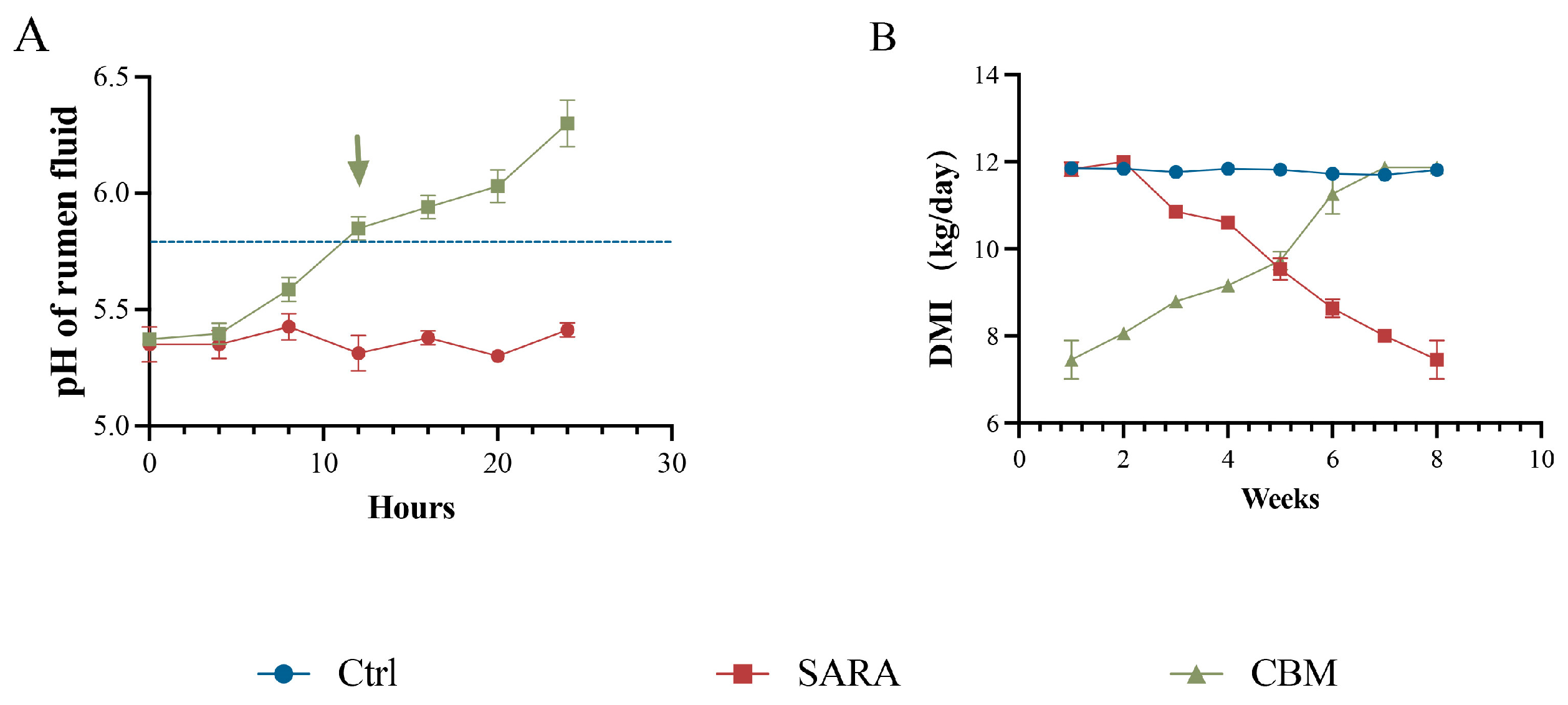
3.1. CBM Alleviates SARA Induced by High-Concentration Diet in Dairy Goats
3.2. The Effects of CBM on CBC in SARA Dairy Goats
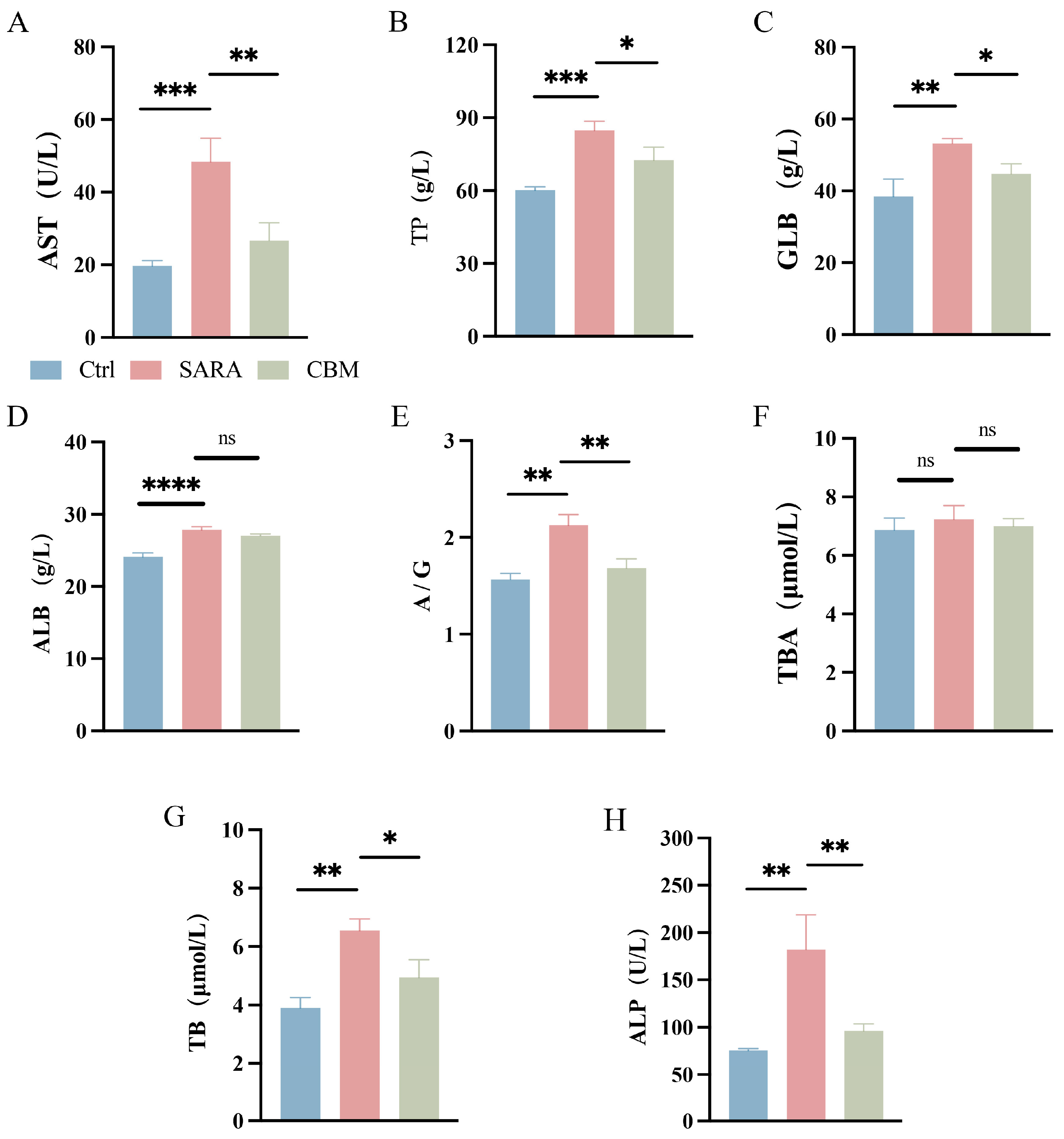
3.3. The Effects of CBM on Blood Biochemical Analysis in SARA Dairy Goats
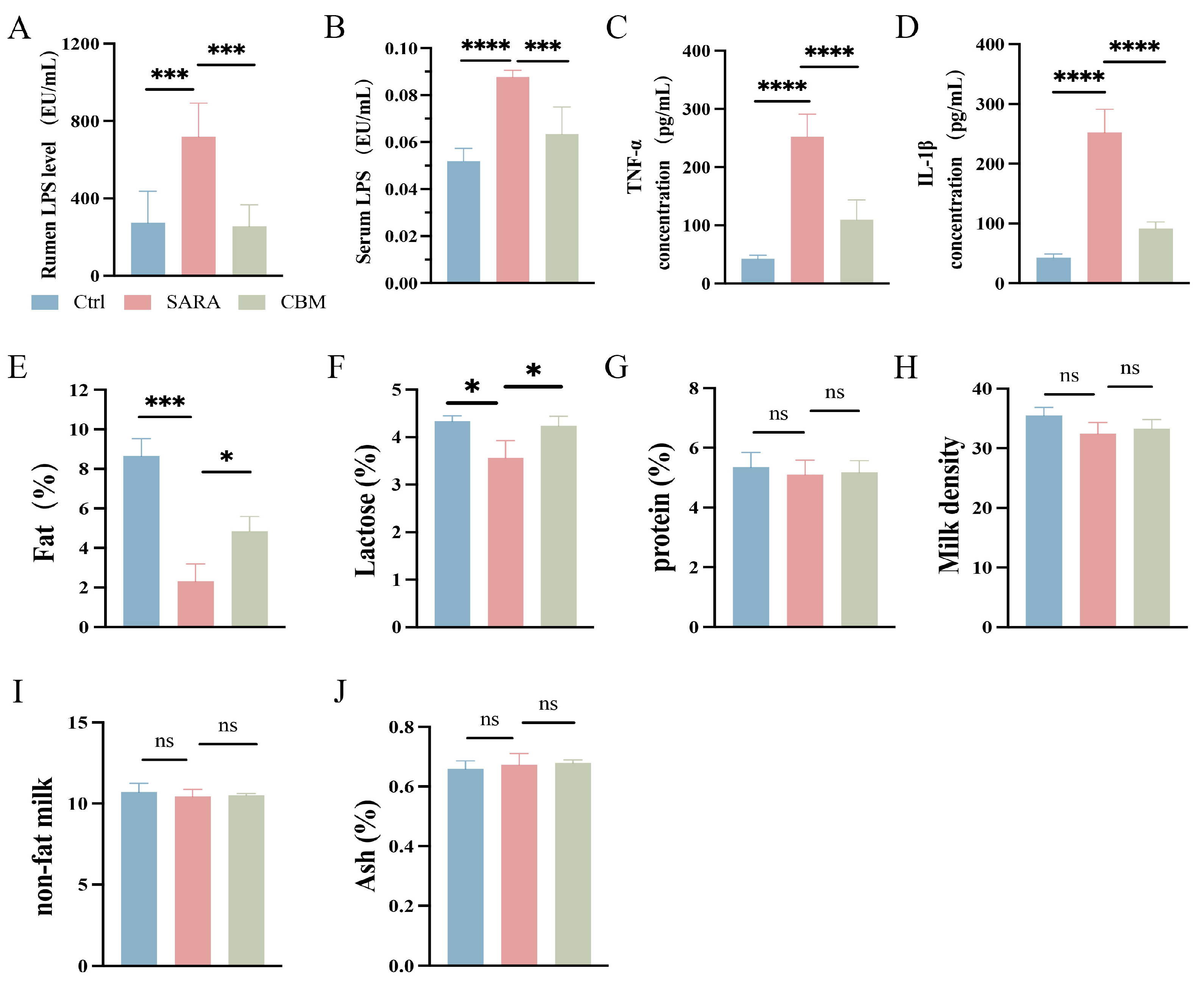
3.4. The Effects of CBM on LPS and the Concentration of Inflammatory Cytokines in SARA Dairy Goats
3.5. The Effects of CBM on Milk Composition in SARA Dairy Goats
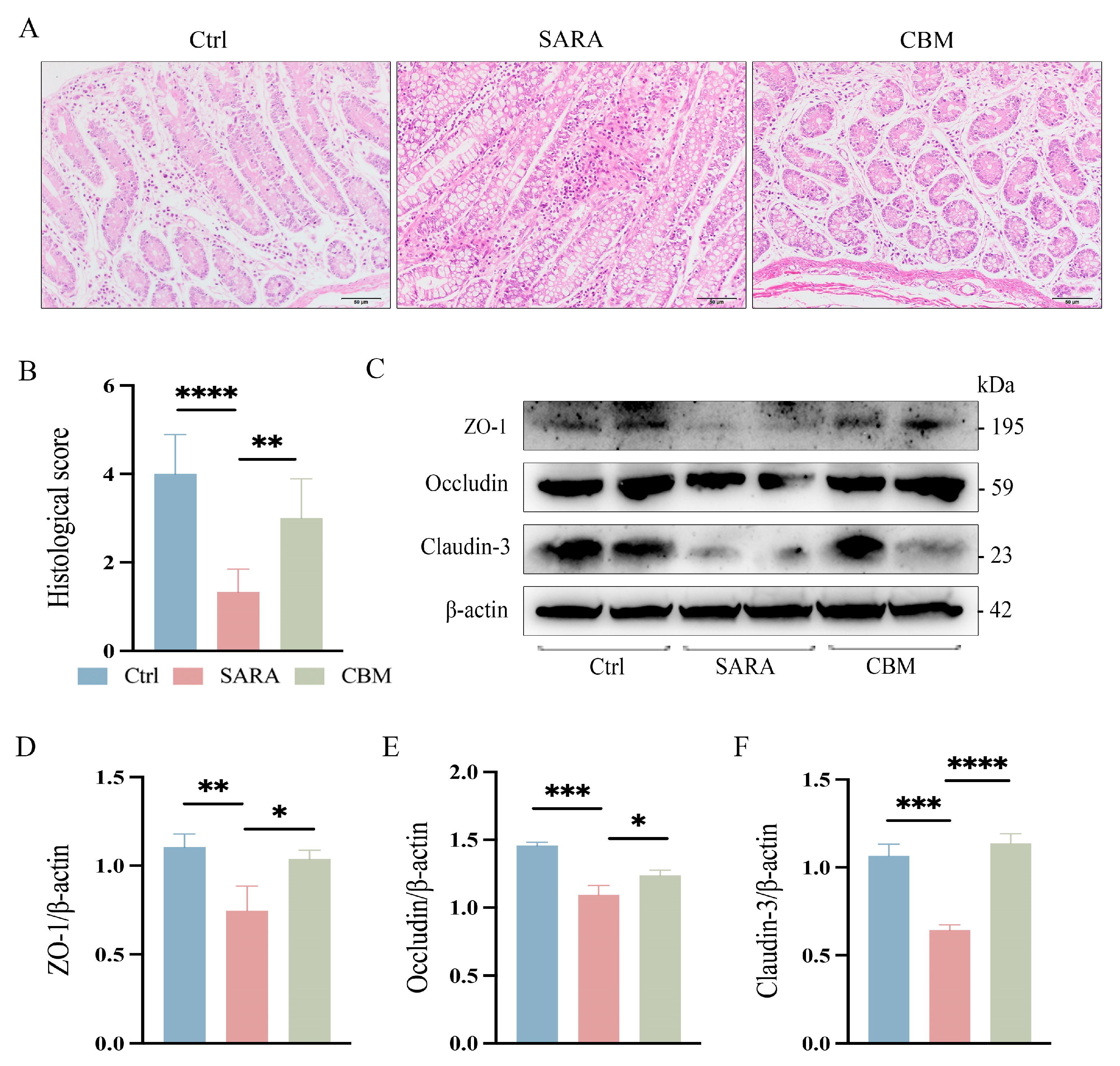
3.6. The Effects of CBM on Rumen and Gut Barrier During SARA in Dairy Goats
3.7. CBM Treatment Reversed Rumen Microbiota Dysbiosis SARA in Dairy Goat
3.8. CBM Treatment Altered Rumen Microbiota Function and Phenotype in SARA Dairy Goat
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Orton, T.; Rohn, K.; Breves, G.; Brede, M. Alterations in fermentation parameters during and after induction of a subacute rumen acidosis in the rumen simulation technique. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1678–1689. [Google Scholar] [CrossRef] [PubMed]
- Adesogan, A.T.; Dahl, G.E. MILK Symposium Introduction: Dairy production in developing countries. J. Dairy Sci. 2020, 103, 9677–9680. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, S.; Wang, H.; Wang, M.; Yu, L. Relative significances of pH and substrate starch level to roles of Streptococcus bovis S1 in rumen acidosis. AMB Express 2016, 6, 80. [Google Scholar] [CrossRef]
