Identification of Released Bacterial Extracellular Vesicles Containing Lpp20 from Helicobacter pylori
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
2.2. Generation of lpp20 Gene (HP1456) Disruption Strains
2.3. Preparation of H. pylori Cell Lysates
2.4. Preparation of H. pylori bEVs
2.5. Visualization and Quantitative Analysis of bEVs’ Size and Concentration
2.6. Immunoprecipitation
2.7. Immunoblotting
2.8. Surface Plasmon Resonance Imaging (SPRi) Analysis
3. Results
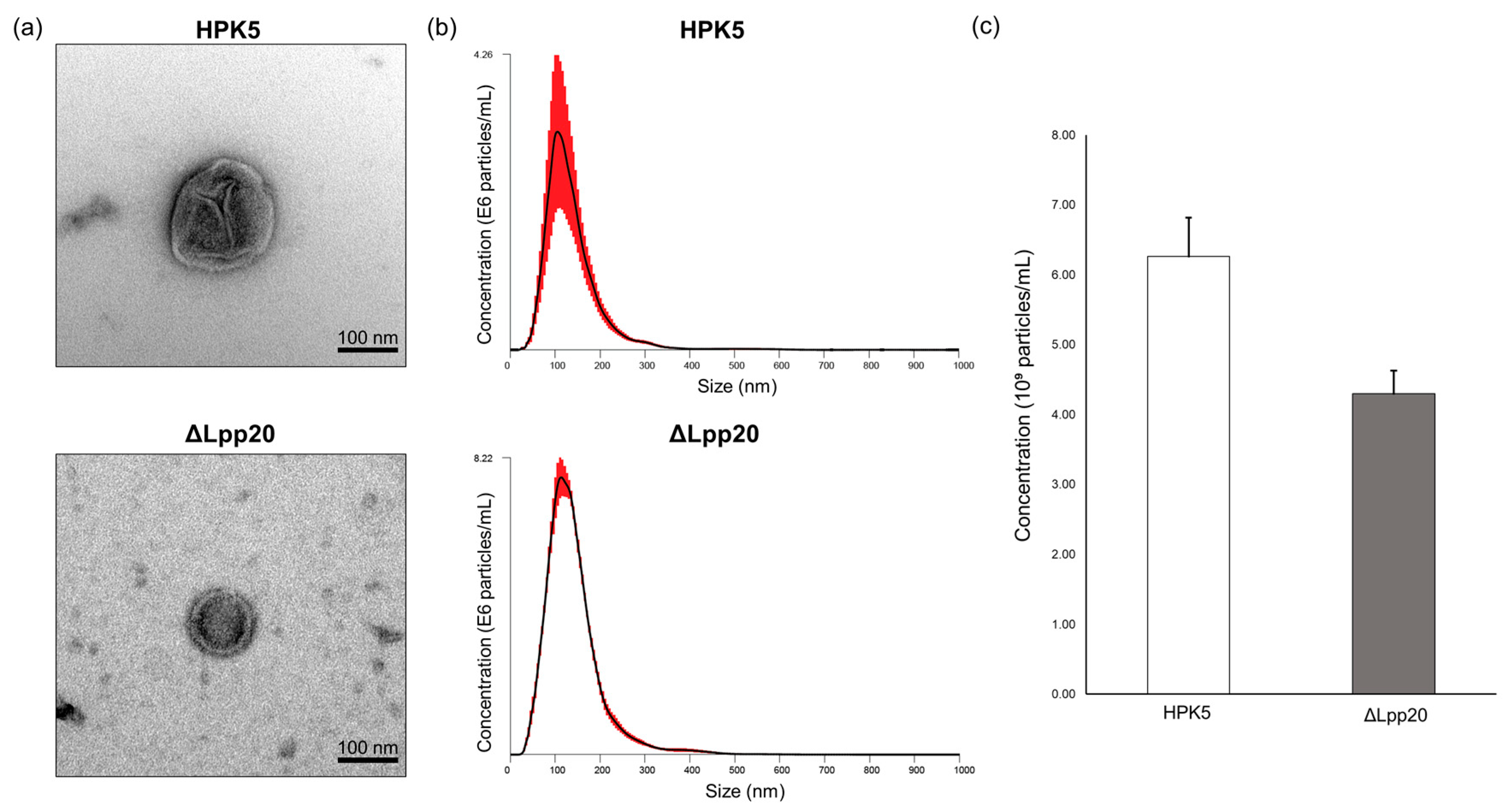
3.1. Identification and Characterization of bEVs of the Samples Used in This Study
3.2. Analysis of Lpp20 in Cell Lysates and bEVs from Seven Clinical H. pylori Strains and Two lpp20-Disrupted Strains
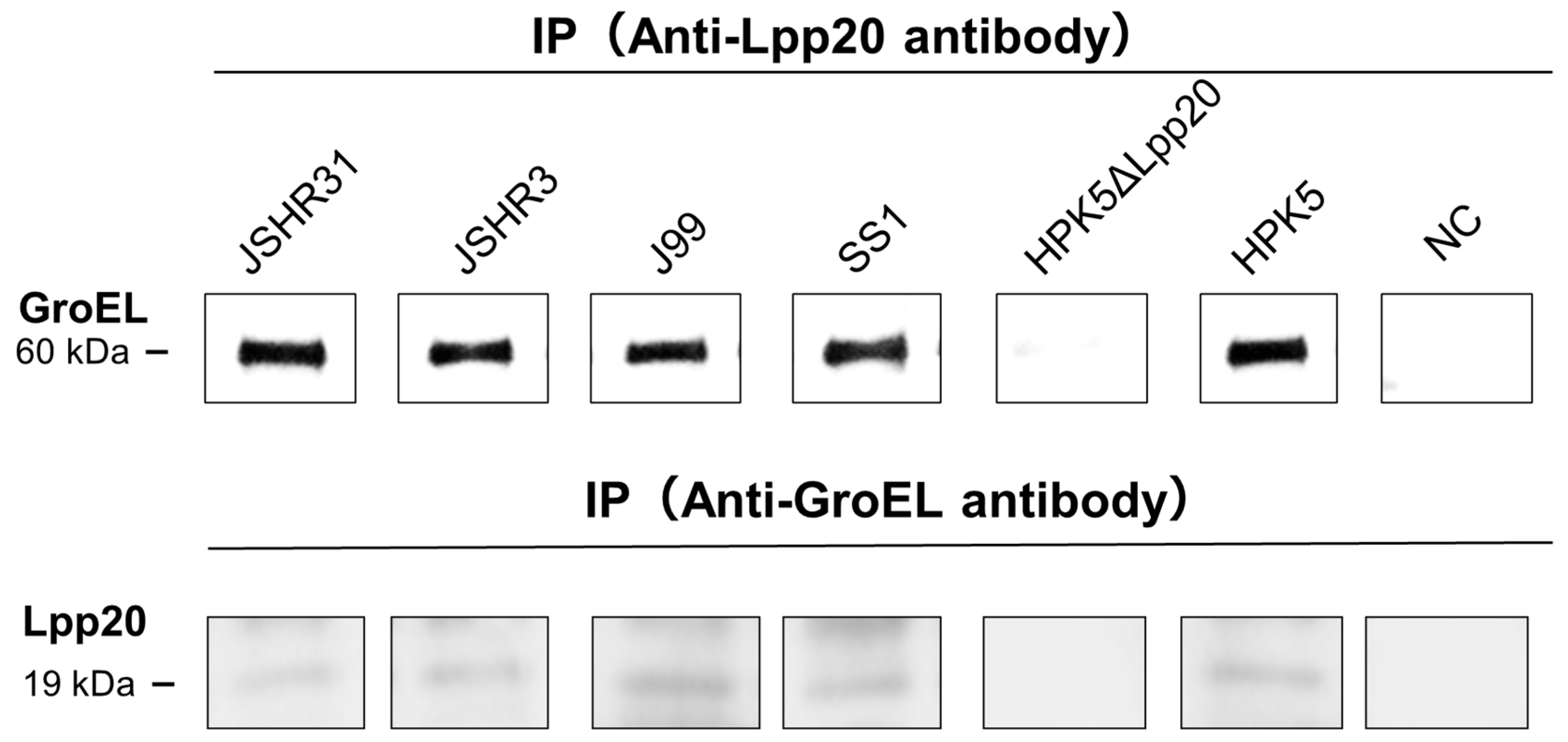
3.3. IP–IB Analysis
3.4. SPRi Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Warren, J.R.; Marshall, B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet 1983, 321, 1273–1275. [Google Scholar]
- Fischbach, W.; Malfertheiner, P. Helicobacter pylori infection: When to eradicate, how to diagnose and treat. Dtsch. Arztebl. Int. 2018, 115, 429–436. [Google Scholar] [PubMed]
- Takeuchi, H.; Okamoto, A. Helicobacter pylori Infection and Chronic Immune Thrombocytopenia. J. Clin. Med. 2022, 11, 4822. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, A.; Ueda, K.; Nishiumi, S.; Murata-Kamiya, N.; Mukai, S.A.; Sawada, S.I.; Azuma, T.; Hatakeyama, M.; Akiyoshi, K. Exosomes as nanocarriers for systemic delivery of the Helicobacter pylori virulence factor CagA. Sci. Rep. 2016, 6, 18346–18351. [Google Scholar]
- Qiang, L.; Hu, J.; Tian, M.; Li, Y.; Ren, C.; Deng, Y.; Jiang, Y. Extracellular vesicles from helicobacter pylori-infected cells and helicobacter pylori outer membrane vesicles in atherosclerosis. Helicobacter 2022, 27, e12877. [Google Scholar] [CrossRef]
- Wang, F.; Yao, Z.; Jin, T.; Mao, B.; Shao, S.; Shao, C. Research progress on Helicobacter pylori infection related neurological diseases. Ageing Res. Rev. 2024, 99, 102399. [Google Scholar]
