Outbreak of Vancomycin-Resistant Enterococcus in a NICU: Insights into Molecular Detection and Infection Control †
Abstract
1. Introduction
2. Materials and Methods
2.1. Hospital Setting
2.2. Study Design, Patients and Data Collection
2.3. Definitions
2.4. Surveillance Program
2.5. Microbiological Procedure
Culture and Susceptibility Test
2.6. Molecular Test
2.7. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics
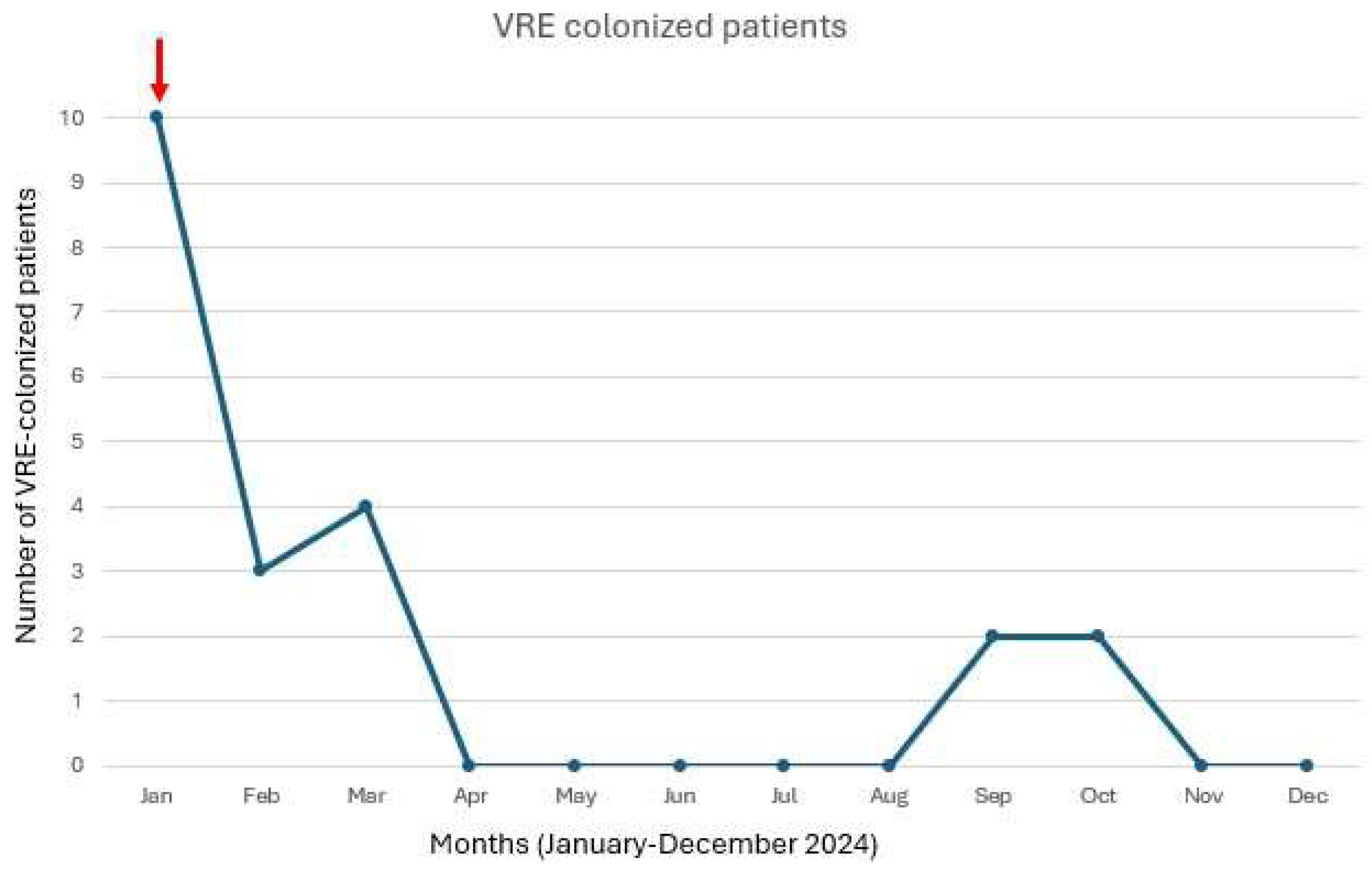
3.2. Outbreak Management
3.2.1. Isolation and Surveillance
3.2.2. Environmental Cleaning Enhancements
3.2.3. Infection Control Measures and Reorganization of the Ward
- Temporary closure of NICU admissions from 30 January to 12 February.