- Chen, L.; Luo, Y.; Wang, H.; Liu, S.; Shen, Y.; Wang, M. Effects of Glucose and Starch on Lactate Production by Newly Isolated Streptococcus bovis S1 from Saanen Goats. Appl. Environ. Microbiol. 2016, 82, 5982–5989. [Google Scholar] [CrossRef]
- Mickdam, E.; Khiaosa-Ard, R.; Metzler-Zebeli, B.U.; Klevenhusen, F.; Chizzola, R.; Zebeli, Q. Rumen microbial abundance and fermentation profile during severe subacute ruminal acidosis and its modulation by plant derived alkaloids in vitro. Anaerobe 2016, 39, 4–13. [Google Scholar] [CrossRef]
- Chen, H.; Wang, C.; Huasai, S.; Chen, A. Effects of dietary forage to concentrate ratio on nutrient digestibility, ruminal fermentation and rumen bacterial composition in Angus cows. Sci. Rep. 2021, 11, 17023. [Google Scholar] [CrossRef]
- He, B.; Fan, Y.; Wang, H. Lactate uptake in the rumen and its contributions to subacute rumen acidosis of goats induced by high-grain diets. Front. Vet. Sci. 2022, 9, 964027. [Google Scholar] [CrossRef]
- Feng, Y.; Ren, L.; Wei, P.; Tong, Q.; Ji, X.; Huang, H. Progress in metabolic mechanism of docosahexenoic acid production by fermentation. Sheng Wu Gong Cheng Xue Bao/Chin. J. Biotechnol. 2010, 26, 1225–1231. [Google Scholar]
- Hu, X.; Li, S.; Mu, R.; Guo, J.; Zhao, C.; Cao, Y.; Zhang, N.; Fua, Y. The Rumen Microbiota Contributes to the Development of Mastitis in Dairy Cows. Microbiol. Spectr. 2022, 10, e02512-21. [Google Scholar] [CrossRef]
- Zeng, J.; Lv, J.; Duan, H.; Yang, S.; Wu, J.; Yan, Z.; Zhang, R.; Hu, J.; Zhang, Y. Subacute Ruminal Acidosis as a Potential Factor that Induces Endometrium Injury in Sheep. Int. J. Mol. Sci. 2023, 24, 1192. [Google Scholar] [CrossRef]
