Abstract
Candida infective endocarditis presents therapeutic challenges with high mortality. A complex case of Candida prosthetic valve endocarditis refractory to standard antifungals (anidulafungin and fluconazole) and high-dose caspofungin was successfully treated with heart transplantation. The literature review revealed a few cases of bacterial endocarditis successfully treated with heart transplantation, but with only two transplanted cases of fungal endocarditis. This report explores heart transplantation as a last resort for managing refractory infective endocarditis. The patient is still alive and free of infection, two and a half years after transplantation.
1. Introduction
Candida infective endocarditis (IE) remains a rare entity, with scarce literary evidence to orient its treatment. Causative agents are evenly distributed between all candida species, although more recent findings suggest an increase in the non-albicans group. Candida species, including Candida tropicalis, exhibit virulence factors such as biofilm formation, adhesins, secretion of phospholipases and proteinases, phenotypic switching, and immune evasion through interference with phagocytosis which contribute to its pathogenicity in cardiovascular infections [1]. Current guidelines are based on expert opinions rather than clinical trials. The current recommendation regarding Candida IE is to associate surgery with an antifungal agent like amphotericin B, followed by a lifelong suppressive oral antifungal agent, due to a high risk of relapse. Even with treatment, mortality rates remain unacceptably high, exceeding 50% [2].
2. Case Presentation
We present a complicated case of fungal endocarditis in a 63-year-old male patient admitted for palpitations and dyspnea. Six months prior to presentation, the patient underwent a mitral biological valve replacement in another hospital for decompensated heart failure due to a native mitral valve Streptococcus gordonii endocarditis. His past medical history revealed cases of hemochromatosis, arterial hypertension, hypercholesterolemia, malignant melanoma, and chronic kidney disease with a glomerular filtration rate of 56 mL/min/1.73 m2 according to the 2021 Chronic Kidney Disease Epidemiology Collaboration equation. He had no known allergies. His mitral valve replacement was complicated on day 36 post-operation by fungemia due to Candida tropicalis, which was treated by anidulafungin for 18 days (first documented negative culture on D8 of treatment, no blood cultures performed before 8 days of treatment). Upon admission 6 months after his mitral valve replacement, his blood cultures tested positive over five consecutive days for Candida tropicalis with the same previous resistance profile according to the Clinical and Laboratory Standards Institute guidelines of 2021 (fluconazole MIC = 1 μg/mL susceptible, anidulafungin MIC = 0.12 μg/mL susceptible, caspofungin MIC = 0.06 μg/mL susceptible) [3]. Trans-thoracic echocardiography showed a 20 mm vegetation on the mitral prosthetic valve with the thickening of its leaflets (Figure 1). Anidulafungin 100 mg per day was initiated (no loading dose given). Further workup showed a spondylodiscitis L2–L3 extending one month later to Th12-L1 on a positron emission tomography scan (PET-scan), with suspicions of septic emboli due to the presence of hypermetabolic lesions on the seventh costal rib and bilateral hilar lymph nodes. A small ischemic embolic cerebral lesion was also documented in the right occipital lobe via magnetic resonance imaging (MRI). Surgical replacement of the prosthetic biological valve with a mechanical one was performed on D5 of anidulafungin; surgical samples were culture-positive for Candida tropicalis. After six weeks of Anidulafungin, the patient still had night sweats. A new trans-esophageal echocardiography showed a new 3 mm vegetation with mitral valve insufficiency (Figure 2). His CRP increased from 21 to 74 mg/L. A new MRI of the spine was performed, showing no signs of improvement of his spondylodiscitis. The patient was readmitted, and therapy was changed. Combination therapy with amphotericin B was not given due to his decreased kidney function. Anidulafungin was switched to caspofungin 150 mg/day (high-dose) for a body weight of 74 kg, followed by positive clinical, biological, and radiological responses (Figure 3). After 3 months of high-dose caspofungin, treatment was shifted to high oral dose fluconazole (800 mg per day). Eight weeks after the switch, a follow-up echocardiography showed significant mitral valve dehiscence. The patient reported progressive dyspnea evolving to heart failure, requiring hospitalizations for acute decompensated heart failure. New blood cultures and a discitis biopsy remained negative. Caspofungin (150 mg per day) was reintroduced. A PET-scan, repeated three months after initiation of dual antifungal therapy, showed the resolution of the cardiac metabolic abnormalities, regression of the spondylodiscitis lesions, and observed regression of metabolic activity in the bilateral mediastino-hilar and hepatic peri-hilar lymph nodes. Faced with mitral valve dehiscence, and with no other surgical options in a patient with a controlled disseminated fungal infection, our multidisciplinary team decided to list the patient for a heart transplantation. Concomitantly to this decision, the new PET-scan revealed new diffuse and heterogenous hepatic hypermetabolism, which is suggestive of drug-induced hepatitis. However, liver echography remained normal.