- Physical unit separation of the NICU (Area A) from the sub-intensive care unit (Area B) using closed, fire-rated REI.
- Staff cohorting to prevent cross-contact with nurses performing a key role.
- Enhanced hand hygiene protocol, including training and direct observation of hand hygiene compliance from 31 January to 15 April.
- Improved diaper management, including immediate handwashing after diaper changes and contact with patients, glove removal, and avoiding overflowing diaper bins.
3.3. Infections Course
3.4. Antimicrobial Susceptibility
4. Discussion
4.1. VRE Colonization and Pathogenicity
4.2. Molecular Testing
4.3. Infection Control
4.4. Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bizot, E.; Truong, J.; Mariani-Kurkdjian, P.; Bonacorsi, S.; Faye, A.; Caseris, M. Pediatric Enterococcal Bacteremia: A 12-Year Retrospective Study in a French Pediatric Center. Pediatr. Infect. Dis. J. 2022, 41, e346–e350. [Google Scholar] [CrossRef] [PubMed]
- Christie, C.; Hammond, J.; Reising, S.; Evans-Patterson, J. Clinical and molecular epidemiology of enterococcal bacteremia in a pediatric teaching hospital. J. Pediatr. 1994, 125, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Lebreton, F.; van Schaik, W.; McGuire, A.M.; Godfrey, P.; Griggs, A.; Mazumdar, V.; Corander, J.; Cheng, L.; Saif, S.; Young, S.; et al. Emergence of epidemic multidrug-resistant Enterococcus faecium from animal and commensal strains. MBio 2013, 4, 10–1128. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Courvalin, P. Vancomycin resistance in gram-positive cocci. Clin. Infect. Dis. 2006, 42, S25–S34. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Pang, S.; Abraham, S.; Coombs, G.W. Molecular characterization and evolution of the first outbreak of vancomycin-resistant Enterococcus faecium in Western Australia. Int. J. Antimicrob. Agents 2019, 53, 814–819. [Google Scholar] [CrossRef] [PubMed]
- Furuichi, M.; Furuichi, M.; Horikoshi, Y.; Miyairi, I. Infectious Diseases Consultation Improves Treatment and Decreases Mortality by Enterococcal Bacteremia in Children. Pediatr. Infect. Dis. J. 2018, 37, 856–860. [Google Scholar] [CrossRef] [PubMed]
- Kara, A.; Devrim, İ.; Bayram, N.; Katipoğlu, N.; Kıran, E.; Oruç, Y.; Demiray, N.; Apa, H.; Gülfidan, G. Risk of vancomycin-resistant enterococci bloodstream infection among patients colonized with vancomycin-resistant enterococci. Braz. J. Infect. Dis. 2015, 19, 58–61. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Özkaya-Parlakay, A.; Cengiz, A.B.; Ceyhan, M.; Bağdat, A.; Barın-Kurtoğlu, Ç.; Gürbüz, V.; Aycan, A.E.; Kara, A. Vancomycin-resistant enterococcus colonization and infection in children: Six-year follow-up. Turk. J. Pediatr. 2014, 56, 618–625. [Google Scholar] [PubMed]
- Khan, A.M.; Morris, S.K.; Bhutta, Z.A. Neonatal and Perinatal Infections. Pediatr. Clin. 2017, 64, 785–798. [Google Scholar] [CrossRef] [PubMed]
