Abstract
Viral hepatitis E represents an important global health problem caused by the hepatitis E virus (HEV). Cases of HEV infection are increasingly associated with food-borne transmissions after the consumption of raw or undercooked food products from infected animals in high-income regions. Although most cases of infection are asymptomatic, severe courses of infection have been reported in specific groups of people, predominantly among pregnant women and immunocompromised patients. The viral nucleic acid of HEV is increasingly being reported in food-producing animals and different products of an animal origin. Even though the incubation period for HEV infection is long, several direct epidemiological links between human cases and the consumption of HEV-contaminated meat and meat products have been described. In this article, we review the current knowledge on human HEV infections, HEV in different food-producing animals and products of an animal origin, as well as the accumulation and resistance to HEV in farm and slaughterhouse environments. We also provide preventive measures to help eliminate HEV from animals, the human population, and the environment.
1. Introduction
The hepatitis E virus (HEV) is a small virus, ranging from 27 to 34 nm in diameter, with its genome composed of a single-stranded, positive-sense RNA [1]. Two different infectious HEV particles exist, namely the non-enveloped particles shed in the faeces and the quasi-enveloped particles found in the bloodstream [2]. The quasi-enveloped particles are cloaked in host cell membranes that are thought to protect the virus from neutralisation by the host antibodies [3].
HEV belongs to the family Hepeviridae within the genus Paslahepevirus. Eight genotypes have been described in the species Paslahepevirus balayani, five of which are significant causative agents of human diseases [4]. Genotypes 1 and 2 (HEV-1 and HEV-2) infect only humans, whereas HEV-3 and HEV-4 are zoonotic and are naturally present in several animal species. One zoonotic case was also described with HEV-7 after the consumption of camel meat and milk [5]. Until now, genotypes HEV-5, HEV-6, and HEV-8 have only been detected in animal populations [6]. The majority of human viral hepatitis E cases in Europe are linked to the zoonotic genotype HEV-3 [7]. This genotype’s main transmission route is through the consumption of raw or undercooked meat and meat products [8].
The presence of HEV has been genetically detected in various naturally infected domestic and wild animals [9]. HEV infections in animals result in a subclinical course of infection, with only mild microscopic lesions [10]. The presence of HEV RNA has also been repeatedly reported in different products of an animal origin [11,12,13].
Infection in the human population can be highly underdiagnosed, since it is usually asymptomatic in immunocompetent patients [2]. However, the clinical manifestation of infection depends on the HEV genotypes and the patient’s immune system. Two population groups are particularly susceptible to developing symptomatic HEV infections, including pregnant women and immunocompromised patients [14,15].
As most cases in Europe are locally acquired infections caused by the zoonotic genotype HEV-3, it is crucial to identify the possible sources of HEV infection and prevent food-borne outbreaks. The aim of the present study is to summarise the current knowledge about the occurrence of HEV in different food-producing animals and their products that represent possible risks of food-borne transmissions for consumers.
2. Epidemiology of HEV
Previously, HEV was thought to be an infection limited to several low-income regions in Asia, Africa, the Middle East, and Mexico, along with travel-associated infection in high-income regions in Asia, Europe, and North America [2]. Currently, it is known that HEV is an important infectious agent worldwide. Epidemics of viral hepatitis E are typical for low-income regions, especially during rainy seasons. The first recorded outbreak was in India between 1955 and 1956, with more than 29,000 symptomatic cases [16]. Numerous outbreaks have also been described in China, Bangladesh, Nepal, and Uganda [17,18,19,20]. However, sporadic autochthonous HEV infections have been reported in many high-income regions [2,7].
The true prevalence of HEV among humans is unknown due to frequent asymptomatic or unrecognised infections. The number of subclinical human infections is at least two times greater than clinical infections among sporadic cases and during outbreaks in low-income regions [19]. Clinical symptoms of HEV infection can only be seen in a small population of adults (1–2%) [21]. The worldwide HEV seroprevalence in the human population varies depending on the population group being tested or the geographical region. Every year, approximately 20 million people are infected with HEV, leading to over three million clinical cases and 70,000 deaths [22]. A recent meta-analysis reported the global HEV prevalence by retrieving data from 75 countries. It was found that more than 900 million individuals have experienced past HEV infections based on their seropositivity for anti-HEV antibodies. A high seroprevalence has been described in Africa (21.76%) and Asia (15.80%), with a lower prevalence in Europe (9.31%), North America (8.05%), and South America (7.28%) [23]. A positive correlation exists between age and HEV seropositivity, where the highest seropositivity rates are observed in adults over sixty years of age [24]. Over recent years in Europe, the number of confirmed cases has risen continuously, with a tenfold surge from 514 cases in 2005 to 5617 in 2015 [25]. Data from more seroprevalence studies has been published in Europe, and some specific areas in the United Kingdom, France, and Germany are considered to be endemic [26,27,28]. There is limited information regarding the variation in the awareness and testing in many European countries. Moreover, HEV is not subject to EU-wide surveillance, which further leads to difficulties in determining the prevalence of HEV infections in Europe [29].
3. Transmission of HEV
HEV genotypes that cause viral hepatitis E in the human population can be transmitted in many ways, depending on the region and the HEV genotype. In low-income regions, the main transmission route is water-borne infections caused by genotypes HEV-1 and HEV-2 [22,30]. Until now, the vertical transmission of HEV from a mother to the foetus during pregnancy and blood-borne infections have also been described [31,32].
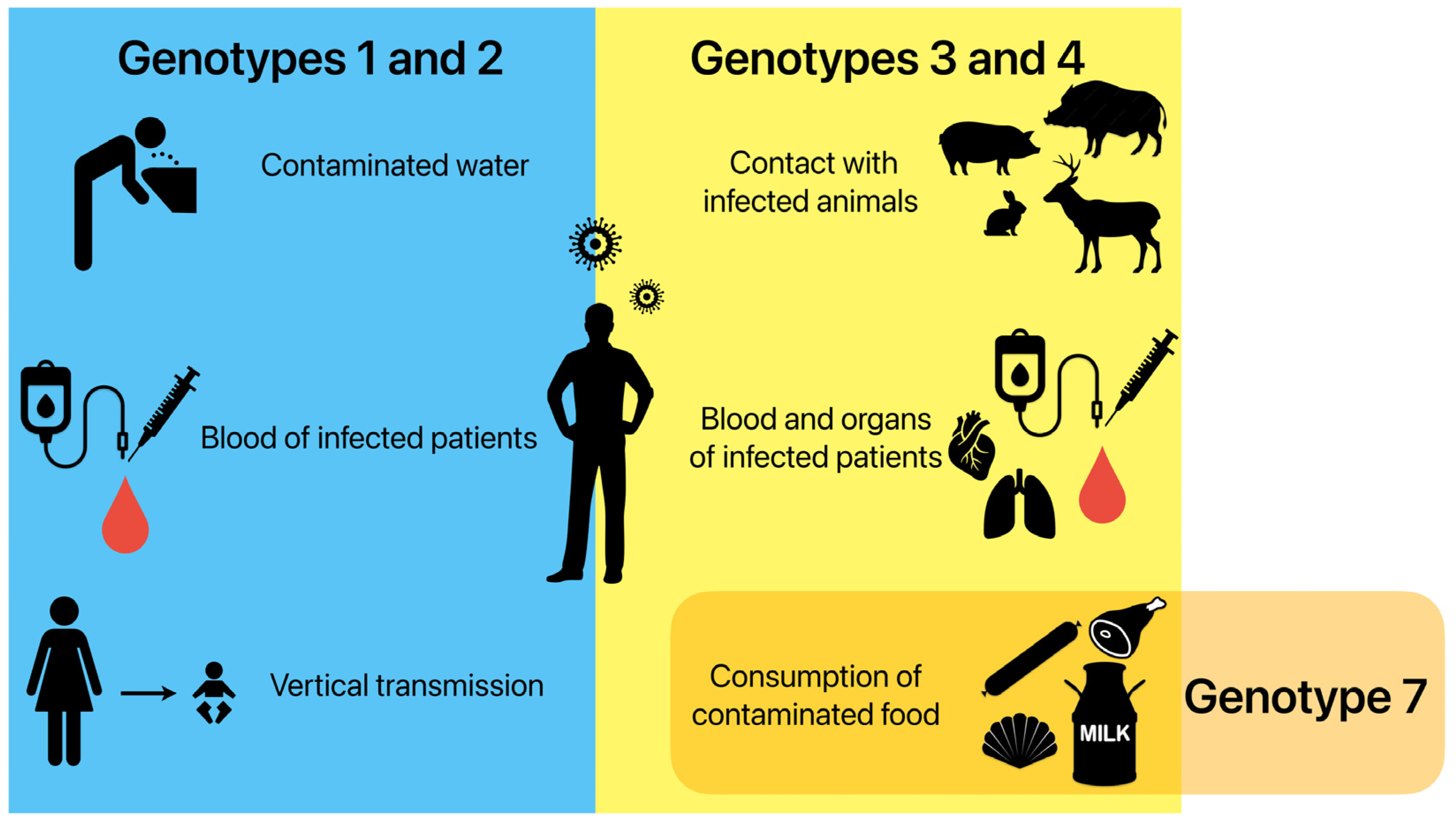
In high-income regions, most infections are caused by genotypes HEV-3 and HEV-4 being transmitted through animal sources. A high identity has been often described between the nucleotide sequences of the HEV strains obtained from humans and animals [33,34]. Zoonotic transmission occurs via direct contact with infected animals and environmental contamination by animal faeces in an agricultural sphere [35]. Food-borne transmissions through raw or undercooked products of an animal origin are considered the most important ways of transmitting these genotypes [34,36]. Sporadic cases of infection have also been linked to blood-borne infections and the transplanted organs of infected humans [37,38]. Infections caused by HEV-7 have been reported in patients after the consumption of camel meat and milk [5]. Figure 1 displays the different HEV transmission routes in the human population.
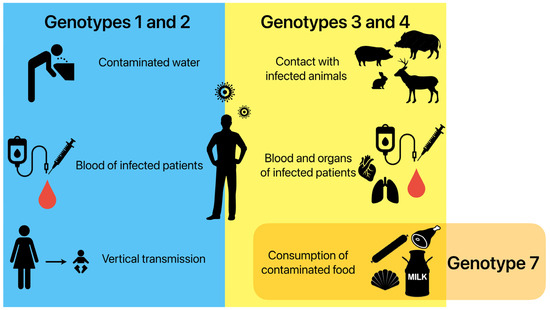
Figure 1.
Possible ways of HEV transmission in the human population depending on genotypes. Genotypes 1 and 2 are mainly transmitted by drinking contaminated water, as well as transfusions of infected blood and from the mother to the foetus during pregnancy. Genotypes 3 and 4 are mainly transmitted by direct contact with infected animals, transfusions of infected blood and transplantations of infected organs, and the consumption of contaminated food. Genotype 7 is connected to the consumption of contaminated food.
3.1. Food-Borne Transmission of HEV
Most cases of HEV infection in high-income regions have been linked to the ingestion of raw or undercooked meat, liver, and liver sausages from infected domestic or wild animals. The long incubation period creates difficulties in proving a causal link, because the food has often been eaten or discarded before the clinical symptoms of infection appear. However, there are several direct epidemiological links between human cases and the consumption of HEV-contaminated meat and meat products.
- Four patients with HEV infections have been reported as consuming raw meat from a wild sika deer in Japan. In three cases, there was a 100% nucleotide identity, and in one case, a 99.7% identity with the consumed meat [36].
- Consumption of grilled wild boar meat has been confirmed as a source of infection in patients from Japan. The similarity between the patient and the meat sequences was 99.95% [39].
- A mini outbreak of HEV infection in family members has also been described in Japan. The source of the infection was grilled pork meat [40].
- Two cases of infection caused by the genotype HEV-4 have been described in France. Patients confirmed the consumption of uncooked pork liver sausages. The genetic sequences were 96.7% identical to the European swine sequences [33].
- A direct HEV transmission through ingesting raw pig liver sausage figatelli has been described in France. Sequences from the case of HEV infection and the consumed sausages were 100% identical [41].
- A patient described an acute infection of HEV after consuming pork meat from a local family farm in Spain. The same strain of the genotype HEV-3 was sequenced from meat consumed by the patient [42].
- A locally acquired outbreak of HEV infection caused by the genotype HEV-3 has been described in Australia [43].
- Chronic infections of HEV-7 have been reported in patients after the consumption of camel meat and milk [5].
- A family outbreak has been described in Spain. Eight members of a family were all infected with HEV-3 after consuming wild boar meat. The HEV sequences from a patient were 100% identical to the wild boar meat [34].
Although there are only few direct epidemiological links between cases, HEV is considered to be an emerging food-borne pathogen. The data concerning HEV in animal populations are expanding annually. Numerous studies have reported the circulation of HEV in different food-producing animals and products of animal origin worldwide (Supplementary Materials, Table S1).
3.1.1. Presence of HEV in Food-Producing Animals
Zoonotic HEV was first discovered in 1997 among domestic pigs in the United States [44]. Pigs have been considered as the most important reservoirs of HEV. Anti-HEV antibodies and HEV RNA have been detected in pigs from the countries of all continents except Antarctica. The circulation of HEV has been confirmed in pig’s sera or faeces from Africa [45,46,47,48], Asia [49,50,51,52,53], North America [54,55,56], South America [57,58,59,60,61], and Australia [62]. Anti-HEV antibodies have been reported in the pig farms of many European countries [63,64,65,66,67]. Similarly, the presence of HEV RNA has been described in pigs [68,69,70,71,72,73]. HEV shedding occurs not only in faeces but also in urine, which may contribute to the spread of the virus in the environment and pose the risk of HEV transmission [74,75].
