Strategies of Environmental Adaptation in the Haloarchaeal Genera Haloarcula and Natrinema
Abstract
1. Introduction
2. Materials and Methods
2.1. Genomic and Metabolic Profiling
2.2. Assessment of Heavy Metal Tolerance
3. Results and Discussion
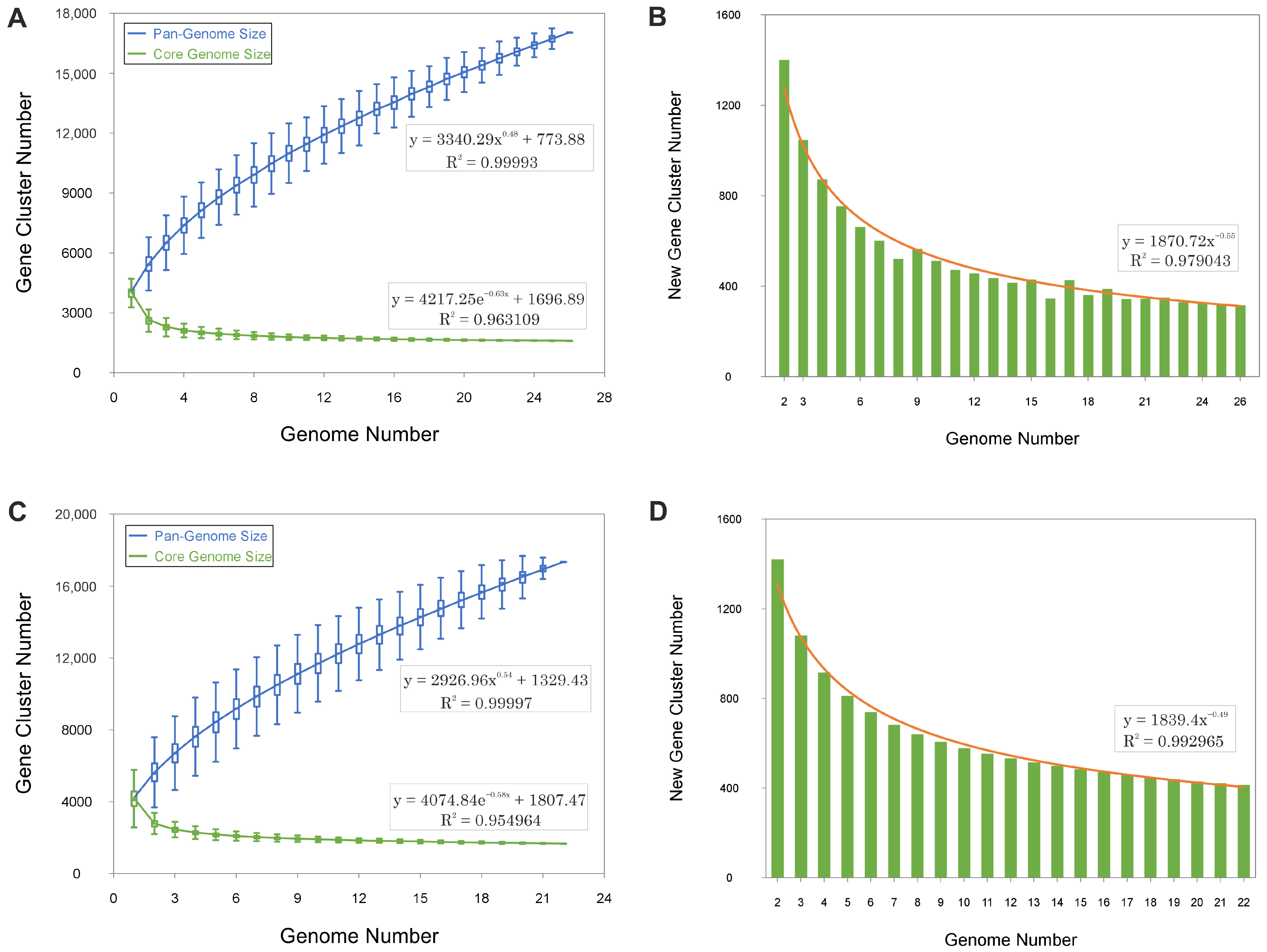
3.1. Comparative Genomic Analysis and Pan-Genome Dynamics of the Genera Haloarcula and Natrinema
3.2. Metabolic Potential and Pathway Analysis
3.2.1. Carbohydrate Metabolism
3.2.2. Nitrogen Metabolism
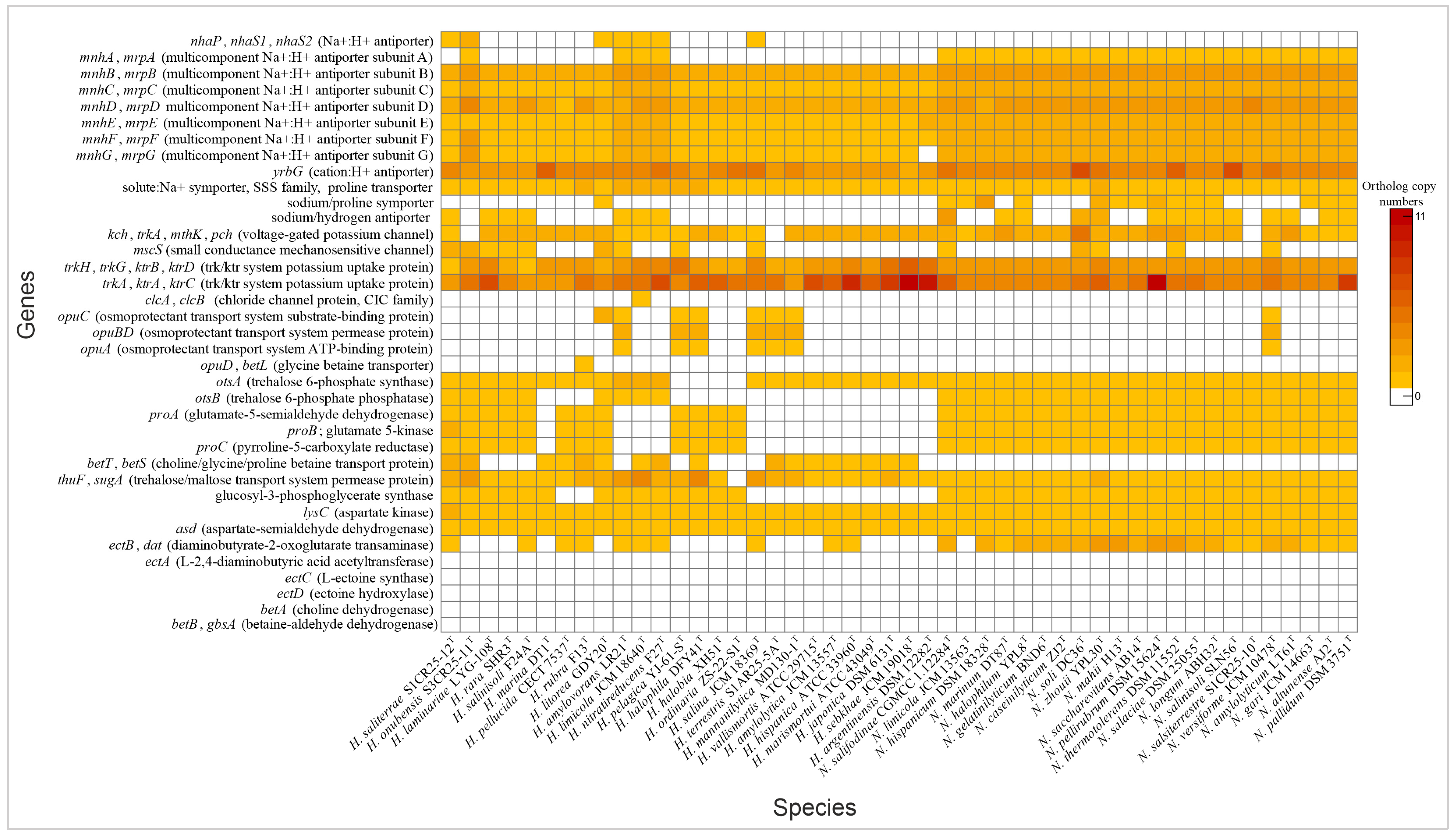
3.2.3. Transporters
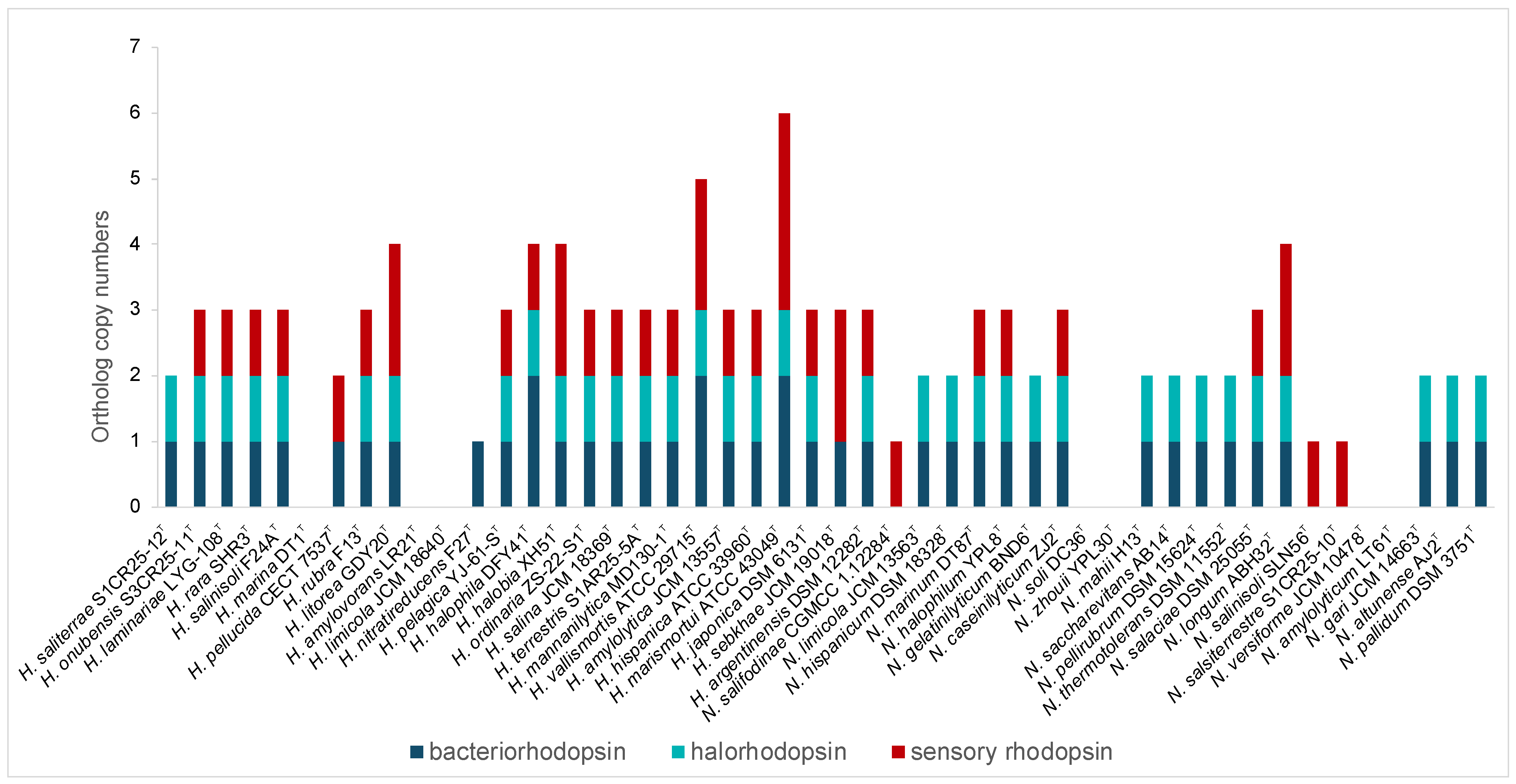
3.2.4. Phototrophy
3.3. Osmoregulatory Mechanisms and Proteomic Adaptations
3.3.1. “Salt-In” Strategy
3.3.2. “Salt-Out” Strategy
3.3.3. Proteome and Genomic Adaptation
3.4. Mechanisms of Stress Tolerance
3.4.1. Adaptation to High UV Radiation
3.4.2. Defense Against Oxidative Stress
3.4.3. Response to Temperature Fluctuations
3.4.4. Protection Against Genetic and Environmental Threats
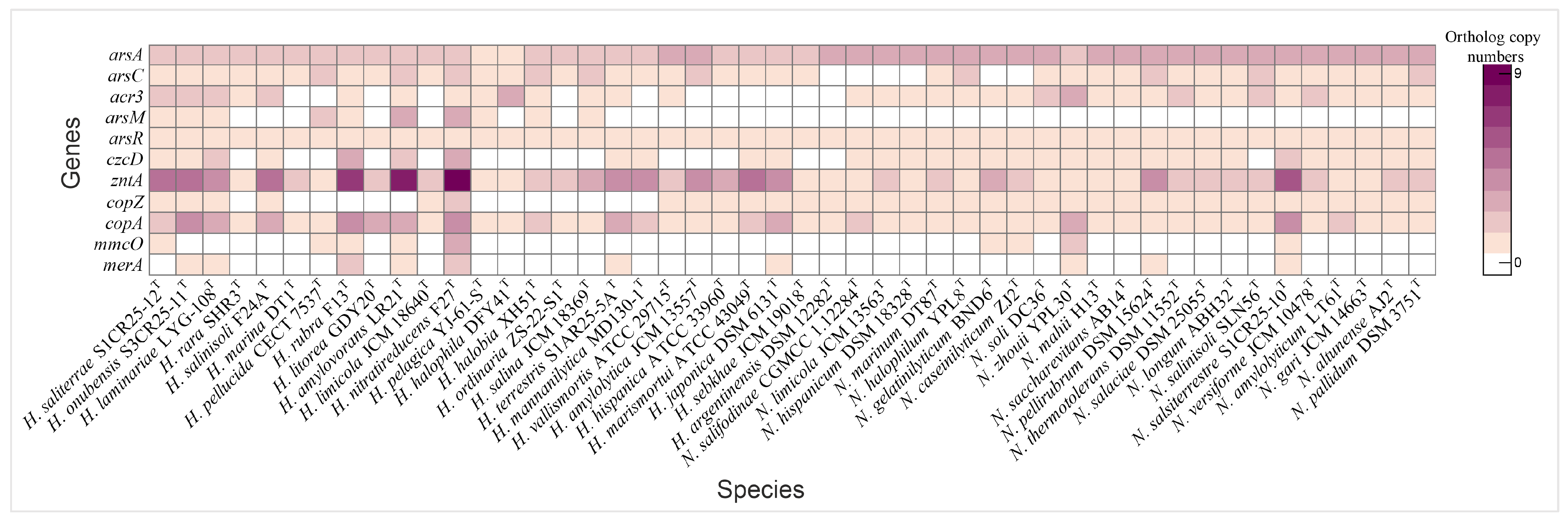
3.5. Heavy Metal Tolerance Mechanisms in Haloarcula and Natrinema
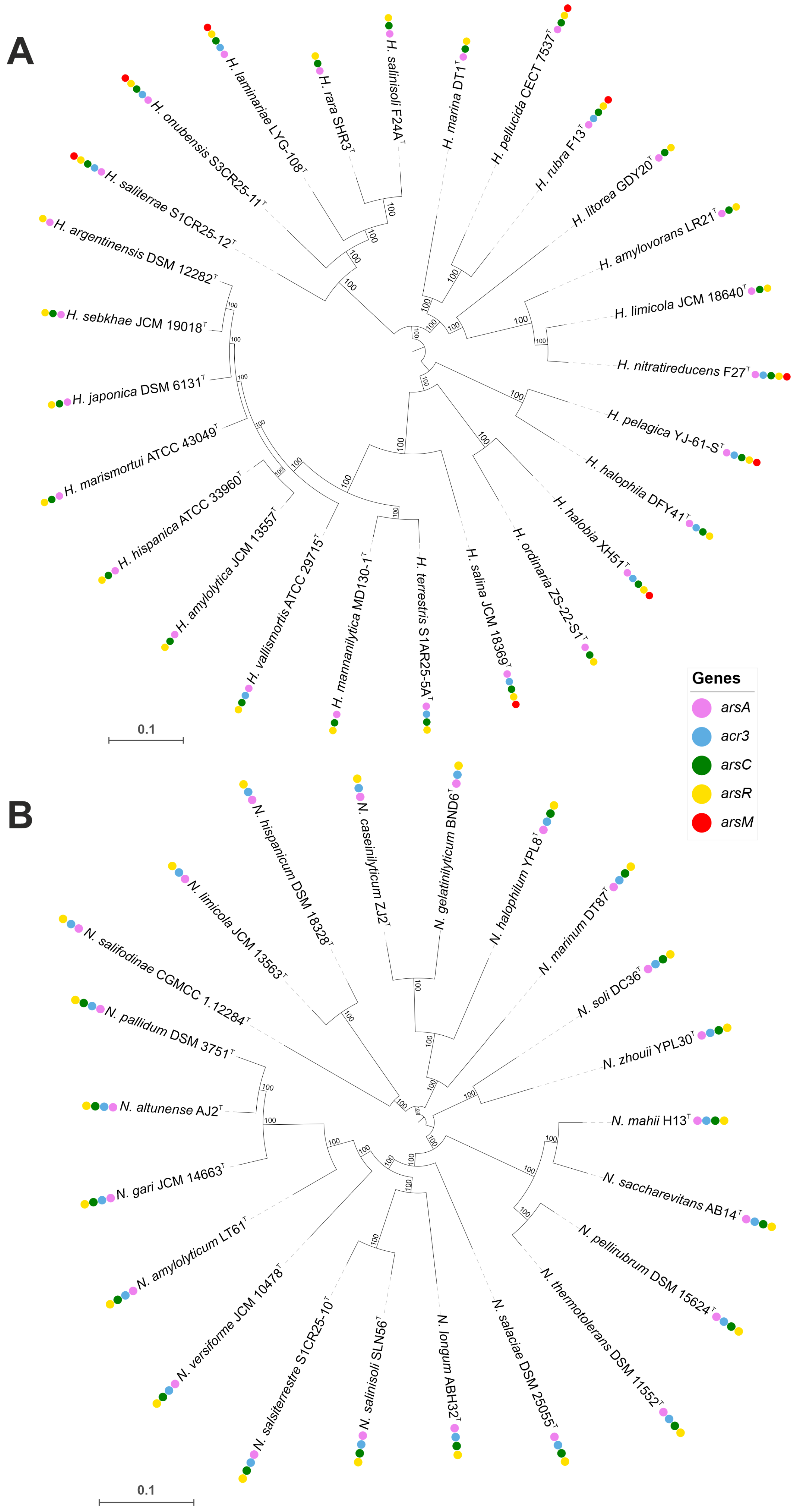
3.5.1. Arsenic Resistance Mechanisms
3.5.2. Copper Detoxification Pathways
3.5.3. Zinc, Cadmium, and Cobalt Efflux Systems
3.5.4. Determination of Minimum Inhibitory Concentrations (MICs) for Heavy Metal Tolerance
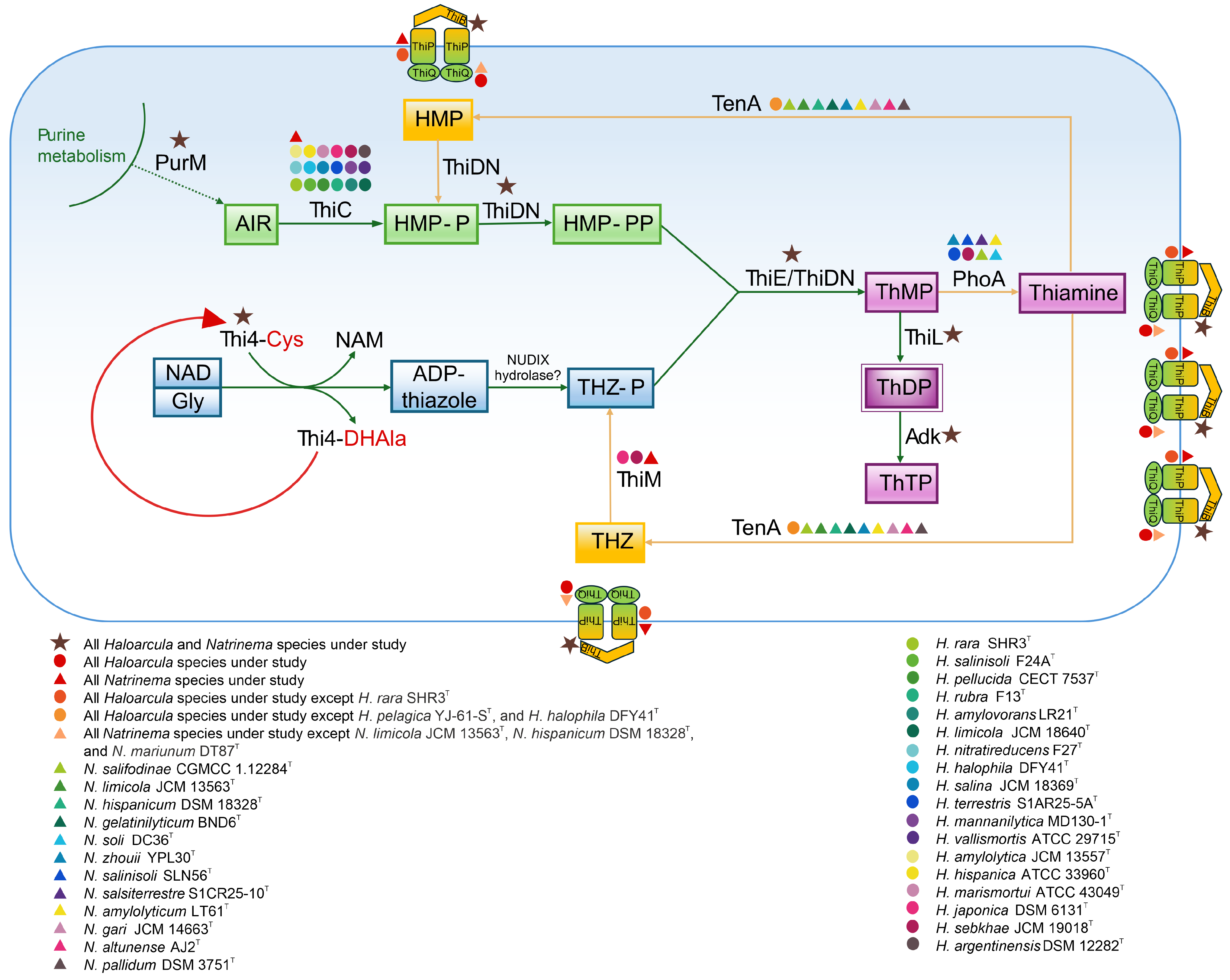
3.6. Functional Analysis of Thiamine Biosynthesis Pathway
3.7. Research Implications
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ventosa, A. Unusual micro-organisms from unusual habitats: Hypersaline environments. In Prokaryotic Diversity: Mechanisms and Significance; Logan, N.A., Lappin-Scott, H.M., Oyston., P.C.F., Eds.; Cambridge University Press: Cambridge, UK, 2006; pp. 223–254. [Google Scholar] [CrossRef]