- Flokas, M.E.; Karageorgos, S.A.; Detsis, M.; Alevizakos, M.; Mylonakis, E. Vancomycin-resistant enterococci colonisation, risk factors and risk for infection among hospitalised paediatric patients: A systematic review and meta-analysis. Int. J. Antimicrob. Agents 2017, 49, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Farhadi, R.; Saffar, M.J.; Monfared, F.T.; Larijani, L.V.; Kenari, S.A.; Charati, J.Y. Prevalence, risk factors, and molecular analysis of vancomycin-resistant Enterococci colonization in a referral neonatal intensive care unit: A prospective study in northern Iran. J. Glob. Antimicrob. Resist. 2022, 30, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Andersson, P.; Beckingham, W.; Gorrie, C.L.; Kennedy, K.; Daveson, K.; Ballard, S.A.; Chen, M.; Roper, K.; Coatsworth, N. Vancomycin-resistant Enterococcus (VRE) outbreak in a neonatal intensive care unit and special care nursery at a tertiary-care hospital in Australia-A retrospective case-control study. Infect. Control. Hosp. Epidemiol. 2019, 40, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Sutcu, M.; Akturk, H.; Acar, M.; Salman, N.; Aydın, D.; Akgun Karapınar, B.; Ozdemir, A.; Cihan, R.; Citak, A.; Somer, A. Impact of vancomycin-resistant enterococci colonization in critically ill pediatric patients. Am. J. Infect. 2016, 44, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Al-Balawi, M.; Morsy, F.M. Enterococcus Faecalis is a Better Competitor Than Other Lactic Acid Bacteria in the Initial Colonization of Colon of Healthy Newborn Babies at First Week of Their Life. Front. Microbiol. 2020, 11, 2017. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marom, R.; Mandel, D.; Haham, A.; Berger, I.; Ovental, A.; Raskind, C.; Grisaru-Soen, G.; Adler, A.; Lellouche, J.; Schwartz, D.; et al. A silent outbreak of vancomycin-resistant Enterococcus faecium in a neonatal intensive care unit. Antimicrob. Resist. Infect. Control. 2020, 9, 87. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Prematunge, C.; MacDougall, C.; Johnstone, J.; Adomako, K.; Lam, F.; Robertson, J.; Garber, G. VRE and VSE Bacteremia Outcomes in the Era of Effective VRE Therapy: A Systematic Review and Meta-analysis. Infect. Control. Hosp. Epidemiol. 2016, 37, 26–35. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Iosifidis, E.; Evdoridou, I.; Agakidou, E.; Chochliourou, E.; Protonotariou, E.; Karakoula, K.; Stathis, I.; Sofianou, D.; Drossou-Agakidou, V.; Pournaras, S.; et al. Vancomycin-resistant Enterococcus outbreak in a neonatal intensive care unit: Epidemiology, molecular analysis and risk factors. Am. J. Infect. Control. 2013, 41, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Tanyeri-Bayraktar, B.; Bayraktar, S. Vancomycin-resistant enterococci colonization in a neonatal intensive care unit: Case-control study. J. Clin. Anal. Med. 2017, 8, 276–279. [Google Scholar]
- Hufnagel, M.; Liese, C.; Loescher, C.; Kunze, M.; Proempeler, H.; Berner, R.; Krueger, M. Enterococcal colonization of infants in a neonatal intensive care unit: Associated predictors, risk factors and seasonal patterns. BMC. Infect. Dis. 2007, 7, 107. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miedema, C.J.; Kerkhof, M.; Arends, J.P.; Bergman, K.A.; Kimpen, J.L. Risk factors for colonization with enterococci in a neonatal intensive care unit. Clin. Microbiol. Infect. 2000, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- Topcuoglu, S.; Gursoy, T.; Ovalı, F.; Serce, O.; Karatekin, G. A new risk factor for neonatal vancomycin-resistant Enterococcus colonisation: Bacterial probiotics. J. Matern. Fetal Neonatal Med. 2015, 28, 1491–1494. [Google Scholar] [CrossRef] [PubMed]