- Monteiro, H.F.; Faciola, A.P. Ruminal acidosis, bacterial changes, and lipopolysaccharides. J. Anim. Sci. 2020, 98, skaa248. [Google Scholar] [CrossRef] [PubMed]
- Plaizier, J.C.; Li, S.C.; Danscher, A.M.; Derakshani, H.; Andersen, P.H.; Khafipour, E. Changes in Microbiota in Rumen Digesta and Feces Due to a Grain-Based Subacute Ruminal Acidosis (SARA) Challenge. Microb. Ecol. 2017, 74, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Ye, H.; Liu, J.; Mao, S. High-grain diets altered rumen fermentation and epithelial bacterial community and resulted in rumen epithelial injuries of goats. Appl. Microbiol. Biotechnol. 2017, 101, 6981–6992. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Su, X.; Li, J.; Yang, Y.; Wang, P.; Yan, F.; Yao, J.; Wu, S. Real-time monitoring of ruminal microbiota reveals their roles in dairy goats during subacute ruminal acidosis. NPJ Biofilms Microbiomes 2021, 7, 45. [Google Scholar] [CrossRef]
- Mao, S.Y.; Zhang, R.Y.; Wang, D.S.; Zhu, W.Y. Impact of subacute ruminal acidosis (SARA) adaptation on rumen microbiota in dairy cattle using pyrosequencing. Anaerobe 2013, 24, 12–19. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, Z.; Guan, L.L.; Yoon, I.; Plaizier, J.C.; Khafipour, E. Postbiotics from Saccharomyces cerevisiae fermentation stabilize microbiota in rumen liquid digesta during grain-based subacute ruminal acidosis (SARA) in lactating dairy cows. J. Anim. Sci. Biotechnol. 2024, 15, 101. [Google Scholar] [CrossRef]