- Zawada, A.E.; Naskręt, D.; Piłaciński, S.; Adamska, A.; Grzymisławski, M.; Eder, P.; Grzelka-Woźniak, A.; Zozulińska-Ziółkiewicz, D.; Dobrowolska, A. Helicobacter pylori infection is associated with increased accumulation of advanced glycation end products in the skin in patients with type 1 diabetes: A preliminary study. Adv. Clin. Exp. Med. 2023, 32, 1009–1016. [Google Scholar]
- Ye, J.; Feng, T.; Su, L.; Li, J.; Gong, Y.; Ma, X. Interactions between Helicobacter pylori infection and host metabolic homeostasis: A comprehensive review. Helicobacter 2023, 28, e13030. [Google Scholar]
- Pellicano, R.; Ianiro, G.; Fagoonee, S.; Settanni, C.R.; Gasbarrini, A. Review: Extragastric diseases and Helicobacter pylori. Helicobacter 2020, 25, e12741. [Google Scholar] [CrossRef]
- Pérez-Cano, H.J.; Ceja-Martínez, J.; Tellezgiron-Lara, V.; Voorduin-Ramos, S.; Morales-López, O.; Somilleda-Ventura, S.A. Relationship between Helicobacter pylori and undifferentiated non-granulomatous anterior uveitis. Infection 2023, 51, 765–768. [Google Scholar] [CrossRef]
- de Korwin, J.; Ianiro, G.; Gibiino, G.; Gasbarrini, A. Helicobacter pylori infection and extragastric diseases in 2017. Helicobacter 2017, 22, e12411. [Google Scholar]
- Takahashi, T.; Yujiri, T.; Shinohara, K.; Inoue, Y.; Sato, Y.; Fujii, Y.; Okubo, M.; Zaitsu, Y.; Ariyoshi, K.; Nakamura, Y.; et al. Molecular mimicry by Helicobacter pylori CagA protein may be involved in the pathogenesis of H. pylori-associated chronic idiopathic thrombocytopenic purpura. Br. J. Haematol. 2004, 124, 91–96. [Google Scholar] [PubMed]
- Kashiwagi, H.; Kuwana, M.; Hato, T.; Takafuta, T.; Fujimura, K.; Kurata, Y.; Murata, M.; Tomiyama, Y. Reference guide for management of adult immune thrombocytopenia in Japan: 2019 Revision. Int. J. Hematol. 2020, 111, 329–351. [Google Scholar] [PubMed]
- Fujimura, K.; Kuwana, M.; Kurata, Y.; Imamura, M.; Harada, H.; Sakamaki, H.; Teramura, M.; Koda, K.; Nomura, S.; Sugihara, S.; et al. Is eradication therapy useful as the first line of treatment in Helicobacter pylori-positive idiopathic thrombocytopenic purpura? Analysis of 207 eradicated chronic ITP cases in Japan. Int. J. Hematol. 2005, 81, 162–168. [Google Scholar]