- Akturk, H.; Sutcu, M.; Somer, A.; Acar, M.; Akgun Karapınar, B.; Aydin, D.; Cihan, R.; Ince, Z.; Çoban, A.; Salman, N. Vancomycin-resistant enterococci colonization in a neonatal intensive care unit: Who will be infected? J. Matern. Fetal Neonatal Med. 2016, 29, 3478–3482. [Google Scholar] [CrossRef] [PubMed]
- Derde, L.P.G.; Cooper, B.S.; Goossens, H.; Malhotra-Kumar, S.; Willems, R.J.L.; Gniadkowski, M.; Hryniewicz, W.; Empel, J.; Dautzenberg, M.J.D.; Annane, D.; et al. Interventions to reduce colonisation and transmission of antimicrobial-resistant bacteria in intensive care units: An interrupted time series study and cluster randomised trial. Lancet Infect. Dis. 2014, 14, 31–39. [Google Scholar] [CrossRef]
- Jernigan, J.A.; Clemence, M.A.; Stott, G.A.; Titus, M.G.; Alexander, C.H.; Palumbo, C.M.; Farr, B.M. Control of methicillin-resistant Staphylococcus aureus at a university hospital: One decade later. Infect. Control. Hosp. Epidemiol. 1995, 16, 686–696. [Google Scholar] [CrossRef] [PubMed]
- Muto, C.A.; Jernigan, J.H.; Ostrowsky, B.E.; Richet, H.M.; Jarvis, W.R.; Boyce, J.M.; Farr, B.M. SHEA guideline Active surveillance cultures are essential to identify the reservoir for spread of MRSA and VRE infections and make control possible using the CDC’s long recommended contact precautions. Infect. Control. Hosp. Epidemiol. 2003, 24, 362–386. [Google Scholar]
- Ostrowsky, B.E.; Trick, W.E.; Sohn, A.H.; Quirk, S.B.; Holt, S.; Carson, L.A.; Hill, B.C.; Arduino, M.J.; Kuehnert, M.J.; Jarvis, W.R. Control of vancomycin-resistant enterococcus in health care facilities in a region. N. Engl. J. Med. 2001, 344, 1427–1433. [Google Scholar] [CrossRef] [PubMed]
- Saliba, R.; Neulier, C.; Seytre, D.; Fiacre, A.; Faibis, F.; Leduc, P.; Amara, M.; Jauréguy, F.; Carbonnelle, E.; Zahar, J.R.; et al. Can real-time polymerase chain reaction allow a faster recovery of hospital activity in cases of an incidental discovery of carbapenemase-producing Enterobacteriaceae and vancomycin-resistant Enterococci carriers? J. Hosp. Infect. 2019, 103, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Hoyos-Mallecot, Y.; Ouzani, S.; Dortet, L.; Fortineau, N.; Naas, T. Performance of the Xpert® Carba-R v2 in the daily workflow of a hygiene unit in a country with a low prevalence of carbapenemase-producing Enterobacteriaceae. Int. J. Antimicrob. Agents 2017, 49, 774–777. [Google Scholar] [CrossRef] [PubMed]