Several studies have also detected anti-HEV antibodies and HEV RNA in domesticated ruminants [55,76,77,78,79,80]. Even though HEV has been confirmed in the cow population, a direct detection of viral RNA in Europe was unsuccessful [70,81,82]. The susceptibility of sheep and goats to HEV infection has been assessed in more molecular surveys. The detection of viral RNA has been reported in Italy, Mongolia, and China [83,84,85,86]. Cross-species transmissions between ruminants and pigs are possible due to the detection of genetically closely related HEV strains in the same geographical areas [83]. The relatively low prevalence rates in domesticated ruminants suggest that they are not a reservoir of HEV. Infections of HEV can also be caused by the spillover events [83]. However, more studies are needed to evaluate the presence of HEV in the ruminant population.
HEV circulation has also been described in wildlife species, mainly in wild boar and deer populations [36,87]. Wild boars are considered to be another vital reservoir of HEV, which has been confirmed by an increasing number of studies in Europe [88,89,90,91,92]. Anti-HEV antibodies and HEV RNA have been found in red deer, roe deer, and fallow deer [78,93,94]. Identical HEV sequences in wild boar, deer, and other animal species in the same geographical areas are common [8,95]. This is probably caused by interspecific interactions in the same area, especially those with a high animal density. Aggregations around feeding sites often occur during the year when feeding supplies are reduced [70].
Rabbits are natural hosts of the rabbit HEV strain (HEV-3ra), which is classified within HEV-3. The first detection of HEV-3ra was published in China [96]. The presence of HEV-3ra has also been described in the United States, Egypt, Japan, The Netherlands, France, and Germany [97,98,99,100,101,102]. Studies from Italy and Portugal have confirmed anti-HEV antibodies, but none of the samples were positive for HEV RNA [103,104]. Currently, the awareness of the possible transmission of this strain has risen, because human cases of viral hepatitis E have been connected with HEV-3ra in France, Ireland, Switzerland, and Germany [105,106,107,108].
3.1.2. Presence of HEV in Meat and Offal
Animal by-products (offal) include all parts of an animal that are not part of the dressed carcass, such as the liver, heart, rumen contents, kidney, blood, fats, and spleen, and are suitable for human consumption [109]. Since HEV primarily replicates in hepatocytes, the liver can be a significant source of infection, especially in reservoir animal species [110]. In contrast to many other food-borne viruses, the viral contamination of HEV in meat products is not restricted to the food surface and is localised inside many different organs [75]. The detection of HEV RNA has also been described in the muscles, blood, kidney, spleen, and the heart [12,94,111,112]. A wide range of animals are susceptible to infections without clinical disease symptoms. Therefore, visual inspections cannot detect infected animals during slaughter [113].
The human risk of food-borne HEV infection depends on the infection status of the slaughtered animals [35]. Several risk factors seem important, such as the slaughter age, genetic background, lack of hygiene measures, and drinking water origins [114]. The immune status of animals is another critical factor. Co-infections of pigs with HEV and the porcine reproductive and respiratory syndrome virus (PRRSV) increase the possible risk, and HEV-containing livers can be present at the slaughter time [115].
Since pigs are considered to be the most essential HEV reservoirs, viral RNA can be detected throughout the complete pork food chain [1]. Generally, HEV RNA is more prevalent in pork liver and blood than in pork muscle [12,111,116]. HEV positivity in pork liver tissue has been described in numerous countries worldwide, including in North and South America, Asia, and Africa [60,61,117,118,119,120,121]. The prevalence of HEV RNA varies considerably, especially across many European countries (0.3–30.8%). Pig livers have been described as being positive in Switzerland (0.3%), Poland (1.0%), Spain (3%), the United Kingdom (3%), Germany (4%), France (4%), Czech Republic (5%), Italy (6%), The Netherlands (6.5%), Ireland (24%), and Hungary (30.8%) [12,81,122,123,124,125,126,127,128]. Viral RNA was also described in the blood and serum samples of slaughtered animals, being highly prevalent in blood and blood-derived products such as haemoglobin, fibrinogen, and plasma [111,112,129].
A direct detection of HEV RNA in the liver tissue of domesticated ruminants is relatively rare. It has been reported in bovine livers from Korea and Brazil [130,131]. Few studies have been published in Europe, but no positivity of HEV RNA has been detected in liver and blood samples [70,81,82]. The presence of viral RNA was confirmed in sheep livers from China and Mongolia, but there is no evidence from sheep and goat livers in Europe [86,132].
Another important category of animal products is from non-domesticated animals. Wild boar, red deer, roe deer, and fallow deer are usually hunted and slaughtered, mostly for private consumption, but their meat and meat products can also be found in specific markets or restaurants. Numerous studies have confirmed the widespread circulation of HEV in meat and offal from wild boars. Genotype HEV-3 is commonly isolated from wild boars in many European countries (1.9–33.5%) [65,70,81,94,133,134,135,136]. HEV RNA was found in the livers, blood, spleens, kidneys, and muscles. Viral RNA has also been detected in the meat and offal from red deer, roe deer, and fallow deer populations. Several studies have detected the virus in deer livers, with the prevalence of HEV RNA varying between 1.7 and 34.4% [70,81,94,133,137]. A study from Germany has confirmed that HEV RNA could also be present in deer kidneys, spleens, and muscles [94].
The presence of HEV-3ra has been described in the liver tissue of wild rabbits in The Netherlands, France, and Germany (17.1–60%) [98,99,100].
3.1.3. Presence of HEV in Meat Products
The viral nucleic acid of HEV has been repeatedly detected in numerous meat products, especially in pork liver foods. After the consumption of raw pig liver sausages, which were directly linked to human cases, it was confirmed that meat products could be an important source of infection [41]. More studies have identified that eating raw or undercooked meat products is a higher risk factor for HEV infection. Still, the results can be influenced by the study methodology, geographical location, and local cuisines. The consumption of pork liver sausages and offal was found to be a significant risk factor in France [138,139]. A study in the United Kingdom described that ready-to-eat pork products may be an essential transmission source [140]. In Germany, uncooked wild boar meat and the consumption of offal were significantly associated with HEV infections [141].
The concentration of HEV in animal livers can be high, so a few livers from viraemic animals can contaminate a whole batch of sausages [142]. The risk of infection can also be partially influenced by the ingredients of meat products, traditional food processing, and different consumption habits occurring in different regions. In some countries, such as France or Germany, liver is an essential ingredient in the processing of sausages. The prevalence among the resulting products was found to range from 17 to 58% [142,143,144]. Food processing technologies, such as smoking, drying, or curing, can affect the infectivity of HEV. Another critical point can be small traditional homemade food processing practices that can raise the possible risk of infection [145]. Consumption habits are also important. In some countries, specific meat products are typically eaten raw or briefly cooked [12].
The occurrence of HEV RNA has been confirmed in meat products from South Africa, Canada, and Brazil [120,146,147,148], whereas no samples have tested positive in Argentina [149]. In contrast, HEV RNA has been described in raw liver sausages, raw pork sausages, salami, raw dried ham, liver pâté, raw wild boar sausages, and wild boar homemade liver sausages in many European countries. Two studies from The Netherlands have detected the nucleic acid of HEV in their typical meat products, including liver pâtés, liverwurst, cervelaat, salami, metworst, and snijworst [150,151]. HEV RNA was also found in liver sausages and liver quenelles in France, as well as in raw pork and wild boar sausages in Germany, wild boar sausages, raw and dry liver sausages in Italy, liver pâtés and raw dry hams in Belgium, pork sausages in Ireland, and liver and raw meat sausages in Switzerland [128,142,144,145,152,153].
3.1.4. Presence of HEV in Milk
Recently, awareness of the possible milk-borne transmission of HEV has risen. Even though the knowledge of HEV within milk is relatively limited, the role of mammary glands as a source of HEV RNA excretion has been investigated, and it was found that HEV can be present in breast milk during the acute phase of HEV infection [154]. The risk of the milk-borne transmission of HEV has also been confirmed by the fact that primates (rhesus macaques) can be experimentally infected by milk from HEV-infected cows [77]. Possible milk-borne infection has been described after camel milk consumption in the United Arab Emirates [5].
A growing number of reports demonstrate the presence of HEV RNA in the milk of domesticated ruminants, such as cows, sheep, and goats. However, the occurrence of HEV in animal milk is variable, and a higher prevalence of HEV RNA can be visible in regions of Asia and Africa while being lower in Europe and America [155]. This is probably connected to the level of hygiene standards and breeding techniques. The regions with traditional small mixed farms with more animal species (pigs, cows, goats, and sheep) can be at a higher risk of cross-species infections [77,84,155].
Up to now, the knowledge of viral RNA within milk has been limited. Only a few studies about HEV RNA in the milk of ruminants have been published, and the presence of viral RNA in cow milk has been confirmed in Egypt (0.2%), Turkey (29.2%), and China (37%) [77,156,157]. In these studies, different genotypes have been detected as follows: in Egypt, HEV-3; in China, HEV-4; and surprisingly in Turkey, the genotypes HEV-1, HEV-3, and HEV-4. The detection of HEV-1 in animal milk is an unusual finding. Further studies are required to confirm the possible presence of this genotype in animal samples. Cow milk was also screened for HEV RNA in Germany and Belgium, but the samples were all found to be negative [155,158]. HEV positivity has been observed in milk samples from small ruminants in Egypt, Turkey, the Czech Republic, and Italy. In a study from Egypt, 0.7% samples of goat milk were positive for the genotype HEV-3 [159]. In Turkey, 12.3% of ovine milk samples and 18.46% of goat milk samples were found to be positive [156]. In the Czech Republic, 2.8% of mixed ovine and goat milk samples were positive [13], while in Italy, positivity in ovine milk was 2.27% with the detection of HEV-3 [160]. As viral particles of HEV can be present in milk, the production of traditional milk products from raw milk in specific European regions represents a significant public health concern.
3.1.5. Presence of HEV in Shellfish
A marine environment contaminated by HEV can lead to the accumulation of the virus in the digestive tissues of shellfish. This poses a risk of the shellfish-borne transmission of this virus in the human population [11]. Shellfish represent an important HEV transmission source, since during their filter-feeding process, they can concentrate HEV viral particles from the surrounding marine environment. Therefore, they can be an ideal natural indicator of the presence of HEV in environmental waters [161]. As most shellfish are usually eaten raw, viable virus particles can pose a risk to public health. Although relatively rare, the consumption of raw or undercooked shellfish has been identified as a possible source of HEV infection. In 2004, the presence of viral hepatitis E in some Japanese patients was connected with shellfish consumption [162]. Later, an outbreak of HEV was confirmed in passengers returning from a world cruise trip, where the potential source of the infection was considered to be of a shellfish origin [163].
Prevalence studies of HEV in shellfish have been reported in more European countries. Whilst a few studies point to the absence of HEV in shellfish [164,165,166,167], other studies have documented a variable HEV presence. A high prevalence of HEV RNA was observed in the United Kingdom, where 85.4% of mussels were found to be positive for HEV RNA. A phylogenetic analysis showed that most sequences were clustered with the genotype HEV-3 from humans and swine [11]. The occurrence of HEV-3 in different kinds of shellfish was also reported in Spain, Italy, and Scotland [168,169,170].
Differences found in the HEV RNA prevalence in shellfish seem to be influenced by the following factors: type of the examined shellfish, season of the sampling, place of the sampling, contamination of the water environment, seasonality, and age of the shellfish (with a longer period, a higher accumulation of viral particles can be found) [161,166,171].
4. Clinical Manifestations of HEV Infection in the Human Population
4.1. Acute HEV Infection
An HEV infection is usually a self-limiting asymptomatic infection in the majority of patients. The incubation period of the HEV infection ranges from 2 to 8 weeks, with an average of 5 to 6 weeks [172]. During the acute phase of the infection, hepatic and extra-hepatic manifestations have been described [173]. Typical symptoms of a prodromal phase of an acute infection are malaise, fever, nausea, vomiting, pruritus, and a subsequent icteric phase, which includes dark urine and jaundice. In immunocompetent patients, the viremia can be cleared without treatment [174].