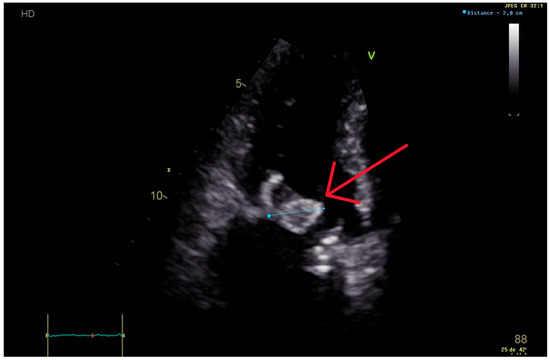
Figure 1.
Trans-thoracic echocardiography performed 6 months after the first valvular replacement, showing a 20 mm vegetation on the mitral prosthetic valve (red arrow), causing severe intraprosthetic obstruction.
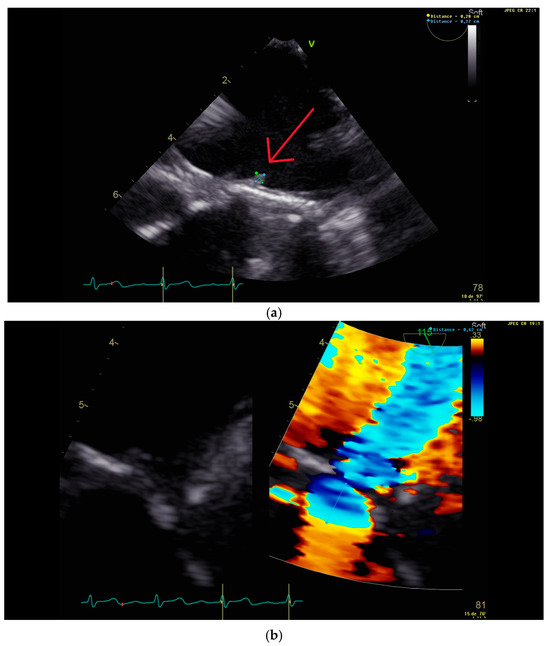
Figure 2.
Trans-esophageal echocardiography performed 2 months after the second valvular replacement, showing (a) a 3 mm vegetation (red arrow) on the new prosthetic mitral valve (below) and (b) mitral insufficiency with a proximal isovelocity surface area of 6 mm.
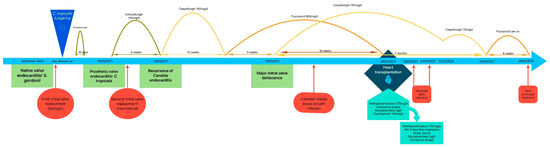
Figure 3.
Timetable showing the different main events and treatments leading to heart transplantation, with follow up.