- DasSarma, S.; DasSarma, P.; Laye, V.; Schwieterman, E. Extremophilic models for astrobiology: Haloarchaeal survival strategies and pigments for remote sensing. Extremophiles 2019, 24, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Falb, M.; Müller, K.; Königsmaier, L.; Oberwinkler, T.; Horn, P.; von Gronau, S.; Gonzalez, O.; Pfeiffer, F.; Bornberg-Bauer, E.; Oesterhelt, D. Metabolism of halophilic archaea. Extremophiles 2008, 12, 177–196. [Google Scholar] [CrossRef] [PubMed]
- Papke, R.; Douady, C.; Doolittle, W.; Rodríguez-Valera, F. Diversity of bacteriorhodopsins in different hypersaline waters from a single Spanish saltern. Environ. Microbiol. 2003, 5, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- Bonete, M.J.; Martínez-Espinosa, R.M.; Pire, C.; Zafrilla, B.; Richardson, D.J. Nitrogen metabolism in haloarchaea. Saline Syst. 2008, 4, 9. [Google Scholar] [CrossRef]
- Ventosa, A.; de la Haba, R.R.; Sánchez-Porro, C.; Papke, R.T. Microbial diversity of hypersaline environments: A metagenomic approach. Curr. Opin. Microbiol. 2015, 25, 80–87. [Google Scholar] [CrossRef]
- Andrei, A.; Banciu, H.; Oren, A. Living with salt: Metabolic and phylogenetic diversity of archaea inhabiting saline ecosystems. FEMS Microbiol. Lett. 2012, 330, 1–9. [Google Scholar] [CrossRef]
- Matarredona, L.; Zafrilla, B.; Camacho, M.; Bonete, M.J.; Esclapez, J. Understanding the tolerance of halophilic archaea to stress landscapes. Environ. Microbiol. Rep. 2024, 16, e70039. [Google Scholar] [CrossRef]
- Moopantakath, J.; Imchen, M.; Anju, V.T.; Busi, S.; Dyavaiah, M.; Martínez-Espinosa, R.M.; Kumavath, R. Bioactive molecules from haloarchaea: Scope and prospects for industrial and therapeutic applications. Front. Microbiol. 2023, 14, 1113540. [Google Scholar] [CrossRef]
- Li, J.; Gao, Y.; Dong, H.; Sheng, G. Haloarchaea, excellent candidates for removing pollutants from hypersaline wastewater. Trends Biotechnol. 2022, 40, 226–239. [Google Scholar] [CrossRef]
- Amoozegar, M.A.; Safarpour, A.; Noghabi, K.A.; Bakhtiary, T.; Ventosa, A. Halophiles and their vast potential in biofuel production. Front. Microbiol. 2019, 10, 1895. [Google Scholar] [CrossRef]