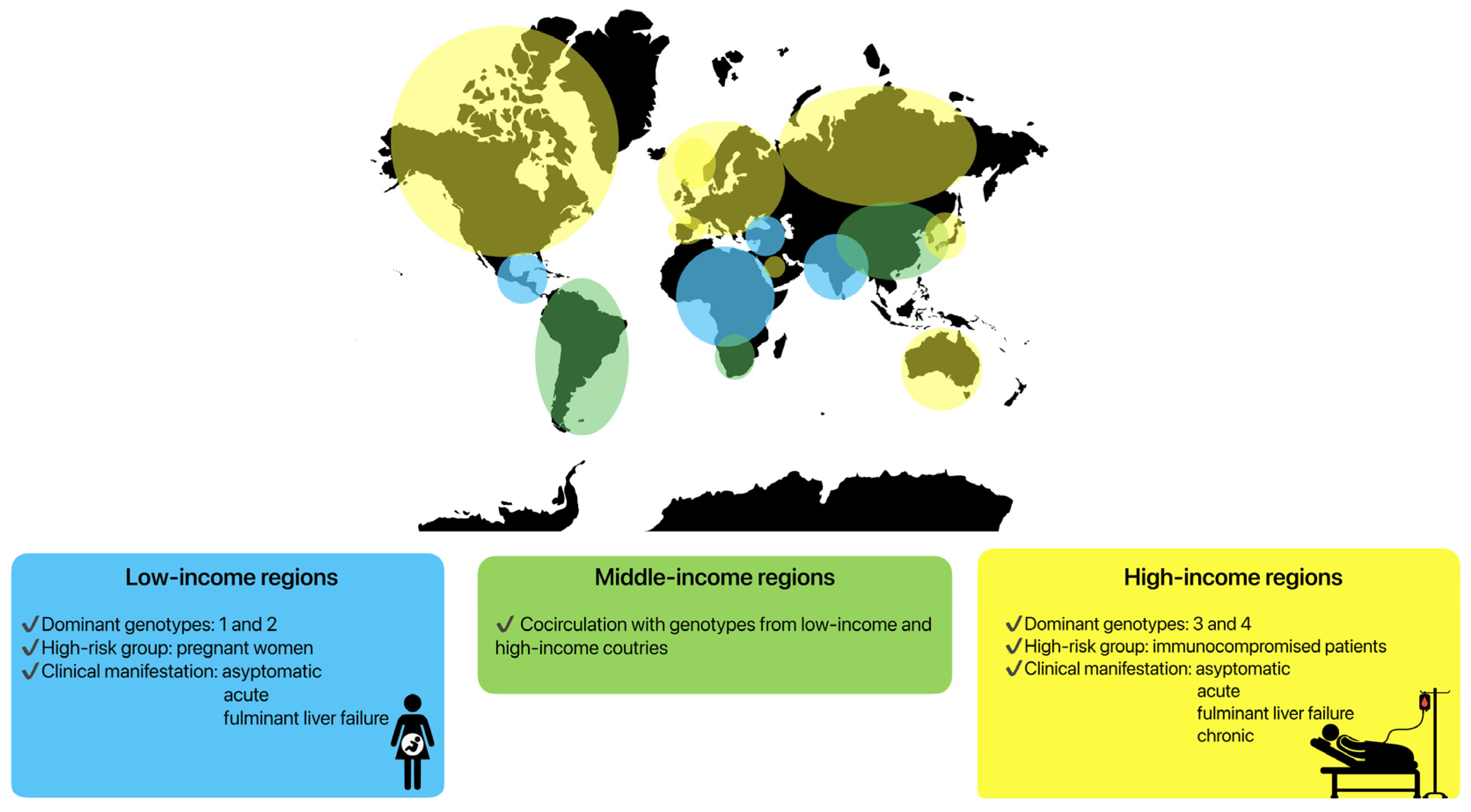
Clinical manifestations are highly variable in specific groups of patients, depending on the HEV genotypes and host immune system. Genotypes HEV-1 and HEV-2 are predominant in low-income countries in Asia or Africa (Figure 2), where the incidence of symptomatic infections is higher [22,175]. The hepatic manifestations following HEV-1 and HEV-2 infections vary from an entirely asymptomatic disease course or a mild systemic infection to icteric acute hepatitis and fulminant liver failure. A severe course of infection caused by these genotypes is visible in pregnant women. During pregnancy, the mortality rate may reach 30%, mainly due to obstetric complications such as haemorrhages or eclampsia [176]. Fulminant liver failure can also occur, with a ten-fold higher risk compared to non-pregnant patients [177]. Vertical transmission to infants also leads to increased neonatal morbidity and mortality [178].
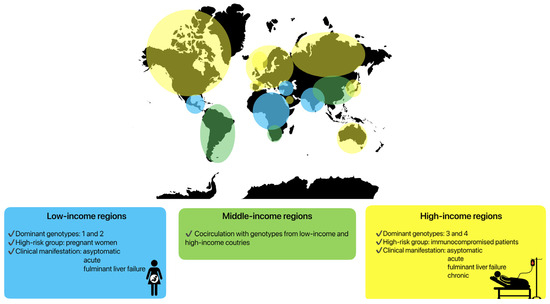
Figure 2.
Clinical manifestations of HEV infection depending on specific regions and genotypes.
The genotype HEV-3 is predominant in Europe and the United States (high-income regions), while the genotype HEV-4 has been documented primarily in Asia. Both genotypes have lower rates of symptomatic presentation [14,179,180]. However, specific patient groups seem to carry a higher risk for HEV infection, especially with regard to HEV-3 [7]. In Europe, predominantly immunocompromised patients suffer from severe symptoms of infection. The main contributing factors have been identified as the male sex, age above 50 years, pre-existing liver disease, immunocompromised status, diabetes mellitus, and alcohol consumption [181,182,183]. In these patients, acute hepatitis, fulminant liver failure, and also chronic HEV infections can be observed.
In some parts of world, most often in middle-income regions, cocirculation between genotypes can be seen. In South America, the predominant genotype is HEV-3, but cases of HEV infection caused by HEV-1 have also been described [184]. Similarly, in some regions of Asia, HEV-4 in cocirculation with HEV-1 can be seen [185] (Figure 2).
Extra-hepatic manifestations of HEV infection are poorly understood. However, its occurrence is probably caused by the host’s systemic immune response in extra-hepatic organs and its viral replication in non-hepatic tissues [186]. Neurological disorders have been the most widely documented, especially Guillain–Barré syndrome, neuralgic amyotrophy, encephalitis, meningoencephalitis, and myositis [187,188]. In France, 16.5% of patients infected with HEV reported neurologic symptoms [189]. Guillain–Barré syndrome associated with an acute HEV infection has been described in 5% of patients in The Netherlands [190]. Acute hepatitis E has also been found in 10% of patients with neuralgic amyotrophy in the United Kingdom and The Netherlands [191]. In more studies, patients with HEV-associated neurological manifestations generally had a normal liver function, and the neurological symptoms were dominant in these patients. Thus, there is a possibility that HEV is directly neurotropic and can possibly be replicated in the nervous system [188]. In addition to neurological diseases, various extra-hepatic manifestations such as renal disorders, haematologic complications, pancreatitis, thyroiditis, myositis, and myocarditis have been associated with HEV [173,192,193,194].
4.2. Chronic HEV Infection
Chronic HEV infections have become a serious problem in immunocompromised patients, resulting in substantial morbidity and even mortality [195]. The first cases of chronic HEV infections were reported in organ transplant recipients in 2008 [196]. According to this study, patients who are viremic for more than 3 months can be regarded as being chronically infected. Cases of chronic HEV infections have also been described in patients infected with the human immunodeficiency virus (HIV), patients with autoimmune diseases, and oncological patients receiving chemotherapy and immunotherapy [197,198,199]. The genotype HEV-3 was the cause in all of these cases. Chronic infection with the genotype HEV-4 was first reported in patients with leukaemia during chemotherapy treatments in China [200]. Until now, chronic HEV infections caused by HEV-1 and HEV-2 have not been observed [201].
Although in most patients with chronic HEV infections, no symptoms and only a mild elevation of the liver enzymes were described, a severe course of infection can be seen in some patients. The most common symptoms are fatigue, diarrhoea, abdominal pain, arthralgia, and rarely, jaundice [15]. Even more serious are irreversible changes in the liver tissue, including nodules, fibrotic remodelling, cirrhosis, and death due to decompensated cirrhosis [202]. Approximately 10% of patients can develop cirrhosis within a few years of a chronic HEV infection [196].
5. Preventive Measures
HEV usually circulates in the environment as a non-enveloped virus, but it can also survive under extreme conditions [203]. The accumulation of viruses in the environment plays a pivotal role in the spread of infection within animal and human populations. The important places where HEV accumulations occur are farms and slaughterhouses, where HEV can persist in the farm environment for an extended period of time. HEV-3 shows a high level of stability on different surfaces, such as wooden, metal, and plastic objects. The infectious virus can be detectable on most surfaces for up to 8 weeks [204]. Infected animals, along with their meat and offal, can contaminate the entire slaughter lines with HEV. Since HEV has been confirmed on different slaughter line surfaces, it can also be found on workers’ hands [72,205]. Surface and hand disinfection are the main components of infection control. Recent studies have confirmed that HEV on surfaces cannot be efficiently inactivated with low concentrations of alcohol-based disinfectants. Therefore, more effective disinfectants based on quaternary ammonium compounds, oxygen radicals, and peracetic acid are recommended to be utilised [204,206]. High-level hygiene standards and the use of effective disinfectants are thus essential preventive measures in all areas of farms and slaughterhouses.
There is limited information about HEV survival in meat, meat products, and shellfish. The infectivity of HEV in food products and its transmission to consumers is not entirely understood. Recent data indicate that HEV can resist food processing techniques [207]. It has been confirmed that a heating process to an internal temperature of 71 °C for 20 min is necessary to inactivate HEV entirely [208]. Experiments with incubating contaminated pig liver homogenates at 56 °C for 1 h did not inactivate the virus. This temperature is equivalent to medium-to-rare cooking conditions in a restaurant, indicating that the consumption of undercooked or raw pig meats could pose a risk of food-borne HEV infections [116].
Similarly, milk heat treatment presents a vital step in preventing food-borne infection. Pasteurisation is standard process in dairy industries; however, in some cases, it is not effective. On the contrary, a milk boiling process with 100 °C for 3 min provides a complete sterilisation [77]. Consequently, there is a possible risk of food-borne infections, which can be connected with consuming unpasteurised milk and milk products, such as cheeses [209]. However, to prevent transmissions by different products of an animal origin, appropriate heat treatment of the food is still required in order to inactivate the virus.
One of the best preventive measures is the existence of an effective vaccine. Up to now, only one vaccine is available. Hecolin is registered and licenced for use in China [210]. The vaccine is administered in three doses with a 0-, 1-, and 6-month schedule. It provides 100% protection against the genotype HEV-1 during the first 12 months. It is also effective against the genotype HEV-4 [210,211,212]. Unfortunately, there is no information about its cross-protection against HEV-3.
Over the past 20 years, numerous essential studies have been published that have changed the understanding of HEV. However, there are still many knowledge gaps. Since there is a lack of surveillance for HEV in humans, animals, and animal products, the true HEV prevalence is unknown. More information is needed about the possible ways of transmission in human and animal populations, HEV survival in the environment, and the development of clinical infections (infective doses, host susceptibility, dose–response relationships, and the virulence of HEV strains). A better understanding of HEV biology, transmission, replication, and pathophysiology could help eliminate the virus in the environment, animal, and human population. Therefore, cooperation between the general population, scientists, human doctors, and veterinary doctors could significantly prevent HEV food-borne outbreaks and protect consumers.
6. Conclusions
The hepatitis E virus is an emerging food-borne pathogen. The presence of HEV RNA has been repeatedly described in food-producing animals, meat, offal, meat products, milk, and shellfish. The number of reported autochthonous sporadic HEV infections in Europe has increased, but standardised methods and the surveillance of HEV infections are still missing. Several direct epidemiological links between human cases and the consumption of HEV-contaminated meat and meat products have been described. Although HEV mainly results in an acute self-limiting infection, severe symptoms can be seen in specific population groups. Preventive actions must be taken to successfully inactivate HEV, prevent food-borne outbreaks, and protect vulnerable consumer populations to ensure the health of humans and animals, along with food and environmental safety.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms13040885/s1, Table S1: Summary of information about foodborne transmission of HEV.
Author Contributions
Conceptualization, A.P. and A.J.; writing—original draft preparation, A.P.; writing—review and editing, B.K., M.U.D. and A.J.; supervision, A.J.; project administration, A.J.; funding acquisition, A.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the VEGA 1/0220/24 project funded by The Scientific Grant Agency of the Ministry of Education, Research, Development and Youth of the Slovak Republic.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analysed in this study.