- Petri, R.M.; Neubauer, V.; Humer, E.; Kröger, I.; Reisinger, N.; Zebeli, Q. Feed Additives Differentially Impact the Epimural Microbiota and Host Epithelial Gene Expression of the Bovine Rumen Fed Diets Rich in Concentrates. Front. Microbiol. 2020, 11, 119. [Google Scholar] [CrossRef]
- Elmhadi, M.E.; Ali, D.K.; Khogali, M.K.; Wang, H. Subacute ruminal acidosis in dairy herds: Microbiological and nutritional causes, consequences, and prevention strategies. Anim. Nutr. 2022, 10, 148–155. [Google Scholar] [CrossRef]
- Ishaq, S.L.; AlZahal, O.; Walker, N.; McBride, B. An Investigation into Rumen Fungal and Protozoal Diversity in Three Rumen Fractions, during High-Fiber or Grain-Induced Sub-Acute Ruminal Acidosis Conditions, with or without Active Dry Yeast Supplementation. Front. Microbiol. 2017, 8, 1943. [Google Scholar] [CrossRef]
- Enemark, J.M. The monitoring, prevention and treatment of sub-acute ruminal acidosis (SARA): A review. Vet. J. 2008, 176, 32–43. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Chiba, E.; Sugino, T.; Kawashima, K.; Hasunuma, T.; Kushibiki, S.; Kim, Y.H.; Sato, S. Notice of RETRACTION: “Changes in rumen fermentation, bacterial community, and predicted functional pathway in Holstein cows with and without subacute ruminal acidosis during the periparturient period” (J. Dairy Sci. 103:4702-4716). J. Dairy Sci. 2021, 104, 1232. [Google Scholar] [CrossRef] [PubMed]
- Sehested, J.; Diernaes, L.; Moller, P.D.; Skadhauge, E. Transport of sodium across the isolated bovine rumen epithelium: Interaction with short-chain fatty acids, chloride and bicarbonate. Exp. Physiol. 