- Parte, A.C.; Sardà Carbasse, J.; Meier-Kolthoff, J.P.; Reimer, L.C.; Göker, M. List of Prokaryotic names with Standing in Nomenclature (LPSN) moves to the DSMZ. Int. J. Syst. Evol. Microbiol. 2020, 70, 5607–5612. [Google Scholar] [CrossRef] [PubMed]
- Torreblanca, M.; Rodriguez-Valera, F.; Juez, G.; Ventosa, A.; Kamekura, M.; Kates, M. Classification of non-alkaliphilic halobacteria based on numerical taxonomy and polar lipid composition, and description of Haloarcula gen. nov. and Haloferax gen. nov. Syst. Appl. Microbiol. 1986, 8, 89–99. [Google Scholar] [CrossRef]
- Ma, X.; Hu, Y.; Li, X.-X.; Tan, S.; Cheng, M.; Hou, J.; Cui, H.-L. Genome-based taxonomy of genera Haloarcula and Halomicroarcula, and description of six novel species of Haloarcula. Extremophiles 2024, 28, 10. [Google Scholar] [CrossRef]
- Ma, X.; Hu, Y.; Li, X.-X.; Tan, S.; Cheng, M.; Hou, J.; Cui, H.-L. Halomicroarcula laminariae sp. nov. and Halomicroarcula marina sp. nov., extremely halophilic archaea isolated from salted brown alga Laminaria and coastal saline-alkali lands. Int. J. Syst. Evol. Microbiol. 2023, 73, 5889. [Google Scholar] [CrossRef] [PubMed]
- Straková, D.; Galisteo, C.; de la Haba, R.R.; Ventosa, A. Characterization of Haloarcula terrestris sp. nov. and reclassification of a Haloarcula species based on a taxogenomic approach. Int. J. Syst. Evol. Microbiol. 2023, 73, 006157. [Google Scholar] [CrossRef]
- McGenity, T.J.; Gemmell, R.T.; Grant, W.D. Proposal of a new halobacterial genus Natrinema gen. nov., with two species Natrinema pellirubrum nom. nov. and Natrinema pallidum nom. nov. Int. J. Syst. Bacteriol. 1998, 48, 1187–1196. [Google Scholar] [CrossRef]
- Ventosa, A.; Gutiérrez, M.C.; Kamekura, M.; Dyall-Smith, M.L. Proposal to transfer Halococcus turkmenicus, Halobacterium trapanicum JCM 9743 and strain GSL-11 to Haloterrigena turkmenica gen. nov., comb. nov. Int. J. Syst. Bacteriol. 1999, 49, 131–136. [Google Scholar] [CrossRef]