Acknowledgments
The authors of this study would like to express gratitude to Kirstin Macdonald for English grammar correction of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| HEV | Hepatitis E Virus |
| HIV | Human Immunodeficiency Virus |
| PRRSV | Porcine Reproductive and Respiratory Syndrome Virus |
References
- Doceul, V.; Bagdassarian, E.; Demange, A.; Pavio, N. Zoonotic Hepatitis E Virus: Classification, Animal Reservoirs and Transmission Routes. Viruses 2016, 3, 270. [Google Scholar] [CrossRef]
- Kamar, N.; Izopet, J.; Pavio, N.; Aggarwal, R.; Labrique, A.; Wedemeyer, H.; Dalton, H.R. Hepatitis E virus infection. Nat. Rev. Dis. Primers 2017, 16, 17086. [Google Scholar] [CrossRef]
- Chapuy-Regaud, S.; Dubois, M.; Plisson-Chastang, C.; Bonnefois, T.; Lhomme, S.; Bertrand-Michel, J.; You, B.; Simoneau, S.; Gleizes, P.E.; Flan, B.; et al. Characterization of the lipid envelope of exosome encapsulated HEV particles protected from the immune response. Biochimie 2017, 141, 70–79. [Google Scholar] [CrossRef]
- Purdy, M.A.; Drexler, J.F.; Meng, X.J.; Norder, H.; Okamoto, H.; Van der Poel, W.H.M.; Reuter, G.; de Souza, W.M.; Ulrich, R.G.; Smith, D.B. ICTV Virus Taxonomy Profile: Hepeviridae. J. Gen. Virol. 2022, 103, 001778. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.H.; Tan, B.H.; Teo, E.C.; Lim, S.G.; Dan, Y.Y.; Wee, A.; Aw, P.P.; Zhu, Y.; Hibberd, M.L.; Tan, C.K.; et al. Chronic Infection With Camelid Hepatitis E Virus in a Liver Transplant Recipient Who Regularly Consumes Camel Meat and Milk. Gastroenterology 2016, 150, 355–357. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.B.; Izopet, J.; Nicot, F.; Simmonds, P.; Jameel, S.; Meng, X.J.; Norder, H.; Okamoto, H.; van der Poel, W.H.M.; Reuter, G.; et al. Update: Proposed reference sequences for subtypes of hepatitis E virus (species Orthohepevirus A). J. Gen. Virol. 2020, 101, 692–698. [Google Scholar] [CrossRef]
- Schemmerer, M.; Wenzel, J.J.; Stark, K.; Faber, M. Molecular epidemiology and genotype-specific disease severity of hepatitis E virus infections in Germany, 2010–2019. Emerg. Microbes Infect. 2022, 11, 1754–1763. [Google Scholar] [CrossRef] [PubMed]
- Pavio, N.; Doceul, V.; Bagdassarian, E.; Johne, R. Recent Knowledge on Hepatitis E Virus in Suidae Reservoirs and Transmission Routes to Human. Vet. Res. 2017, 48, 78. [Google Scholar] [CrossRef]
- Thiry, D.; Mauroy, A.; Pavio, N.; Purdy, M.A.; Rose, N.; Thiry, E.; de Oliveira-Filho, E.F. Hepatitis E Virus and Related Viruses in Animals. Transbound. Emerg. Dis. 2017, 64, 37–52. [Google Scholar] [CrossRef]
- Halbur, P.G.; Kasorndorkbua, C.; Gilbert, C.; Guenette, D.; Potters, M.B.; Purcell, R.H.; Emerson, S.U.; Toth, T.E.; Meng, X.J. Comparative pathogenesis of infection of pigs with hepatitis E viruses recovered from a pig and a human. J. Clin. Microbiol. 2001, 39, 918–923. [Google Scholar] [CrossRef]
- Crossan, C.; Baker, P.J.; Craft, J.; Takeuchi, Y.; Dalton, H.R.; Scobie, L. Hepatitis E virus genotype 3 in shellfish, United Kingdom. Emerg. Infect. Dis. 2012, 18, 2085–2087. [Google Scholar] [CrossRef] [PubMed]
- Di Bartolo, I.; Diez-Valcarce, M.; Vasickova, P.; Kralik, P.; Hernandez, M.; Angeloni, G.; Ostanello, F.; Bouwknegt, M.; Rodríguez-Lázaro, D.; Pavlik, I.; et al. Hepatitis E virus in pork production chain in Czech Republic, Italy, and Spain, 2010. Emerg. Infect. Dis. 2012, 18, 1282–1289. [Google Scholar] [CrossRef]
- Dziedzinska, R.; Krzyzankova, M.; Bena, M.; Vasickova, P. Evidence of Hepatitis E Virus in Goat and Sheep Milk. Viruses 2020, 12, 1429. [Google Scholar] [CrossRef]
- Hartl, J.; Wehmeyer, M.H.; Pischke, S. Acute hepatitis E: Two sides of the same coin. Viruses 2016, 8, 299. [Google Scholar] [CrossRef] [PubMed]
- Kamar, N.; Garrouste, C.; Haagsma, E.B.; Garrigue, V.; Pischke, S.; Chauvet, C.; Dumortier, J.; Cannesson, A.; Cassuto-Viguier, E.; Thervet, E.; et al. Factors associated with chronic hepatitis in patients with hepatitis E virus infection who have received solid organ transplants. Gastroenterology 2011, 140, 1481–1489. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, R. A review of the literature on the epidemiology of infectious hepatitis. Indian J. Med. Res. 1957, 45, 145–155. [Google Scholar]
- Kane, M.A.; Bradley, D.W.; Shrestha, S.M.; Maynard, J.E.; Cook, E.H.; Mishra, R.P.; Joshi, D.D. Epidemic Non-A, Non-B Hepatitis in Nepal: Recovery of a Possible Etiologic Agent and Transmission Studies in Marmosets. JAMA 1984, 252, 3140–3145. [Google Scholar] [CrossRef]
- Zhuang, H.; Cao, X.Y.; Liu, C.B.; Wang, G.M. Epidemiology of hepatitis E in China. Gastroenterol. Jpn. 1991, 11, 135–138. [Google Scholar] [CrossRef]
- Teshale, E.H.; Grytdal, S.P.; Howard, C.; Barry, V.; Kamili, S.; Drobeniuc, J.; Hill, V.R.; Okware, S.; Hu, D.J.; Holmberg, S.D. Evidence of Person-to-Person Transmission of Hepatitis E Virus during a Large Outbreak in Northern Uganda. Clin. Infect. Dis. 2010, 50, 1006–1010. [Google Scholar] [CrossRef]
- Gurley, E.S.; Hossain, M.J.; Paul, R.C.; Sazzas, H.M.S.; Islam, M.S.; Parveen, S.; Faruque, L.I.; Husain, M.; Ara, K.; Jahan, Y.; et al. Outbreak of hepatitis E in urban Bangladesh resulting in maternal and perinatal mortality. Clin. Infect. Dis. 2014, 59, 658–665. [Google Scholar] [CrossRef]
- Wedemeyer, H.; Pischke, S. Hepatitis E vaccination—Is HEV 239 the breakthrough? Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Rein, D.B.; Stevens, G.A.; Theaker, J.; Wittenborn, J.S.; Wiersma, S.T. The global burden of hepatitis E virus genotypes 1 and 2 in 2005. Hepatology 2012, 55, 988–997. [Google Scholar] [CrossRef]
- Li, P.; Liu, J.; Li, Y.; Su, J.; Ma, Z.; Bramer, W.M.; Cao, W.; de Man, R.A.; Peppelenbosch, M.P.; Pan, Q. The global epidemiology of hepatitis E virus infection: A systematic review and meta-analysis. Liver Int. 2020, 40, 1516–1528. [Google Scholar] [CrossRef]
- Faber, M.S.; Wenzel, J.J.; Jilg, W.; Thamm, M.; Höhle, M.; Stark, K. Hepatitis E virus seroprevalence among adults, Germany. Emerg. Infect. Dis. 2012, 18, 1654–1657. [Google Scholar] [CrossRef] [PubMed]
- Aspinall, E.J.; Couturier, E.; Faber, M.; Said, B.; Ijaz, S.; Tavoshi, L.; Takkinen, J.; Adlhoch, C. The Country Experts. Hepatitis E virus infection in Europe: Surveillance and descriptive epidemiology of confirmed cases, 2005 to 2015. Euro. Surveill. 2017, 22, 30561. [Google Scholar] [CrossRef] [PubMed]
- Krumbholz, A.; Joel, S.; Dremsek, P.; Neubert, A.; Johne, R.; Dürrwald, R.; Walther, M.; Müller, T.H.; Kühnel, D.; Lange, J.; et al. Seroprevalence of hepatitis E virus (HEV) in humans living in high pig density areas of Germany. Med. Microbiol. Immunol. 2014, 203, 273–282. [Google Scholar] [CrossRef]
- Dalton, H.R.; Stableforth, W.; Thurairajah, P.; Hazeldine, S.; Remnarace, R.; Usama, W.; Farrington, L.; Hamad, N.; Sieberhagen, C.; Ellis, V.; et al. Autochthonous hepatitis E in Southwest England: Natural history, complications and seasonal variation, and hepatitis E virus IgG seroprevalence in blood donors, the elderly and patients with chronic liver disease. Eur. J. Gastroenterol. Hepatol. 2008, 20, 784–790. [Google Scholar] [CrossRef]
- Mansuy, J.M.; Bendall, R.; Legrand-Abravanel, F.; Sauné, K.; Miédouge, M.; Ellis, V.; Rech, H.; Destruel, F.; Kamar, N.; Dalton, H.R.; et al. Hepatitis E virus antibodies in blood donors, France. Emerg. Infect. Dis. 2011, 17, 2309–2312. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control (ECDC). Communicable Disease Threats Report. Weekly Bulletin. Week 6, 4–10 February 2024. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/communicable-disease-threats-report-week-6-2024.pdf (accessed on 11 March 2025).
- Naik, S.R.; Aggarwal, R.; Salunke, P.N.; Mehrotra, N.N. A large waterborne viral hepatitis E epidemic in Kanpur, India. Bull. World Health Organ. 1992, 70, 597–604. [Google Scholar]
- Khuroo, M.S.; Kamili, S.; Yattoo, G.N. Hepatitis E virus infection may be transmitted through blood transfusions in an endemic area. J. Gastroenterol. Hepatol. 2004, 19, 778–784. [Google Scholar] [CrossRef]
- Khuroo, M.S.; Khuroo, M.S.; Khuroo, N.S. Transmission of Hepatitis E Virus in Developing Countries. Viruses 2016, 8, 253. [Google Scholar] [CrossRef] [PubMed]
- Colson, P.; Romanet, P.; Moal, V.; Borentain, P.; Purgus, R.; Benezech, A.; Motte, A.; Gérolami, R. Autochthonous infections with hepatitis E virus genotype 4, France. Emerg. Infect. Dis. 2012, 18, 1361–1364. [Google Scholar] [CrossRef] [PubMed]
- Rivero-Juarez, A.; Frias, M.; Martinez-Peinado, A.; Risalde, M.A.; Rodriguez-Cano, D.; Camacho, A.; García-Bocanegra, I.; Cuenca-Lopez, F.; Gomez-Villamandos, J.C.; Rivero, A. Familial hepatitis E outbreak linked to wild boar meat consumption. Zoonoses Public Health 2017, 64, 561–565. [Google Scholar] [CrossRef]
- Meester, M.; Tobias, T.J.; Bouwknegt, M.; Kusters, N.E.; Stegeman, J.A.; van der Poel, W.H.M. Infection dynamics and persistence of hepatitis E virus on pig farms—A review. Porcine Health Manag. 2021, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Tei, S.; Kitajima, N.; Takahashi, K.; Mishiro, S. Zoonotic transmission of hepatitis E virus from deer to human beings. Lancet 2003, 362, 371–373. [Google Scholar] [CrossRef]
- Schlosser, B.; Stein, A.; Neuhaus, R.; Pahl, S.; Ramez, B.; Krüger, D.H.; Berg, T.; Hofmann, J. Liver transplant from a donor with occult HEV infection induced chronic hepatitis and cirrhosis in the recipient. J. Hepatol. 2012, 56, 500–502. [Google Scholar] [CrossRef]
- Hewitt, P.E.; Ijaz, S.; Brailsford, S.R.; Brett, R.; Dicks, S.; Haywood, B.; Kennedy, I.T.; Kitchen, A.; Patel, P.; Poh, J.; et al. Hepatitis E virus in blood components: A prevalence and transmission study in southeast England. Lancet 2014, 384, 1766–1773. [Google Scholar] [CrossRef]
- Li, T.C.; Chijiwa, K.; Sera, N.; Ishibashi, T.; Etoh, Y.; Shinohara, Y.; Kurata, Y.; Ishida, M.; Sakamoto, S.; Takeda, N.; et al. Hepatitis E virus transmission from wild boar meat. Emerg. Infect. Dis. 2005, 11, 1958–1960. [Google Scholar] [CrossRef]
- Matsubayashi, K.; Kang, J.-H.; Sakata, H.; Takahashi, K.; Shindo, M.; Kato, M.; Sato, S.; Kato, T.; Nishimori, H.; Tsuji, K.; et al. A case of transfusion-transmitted hepatitis E caused by blood from a donor infected with hepatitis E virus via zoonotic food-borne route. Transfusion 2008, 48, 1368–1375. [Google Scholar] [CrossRef]
- Renou, C.; Roque-Afonso, A.M.; Pavio, N. Foodborne transmission of hepatitis E virus from raw pork liver sausage, France. Emerg. Infect. Dis. 2014, 20, 1945–1947. [Google Scholar] [CrossRef]
- Riveiro-Barciela, M.; Minguez, B.; Girones, R.; Rodriguez-Frias, F.; Quer, J.; Buti, M. Phylogenetic demonstration of hepatitis E infection transmitted by pork meat ingestion. J. Clin. Gastroenterol. 2015, 49, 165–168. [Google Scholar] [CrossRef]