- Meschiari, M.; Kaleci, S.; Monte, M.D.; Dessilani, A.; Santoro, A.; Scialpi, F.; Franceschini, E.; Orlando, G.; Cervo, A.; Monica, M.; et al. Vancomycin resistant enterococcus risk factors for hospital colonization in hematological patients: A matched case-control study. Antimicrob. Resist. Infect. Control. 2023, 12, 126. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ford, C.D.; Gazdik Stofer, M.A.; Coombs, J.; Lopansri, B.K.; Webb, B.J.; Motyckova, G.; Petersen, F.B. Decrease in Vancomycin-Resistant Enterococcus Colonization After Extensive Renovation of a Unit Dedicated to the Treatment of Hematologic Malignancies and Hematopoietic Stem-Cell Transplantation. Infect. Control. Hosp. Epidemiol. 2017, 38, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Orena, B.S.; Liporace, M.F.; Teri, A.; Girelli, D.; Salari, F.; Mutti, M.; Giordano, G.; Alteri, C.; Gentiloni Silverj, F.; Matinato, C.; et al. Active Surveillance of Patients Colonized with CRE: A Single-Center Study Based on a Combined Molecular/Culture Protocol. Antibiotics 2024, 13, 1053. [Google Scholar] [CrossRef]
- Harris, A.D.; Morgan, D.J.; Pineles, L.; Magder, L.; O’Hara, L.M.; Johnson, J.K. Acquisition of Antibiotic-Resistant Gram-negative Bacteria in the Benefits of Universal Glove and Gown (BUGG) Cluster Randomized Trial. Clin. Infect. Dis. 2021, 72, 431–437. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Drees, M.; Snydman, D.R.; Schmid, C.H.; Barefoot, L.; Hansjosten, K.; Vue, P.M.; Cronin, M.; Nasraway, S.A.; Golan, Y. Prior environmental contamination increases the risk of acquisition of vancomycin-resistant enterococci. Clin. Infect. Dis. 2008, 46, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Martínez, J.A.; Ruthazer, R.; Hansjosten, K.; Barefoot, L.; Snydman, D.R. Role of environmental contamination as a risk factor for acquisition of vancomycin-resistant enterococci in patients treated in a medical intensive care unit. Arch. Intern. Med. 2003, 163, 1905–1912. [Google Scholar] [CrossRef] [PubMed]
- Travi, G.; Peracchi, F.; Nicolini, E.; Busni, A.; Tartaglione, L.; Bielli, A.; Proto, A.; Mezzadri, L.; Bana, B.N.; Del Curto, C.; et al. Vancomycin-resistant Enterococcus outbreak in neonatal intensive care unit: A real concern? In Proceedings of the Poster Session—35th ECCMID, Vienna, Austria, 11–15 April 2025. [Google Scholar]
| Patients, n (%) | 21 |
| Male, n (%) | 13 (62) |
| Age at NICU admission (days), median (IQR) | 6 (1–1) |
| Birthweight (g), median (IQR) | 1879 (1150–2360) |
| Gestational age (weeks + days), median (IQR) | 33 + 1 (31 + 3; 36 + 6) |
| Prematurity, n (%) | 16 (76) |
| Prematurity (weeks + days), median (IQR) | 31 + 3 (28 + 4; 34 + 2) |
| Reason for NICU admission, n (%) | |
| Prematurity | 16 (76) |
| Congenital heart disease | 7 (33) |
| Other | 2 (10) |
| Time from admission to VRE colonization (days), median (IQR) | 34 (6–37) |
| Previous BSI, n (%) | 8 (38) |
| Staphylococcus spp. | 7 (33) |
| Streptococcus spp. | 1 (5) |
| Enterobacterales spp. | 2 (10) |
| Previous antimicrobial treatment, n (%) | 8 (38) |
| Vancomycin | 7 (33) |
| Gentamycin | 5 (24) |
| Meropenem | 3 (14) |
| Linezolid | 1 (5) |
| Orotracheal intubation, n (%) | 6 (29) |
| Central venous catheter, n (%) | 8 (38) |
| Surgical intervention, n (%) | 7 (33) |
| Cardio-thorax | 4 (19) |
| Abdominal | 3 (14) |
| Oro-facial | 1 (5) |
| Time from surgical intervention to colonization, n (%) | 26 (16–36) |
| VRE infection, n (%) | 1 (5) |
| Clinical response to infection treatment, n (%) | 1 (100) |
| Molecular detection of van A, n (%) | 8 (38) |
| Death, n (%) | 1 (5) |