- de la Haba, R.R.; Minegishi, H.; Kamekura, M.; Shimane, Y.; Ventosa, A. Phylogenomics of haloarchaea: The controversy of the genera Natrinema-Haloterrigena. Front. Microbiol. 2021, 12, 740909. [Google Scholar] [CrossRef]
- Rodríguez-R, L.M.; Konstantinidis, K.T. The Enveomics collection: A toolbox for specialized analyses of microbial genomes and metagenomes. PeerJ Preprints 2016, 4, e1900v1. [Google Scholar] [CrossRef]
- Tang, D.; Chen, M.; Huang, X.; Zhang, G.; Zeng, L.; Zhang, G.; Wu, S.; Wang, Y. SRplot: A free online platform for data visualization and graphing. PLoS ONE 2023, 18, e0294236. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Jia, X.; Yang, J.; Ling, Y.; Zhang, Z.; Yu, J.; Wu, J.; Xiao, J. PanGP: A tool for quickly analyzing bacterial pan-genome profile. Bioinformatics 2014, 30, 1297–1299. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Sato, Y.; Morishima, K. BlastKOALA and GhostKOALA: KEGG tools for functional characterization of genome and metagenome sequences. J. Mol. Biol. 2016, 428, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Couvin, D.; Bernheim, A.; Toffano-Nioche, C.; Touchon, M.; Michalik, J.; Neéron, B.; Rocha, E.P.C.; Vergnaud, G.; Gautheret, D.; Pourcel, C. CRISPRCasFinder, an update of CRISRFinder, includes a portable version, enhanced performance and integrates search for Cas proteins. Nucleic Acids Res. 2018, 46, W246–W251. [Google Scholar] [CrossRef]
- Rice, P.; Longden, L.; Bleasby, A. EMBOSS: The European molecular biology open software suite. Trends Genet. 2000, 16, 276–277. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Pagès, H.; Aboyoun, P.; Gentleman, R.; DebRoy, S. Biostrings: Efficient Manipulation of Biological Strings; R package version 2.74.1. Available online: https://bioconductor.org/packages/release/bioc/html/Biostrings.html (accessed on 19 September 2024).