- Yapa, C.M.; Furlong, C.; Rosewell, A.; Ward, K.A.; Adamson, S.; Shadbolt, C.; Kok, J.; Tracy, S.L.; Bowden, S.; Smedley, E.J.; et al. First reported outbreak of locally acquired hepatitis E virus infection in Australia. Med. J. Aust. 2016, 204, 274. [Google Scholar] [CrossRef]
- Meng, X.J.; Purcell, R.H.; Halbur, P.G.; Lehman, J.R.; Webb, D.M.; Tsareva, T.S.; Haynes, J.S.; Thacker, B.J.; Emerson, S.U. A novel virus in swine is closely related to the human hepatitis E virus. Proc. Natl. Acad. Sci. USA 1997, 94, 9860–9865. [Google Scholar] [CrossRef] [PubMed]
- Kaba, M.; Colson, P.; Musongela, J.P.; Tshilolo, L.; Davoust, B. Detection of hepatitis E virus of genotype 3 in a farm pig in Kinshasa (Democratic Republic of the Congo). Infect. Genet. Evol. 2010, 10, 154–157. [Google Scholar] [CrossRef]
- Owolodun, O.A.; Gerber, P.F.; Giménez-Lirola, L.G.; Kwaga, J.K.; Opriessnig, T. First report of hepatitis E virus circulation in domestic pigs in Nigeria. Am. J. Trop. Med. Hyg. 2014, 91, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Adelabu, O.A.; Chuks Iweriebor, B.; Nwodo, U.U.; Obi, L.C.; Okoh, A.I. Incidence and Molecular Characterization of Hepatitis E Virus from Swine in Eastern Cape, South Africa. Adv. Virol. 2017, 2017, 1073253. [Google Scholar] [CrossRef]
- Mesquita, J.R.; Istrate, C.; Santos-Ferreira, N.L.; Ferreira, A.S.; Abreu-Silva, J.; Veiga, J.; van der Poel, W.H.M.; Nascimento, M.S.J. Short communication: Detection and molecular characterization of hepatitis E virus in domestic animals of São Tomé and Príncipe. Trop. Anim. Health Prod. 2019, 51, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Conlan, J.V.; Jarman, R.G.; Vongxay, K.; Chinnawirotpisan, P.; Melendrez, M.C.; Fenwick, S.; Thompson, R.C.; Blacksell, S.D. Hepatitis E virus is prevalent in the pig population of Lao People’s Democratic Republic and evidence exists for homogeneity with Chinese Genotype 4 human isolates. Infect. Genet. Evol. 2011, 11, 1306–1311. [Google Scholar] [CrossRef]
- Hinjoy, S.; Nelson, K.E.; Gibbons, R.V.; Jarman, R.G.; Chinnawirotpisan, P.; Fernandez, S.; Tablerk, P.; Labrique, A.B.; Patchanee, P. A cross-sectional study of hepatitis E virus infection in pigs in different-sized farms in northern Thailand. Foodborne Pathog. Dis. 2013, 10, 698–704. [Google Scholar] [CrossRef]
- Liang, H.; Su, S.; Deng, S.; Gu, H.; Ji, F.; Wang, L.; Liang, C.; Wang, H.; Zhang, G. The prevalence of hepatitis E virus infections among swine, swine farmers and the general population in Guangdong Province, China. PLoS ONE 2014, 9, e88106. [Google Scholar] [CrossRef]
- Kim, Y.H.; Park, B.J.; Ahn, H.S.; Han, S.H.; Go, H.J.; Kim, D.H.; Lee, J.B.; Park, S.Y.; Song, C.S.; Lee, S.W.; et al. Detection of hepatitis E virus genotypes 3 and 4 in pig farms in Korea. J. Vet. Sci. 2018, 19, 309–312. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.C.; Ha, L.N.N.; Giang, T.T.; Tiep, V.M.; Chau, N.T.M.; Phuong Anh, T.N.; Duy, P.K.; Nhan, L.P.; Hoai, N.T.T.; Linh, L.T.K.; et al. Characterization of zoonotic hepatitis E virus in domestic pigs and wild boar in Vietnam: Implications for public health. One Health 2024, 19, 100857. [Google Scholar] [CrossRef]
- Yoo, D.; Willson, P.; Pei, Y.; Hayes, M.A.; Deckert, A.; Dewey, C.E.; Friendship, R.M.; Yoon, Y.; Gottschalk, M.; Yason, C.; et al. Prevalence of hepatitis E virus antibodies in Canadian swine herds and identification of a novel variant of swine hepatitis E virus. Clin. Diagn. Lab. Immunol. 2001, 8, 1213–1219. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Meng, J.; Dai, X.; Liang, J.H.; Feagins, A.R.; Meng, X.J.; Belfiore, N.M.; Bradfors, C.; Corn, J.L.; Cray, C.; et al. Restricted enzooticity of hepatitis E virus genotypes 1 to 4 in the United States. J. Clin. Microbiol. 2011, 49, 4164–4172. [Google Scholar] [CrossRef]
- Sooryanarain, H.; Heffron, C.L.; Hill, D.E.; Fredericks, J.; Rosenthal, B.M.; Werre, S.R.; Opriessnig, T.; Meng, X.J. Hepatitis E virus in pigs from slaughterhouses, United States, 2017–2019. Emerg. Infect. Dis. 2020, 26, 354–357. [Google Scholar] [CrossRef]
- Munné, M.S.; Vladimirsky, S.; Otegui, L.; Castro, R.; Brajterman, L.; Soto, S.; Guarnera, E.; Molina, V.; Monfellano, M.; Schlauder, G.G.; et al. Identification of the first strain of swine hepatitis E virus in South America and prevalence of anti-HEV antibodies in swine in Argentina. J. Med. Virol. 2006, 78, 1579–1583. [Google Scholar] [CrossRef]
- Dell’Amico, M.C.; Cavallo, A.; Gonzales, J.L.; Bonelli, S.I.; Valda, Y.; Pieri, A.; Segund, H.; Ibañez, R.; Mantella, A.; Bartalesi, F.; et al. Hepatitis E virus genotype 3 in humans and Swine, Bolivia. Emerg. Infect. Dis. 2011, 17, 1488–1490. [Google Scholar] [CrossRef]
- Amorim, A.R.; Mendes, G.S.; Pena, G.P.A.; Santos, N. Hepatitis E virus infection of slaughtered healthy pigs in Brazil. Zoonoses Public Health 2018, 65, 501–504. [Google Scholar] [CrossRef] [PubMed]
- Forero, J.E.; Gutiérrez-Vergara, C.; Parra Suescún, J.; Correa, G.; Rodríguez, B.; Gutiérrez, L.A.; Díaz, F.J.; López-Herrera, A. Phylogenetic analysis of Hepatitis E virus strains isolated from slaughter-age pigs in Colombia. Infect. Genet. Evol. 2017, 49, 138–145. [Google Scholar] [CrossRef]
- Mirazo, S.; Gardinali, N.R.; Cecilia, D.; Verger, L.; Ottonelli, F.; Ramos, N.; Castro, G.; Pinto, M.A.; Ré, V.; Pisano, B.; et al. Serological and virological survey of hepatitis E virus (HEV) in animal reservoirs from Uruguay reveals elevated prevalences and a very close phylogenetic relationship between swine and human strains. Vet. Microbiol. 2018, 213, 21–27. [Google Scholar] [CrossRef]
- Chandler, J.D.; Riddell, M.A.; Li, F.; Love, R.J.; Anderson, D.A. Serological evidence for swine hepatitis E virus infection in Australian pig herds. Vet. Microbiol. 1999, 68, 95–105. [Google Scholar] [CrossRef]
- Seminati, C.; Mateu, E.; Peralta, B.; de Deus, N.; Martin, M. Distribution of hepatitis E virus infection and its prevalence in pigs on commercial farms in Spain. Vet. J. 2008, 175, 130–132. [Google Scholar] [CrossRef] [PubMed]
- Rutjes, S.A.; Bouwknegt, M.; van der Giessen, J.W.; de Roda Husman, A.M.; Reusken, C.B. Seroprevalence of hepatitis E virus in pigs from different farming systems in The Netherlands. J. Food Prot. 2014, 77, 640–642. [Google Scholar] [CrossRef] [PubMed]
- Jori, F.; Laval, M.; Maestrini, O.; Casabianca, F.; Charrier, F.; Pavio, N. Assessment of domestic pigs, wild boars and feral hybrid pigs as reservoirs of hepatitis E virus in Corsica, France. Viruses 2016, 8, 236. [Google Scholar] [CrossRef]
- Tsachev, I.; Baymakova, M.; Ciccozzi, M.; Pepovich, R.; Kundurzhiev, T.; Marutsov, P.; Dimitrov, K.K.; Gospodinova, K.; Pishmisheva, M.; Pekova, L. Seroprevalence of Hepatitis E Virus Infection in Pigs from Southern Bulgaria. Vector Borne Zoonotic Dis. 2019, 19, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Monini, M.; Di Bartolo, I.; De Sabato, L.; Ianiro, G.; Agostinelli, F.; Ostanello, F. Hepatitis E Virus (HEV) in Heavy Pigs in Slaughterhouses of Northern Italy: Investigation of Seroprevalence, Viraemia, and Faecal Shedding. Animals 2023, 16, 2942. [Google Scholar] [CrossRef]
- Rutjes, S.A.; Lodder, W.J.; Bouwknegt, M.; de Roda Husman, A.M. Increased hepatitis E virus prevalence on Dutch pig farms from 33 to 55% by using appropriate internal quality controls for RT-PCR. J. Virol. Methods 2007, 143, 112–116. [Google Scholar] [CrossRef]
- Vasickova, P.; Psikal, I.F.; Widen, F.; Smitalova, R.; Bendova, J.; Pavlik, I.; Kralik, P. Detection and genetic characterisation of hepatitis E virus in Czech pig production herds. Res. Vet. Sci. 2009, 87, 143–148. [Google Scholar] [CrossRef]
- Forgách, P.; Nowotny, N.; Erdelyi, K.; Boncz, A.; Zentai, J.; Szucs, G.; Reuter, G.; Bakonyi, T. Detection of hepatitis E virus in samples of animal origin collected in Hungary. Vet. Microbiol. 2010, 143, 106–116. [Google Scholar] [CrossRef]
- Kosinova, E.; Bendova, J.; Vasickova, P.; Smitalova, R.; Prodelalova, J. The prevalence of hepatitis E virus in piglets on Czech pig production farms and phylogenetic analysis of recovered isolates. Vet. Med.-Czech. 2012, 57, 115–120. [Google Scholar] [CrossRef]
- Berto, A.; Martelli, F.; Grierson, S.; Banks, M. Hepatitis E virus in pork food chain, United Kingdom, 2009–2010. Emerg. Infect. Dis. 2012, 18, 1358–1360. [Google Scholar] [CrossRef]
- Jackova, A.; Dudasova, K.; Salamunova, S.; Mandelik, R.; Novotny, J.; Vilcek, S. Identification and genetic diversity of hepatitis E virus in domestic swine from Slovakia. BMC Vet. Res. 2021, 17, 232. [Google Scholar] [CrossRef] [PubMed]
- Banks, M.; Heath, G.S.; Grierson, S.S.; King, D.P.; Gresham, A.; Girones, R.; Widen, F.; Harrison, T.J. Evidence for the presence of hepatitis E virus in pigs in the United Kingdom. Vet. Rec. 2004, 154, 223–227. [Google Scholar] [CrossRef]
- Bouwknegt, M.; Rutjes, S.A.; Reusken, C.B.; Stockhofe-Zurwieden, N.; Frankena, K.; de Jong, M.C.; de Roda Husman, A.M.; Poel, W.H. The course of hepatitis E virus infection in pigs after contact-infection and intravenous inoculation. BMC Vet. Res. 2009, 5, 7. [Google Scholar] [CrossRef]
- Geng, Y.; Wang, C.; Zhao, C.; Yu, X.; Harrison, T.J.; Tian, K.; Wang, Y. Serological prevalence of hepatitis E virus in domestic animals and diversity of genotype 4 hepatitis E virus in China. Vector Borne Zoonotic Dis. 2010, 10, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Li, Y.; Yu, W.; Jing, S.; Wang, J.; Long, F.; He, Z.; Yang, C.; Bi, Y.; Cao, W.; et al. Excretion of Infectious Hepatitis E Virus into Milk in Cows Imposes High Risks of Zoonosis. Hepatology 2016, 64, 350. [Google Scholar] [CrossRef] [PubMed]
- Palombieri, A.; Robetto, S.; Di Profio, F.; Sarchese, V.; Fruci, P.; Bona, M.C.; Ru, G.; Orusa, R.; Marsilio, F.; Martella, V.; et al. Surveillance Study of Hepatitis E Virus (HEV) in Domestic and Wild Ruminants in Northwestern Italy. Animals 2020, 10, 2351. [Google Scholar] [CrossRef]
- Caballero-Gómez, J.; García-Bocanegra, I.; Jiménez-Martín, D.; Cano-Terriza, D.; Risalde, M.A.; López-López, P.; Jiménez-Ruiz, S.; Rivero, A.; Rivero-Juarez, A. Epidemiological survey and risk factors associated with hepatitis E virus in small ruminants in southern Spain. Zoonoses Public Health 2022, 69, 387–393. [Google Scholar] [CrossRef]
- Santos-Silva, S.; Santos, N.; López-López, P.; Nascimento, M.S.J.; Gonçalves, H.M.R.; Van der Poel, W.H.M.; Rivero-Juarez, A.; Mesquita, J.R. Hepatitis E virus in wild and domestic rabbits from Portugal: A combined molecular and longitudinal serological study. Vet. Res. Commun. 2024, 48, 3283–3289. [Google Scholar] [CrossRef]
- Reuter, G.; Fodor, D.; Forgách, P.; Kátai, A.; Szucs, G. Characterization and zoonotic potential of endemic hepatitis E virus (HEV) strains in humans and animals in Hungary. J. Clin. Virol. 2009, 44, 277–281. [Google Scholar] [CrossRef]
- Prpić, J.; Černi, S.; Škorić, D.; Keros, T.; Brnić, D.; Cvetnić, Ž.; Jemeršić, L. Distribution and Molecular Characterization of Hepatitis E virus in Domestic Animals and Wildlife in Croatia. Food Environ. Virol. 2015, 7, 195–205. [Google Scholar] [CrossRef]