Heart transplantation was performed 15 weeks after listing the patient in Eurotransplant. Immunosuppressive treatment (IS) regimen differed from the one usually administered in our hospital; no induction treatment by thymoglobuline or basiliximab was administered. Initially, intravenous, followed by oral, methylprednisolone was administered in association with cyclosporin (instead of tacrolimus) and mycophenolate mofetil as IS maintenance treatment. Three days before transplantation, caspofungin dosage was lowered to 70 mg/day and fluconazole was stopped due to probable hepatotoxicity (alkaline phosphatase 634 U/L, gamma-glutamyl transferase 1407 U/L, alanine aminotransferase 279 U/L, aspartate aminotransferase 140 U/L). His liver enzymes improved over the following days and caspofungin dosage increased back to 150 mg three days post-transplantation. Caspofungin drug monitoring was performed while being administered 150 mg per day, with measured serum concentrations of 7.615 µg/mL and 7.678 µg/mL.
Routine endomyocardial biopsy conducted 9 days post-transplantation revealed a grade 2R acute graft rejection with no humoral rejection (pAMR0), according to the International Society for Heart and Lung Transplantation score. Heart transplantation was assessed by echocardiography, presenting a left ventricular hypertrophy with a normal ejection fraction. Cyclosporin was switched to tacrolimus and IV methylprednisolone (10 mg/kg/d) was administered for 3 consecutive days, followed by an increase in oral methylprednisolone doses. Two weeks later, persistent signs of grade 2R acute rejection with no humoral rejection (pAMR0) were noted on endomyocardial biopsy, requiring a new treatment with IV methylprednisolone (10 mg/kg/d) for 3 consecutive days. Control endomyocardial biopsies over time showed improvement, followed by resolution of signs of acute rejection.
A PET-scan performed during acute rejection treatment showed favorable changes in spondylodiscitis and lymph nodes. Caspofungin dosage was lowered to 70 mg/day 10 weeks after transplantation, relayed by oral fluconazole 3 months after transplantation.
A PET-scan 5 months after transplantation showed complete resolution of all signs of infection. Administering of fluconazole was then stopped.
3. Discussion
This is a unique case of cardiac transplantation due to fungal endocarditis. Treatment was only guided by expert opinion. Our patient did not respond to the combination of standard-dose anidulafungin and surgical valve replacement, resulting in our decision to administer higher doses of a different echinocandin, based on the 2016 Infectious Diseases Society of America guidelines’ recommendation of low-quality evidence [4]. We switched to caspofungin, which was not only the first echinocandin approved for clinical use but also the most frequently used in the setting of Candida endocarditis [5].
Echinocandins exert their killing effect in a concentration-dependent fashion. Treatment efficacy is achieved by optimizing Cmax/MIC and AUC/MIC, which differ according to the Candida species [6]. Higher-than-usual doses of echinocandins are thought to be more effective in difficult-to-treat forms of candidiasis, including endocarditis [7]. Higher doses of caspofungin (150 mg per day) are usually well tolerated, most commonly observed side effects being phlebitis and elevated liver enzymes [8].
The choice of an echinocandin over fluconazole was furthermore justified by the need for better anti-biofilm activity. It has been demonstrated that fluconazole along with voriconazole and posaconazole have no activity on C. albicans biofilms. A basic component of the fungal cell wall is the ß-1,3-D-glucan. Since a fungal biofilm’s composition was demonstrated to include cell wall polysaccharides, it was hypothesized that as echinocandins inhibit the ß-1,3-D-glucan, they should have activity against fungal biofilms [5]. Then, in another study comparing the in vitro activity against Candida species in biofilms, echinocandins were favored over the combination of amphotericin and azoles [9].
In this case report, there was an indication for heart transplantation as no other surgical therapeutic option was possible, as recommended in the 2023 European Society of Cardiology guidelines [2]. One case report of a native tricuspid valve endocarditis due to Candida krusei [10] and another one of a mitral valve Candida albicans endocarditis [11] were successfully treated with heart transplantation. Other case reports of bacterial endocarditis treated successfully with heart transplantation were also described in 4 reviews by Guerrero et al. [12], Murphy et al. [13], Givone et al. [14], and Tattevin et al. [11]. In addition to the cases already reported in those studies, an updated review of the literature up to February 2024 presented in Table 1 shows 3 more cases of infective bacterial endocarditis treated by salvage heart transplantation. All cases of fungal endocarditis treated with heart transplantation in the literature are presented in Table 2.