- Nakazawa, M. fmsb: Functions for Medical Statistics Book with Some Demographic Data; R package version 0.7.6. Available online: https://cran.r-project.org/web/packages/fmsb/index.html (accessed on 19 September 2024).
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2–Approximately maximum-likelihood trees for large alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef]
- Subov, N.N. Oceanographical Tables; Oceanographical Institute of USSR, Commissariat of Agriculture of USSR; Hydro-Meteorological Committee of USSR: Moscow, Russia, 1931.
- Nieto, J.J.; Ventosa, A.; Ruiz-Berraquero, F. Susceptibility of halobacteria to heavy metals. Appl. Environ. Microbiol. 1987, 53, 1199–1202. [Google Scholar] [CrossRef]
- Williams, T.J.; Allen, M.; Tschitschko, B.; Cavicchioli, R. Glycerol metabolism of haloarchaea. Environ. Microbiol. 2017, 19, 864–877. [Google Scholar] [CrossRef]
- Pickl, A.; Johnsen, U.; Schönheit, P. Fructose degradation in the haloarchaeon Haloferax volcanii involves a bacterial type phosphoenolpyruvate-dependent phosphotransferase system, fructose-1-phosphate kinase, and class II fructose-1,6-bisphosphate aldolase. J. Bacteriol. 2012, 194, 3088–3097. [Google Scholar] [CrossRef]
- Williams, T.J.; Allen, M.A.; DeMaere, M.Z.; Kyrpides, N.C.; Tringe, S.G.; Woyke, T.; Cavicchioli, R. Microbial ecology of an Antarctic hypersaline lake: Genomic assessment of ecophysiology among dominant haloarchaea. ISME J. 2014, 8, 1645–1658. [Google Scholar] [CrossRef]
- Nobelmann, B.; Lengeler, J.W. Molecular analysis of the gat genes from Escherichia coli and of their roles in galactitol transport and metabolism. J. Bacteriol. 1996, 178, 6790–6795. [Google Scholar] [CrossRef]
- Taran, M. Utilization of petrochemical wastewater for the production of poly(3-hydroxybutyrate) by Haloarcula sp. IRU1. J. Hazard. Mater. 2011, 188, 26–28. [Google Scholar] [CrossRef] [PubMed]
- Morlino, M.S.; Serna García, R.; Savio, F.; Zampieri, G.; Morosinotto, T.; Treu, L.; Campanaro, S. Cupriavidus necator as a platform for polyhydroxyalkanoate production: An overview of strains, metabolism, and modeling approaches. Biotechnol. Adv. 2023, 69, 108264. [Google Scholar] [CrossRef]
- Butler, O.M.; Manzoni, S.; Warren, C.R. Community composition and physiological plasticity control microbial carbon storage across natural and experimental soil fertility gradients. ISME J. 2023, 17, 2259–2269. [Google Scholar] [CrossRef] [PubMed]
- Addagada, L.; Pathak, P.; Shahid, M.K.; Rout, P.R. Microbial polyhydroxyalkanoates (PHAs): A brief overview of their features, synthesis, and agro-industrial applications. In Advances in Agricultural and Industrial Microbiology; Nayak, S.K., Baliyarsingh, B., Mannazzu, I., Singh, A., Mishra, B.B., Eds.; Springer: Singapore, 2022; pp. 217–236. [Google Scholar] [CrossRef]
- Mitra, R.; Xu, T.; Xiang, H.; Han, J. Current developments on polyhydroxyalkanoates synthesis by using halophiles as a promising cell factory. Microb. Cell Fact. 2020, 19, 86. [Google Scholar] [CrossRef]
- Müller, M.C.; Lemaire, O.N.; Kurth, J.M.; Welte, C.U.; Wagner, T. Differences in regulation mechanisms of glutamine synthetases from methanogenic archaea unveiled by structural investigations. Commun. Biol. 2024, 7, 111. [Google Scholar] [CrossRef]
- Beckers, G.; Bendt, A.K.; Krämer, R.; Burkovski, A. Molecular identification of the urea uptake system and transcriptional analysis of urea transporter- and urease-encoding genes in Corynebacterium glutamicum. J. Bacteriol. 2004, 186, 7645–7652. [Google Scholar] [CrossRef]
- Gadda, G.; Francis, K. Nitronate monooxygenase, a model for anionic flavin semiquinone intermediates in oxidative catalysis. Arch. Biochem. Biophys. 2010, 493, 53–61. [Google Scholar] [CrossRef]
- Herrero, A.; Flores, E.; Imperial, J. Nitrogen assimilation in bacteria. In Encyclopedia of Microbiology, 4th ed.; Schmidt, T.M., Ed.; Academic Press: Oxford, UK, 2019; pp. 280–300. [Google Scholar] [CrossRef]
- Seelmann, C.; Willistein, M.; Heider, J.; Boll, M. Tungstoenzymes: Occurrence, catalytic diversity and cofactor synthesis. Inorganics 2020, 8, 44. [Google Scholar] [CrossRef]
- Selvaraj, M.K.; Thakur, A.; Kumar, M.; Pinnaka, A.K.; Suri, C.R.; Siddhardha, B.; Elumalai, S.P. Ion-pumping microbial rhodopsin protein classification by machine learning approach. BMC Bioinform. 2023, 24, 29. [Google Scholar] [CrossRef]
- Zhang, H.; Fang, H.; Liu, D.; Zhang, Y.; Adu-Amankwaah, J.; Yuan, J.; Tan, R.; Zhu, J. Applications and challenges of rhodopsin-based optogenetics in biomedicine. Front. Neurosci. 2022, 16, 966772. [Google Scholar] [CrossRef]
- Tu, W.; Saeed, H.; Huang, W.E. Rhodopsin-based light-harvesting system for sustainable synthetic biology. Microb. Biotechnol. 2024, 17, e14521. [Google Scholar] [CrossRef]
- Roberts, M.F. Osmoadaptation and osmoregulation in archaea: Update 2004. Front. Biosci. 2004, 9, 1999–2019. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Walsh, D.A.; Bapteste, E.; Rodriguez-Valera, F.; Ford Doolittle, W.; Papke, R.T. Evolution of rhodopsin ion pumps in haloarchaea. BMC Evol. Biol. 2007, 7, 79. [Google Scholar] [CrossRef] [PubMed]
- Maksaev, G.; Haswell, E.S. Recent characterizations of MscS and its homologs provide insight into the basis of ion selectivity in mechanosensitive channels. Channels 2013, 7, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Vandrich, J.; Pfeiffer, F.; Alfaro-Espinoza, G.; Kunte, H.J. Contribution of mechanosensitive channels to osmoadaptation and ectoine excretion in Halomonas elongata. Extremophiles 2020, 24, 421–432. [Google Scholar] [CrossRef]