- Di Martino, B.; Di Profio, F.; Melegari, I.; Sarchese, V.; Robetto, S.; Marsilio, F.; Martella, V. Detection of hepatitis E virus (HEV) in goats. Virus Res. 2016, 2, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Long, F.; Yu, W.; Yang, C.; Wang, J.; Li, Y.; Li, Y.; Huang, F. High Prevalence of Hepatitis E Virus Infection in Goats. J. Med. Virol. 2017, 89, 1981–1987. [Google Scholar] [CrossRef] [PubMed]
- Sarchese, V.; Di Profio, F.; Melegari, I.; Palombieri, A.; Sanchez, S.B.; Arbuatti, A.; Ciuffetelli, M.; Marsilio, F.; Martella, V.; Di Martino, B. Hepatitis E virus in sheep in Italy. Transbound. Emerg. Dis. 2019, 66, 1120–1125. [Google Scholar] [CrossRef] [PubMed]
- Batmagnai, E.; Boldbaatar, B.; Sodbayasgalan, A.; Kato-Mori, Y.; Hagiwara, K. Hepatitis E Virus (HEV) Spreads from Pigs and Sheep in Mongolia. Animals 2023, 13, 891. [Google Scholar] [CrossRef]
- Weger, S.; Elkin, B.; Lindsay, R.; Bollinger, T.; Crichton, V.; Andonov, A. Hepatitis E Virus Seroprevalence in Free-Ranging Deer in Canada. Transbound. Emerg. Dis. 2017, 64, 1008–1011. [Google Scholar] [CrossRef]
- Adlhoch, C.; Wolf, A.; Meisel, H.; Kaiser, M.; Ellerbrok, H.; Pauli, G. High HEV presence in four different wild boar populations in East and West Germany. Vet. Microbiol. 2009, 139, 270–278. [Google Scholar] [CrossRef]
- Burri, C.; Vial, F.; Ryser-Degiorgis, M.-P.; Schwermer, H.; Darling, K.; Reist, M.; Wu, N.; Beerli, O.; Schöning, J.; Cavassini, M.; et al. Seroprevalence of hepatitis E virus in domestic pigs and wild boars in Switzerland. Zoonoses Public Health 2014, 61, 537–544. [Google Scholar] [CrossRef]
- Ivanova, A.; Tefanova, V.; Reshetnjak, I.; Kuznetsova, T.; Geller, J.; Lundkvist, Å.; Janson, M.; Neare, K.; Velström, K.; Jokelainen, P.; et al. Hepatitis E virus in domestic pigs, wild boars, pig farm workers, and hunters in Estonia. Food Environ. Virol. 2015, 7, 403–412. [Google Scholar] [CrossRef]
- Mesquita, J.R.; Oliveira, R.M.; Coelho, C.; Vieira-Pinto, M.; Nascimento, M.S. Hepatitis E Virus in Sylvatic and Captive Wild Boar from Portugal. Transbound. Emerg. Dis. 2016, 63, 574–578. [Google Scholar] [CrossRef]
- Roth, A.; Lin, J.; Magnius, L.; Karlsson, M.; Belák, S.; Widén, F.; Norder, H. Markers for Ongoing or Previous Hepatitis E Virus Infection Are as Common in Wild Ungulates as in Humans in Sweden. Viruses 2016, 8, 259. [Google Scholar] [CrossRef] [PubMed]
- Neumann, S.; Hackl, S.S.; Piepenschneider, M.; Vina-Rodriguez, A.; Dremsek, P.; Ulrich, R.G.; Groschup, M.H.; Eiden, M. Serologic and Molecular Survey of Hepatitis E Virus in German Deer Populations. J. Wildl. Dis. 2016, 52, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Anheyer-Behmenburg, H.E.; Szabo, K.; Schotte, U.; Binder, A.; Klein, G.; Johne, R. Hepatitis E virus in wild boars and spillover infection in red and roe deer, Germany, 2013–2015. Emerg. Infect. Dis. 2017, 23, 130–133. [Google Scholar] [CrossRef]
- Boadella, M.; Casas, M.; Martín, M.; Vicente, J.; Segalés, J.; de la Fuente, J.; Gortázar, C. Increasing contact with hepatitis E virus in red deer, Spain. Emerg. Infect. Dis. 2010, 16, 1994–1996. [Google Scholar] [CrossRef]
- Zhao, C.; Ma, Z.; Harrison, T.J.; Feng, R.; Zhang, C.; Qiao, Z.; Fan, J.; Ma, H.; Li, M.; Song, A.; et al. A novel genotype of hepatitis E virus prevalent among farmed rabbits in China. J. Med. Virol. 2009, 81, 1371–1379. [Google Scholar] [CrossRef]
- Cossaboom, C.M.; Córdoba, L.; Dryman, B.A.; Meng, X.J. Hepatitis E virus in rabbits, Virginia, USA. Emerg. Infect. Dis. 2011, 17, 2047–2049. [Google Scholar] [CrossRef]
- Izopet, J.; Dubois, M.; Bertagnoli, S.; Lhomme, S.; Marchandeau, S.; Boucher, S. Hepatitis E virus strains in rabbits and evidence of a closely related strain in humans, France. Emerg. Infect. Dis. 2012, 18, 1274–1281. [Google Scholar] [CrossRef]
- Burt, S.A.; Veltman, J.; Hakze-van der Honing, R.; Schmitt, H.; van der Poel, W.H.M. Hepatitis E virus in farmed rabbits, wild rabbits and petting farm rabbits in The Netherlands. Food Environ. Virol. 2016, 8, 227–229. [Google Scholar] [CrossRef] [PubMed]
- Hammerschmidt, F.; Schwaiger, K.; Dähnert, L.; Vina-Rodriguez, A.; Höper, D.; Gareis, M.; Groschup, M.H.; Eiden, M. Hepatitis E virus in wild rabbits and European brown hares in Germany. Zoonoses Public Health 2017, 64, 612–622. [Google Scholar] [CrossRef]
- Mendoza, M.V.; Yonemitsu, K.; Ishijima, K.; Minami, S.; Supriyono; Tran, N.T.B.; Kuroda, Y.; Tatemoto, K.; Inoue, Y.; Okada, A.; et al. Characterization of rabbit hepatitis E virus isolated from a feral rabbit. Vet. Microbiol. 2021, 263, 109275. [Google Scholar] [CrossRef]
- El-Adly, A.M. Serological and genetic diversity of hepatitis E virus among rabbits population in Egypt. Open Vet. J. 2023, 13, 515–522. [Google Scholar] [CrossRef] [PubMed]
- De Sabato, L.; Ianiro, G.; Filipello, V.; Arnaboldi, S.; Righi, F.; Ostanello, F.; Giammarioli, M.; Lavazza, A.; Di Bartolo, I. Absence of Hepatitis E Virus (HEV) in Italian Lagomorph Species Sampled between 2019 and 2021. Animals 2023, 13, 545. [Google Scholar] [CrossRef]
- Santos-Silva, S.; Romalde, J.L.; Bento, J.T.; Cruz, A.V.S.; López-López, P.; Gonçalves, H.M.R.; Van der Poel, W.H.M.; Nascimento, M.S.J.; Rivero-Juarez, A.; Mesquita, J.R. Serological and Molecular Survey of Hepatitis E Virus in Small Ruminants from Central Portugal. Food Environ. Virol. 2024, 16, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Abravanel, F.; Lhomme, S.; El Costa, H.; Schvartz, B.; Peron, J.M.; Kamar, N.; Izopet, J. Rabbit Hepatitis E Virus Infections in Humans, France. Emerg. Infect. Dis. 2017, 23, 1191–1193. [Google Scholar] [CrossRef]
- Sahli, R.; Fraga, M.; Semela, D.; Moradpour, D.; Gouttenoire, J. Rabbit HEV in immunosuppressed patients with hepatitis E acquired in Switzerland. J. Hepatol. 2019, 70, 1023–1025. [Google Scholar] [CrossRef] [PubMed]
- Baylis, S.A.; O’Flaherty, N.; Burke, L.; Hogema, B.; Corman, V.M. Identification of rabbit hepatitis E virus (HEV) and novel HEV clade in Irish blood donors. J. Hepatol. 2022, 77, 870–872. [Google Scholar] [CrossRef]
- Klink, P.; Harms, D.; Altmann, B.; Dörffel, Y.; Morgera, U.; Zander, S.; Bock, C.T.; Hofmann, J. Molecular characterisation of a rabbit Hepatitis E Virus strain detected in a chronically HEV-infected individual from Germany. One Health 2023, 22, 100528. [Google Scholar] [CrossRef]
- Alao, B.O.; Falowo, A.B.; Chulayo, A.; Muchenje, V. The Potential of Animal By-Products in Food Systems: Production, Prospects and Challenges. Sustainability 2017, 9, 1089. [Google Scholar] [CrossRef]
- Yin, X.; Feng, Z. Hepatitis E Virus Entry. Viruses 2019, 11, 883. [Google Scholar] [CrossRef]
- Boxman, I.L.A.; Jansen, C.C.C.; Hagele, G.; Zwartkruis-Nahuis, A.; Cremer, J.; Vennema, H.; Tijsma, A.S.L. Porcine blood used as ingredient in meat productions may serve as a vehicle for hepatitis E virus transmission. Int. J. Food Microbiol. 2017, 257, 225–231. [Google Scholar] [CrossRef]
- García, N.; Hernández, M.; Gutierrez-Boada, M.; Valero, A.; Navarro, A.; Muñoz-Chimeno, M.; Fernández-Manzano, A.; Escobar, F.M.; Martínez, I.; Bárcena, C.; et al. Occurrence of Hepatitis E Virus in Pigs and Pork Cuts and Organs at the Time of Slaughter, Spain, 2017. Front. Microbiol. 2020, 10, 2990. [Google Scholar] [CrossRef] [PubMed]
- van der Poel, W.H.; Verschoor, F.; van der Heide, R.; Herrera, M.I.; Vivo, A.; Kooreman, M.; de Roda Husman, A.M. Hepatitis E virus sequences in swine related to sequences in humans, The Netherlands. Emerg. Infect. Dis. 2001, 7, 970–976. [Google Scholar] [CrossRef]
- Walachowski, S.; Dorenlor, V.; Lefevre, J.; Lunazzi, A.; Eono, F.; Merbah, T.; Eveno, E.; Pavio, N.; Rose, N. Risk factors associated with the presence of hepatitis E virus in livers and seroprevalence in slaughter-age pigs: A retrospective study of 90 swine farms in France. Epidemiol. Infect. 2014, 142, 1934–1944. [Google Scholar] [CrossRef]
- Salines, M.; Barnaud, E.; Andraud, M.; Eono, F.; Renson, P.; Bourry, O.; Pavio, N.; Rose, N. Hepatitis E virus chronic infection of swine co-infected with Porcine Reproductive and Respiratory Syndrome Virus. Vet. Res. 2015, 46, 55. [Google Scholar] [CrossRef] [PubMed]
- Feagins, A.R.; Opriessnig, T.; Guenette, D.K.; Halbur, P.G.; Meng, X.J. Inactivation of infectious hepatitis E virus present in commercial pig livers sold in local grocery stores in the United States. Int. J. Food Microbiol. 2008, 123, 32–37. [Google Scholar] [CrossRef]
- Jung, K.; Kang, B.; Song, D.S.; Chae, C. Prevalence and genotyping of hepatitis E virus in swine population in Korea between 1995 and 2004: A retrospective study. Vet. J. 2007, 173, 683–687. [Google Scholar] [CrossRef]
- Temmam, S.; Besnard, L.; Andriamandimby, S.F.; Foray, C.; Rasamoelina-Andriamanivo, H.; Héraud, J.M.; Cardinale, E.; Dellagi, K.; Pavio, N.; Pascalis, H.; et al. High prevalence of hepatitis E in humans and pigs and evidence of genotype-3 virus in swine, Madagascar. Am. J. Trop. Med. Hyg. 2013, 88, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Traoré, K.A.; Ouoba, J.B.; Huot, N.; Rogée, S.; Dumarest, M.; Traoré, A.S.; Pavio, N.; Barro, N.; Roques, P. Hepatitis E Virus Exposure is Increased in Pork Butchers from Burkina Faso. Am. J. Trop. Med. Hyg. 2015, 93, 1356–1359. [Google Scholar] [CrossRef]
- Chatonnat, E.; Julien, M.; Jubinville, E.; Goulet-Beaulieu, V.; Pavio, N.; Jean, J. High occurrence of hepatitis E virus in raw pork liver and pork liver pâté produced in the Canadian province of Quebec. Front. Sustain. Food Syst. 2023, 7, 1163507. [Google Scholar] [CrossRef]
- Intharasongkroh, D.; Sa-Nguanmoo, P.; Tuanthap, S.; Thongmee, T.; Duang-In, A.; Klinfueng, S.; Chansaenroj, J.; Vongpunsawad, S.; Theamboonlers, A.; Payungporn, S.; et al. Hepatitis E Virus in Pork and Variety Meats Sold in Fresh Markets. Food Environ. Virol. 2017, 9, 45–53. [Google Scholar] [CrossRef]
- Bouwknegt, M.; Lodder-Verschoor, F.; van der Poel, W.H.; Rutjes, S.A.; de Roda Husman, A.M. Hepatitis E virus RNA in commercial porcine livers in The Netherlands. J. Food Prot. 2007, 70, 2889–2895. [Google Scholar] [CrossRef] [PubMed]
- Rose, N.; Lunazzi, A.; Dorenlor, V.; Merbah, T.; Eono, F.; Eloit, M.; Madec, F.; Pavio, N. High prevalence of Hepatitis E virus in French domestic pigs. Comp. Immunol. Microbiol. Infect. Dis. 2011, 34, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Berto, A.; Backer, J.A.; Mesquita, J.R.; Nascimento, M.S.; Banks, M.; Martelli, F.; Ostanello, F.; Angeloni, G.; Di Bartolo, I.; Ruggeri, F.M.; et al. Prevalence and transmission of hepatitis E virus in domestic swine populations in different European countries. BMC Res. Notes 2012, 5, 190. [Google Scholar] [CrossRef]