- Takeuchi, H.; Islam, J.M.; Kaneko, A.; Kimura, A.; Shida, T.; Oboshi, W.; Katayama, H.; Oishi, T.; Fujieda, M.; Morimoto, N. Helicobacter pylori protein that binds to and activates platelet specifically reacts with sera of H. pylori-associated chronic immune thrombocytopenia. Platelets 2021, 32, 1120–1123. [Google Scholar] [CrossRef]
- Morimoto, N.; Takeuchi, H.; Takahashi, T.; Ueta, T.; Tanizawa, Y.; Kumon, Y.; Kobayashi, M.; Sugiura, T. Helicobacter pylori-associated chronic idiopathic thrombocytopenic purpura and low molecular weight H. pylori proteins. Scand. J. Infect. Dis. 2007, 39, 409–416. [Google Scholar]
- Bakos, N.; Fekete, B.; Prohászka, Z.; Füst, G.; Kalabay, L. High prevalence of IgG and IgA antibodies to 19-kDa Helicobacter pylori-associated lipoprotein in chronic urticaria. Allergy 2003, 58, 663–667. [Google Scholar]
- Welsh, J.A.; Goberdhan, D.C.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.; Erdbrügger, U.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar]
- Zavan, L.; Bitto, N.J.; Johnston, E.L.; Greening, D.W.; Kaparakis-Liaskos, M. Helicobacter pylori Growth Stage Determines the Size; Protein Composition; and Preferential Cargo Packaging of Outer Membrane Vesicles. Proteomics 2019, 19, e1800209. [Google Scholar]
- Wei, S.; Li, X.; Wang, J.; Wang, Y.; Zhang, C.; Dai, S.; Wang, X.; Deng, X.; Zhao, L.; Shan, B. Outer Membrane Vesicles Secreted by Helicobacter pylori Transmitting Gastric Pathogenic Virulence Factors. ACS Omega 2021, 7, 240–258. [Google Scholar]
- Parker, H.; Chitcholtan, K.; Hampton, M.B.; Keenan, J.I. Uptake of Helicobacter pylori outer membrane vesicles by gastric epithelial cells. Infect. Immun. 2010, 78, 5054–5061. [Google Scholar] [CrossRef] [PubMed]
- Jarzab, M.; Posselt, G.; Meisner-Kober, N.; Wessler, S. Helicobacter pylori-Derived Outer Membrane Vesicles (OMVs): Role in Bacterial Pathogenesis? Microorganisms 2020, 8, 1328. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Mandujano, A.; Hernández-Cortez, C.; Ibarra, J.A.; Castro-Escarpulli, G. The outer membrane vesicles: Secretion system type zero. Traffic 2017, 18, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Furuyama, N.; Sircili, M.P. Outer Membrane Vesicles (OMVs) Produced by Gram-Negative Bacteria: Structure, Functions, Biogenesis, and Vaccine Application. Biomed. Res. Int. 2021, 2021, 1490732. [Google Scholar] [CrossRef]