- Gunde-Cimerman, N.; Plemenitaš, A.; Oren, A. Strategies of adaptation of microorganisms of the three domains of life to high salt concentrations. FEMS Microbiol. Rev. 2018, 42, 353–375. [Google Scholar] [CrossRef]
- Youssef, N.H.; Savage-Ashlock, K.N.; McCully, A.L.; Luedtke, B.; Shaw, E.I.; Hoff, W.D.; Elshahed, M.S. Trehalose/2-sulfotrehalose biosynthesis and glycine-betaine uptake are widely spread mechanisms for osmoadaptation in the Halobacteriales. ISME J. 2014, 8, 636–649. [Google Scholar] [CrossRef]
- Fichman, Y.; Gerdes, S.Y.; Kovács, H.; Szabados, L.; Zilberstein, A.; Csonka, L.N. Evolution of proline biosynthesis: Enzymology, bioinformatics, genetics, and transcriptional regulation. Biol. Rev. Camb. Philos. Soc. 2015, 90, 1065–1099. [Google Scholar] [CrossRef]
- Østerås, M.; Boncompagni, E.; Vincent, N.; Poggi, M.C.; Le Rudulier, D. Presence of a gene encoding choline sulfatase in Sinorhizobium meliloti bet operon: Choline-O-sulfate is metabolized into glycine betaine. Proc. Natl. Acad. Sci. USA 1998, 95, 11394–11399. [Google Scholar] [CrossRef]
- Peters, P.; Galinski, E.A.; Trüper, H.G. The biosynthesis of ectoine. FEMS Microbiol. Lett. 1990, 71, 157–162. [Google Scholar] [CrossRef]
- Czech, L.; Hermann, L.; Stöveken, N.; Richter, A.A.; Höppner, A.; Smits, S.H.J.; Heider, J.; Bremer, E. Role of the extremolytes ectoine and hydroxyectoine as stress protectants and nutrients: Genetics, phylogenomics, biochemistry, and structural analysis. Genes 2018, 9, 177. [Google Scholar] [CrossRef] [PubMed]
- Richter, A.A.; Mais, C.N.; Czech, L.; Geyer, K.; Hoeppner, A.; Smits, S.H.J.; Erb, T.J.; Bange, G.; Bremer, E. Biosynthesis of the stress-protectant and chemical chaperon ectoine: Biochemistry of the transaminase EctB. Front Microbiol. 2019, 10, 2811. [Google Scholar] [CrossRef] [PubMed]
- Nunes-Costa, D.; Maranha, A.; Costa, M.; Alarico, S.; Empadinhas, N. Glucosylglycerate metabolism, bioversatility and mycobacterial survival. Glycobiology 2017, 27, 213–227. [Google Scholar] [CrossRef]
- Becker, E.A.; Seitzer, P.M.; Tritt, A.; Larsen, D.; Krusor, M.; Yao, A.I.; Wu, D.; Madern, D.; Eisen, J.A.; Darling, A.E.; et al. Phylogenetically driven sequencing of extremely halophilic archaea reveals strategies for static and dynamic osmo-response. PLoS Genet. 2014, 1, e1004784. [Google Scholar] [CrossRef]
- Li, J.; Zhou, J.; Wu, Y.; Yang, S.; Tian, D. GC-content of synonymous codons profoundly influences amino acid usage. G3 Genes Genomes Genet. 2015, 5, 2027–2036. [Google Scholar] [CrossRef]
- Matarredona, L.; Camacho, M.; Zafrilla, B.; Bonete, M.J.; Esclapez, J. The role of stress proteins in haloarchaea and their adaptive response to environmental shifts. Biomolecules 2020, 10, 1390. [Google Scholar] [CrossRef]
- Jones, D.L.; Baxter, B.K. DNA repair and photoprotection: Mechanisms of overcoming environmental ultraviolet radiation exposure in halophilic archaea. Front. Microbiol. 2017, 8, 1882. [Google Scholar] [CrossRef]
- White, M.F.; Allers, T. DNA repair in the archaea—An emerging picture. FEMS Microbiol. Rev. 2018, 42, 514–526. [Google Scholar] [CrossRef]
- Hogrel, G.; Lu, Y.; Alexandre, N.; Bossé, A.; Dulermo, R.; Ishino, S.; Ishino, Y.; Flament, D. Role of RadA and DNA polymerases in recombination-associated DNA synthesis in hyperthermophilic archaea. Biomolecules 2020, 10, 1045. [Google Scholar] [CrossRef]
- Krokan, H.E.; Bjørås, M. Base excision repair. Cold Spring Harb. Perspect. Biol. 2013, 5, a012583. [Google Scholar] [CrossRef]
- Denver, D.R.; Swenson, S.L.; Lynch, M. An evolutionary analysis of the helix-hairpin-helix superfamily of DNA repair glycosylases. Mol. Biol. Evol. 2003, 20, 1603–1611. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell. Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef]
- Gleason, F.K.; Holmgren, A. Thioredoxin and related proteins in procaryotes. FEMS Microbiol. Rev. 1988, 4, 271–297. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Holmgren, A. The thioredoxin antioxidant system. Free Radic. Biol. Med. 2014, 66, 75–87. [Google Scholar] [CrossRef]
- Grininger, M.; Staudt, H.; Johansson, P.; Wachtveitl, J.; Oesterhelt, D. Dodecin is the key player in flavin homeostasis of archaea. J. Biol. Chem. 2009, 284, 13068–13076. [Google Scholar] [CrossRef] [PubMed]
- Giani, M.; Pire, C.; Martínez-Espinosa, R.M. Bacterioruberin: Biosynthesis, antioxidant activity, and therapeutic applications in cancer and immune pathologies. Mar. Drugs 2024, 22, 167. [Google Scholar] [CrossRef]
- Guerra, J.P.L.; Penas, D.; Tavares, P.; Pereira, A.S. Influence of cupric (Cu2+) ions on the iron oxidation mechanism by DNA-binding protein from starved cells (Dps) from Marinobacter nauticus. Int. J. Mol. Sci. 2023, 24, 10256. [Google Scholar] [CrossRef]
- Bai, L.; Xie, T.; Hu, Q.; Deng, C.; Zheng, R.; Chen, W. Genome-wide comparison of ferritin family from Archaea, Bacteria, Eukarya, and Viruses: Its distribution, characteristic motif, and phylogenetic relationship. Sci. Nat. 2015, 102, 64. [Google Scholar] [CrossRef]
- Diamant, S.; Goloubinoff, P. Temperature-controlled activity of DnaK-DnaJ-GrpE chaperones: Protein-folding arrest and recovery during and after heat shock depends on the substrate protein and the GrpE concentration. Biochemistry 1998, 37, 9688–9694. [Google Scholar] [CrossRef]