- Wenzel, J.J.; Preiss, J.; Schemmerer, M.; Huber, B.; Plentz, A.; Jilg, W. Detection of hepatitis E virus (HEV) from porcine livers in Southeastern Germany and high sequence homology to human HEV isolates. J. Clin. Virol. 2011, 52, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Bigoraj, E.; Paszkiewicz, W.; Rzeżutka, A. Porcine Blood and Liver as Sporadic Sources of Hepatitis E Virus (HEV) in the Production Chain of Offal-Derived Foodstuffs in Poland. Food Environ. Virol. 2021, 13, 347–356. [Google Scholar] [CrossRef]
- Vonlanthen-Specker, I.; Stephan, R.; Sidler, X.; Moor, D.; Fraefel, C.; Bachofen, C. Genetic Diversity of Hepatitis E Virus Type 3 in Switzerland—From Stable to Table. Animals 2021, 11, 3177. [Google Scholar] [CrossRef]
- Bennett, C.; Coughlan, S.; Hunt, K.; Butler, F.; Fanning, S.; Ryan, E.; De Gascun, C.; O’Gorman, J. Detection of hepatitis E RNA in pork products at point of retail in Ireland—Are consumers at risk? Int. J. Food Microbiol. 2024, 410, 110492. [Google Scholar] [CrossRef]
- Geng, Y.; Zhao, C.; Guo, T.; Xu, Y.; Wang, X.; Huang, W.; Liu, H.; Wang, Y. Detection of Hepatitis E Virus in Raw Pork and Pig Viscera As Food in Hebei Province of China. Foodborne Pathog. Dis. 2019, 16, 325–330. [Google Scholar] [CrossRef]
- Go, H.J.; Park, B.J.; Ahn, H.S.; Lyoo, E.L.; Kim, D.H.; Lee, J.B.; Park, S.Y.; Song, C.S.; Lee, S.W.; Choi, I.S. Identification of Hepatitis E Virus in Bovine and Porcine Raw Livers. J. Microbiol. Biotechnol. 2019, 29, 2022–2025. [Google Scholar] [CrossRef]
- Bastos, C.; Eisen, A.K.A.; Demoliner, M.; Heldt, F.H.; Filippi, M.; de Abreu Góes Pereira, V.M.; Teixeira, T.A.M.; Roth, L.O.; Gularte, J.S.; Spilki, F.R. Hepatitis E virus genotype 3 in bovine livers slaughtered in the state of Rio Grande do Sul, Brazil. Braz. J. Microbiol. 2022, 53, 1115–1120. [Google Scholar] [CrossRef]
- Wu, J.; Si, F.; Jiang, C.; Li, T.; Jin, M. Molecular detection of hepatitis E virus in sheep from southern Xinjiang, China. Virus Genes 2015, 50, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Lhomme, S.; Top, S.; Bertagnoli, S.; Dubois, M.; Guerin, J.L.; Izopet, J. Wildlife reservoir for hepatitis E virus, Southwestern France. Emerg. Infect. Dis. 2015, 21, 1224–1226. [Google Scholar] [CrossRef]
- Montagnaro, S.; De Martinis, C.; Sasso, S.; Ciarcia, R.; Damiano, S.; Auletta, L.; Iovane, V.; Zottola, T.; Pagnini, U. Viral and antibody prevalence of hepatitis E in European wild boars (Sus scrofa) and hunters at zoonotic risk in the Latium Region. J. Comp. Pathol. 2015, 153, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Schielke, A.; Ibrahim, V.; Czogiel, I.; Faber, M.; Schrader, C.; Dremsek, P.; Ulrich, R.G.; Johne, R. Hepatitis E virus antibody prevalence in hunters from a district in Central Germany, 2013: A cross-sectional study providing evidence for the benefit of protective gloves during disembowelling of wild boars. BMC Infect. Dis. 2015, 15, 440. [Google Scholar] [CrossRef]
- Serracca, L.; Battistini, R.; Rossini, I.; Mignone, W.; Peletto, S.; Boin, C.; Pistone, G.; Ercolini, R.; Ercolini, C. Molecular investigation on the presence of hepatitis E virus (HEV) in wild game in north-western Italy. Food Environ. Virol. 2015, 7, 206–212. [Google Scholar] [CrossRef]
- Fonti, N.; Pacini, M.I.; Forzan, M.; Parisi, F.; Periccioli, M.; Mazzei, M.; Poli, A. Molecular and Pathological Detection of Hepatitis E Virus in Roe Deer (Capreolus capreolus) and Fallow Deer (Dama dama) in Central Italy. Vet. Sci. 2022, 9, 100. [Google Scholar] [CrossRef] [PubMed]
- Chaussade, H.; Rigaud, E.; Allix, A.; Carpentier, A.; Touze, A.; Delzescaux, D.; Choutet, P.; Garcia-Bonnet, N.; Coursaget, P. Hepatitis E virus seroprevalence and risk factors for individuals in working contact with animals. J. Clin. Virol. 2013, 58, 504–508. [Google Scholar] [CrossRef]
- Mansuy, J.M.; Saune, K.; Rech, H.; Abravanel, F.; Mengelle, C.; L’Homme, S.; Destruel, F.; Kamar, N.; Izopet, J. Seroprevalence in blood donors reveals widespread, multi-source exposure to hepatitis E virus, southern France, October 2011. Euro. Surveill. 2015, 20, 27–34. [Google Scholar] [CrossRef]
- Said, B.; Ijaz, S.; Chand, M.A.; Kafatos, G.; Tedder, R.; Morgan, D. Hepatitis E virus in England and Wales: Indigenous infection is associated with the consumption of processed pork products. Epidemiol. Infect. 2014, 142, 1467–1475. [Google Scholar] [CrossRef]
- Wichmann, O.; Schimanski, S.; Koch, J.; Kohler, M.; Rothe, C.; Plentz, A.; Jilg, W.; Stark, K. Phylogenetic and case-control study on hepatitis E virus infection in Germany. J. Infect. Dis. 2008, 198, 1732–1741. [Google Scholar] [CrossRef]
- Pavio, N.; Merbah, T.; Thébault, A. Frequent hepatitis E virus contamination in food containing raw pork liver, France. Emerg. Infect. Dis. 2014, 20, 1925–1927. [Google Scholar] [CrossRef] [PubMed]
- Colson, P.; Borentain, P.; Queyriaux, B.; Kaba, M.; Moal, V.; Gallian, P.; Heyries, L.; Raoult, D.; Gerolami, R. Pig liver sausage as a source of hepatitis E virus transmission to humans. J. Infect. Dis. 2010, 202, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Szabo, K.; Trojnar, E.; Anheyer-Behmenburg, H.; Binder, A.; Schotte, U.; Ellerbroek, L.; Klein, G.; Johne, R. Detection of hepatitis E virus RNA in raw sausages and liver sausages from retail in Germany using an optimized method. Int. J. Food Microbiol. 2015, 215, 149–156. [Google Scholar] [CrossRef]
- Montone, A.M.I.; De Sabato, L.; Suffredini, E.; Alise, M.; Zaccherini, A.; Volzone, P.; Di Maro, O.; Neola, B.; Capuano, F.; Di Bartolo, I. Occurrence of HEV-RNA in Italian Regional Pork and Wild Boar Food Products. Food Environ. Virol. 2019, 11, 420–426. [Google Scholar] [CrossRef]
- Heldt, F.H.; Staggmeier, R.; Gularte, J.S.; Demoliner, M.; Henzel, A.; Spilki, F.R. Hepatitis E Virus in Surface Water, Sediments, and Pork Products Marketed in Southern Brazil. Food Environ. Virol. 2016, 8, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Mykytczuk, O.; Harlow, J.; Bidawid, S.; Corneau, N.; Nasheri, N. Prevalence and Molecular Characterization of the Hepatitis E Virus in Retail Pork Products Marketed in Canada. Food Environ. Virol. 2017, 9, 208–218. [Google Scholar] [CrossRef]
- Korsman, S.; Bloemberg, J.; Brombacher, M.; Giuricich, A.; Halley-Stott, R.P.; Kaba, M. Hepatitis E in pig-derived food products in Cape Town, South Africa, 2014. S. Afr. Med. J. 2019, 26, 584–586. [Google Scholar] [CrossRef]
- Di Cola, G.; Di Cola, G.; Fantilli, A.; Mamani, V.; Tamiozzo, P.; Martínez Wassaf, M.; Nates, S.V.; Ré, V.E.; Pisano, M.B. High circulation of hepatitis E virus (HEV) in pigs from the central region of Argentina without evidence of virus occurrence in pork meat and derived products. Res. Vet. Sci. 2023, 164, 105000. [Google Scholar] [CrossRef]
- Boxman, I.L.A.; Jansen, C.C.C.; Hagele, G.; Zwartkruis-Nahuis, A.; Tijsma, A.S.L.; Vennema, H. Monitoring of pork liver and meat products on the Dutch market for the presence of HEV RNA. Int. J. Food Microbiol. 2019, 296, 58–64. [Google Scholar] [CrossRef]
- Boxman, I.L.A.; Jansen, C.C.C.; Zwartkruis-Nahuis, A.J.T.; Hägele, G.; Sosef, N.P.; Dirks, R.A.M. Detection and quantification of hepatitis E virus RNA in ready to eat raw pork sausages in The Netherlands. Int. J. Food Microbiol. 2020, 333, 108791. [Google Scholar] [CrossRef]
- Moor, D.; Liniger, M.; Baumgartner, A.; Felleisen, R. Screening of Ready-to-Eat Meat Products for Hepatitis E Virus in Switzerland. Food Environ. Virol. 2018, 10, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Locus, T.; Lambrecht, E.; Peeters, M.; Suin, V.; Verhaegen, B.; Van Hoorde, K.; Lamoral, S.; Vanwolleghem, T.; Van Gucht, S. Hepatitis E virus in pork meat products and exposure assessment in Belgium. Int. J. Food Microbiol. 2023, 397, 110198. [Google Scholar] [CrossRef]
- Rivero-Juarez, A.; Frias, M.; Rodriguez-Cano, D.; Cuenca-López, F.; Rivero, A. Isolation of hepatitis E virus from breast milk during acute infection. Clin. Infect. Dis. 2016, 62, 1464. [Google Scholar] [CrossRef] [PubMed]
- Baechlein, C.; Becher, P. No Evidence for Zoonotic Hepatitis E Virus Infection through Dairy Milk in Germany. Hepatology 2017, 65, 394. [Google Scholar] [CrossRef] [PubMed]
- Demirci, M.; Yiğin, A.; Ünlü, Ö.; Altun, S.K. Detection of HEV RNA Amounts and Genotypes in Raw Milks Obtained from Different Animals. Mikrobiyol. Bul. 2019, 53, 43–52. [Google Scholar] [CrossRef]
- Sayed, I.M.; Elkhawaga, A.A.; El-Mokhtar, M.A. Circulation of hepatitis E virus (HEV) and/or HEV-like agent in non-mixed dairy farms could represent a potential source of infection for Egyptian people. Int. J. Food Microbiol. 2020, 317, 108479. [Google Scholar] [CrossRef]
- Vercouter, A.S.; Sayed, I.M.; Lipkens, Z.; De Bleecker, K.; De Vliegher, S.; Colman, R.; Koppelman, M.; Supré, K.; Meuleman, P. Absence of Zoonotic Hepatitis E Virus Infection in Flemish Dairy Cows. Int. J. Food Microbiol. 2018, 281, 54–59. [Google Scholar] [CrossRef]
- El-Mokhtar, M.A.; Elkhawaga, A.A.; Sayed, I.M. Assessment of hepatitis E virus (HEV) in the edible goat products pointed out a risk for human infection in Upper Egypt. Int. J. Food Microbiol. 2020, 330, 108784. [Google Scholar] [CrossRef]
- Ferri, G.; Pennisi, L.; Malatesta, F.; Vergara, A. First Detection of Hepatitis E Virus RNA in Ovine Raw Milk from Herds in Central Italy. Foods 2024, 13, 3218. [Google Scholar] [CrossRef]
- Gao, S.; Li, D.; Zha, E.; Zhou, T.; Wang, S.; Yue, X. Surveillance of hepatitis E virus contamination in shellfish in China. Int. J. Environ. Res. Public Health 2015, 12, 2026–2036. [Google Scholar] [CrossRef]
- Koizumi, Y.; Isoda, N.; Sato, Y.; Iwaki, T.; Ono, K.; Ido, K.; Sugano, K.; Takahashi, M.; Nishizawa, T.; Okamoto, H. Infection of a Japanese patient by genotype 4 hepatitis e virus while traveling in Vietnam. J. Clin. Microbiol. 2004, 42, 3883–3885. [Google Scholar] [CrossRef] [PubMed]
- Said, B.; Ijaz, S.; Kafatos, G.; Booth, L.; Thomas, H.L.; Walsh, A.; Ramsay, M.E.; Morgan, D. Hepatitis E outbreak on cruise ship. Emerg. Infect. Dis. 2009, 15, 1738–1744. [Google Scholar] [CrossRef]
- Grodzki, M.; Schaeffer, J.; Piquet, J.-C.; Le Saux, J.-C.; Chevé, J.; Ollivier, J.; Le Pendu, J.; Le Guyader, F.S. Bioaccumulation Efficiency, Tissue Distribution, and Environmental Occurrence of Hepatitis E Virus in Bivalve Shellfish from France. Appl. Environ. Microbiol. 2014, 80, 4269–4276. [Google Scholar] [CrossRef]
- La Rosa, G.; Fratini, M.; Spuri Vennarucci, V.; Guercio, A.; Purpari, G.; Muscillo, M. GIV noroviruses and other enteric viruses in bivalves: A preliminary study. New Microbiol. 2012, 35, 27–34. [Google Scholar] [PubMed]
- Krog, J.S.; Larsen, L.E.; Schultz, A.C. Enteric porcine viruses in farmed shellfish in Denmark. Int. J. Food Microbiol. 2014, 186, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Fusco, G.; Di Bartolo, I.; Cioffi, B.; Ianiro, G.; Palermo, P.; Monini, M.; Amoroso, M.G. Prevalence of foodborne viruses in mussels in Southern Italy. Food Environ. Virol. 2017, 9, 187–194. [Google Scholar] [CrossRef]