Table 1.
Case reports of bacterial endocarditis treated with heart transplantation (complement to Guerrero et al. [12], Murphy et al. [13], Givone et al. [14] and Tattevin et al. [11]). Abbreviations: M = male; F = female.

Table 2.
Case reports of fungal endocarditis treated with heart transplantation in the literature. Abbreviations: M = male; F = female; UK = unknown.
Several multidisciplinary team discussions were needed before deciding to list our patient for a heart transplantation. Arguments in favor of transplantation were that the infection appeared to be only locally uncontrolled (no fever, negative blood cultures, no evidence of new sites of infection, and a Charlson Comorbidity Index of 3 with 77% estimated 10-year survival) and good clinical status of the patient. Arguments against transplantation included the relative contraindication of an active infection, the lack of similar reported cases, shortage of organ donors, and the plan to administer less intensive IS treatment than usual. It should be noted that immunosuppression is also a risk factor when acquiring new fungal and non-fungal infections. Heart transplantation in the case of endocarditis remains limited due to the risk of infection relapse, graft lost, and death after transplantation.
To the best of our knowledge, this is the first case of Candida tropicalis prosthetic valve endocarditis, and the third case of fungal infective endocarditis, to be successfully treated with heart transplantation.
To the date of writing this article our patient is alive, doing well and living at home.
Author Contributions
Conceptualization, R.E.N. and M.H.; methodology, R.E.N. and M.H.; validation, all authors; formal analysis, R.E.N. and M.H.; investigation, R.E.N., E.L.L., F.V.E., A.R. and M.H.; writing—original draft preparation, R.E.N. and M.H.; writing—review and editing, all authors; supervision, M.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Erasme Hospital (protocol code P2024/086 and approved on 7 March 2024).
Informed Consent Statement
Written informed consent has been obtained from the patient to publish this paper.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Deorukhkar, S.C.; Saini, S.; Mathew, S. Virulence Factors Contributing to Pathogenicity of Candida tropicalis and Its Antifungal Susceptibility Profile. Int. J. Microbiol. 2014, 2014, 456878. [Google Scholar] [CrossRef] [PubMed]
- Delgado, V.; Ajmone Marsan, N.; de Waha, S.; Bonaros, N.; Brida, M.; Burri, H.; Caselli, S.; Doenst, T.; Ederhy, S.; Erba, P.A.; et al. 2023 ESC Guidelines for the management of endocarditis: Developed by the task force on the management of endocarditis of the European Society of Cardiology (ESC) Endorsed by the European Association for Cardio-Thoracic Surgery (EACTS) and the European Association of Nuclear Medicine (EANM). Eur. Heart J. 2023, 44, 3948–4042. [Google Scholar] [PubMed]
- Mycology|University of Adelaide [Internet]. CLSI Break Points. Available online: https://www.adelaide.edu.au/mycology/clsi-break-points (accessed on 13 February 2025).