| Strains | Molecular Test | Antibiogram | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ampicillin | Tigecycline | Vancomycin | Teicoplanin | Linezolid | |||||||
| MIC (mg/L) | Interpretation | MIC (mg/L) | Interpretation | MIC (mg/L) | Interpretation | MIC (mg/L) | Interpretation | MIC (mg/L) | Interpretation | ||
| 1 | van A | >8 | R | ≤0.25 | S | >256 | R | 96 | R | 2 | S |
| 2 | >8 | R | ≤0.25 | S | >256 | R | 48 | R | ≤1 | S | |
| 3 | >8 | R | ≤0.25 | S | >256 | R | 96 | R | ≤1 | S | |
| 4 | >8 | R | ≤0.25 | S | >256 | R | >256 | R | ≤1 | S | |
| 5 | >8 | R | ≤0.25 | S | >256 | R | 64 | R | ≤1 | S | |
| 6 | >8 | R | ≤0.25 | S | >256 | R | 96 | R | ≤1 | S | |
| 7 | >8 | R | ≤0.25 | S | >256 | R | 96 | R | ≤1 | S | |
| 8 | >8 | R | ≤0.25 | S | >256 | R | 64 | R | 2 | S | |
| 9 | >8 | R | ≤0.25 | S | >256 | R | 64 | R | ≤1 | S | |
| 10 | >8 | R | ≤0.25 | S | >256 | R | 96 | R | ≤1 | S | |
| 11 | >8 | R | ≤0.25 | S | >256 | R | 64 | R | 2 | S | |
| 12 | >8 | R | ≤0.25 | S | >256 | R | 96 | R | ≤1 | S | |
| 13 | van A | >8 | R | ≤0.25 | S | >256 | R | 96 | R | ≤1 | S |
| 14 | >8 | R | ≤0.25 | S | >256 | R | >256 | R | 2 | S | |
| 15 | van A | >8 | R | ≤0.25 | S | >256 | R | 64 | R | 2 | S |
| 16 | van A | >8 | R | ≤0.25 | S | >256 | R | >256 | R | 2 | S |
| 17 | van A | >8 | R | ≤0.25 | S | >256 | R | >256 | R | 2 | S |
| 18 | >8 | R | ≤0.25 | S | >32 | R | >16 | R | 2 | S | |
| 19 | van A | >8 | R | ≤0.25 | S | >32 | R | >16 | R | 2 | S |
| 20 | van A | >8 | R | ≤0.25 | S | >32 | R | >16 | R | 2 | S |
| 21 | van A | >8 | R | ≤0.25 | S | >32 | R | >16 | R | 2 | S |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peracchi, F.; Travi, G.; Proto, A.; Nicolini, E.; Busni, A.; Mezzadri, L.; Tartaglione, L.; Bielli, A.; Matarazzo, E.; Casalicchio, G.; et al. Outbreak of Vancomycin-Resistant Enterococcus in a NICU: Insights into Molecular Detection and Infection Control. Microorganisms 2025, 13, 822. https://doi.org/10.3390/microorganisms13040822
Peracchi F, Travi G, Proto A, Nicolini E, Busni A, Mezzadri L, Tartaglione L, Bielli A, Matarazzo E, Casalicchio G, et al. Outbreak of Vancomycin-Resistant Enterococcus in a NICU: Insights into Molecular Detection and Infection Control. Microorganisms. 2025; 13(4):822. https://doi.org/10.3390/microorganisms13040822
Chicago/Turabian StylePeracchi, Francesco, Giovanna Travi, Alice Proto, Elena Nicolini, Andrea Busni, Luca Mezzadri, Livia Tartaglione, Alessandra Bielli, Elisa Matarazzo, Giorgia Casalicchio, and et al. 2025. "Outbreak of Vancomycin-Resistant Enterococcus in a NICU: Insights into Molecular Detection and Infection Control" Microorganisms 13, no. 4: 822. https://doi.org/10.3390/microorganisms13040822
APA StylePeracchi, F., Travi, G., Proto, A., Nicolini, E., Busni, A., Mezzadri, L., Tartaglione, L., Bielli, A., Matarazzo, E., Casalicchio, G., Del Curto, C., Rossotti, R., Merli, M., Vismara, C., Crippa, F., Martinelli, S., & Puoti, M. (2025). Outbreak of Vancomycin-Resistant Enterococcus in a NICU: Insights into Molecular Detection and Infection Control. Microorganisms, 13(4), 822. https://doi.org/10.3390/microorganisms13040822