- Rivadulla, E.; Varela, M.F.; Mesquita, J.R.; Nascimento, M.S.; Romalde, J.L. Detection of Hepatitis E Virus in Shellfish Harvesting Areas from Galicia (Northwestern Spain). Viruses 2019, 11, 618. [Google Scholar] [CrossRef]
- La Rosa, G.; Proroga, Y.T.R.; De Medici, D.; Capuano, F.; Iaconelli, M.; Della Libera, S.; Suffredini, E. First Detection of Hepatitis E Virus in Shellfish and in Seawater from Production Areas in Southern Italy. Food Environ. Virol. 2018, 10, 127–131. [Google Scholar] [CrossRef]
- O’Hara, Z.; Crossan, C.; Craft, J.; Scobie, L. First Report of the Presence of Hepatitis E Virus in Scottish-Harvested Shellfish Purchased at Retail Level. Food Environ. Virol. 2018, 10, 217–221. [Google Scholar] [CrossRef]
- Alipoor Amroabadi, M.; Rahimi, E.; Shakerian, A. Presence of Enteric Viruses in Shellfish Samples from the Persian Gulf. Arch. Razi Inst. 2024, 79, 129–137. [Google Scholar] [CrossRef]
- Christou, L.; Kosmidou, M. Hepatitis E virus in the Western world--a pork-related zoonosis. Clin. Microbiol. Infect. 2013, 19, 600–604. [Google Scholar] [CrossRef]
- Aslan, A.T.; Balaban, H.Y. Hepatitis E virus: Epidemiology, diagnosis, clinical manifestations, and treatment. World J. Gastroenterol. 2020, 7, 5543–5560. [Google Scholar] [CrossRef] [PubMed]
- Wedemeyer, H.; Pischke, S.; Manns, M.P. Pathogenesis and treatment of hepatitis E virus infection. Gastroenterology 2012, 142, 1388–1397. [Google Scholar] [CrossRef]
- Dagnew, M.; Belachew, A.; Tiruneh, M.; Moges, F. Hepatitis E virus infection among pregnant women in Africa: Systematic review and meta-analysis. BMC Infect. Dis. 2019, 13, 519. [Google Scholar] [CrossRef] [PubMed]
- Navaneethan, U.; Al Mohajer, M.; Shata, M.T. Hepatitis E and pregnancy: Understanding the pathogenesis. Liver Int. 2008, 28, 1190–1199. [Google Scholar] [CrossRef]
- Khuroo, M.S.; Teli, M.R.; Skidmore, S.; Sofi, M.A.; Khuroo, M.I. Incidence and severity of viral hepatitis in pregnancy. Am. J. Med. 1981, 70, 252–255. [Google Scholar] [CrossRef] [PubMed]
- Khuroo, M.S.; Kamili, S.; Khuroo, M.S. Clinical course and duration of viremia in vertically transmitted hepatitis E virus (HEV) infection in babies born to HEV-infected mothers. J. Viral Hepat. 2009, 16, 519–523. [Google Scholar] [CrossRef]
- Sridhar, S.; Cheng, V.C.C.; Wong, S.C.; Yip, C.C.Y.; Wu, S.; Lo, A.W.I.; Leung, K.H.; Mak, W.W.N.; Cai, J.; Li, X.; et al. Donor-Derived Genotype 4 Hepatitis E Virus Infection, Hong Kong, China, 2018. Emerg. Infect. Dis. 2019, 25, 425–433. [Google Scholar] [CrossRef]
- Pisano, M.B.; Campbell, C.; Anugwom, C.; Re, V.E.; Debes, J.D. Hepatitis E virus infection in the United States: Seroprevalence, risk factors and the influence of immunological assays. PLoS ONE 2022, 17, e0272809. [Google Scholar] [CrossRef]
- Dalton, H.R.; Bendall, R.P.; Rashid, M.; Ellis, V.; Ali, R.; Ramnarace, R.; Stableforth, W.; Headdon, W.; Abbott, R.; McLaughlim, C.; et al. Host risk factors and autochthonous hepatitis E infection. Eur. J. Gastroenterol. Hepatol. 2011, 23, 1200–1205. [Google Scholar] [CrossRef]
- Wallace, S.J.; Swann, R.; Donnelly, M.; Kemp, L.; Guaci, J.; Murray, A.; Spoor, J.; Lin, N.; Miller, M.; Dalton, H.R.; et al. Mortality and morbidity of locally acquired hepatitis E in the national Scottish cohort: A multicentre retrospective study. Aliment. Pharmacol. Ther. 2020, 51, 974–986. [Google Scholar] [CrossRef] [PubMed]
- Lenggenhager, D.; Pawel, S.; Honcharova-Biletska, H.; Evert, K.; Wenzel, J.J.; Montani, M.; Furrer, E.; Fraga, M.; Moradpour, D.; Sempoux, C.; et al. The histologic presentation of hepatitis E reflects patients’ immune status and pre-existing liver condition. Mod. Pathol. 2021, 34, 233–248. [Google Scholar] [CrossRef] [PubMed]
- Pisano, M.B.; Mirazo, S.; Re, V.E. Hepatitis E Virus Infection: Is It Really a Problem in Latin America? Clin. Liver Dis. 2020, 22, 108–113. [Google Scholar] [CrossRef]
- Liu, P.; Li, L.; Wang, L.; Bu, Q.; Fu, H.; Han, J.; Zhu, Y.; Lu, F.; Zhuang, H. Phylogenetic analysis of 626 hepatitis E virus (HEV) isolates from humans and animals in China (1986–2011) showing genotype diversity and zoonotic transmission. Infect. Genet. Evol. 2012, 12, 428–434. [Google Scholar] [CrossRef]
- Fousekis, F.S.; Mitselos, I.V.; Christodoulou, D.K. Extrahepatic manifestations of hepatitis E virus: An overview. Clin. Mol. Hepatol. 2020, 26, 16–23. [Google Scholar] [CrossRef]
- Woolson, K.L.; Forbes, A.; Vine, L.; Beynon, L.; McElhinney, L.; Panayi, V.; Hunter, J.G.; Madden, R.G.; Glasgow, T.; Kotecha, A.; et al. Extra-hepatic manifestations of autochthonous hepatitis E infection. Aliment. Pharmacol. Ther. 2014, 40, 1282–1291. [Google Scholar] [CrossRef]
- Dalton, H.R.; Kamar, N.; van Eijk, J.J.; McLean, B.N.; Cintas, P.; Bendall, R.P.; Jacobs, B.C. Hepatitis E virus and neurological injury. Nat. Rev. Neurol. 2016, 12, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Abravanel, F.; Pique, J.; Couturier, E.; Nicot, F.; Dimeglio, C.; Lhomme, S.; Chiabrando, J.; Saune, K.; Peron, J.M.; Kamar, N.; et al. Acute hepatitis E in French patients and neurological manifestations. J. Infect. 2018, 77, 220–226. [Google Scholar] [CrossRef]
- van den Berg, B.; van der Eijk, A.A.; Pas, S.D.; Hunter, J.G.; Madden, R.G.; Tio-Gillen, A.P.; Dalton, H.R.; Jacobs, B.C. Guillain-Barre syndrome associated with preceding hepatitis E virus infection. Neurology 2014, 82, 491–497. [Google Scholar] [CrossRef]
- van Eijk, J.J.; Madden, R.G.; van der Eijk, A.A.; Hunter, J.G.; Reimerink, J.H.; Bendall, R.P.; Pas, S.D.; Ellis, V.; van Alfen, N.; Beynon, L.; et al. Neuralgic amyotrophy and hepatitis E virus infection. Neurology 2014, 82, 498–503. [Google Scholar] [CrossRef]
- Rauff, B.; Idrees, M.; Shah, S.A.; Butt, S.; Butt, A.M.; Ali, L.; Hussain, A.; Irshad Ur, R.; Ali, M. Hepatitis associated aplastic anemia. A review. Virol. J. 2011, 8, 87. [Google Scholar] [CrossRef] [PubMed]
- Taton, B.; Moreau, K.; Lepreux, S.; Bachelet, T.; Trimoulet, P.; De Ledinghen, V.; Pommereau, A.; Ronco, P.; Kamar, N.; Merville, P.; et al. Hepatitis E virus infection as a new probable cause of de novo membranous nephropathy after kidney transplantation. Transpl. Infect. Dis. 2013, 15, E211–E215. [Google Scholar] [CrossRef] [PubMed]
- Bazerbachi, F.; Haffar, S.; Garg, S.K.; Beynon, L.; McElhinney, L.; Panayi, V.; Hunter, J.G.; Madden, R.G.; Glasgow, T.; Kotecha, A.; et al. Extra-hepatic manifestations associated with hepatitis E virus infection: A comprehensive review of the literature. Gastroenterol. Rep. 2016, 4, 1–15. [Google Scholar] [CrossRef]
- Tseng, T.C.; Liu, C.J.; Chang, C.T.; Su, T.H.; Yang, W.T.; Tsai, C.H.; Chen, C.L.; Yang, H.C.; Liu, C.H.; Chen, P.J.; et al. HEV superinfection accelerates disease progression in patients with chronic HBV infection and increases mortality in those with cirrhosis. J. Hepatol. 2020, 72, 1105–1111. [Google Scholar] [CrossRef]
- Kamar, N.; Selves, J.; Mansuy, J.M.; Ouezzani, L.; Péron, J.M.; Guitard, J.; Cointault, O.; Esposito, L.; Abravanel, F.; Danjoux, M.; et al. Hepatitis E virus and chronic hepatitis in organ-transplant recipients. N. Engl. J. Med. 2008, 358, 811–817. [Google Scholar] [CrossRef]
- Dalton, H.R.; Bendall, R.P.; Keane, F.E.; Tedder, R.S.; Ijaz, S. Persistent carriage of hepatitis E virus in patients with HIV infection. N. Engl. J. Med. 2009, 361, 1025–1027. [Google Scholar] [CrossRef]
- von Felden, J.; Alric, L.; Pischke, S.; Aitken, C.; Schlabe, S.; Spengler, U.; Giordani, M.T.; Schnitzler, P.; Bettinger, D.; Thimme, R.; et al. The burden of hepatitis E among patients with haematological malignancies: A retrospective European cohort study. J. Hepatol. 2019, 71, 465–472. [Google Scholar] [CrossRef]
- Di Bartolomeo, S.; Carubbi, F.; Cipriani, P. Hepatitis E Virus and rheumatic diseases: What do rheumatologists need to know? BMC Rheumatol. 2020, 4, 51. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Zhang, H.; Huang, W.; Harrison, T.J.; Geng, K.; Li, Z.; Wang, Y. Persistent hepatitis e virus genotype 4 infection in a child with acute lymphoblastic leukemia. Hepat. Mon. 2014, 14, e15618. [Google Scholar] [CrossRef]
- Horvatits, T.; Schulze Zur Wiesch, J.; Lütgehetmann, M.; Lohse, A.W.; Pischke, S. The Clinical Perspective on Hepatitis E. Viruses 2019, 11, 617. [Google Scholar] [CrossRef]
- Gerolami, R.; Moal, V.; Colson, P. Chronic hepatitis e with cirrhosis in a kidney-transplant recipient. N. Engl. J. Med. 2008, 358, 859–860. [Google Scholar] [CrossRef] [PubMed]
- Behrendt, P.; Friesland, M.; Wißmann, J.E.; Kinast, V.; Stahl, Y.; Praditya, D.; Hueffner, L.; Nörenberg, P.M.; Bremer, B.; Maasoumy, B.; et al. Hepatitis E virus is highly resistant to alcohol-based disinfectants. J. Hepatol. 2022, 76, 1062–1069. [Google Scholar] [CrossRef]
- Wolff, A.; Günther, T.; Johne, R. Stability of Hepatitis E Virus After Drying on Different Surfaces. Food Environ. Virol. 2022, 14, 138–148. [Google Scholar] [CrossRef]
- Milojević, L.; Velebit, B.; Teodorović, V.; Kirbiš, A.; Petrović, T.; Karabasil, N.; Dimitrijević., M. Screening and molecular characterization of hepatitis E virus in slaughter pigs in Serbia. Food Environ. Virol. 2019, 11, 410–419. [Google Scholar] [CrossRef]
- Wißmann, J.E.; Brüggemann, Y.; Todt, D.; Steinmann, J.; Steinmann, E. Survival and inactivation of hepatitis E virus on inanimate surfaces. J. Hosp. Infect. 2023, 134, 57–62. [Google Scholar] [CrossRef]
- Wolff, A.; Günther, T.; Albert, T.; Schilling-Loeffler, K.; Gadicherla, A.K.; Johne, R. Stability of hepatitis E virus at different pH values. Int. J. Food Microbiol. 2020, 325, 108625. [Google Scholar] [CrossRef] [PubMed]
- Barnaud, E.; Rogée, S.; Garry, P.; Rose, N.; Pavio, N. Thermal inactivation of infectious hepatitis E virus in experimentally contaminated food. Appl. Environ. Microbiol. 2012, 78, 5153–5159. [Google Scholar] [CrossRef]
- Sakaridis, I.; Psomas, E.; Karatzia, M.A.; Samouris, G. Hygiene and Safety of Hard Cheese Made from Raw Cows’ Milk. Vet. Sci. 2022, 9, 569. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.C.; Zhang, J.; Zhang, X.F.; Zhou, C.; Wang, Z.Z.; Huang, S.J.; Wang, H.; Yang, C.L.; Jiang, H.M.; Cai, J.P.; et al. Efficacy and safety of a recombinant hepatitis E vaccine in healthy adults: A large-scale, randomised, double-blind placebo-controlled, phase 3 trial. Lancet 2010, 376, 895–902. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.F.; Huang, S.J.; Wu, T.; Hu, Y.M.; Wang, Z.Z.; Wang, H.; Jiang, H.M.; Wang, Y.J.; Yan, Q.; et al. Long-term efficacy of a hepatitis E vaccine. N. Engl. J. Med. 2015, 372, 914–922. [Google Scholar] [CrossRef]
- Lynch, J.A.; Lim, J.K.; Asaga, P.E.P.; Wartel, T.A.; Marti, M.; Yakubu, B.; Rees, H.; Talaat, K.; Kmush, B.; Aggarwal, R.; et al. Hepatitis E vaccine-Illuminating the barriers to use. PLoS Negl. Trop. Dis. 2023, 17, e0010969. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).