- Motohashi, K.; Watanabe, Y.; Yohda, M.; Yoshida, M. Heat-inactivated proteins are rescued by the DnaK.J-GrpE set and ClpB chaperones. Proc. Natl. Acad. Sci. USA 1999, 96, 7184–7189. [Google Scholar] [CrossRef]
- Saini, A.; Kumar, A.; Singh, G.; Giri, S. Survival strategies and stress adaptations in halophilic archaebacteria. In Microbial Stress Response: Mechanisms and Data Science; Dhiman, S.S., Gnimpieba, E.Z., Gadhamshetty, V., Eds.; American Chemical Society: Washington, DC, USA, 2023; pp. 1–21. [Google Scholar] [CrossRef]
- Lim, S.; Glover, D.J.; Clark, D.S. Prefoldins in Archaea. In Prefoldins: The New Chaperones; Djouder, N., Ed.; Springer Nature: Cham, Switzerland, 2018; pp. 11–23. [Google Scholar] [CrossRef]
- Bhowmick, A.; Bhakta, K.; Roy, M.; Gupta, S.; Das, J.; Samanta, S.; Patranabis, S.; Ghosh, A. Heat shock response in Sulfolobus acidocaldarius and first implications for cross-stress adaptation. Res. Microbiol. 2023, 174, 104106. [Google Scholar] [CrossRef]
- Anchal; Kaushik, V.; Goel, M. Distribution of peptidyl-prolyl isomerase (PPIase) in the Archaea. Front. Microbiol. 2021, 12, 751049. [Google Scholar] [CrossRef]
- Pazicky, S.; Werle, A.A.; Lei, J.; Löw, C.; Weininger, U. Impact of distant peptide substrate residues on enzymatic activity of SlyD. Cell. Mol. Life Sci. 2022, 79, 138. [Google Scholar] [CrossRef] [PubMed]
- Phadtare, S.; Severinov, K. RNA remodeling and gene regulation by cold shock proteins. RNA Biol. 2010, 7, 788–795. [Google Scholar] [CrossRef]
- Keto-Timonen, R.; Hietala, N.; Palonen, E.; Hakakorpi, A.; Lindström, M.; Korkeala, H. Cold shock proteins: A minireview with special emphasis on Csp-family of enteropathogenic Yersinia. Front. Microbiol. 2016, 7, 1151. [Google Scholar] [CrossRef]
- Maier, L.K.; Alkhnbashi, O.S.; Backofen, R.; Marchfelder, A. CRISPR and salty: CRISPR-Cas systems in haloarchaea. In RNA Metabolism and Gene Expression in Archaea; Clouet-d’Orval, B., Ed.; Springer: Cham, Switzerland, 2017; pp. 243–269. [Google Scholar] [CrossRef]
- Pu, T.; Mei, Z.; Zhang, W.; Liang, W.J.; Zhou, X.; Liang, J.; Deng, Z.; Wang, Z. An in vitro DNA phosphorothioate modification reaction. Mol. Microbiol. 2020, 113, 452–463. [Google Scholar] [CrossRef]
- Castillo, R.; Saier, M.H. Functional promiscuity of homologues of the bacterial ArsA ATPases. Int. J. Microbiol. 2010, 2010, 187373. [Google Scholar] [CrossRef]
- Ben Fekih, I.; Zhang, C.; Li, Y.P.; Zhao, Y.; Alwathnani, H.A.; Saquib, Q.; Rensing, C.; Cervantes, C. Distribution of arsenic resistance genes in prokaryotes. Front. Microbiol. 2018, 9, 2473. [Google Scholar] [CrossRef]
- Firrincieli, A.; Presentato, A.; Favoino, G.; Marabottini, R.; Allevato, E.; Stazi, S.R.; Scarascia Mugnozza, G.; Harfouche, A.; Petruccioli, M.; Turner, R.J.; et al. Identification of resistance genes and response to arsenic in Rhodococcus aetherivorans BCP1. Front. Microbiol. 2019, 10, 888. [Google Scholar] [CrossRef]
- Markossian, K.A.; Kurganov, B.I. Copper chaperones, intracellular copper trafficking proteins. Function, structure, and mechanism of action. Biochemistry 2003, 68, 827–837. [Google Scholar] [CrossRef]
- Ettema, T.J.G.; Brinkman, A.B.; Lamers, P.P.; Kornet, N.G.; de Vos, W.M.; van der Oost, J. Molecular characterization of a conserved archaeal copper resistance (cop) gene cluster and its copper-responsive regulator in Sulfolobus solfataricus P2. Microbiology 2006, 152, 1969–1979. [Google Scholar] [CrossRef] [PubMed]
- Gräff, M.; Buchholz, P.C.F.; Le Roes-Hill, M.; Pleiss, J. Multicopper oxidases: Modular structure, sequence space, and evolutionary relationships. Proteins 2020, 88, 1329–1339. [Google Scholar] [CrossRef] [PubMed]
- Anton, A.; Große, C.; Reißmann, J.; Pribyl, T.; Nies, D.H. CzcD is a heavy metal ion transporter involved in regulation of heavy metal resistance in Ralstonia sp. strain CH34. J. Bacteriol. 1999, 181, 6876–6881. [Google Scholar] [CrossRef] [PubMed]
- Noll, M.; Lutsenko, S. Expression of ZntA, a zinc-transporting P1-type ATPase, is specifically regulated by zinc and cadmium. IUBMB Life 2000, 49, 297–302. [Google Scholar] [CrossRef]
- Straková, D.; Sánchez-Porro, C.; de la Haba, R.R.; Ventosa, A. Decoding the genomic profile of the Halomicroarcula genus: Comparative analysis and characterization of two novel species. Microorganisms 2024, 12, 334. [Google Scholar] [CrossRef]
- Galisteo, C.; Puente-Sánchez, F.; de la Haba, R.R.; Bertilsson, S.; Sánchez-Porro, C.; Ventosa, A. Metagenomic insights into the prokaryotic communities of heavy metal-contaminated hypersaline soils. Sci. Total. Environ. 2024, 951, 175497. [Google Scholar] [CrossRef]
- Straková, D.; Sánchez-Porro, C.; de la Haba, R.R.; Ventosa, A. Unveiling the genomic landscape and adaptive mechanisms of the haloarchaeal genus Halogeometricum: Spotlight on thiamine biosynthesis. Front. Mar. Sci. 2024, 11, 1421769. [Google Scholar] [CrossRef]
- Ordoñez, O.F.; Rasuk, M.C.; Soria, M.N.; Contreras, M.; Farías, M.E. Haloarchaea from the Andean Puna: Biological role in the energy metabolism of arsenic. Microb. Ecol. 2018, 76, 695–705. [Google Scholar] [CrossRef]
- Tavoosi, N.; Akhavan Sepahi, A.; Amoozegar, M.A.; Kiarostami, V. Toxic heavy metal/oxyanion tolerance in haloarchaea from some saline and hypersaline ecosystems. J. Basic. Microbiol. 2023, 63, 558–569. [Google Scholar] [CrossRef]