1996, 81, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, K.; Song, X.; Huang, Q.; Lin, S.; Deng, S.; Qi, M.; Yang, Y.; Lu, Q.; Zhao, D.; et al. TLR4 Overexpression Aggravates Bacterial Lipopolysaccharide-Induced Apoptosis via Excessive Autophagy and NF-κB/MAPK Signaling in Transgenic Mammal Models. Cells 2023, 12, 1769. [Google Scholar] [CrossRef]
- Li, L.; Tang, W.; Zhao, M.; Gong, B.; Cao, M.; Li, J. Study on the regulation mechanism of lipopolysaccharide on oxidative stress and lipid metabolism of bovine mammary epithelial cells. Physiol. Res. 2021, 70, 777–785. [Google Scholar] [CrossRef]
- Luo, S.; Shi, Q.; Chen, J.; Wang, H.; Wu, W.; Zha, Z. Expression and Significance of MMPs in Synovial Fluid, Serum and PBMC Culture Supernatant Stimulated by LPS in Osteoarthritis Patients With or Without Diabetes. Exp. Clin. Endocrinol. Diabetes 2019, 127, 195–202. [Google Scholar] [CrossRef]
- Kvidera, S.K.; Horst, E.A.; Abuajamieh, M.; Mayorga, E.J.; Fernandez, M.V.; Baumgard, L.H. Glucose requirements of an activated immune system in lactating Holstein cows. J. Dairy Sci. 2017, 100, 2360–2374. [Google Scholar] [CrossRef]
- Del Chierico, F.; Rapini, N.; Deodati, A.; Matteoli, M.C.; Cianfarani, S.; Putignani, L. Pathophysiology of Type 1 Diabetes and Gut Microbiota Role. Int. J. Mol. Sci. 2022, 23, 14650. [Google Scholar] [CrossRef]
- Radjabzadeh, D.; Bosch, J.A.; Uitterlinden, A.G.; Zwinderman, A.H.; Ikram, M.A.; van Meurs, J.B.J.; Luik, A.I.; Nieuwdorp, M.; Lok, A.; van Duijn, C.M.; et al. Gut microbiome-wide association study of depressive symptoms. Nat. Commun. 2022, 13, 7128. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, D.; Miao, J.; Zhang, C.; Li, X.; Feng, H.; Xing, Y.; Zhang, Z.; Bao, C.; Lin, Z.; et al. Microbiome and metabolome dysbiosis of the gut-lung axis in pulmonary hypertension. Microbiol. Res. 2022, 265, 127205. [Google Scholar] [CrossRef]
- Firrman, J.; Liu, L.; Mahalak, K.; Tanes, C.; Bittinger, K.; Tu, V.; Bobokalonov, J.; Mattei, L.; Zhang, H.; Van den Abbeele, P. The impact of environmental pH on the gut microbiota community structure and short chain fatty acid production. FEMS Microbiol. Ecol. 2022, 98, fiac038. [Google Scholar] [CrossRef]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The Effects of the Mediterranean Diet on Health and Gut Microbiota. Nutrients 2023, 15, 2150. [Google Scholar] [CrossRef] [PubMed]
- He, K.Y.; Lei, X.Y.; Wu, D.H.; Zhang, L.; Li, J.Q.; Li, Q.T.; Yin, W.T.; Zhao, Z.L.; Liu, H.; Xiang, X.Y.; et al. Akkermansia muciniphila protects the intestine from irradiation-induced injury by secretion of propionic acid. Gut Microbes 2023, 15, 2293312. [Google Scholar] [CrossRef] [PubMed]
- Su, X.L.; Tian, Q.; Zhang, J.; Yuan, X.Z.; Shi, X.S.; Guo, R.B.; Qiu, Y.L. Acetobacteroides hydrogenigenes gen. nov., sp. nov., an anaerobic hydrogen-producing bacterium in the family Rikenellaceae isolated from a reed swamp. Int. J. Syst. Evol. Microbiol. 2014, 64, 2986–2991. [Google Scholar] [CrossRef] [PubMed]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottière, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and functional importance in the gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef]