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2016, 62, e1–e50. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, D.M.; George, T.; Chandra, J.; Mukherjee, P.K.; Ghannoum, M.A. Antifungal Susceptibility of Candida Biofilms: Unique Efficacy of Amphotericin B Lipid Formulations and Echinocandins. Antimicrob. Agents Chemother. 2002, 46, 1773–1780. [Google Scholar] [CrossRef] [PubMed]
- Andes, D.; Diekema, D.J.; Pfaller, M.A.; Bohrmuller, J.; Marchillo, K.; Lepak, A. In Vivo Comparison of the Pharmacodynamic Targets for Echinocandin Drugs against Candida Species. Antimicrob. Agents Chemother. 2010, 54, 2497–2506. [Google Scholar] [CrossRef] [PubMed]
- Lepak, A.J.; Andes, D.R. Antifungal Pharmacokinetics and Pharmacodynamics. Cold Spring Harb. Perspect. Med. 2015, 5, a019653. [Google Scholar] [CrossRef] [PubMed]
- Betts, R.F.; Nucci, M.; Talwar, D.; Gareca, M.; Queiroz-Telles, F.; Bedimo, R.J.; Herbrecht, R.; Ruiz-Palacios, G.; Young, J.-A.H.; Baddley, J.W.; et al. A Multicenter, Double-Blind Trial of a High-Dose Caspofungin Treatment Regimen versus a Standard Caspofungin Treatment Regimen for Adult Patients with Invasive Candidiasis. Clin. Infect. Dis. 2009, 48, 1676–1684. [Google Scholar] [CrossRef] [PubMed]
- Tumbarello, M.; Posteraro, B.; Trecarichi, E.M.; Fiori, B.; Rossi, M.; Porta, R.; Donati, K.d.G.; La Sorda, M.; Spanu, T.; Fadda, G.; et al. Biofilm production by Candida species and inadequate antifungal therapy as predictors of mortality for patients with candidemia. J. Clin. Microbiol. 2007, 45, 1843–1850. [Google Scholar] [CrossRef] [PubMed]
- Checchia, T.; Moura, L.; Colatusso, C.; Veiga, S.; Fortes, J.; Tuon, F. Heart transplantation and Candida endocarditis. Transpl. Infect. Dis. 2016, 18, 483–484. [Google Scholar] [CrossRef] [PubMed]
- Tattevin, P.; Muñoz, P.; Moreno, A.; Hékimian, G.; Delahaye, F.; Duval, X.; Castel, M.Á.; Hasse, B.; Jaramillo, N.; Vincelj, J.; et al. Heart transplantation as salvage treatment of intractable infective endocarditis. Infect. Dis. Lond. Engl. 2023, 55, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, M.L.F.; Aldámiz, G.; Bayón, J.; Cohen, V.A.; Fraile, J. Long-term survival of salvage cardiac transplantation for infective endocarditis. Ann. Thorac. Surg. 2011, 92, e93–e94. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.M.; Vikram, H.R. Heart transplantation for infective endocarditis: Viable option for a limited few? Transpl. Infect. Dis. Off. J. Transplant. Soc. 2019, 21, e13006. [Google Scholar] [CrossRef] [PubMed]
- Givone, F.; Peghin, M.; Vendramin, I.; Carletti, S.; Tursi, V.; Pasciuta, R.; Livi, U.; Bassetti, M. Salvage heart transplantation for Mycoplasma hominis prosthetic valve endocarditis: A case report and review of the literature. Transpl. Infect. Dis. Off. J. Transplant. Soc. 2020, 22, e13249. [Google Scholar] [CrossRef] [PubMed]
- Rzucidło-Resil, J.; Golińska-Grzybała, K.; Przybylski, R.; Kapelak, B.; Gajos, G.; Gackowski, A. A complicated course of Salmonella endocarditis leading to heart transplantation. Pol. Heart J. Kardiol. Pol. 2022, 80, 945–946. [Google Scholar] [CrossRef] [PubMed]
- van ‘t Veer, M.; Otterspoor, L.; De Regt, M.; Peels, K.; Evens, J.; Vink, A.; de Jonge, N. Heart transplantation for end-stage heart failure combined with Q fever isolated to the heart: A case report. Eur. Heart J. Case Rep. 2020, 4, 1–4. [Google Scholar] [CrossRef]
- Beliaev, A.M.; Ruygrok, P.; Bergin, C.; Haydock, D.A.; Sibal, A.K. Heart transplantation for recurrent Cutibacterium (Propionibacterium) acnes prosthetic heart valve endocarditis. ANZ J. Surg. 2021, 91, 196–197. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).