- Salgaonkar, B.; Das, D.; Braganca, J. Resistance of extremely halophilic archaea to zinc and zinc oxide nanoparticles. Appl. Nanosci. 2016, 6, 251–258. [Google Scholar] [CrossRef]
- Amoozegar, M.A.; Hamedi, J.; Dadashipour, M.; Shariatpanahi, S. Effect of salinity on the tolerance to toxic metals and oxyanions in native moderately halophilic spore-forming bacilli. World J. Microbiol. Biotechnol. 2005, 21, 1237–1243. [Google Scholar] [CrossRef]
- Al-Mailem, D.M.; Eliyas, M.; Radwan, S.S. Ferric sulfate and proline enhance heavy-metal tolerance of halophilic/halotolerant soil microorganisms and their bioremediation potential for spilled-oil under multiple stresses. Front. Microbiol. 2018, 9, 394. [Google Scholar] [CrossRef] [PubMed]
- Lawhorn, B.G.; Mehl, R.A.; Begley, T.P. Biosynthesis of the thiamin pyrimidine: The reconstitution of a remarkable rearrangement reaction. Org. Biomol. Chem. 2004, 2, 2538–2546. [Google Scholar] [CrossRef] [PubMed]
- Rodionov, D.A.; Leyn, S.A.; Li, X.; Rodionova, I.A. A novel transcriptional regulator related to thiamine phosphate synthase controls thiamine metabolism genes in archaea. J. Bacteriol. 2017, 199, e00743–16. [Google Scholar] [CrossRef]
- Hayashi, M.; Kobayashi, K.; Esaki, H.; Konno, H.; Akaji, K.; Tazuya, K.; Yamada, K.; Nakabayashi, T.; Nosaka, K. Enzymatic and structural characterization of an archaeal thiamine phosphate synthase. Biochim. Biophys. Acta 2014, 1844, 803–809. [Google Scholar] [CrossRef]
- Hwang, S.; Cordova, B.; Abdo, M.; Pfeiffer, F.; Maupin-Furlow, J.A. ThiN as a versatile domain of transcriptional repressors and catalytic enzymes of thiamine biosynthesis. J. Bacteriol. 2017, 199, e00810–16. [Google Scholar] [CrossRef]
- Maupin-Furlow, J.A. Vitamin B1 (Thiamine) metabolism and regulation in archaea. In B Group Vitamins-Current Uses and Perspectives; LeBlanc, J.G., de Giori, G.S., Eds.; IntechOpen: London, UK, 2018; pp. 9–31. [Google Scholar] [CrossRef]
- Jurgenson, C.T.; Begley, T.P.; Ealick, S.E. The structural and biochemical foundations of thiamin biosynthesis. Annu. Rev. Biochem. 2009, 78, 569–603. [Google Scholar] [CrossRef]
- Hwang, S.; Cordova, B.; Chavarria, N.; Elbanna, D.; McHugh, S.; Rojas, J.; Pfeiffer, F.; Maupin-Furlow, J.A. Conserved active site cysteine residue of archaeal THI4 homolog is essential for thiamine biosynthesis in Haloferax volcanii. BMC Microbiol. 2014, 14, 260. [Google Scholar] [CrossRef]
- Bettendorff, L.; Wins, P. Thiamine diphosphate in biological chemistry: New aspects of thiamine metabolism, especially triphosphate derivatives acting other than as cofactors. FEBS J. 2009, 276, 2917–2925. [Google Scholar] [CrossRef]
- Bettendorff, L. Update on thiamine triphosphorylated derivatives and metabolizing enzymatic complexes. Biomolecules 2021, 11, 1645. [Google Scholar] [CrossRef]
- Jenkins, A.H.; Schyns, G.; Potot, S.; Sun, G.; Begley, T.P. A new thiamin salvage pathway. Nat. Chem. Biol. 2007, 3, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Begley, T.P.; Downs, D.M.; Ealick, S.E.; McLafferty, F.W.; Van Loon, A.P.; Taylor, S.; Campobasso, N.; Chiu, H.J.; Kinsland, C.; Reddick, J.J.; et al. Thiamin biosynthesis in prokaryotes. Arch. Microbiol. 1999, 171, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Martínez Martínez, G.; Pire, C.; Martínez-Espinosa, R.M. Hypersaline environments as natural sources of microbes with potential applications in biotechnology: The case of solar evaporation systems to produce salt in Alicante County (Spain). Curr. Res. Microb. Sci. 2022, 3, 100136. [Google Scholar] [CrossRef]
- Mrowicka, M.; Mrowicki, J.; Dragan, G.; Majsterek, I. The importance of thiamine (vitamin B1) in humans. Biosci. Rep. 2023, 43, BSR20230374. [Google Scholar] [CrossRef]
- Tylicki, A.; Łotowski, Z.; Siemieniuk, M.; Ratkiewicz, A. Thiamine and selected thiamine antivitamins—Biological activity and methods of synthesis. Biosci. Rep. 2018, 38, BSR20171148. [Google Scholar] [CrossRef]



| Species | MIC (mM) of: | ||||
|---|---|---|---|---|---|
| As5+ | Cd2+ | Cu2+ | Pb2+ | Zn2+ | |
| Haloarcula terrestris S1AR25-5AT | >700 | 80 | 4 | 10 | 1 |
| Haloarcula saliterrae S1CR25-12T | >700 | 10 | 5 | 10 | 1 |
| Haloarcula onubensis S3CR25-11T | 500 | 50 | 20 | 10 | 1 |
| Natrinema salsiterrestre S1CR25-10T | >700 | 50 | 20 | 10 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Straková, D.; Sánchez-Porro, C.; de la Haba, R.R.; Ventosa, A. Strategies of Environmental Adaptation in the Haloarchaeal Genera Haloarcula and Natrinema. Microorganisms 2025, 13, 761. https://doi.org/10.3390/microorganisms13040761
Straková D, Sánchez-Porro C, de la Haba RR, Ventosa A. Strategies of Environmental Adaptation in the Haloarchaeal Genera Haloarcula and Natrinema. Microorganisms. 2025; 13(4):761. https://doi.org/10.3390/microorganisms13040761
Chicago/Turabian StyleStraková, Dáša, Cristina Sánchez-Porro, Rafael R. de la Haba, and Antonio Ventosa. 2025. "Strategies of Environmental Adaptation in the Haloarchaeal Genera Haloarcula and Natrinema" Microorganisms 13, no. 4: 761. https://doi.org/10.3390/microorganisms13040761
APA StyleStraková, D., Sánchez-Porro, C., de la Haba, R. R., & Ventosa, A. (2025). Strategies of Environmental Adaptation in the Haloarchaeal Genera Haloarcula and Natrinema. Microorganisms, 13(4), 761. https://doi.org/10.3390/microorganisms13040761