- Bach Knudsen, K.E.; Lærke, H.N.; Hedemann, M.S.; Nielsen, T.S.; Ingerslev, A.K.; Gundelund Nielsen, D.S.; Theil, P.K.; Purup, S.; Hald, S.; Schioldan, A.G.; et al. Impact of Diet-Modulated Butyrate Production on Intestinal Barrier Function and Inflammation. Nutrients 2018, 10, 1499. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, G.; Su, N.; He, Y.; Yuan, C.; Zhao, C.; Hu, X.; Fu, Y.; Zhang, N. Carbonate Buffer Mixture Alleviates Subacute Rumen Acidosis Induced by Long-Term High-Concentrate Feeding in Dairy Goats by Regulating Rumen Microbiota. Microorganisms 2025, 13, 945. https://doi.org/10.3390/microorganisms13040945
Fan G, Su N, He Y, Yuan C, Zhao C, Hu X, Fu Y, Zhang N. Carbonate Buffer Mixture Alleviates Subacute Rumen Acidosis Induced by Long-Term High-Concentrate Feeding in Dairy Goats by Regulating Rumen Microbiota. Microorganisms. 2025; 13(4):945. https://doi.org/10.3390/microorganisms13040945
Chicago/Turabian StyleFan, Guyue, Nier Su, Yuhong He, Chongshan Yuan, Caijun Zhao, Xiaoyu Hu, Yunhe Fu, and Naisheng Zhang. 2025. "Carbonate Buffer Mixture Alleviates Subacute Rumen Acidosis Induced by Long-Term High-Concentrate Feeding in Dairy Goats by Regulating Rumen Microbiota" Microorganisms 13, no. 4: 945. https://doi.org/10.3390/microorganisms13040945
APA StyleFan, G., Su, N., He, Y., Yuan, C., Zhao, C., Hu, X., Fu, Y., & Zhang, N. (2025). Carbonate Buffer Mixture Alleviates Subacute Rumen Acidosis Induced by Long-Term High-Concentrate Feeding in Dairy Goats by Regulating Rumen Microbiota. Microorganisms, 13(4), 945. https://doi.org/10.3390/microorganisms13040945