- Rueter, C.; Bielaszewska, M. Secretion and Delivery of Intestinal Pathogenic Escherichia coli Virulence Factors via Outer Membrane Vesicles. Front. Cell Infect. Microbiol. 2020, 10, 91. [Google Scholar] [CrossRef]
- Eletto, D.; Mentucci, F.; Voli, A.; Petrella, A.; Porta, A.; Tosco, A. Helicobacter pylori Pathogen-Associated Molecular Patterns: Friends or Foes? Int. J. Mol. Sci. 2022, 23, 3531. [Google Scholar] [CrossRef]
- Schwechheimer, C.; Kuehn, M.J. Outer-membrane vesicles from Gram-negative bacteria: Biogenesis and functions. Nat. Rev. Microbiol. 2015, 13, 605–619. [Google Scholar] [CrossRef]
- Parker, H.; Keenan, J.I. Composition and function of Helicobacter pylori outer membrane vesicles. Microbes Infect. 2012, 14, 9–16. [Google Scholar] [CrossRef]
- Keenan, J.I.; Allardyce, R.A.; Bagshaw, P.F. Dual silver staining to characterise Helicobacter spp. outer membrane components. J. Immunol. Methods 1997, 209, 17–24. [Google Scholar] [CrossRef]
- Yokota, K.; Osaki, T.; Hayashi, S.; Yokota, S.I.; Takeuchi, H.; Rimbara, E.; Ojima, H.; Sato, T.; Yonezawa, H.; Shibayama, K.; et al. Establishment of a reference panel of Helicobacter pylori strains for antimicrobial susceptibility testing. Helicobacter 2022, 27, e12874. [Google Scholar] [CrossRef]
- Tomb, J.F.; White, O.; Kerlavage, A.R.; Clayton, R.A.; Sutton, G.G.; Fleischmann, R.D.; Ketchum, K.A.; Klenk, H.P.; Gill, S.; Dougherty, B.A.; et al. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 1997, 388, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Alm, R.A.; Ling, L.-S.L.; Moir, D.T.; King, B.L.; Brown, E.D.; Doig, P.C.; Smith, D.R.; Noonan, B.; Guild, B.C.; Dejonge, B.L.; et al. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature 1999, 397, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; O’Rourke, J.; De Ungria, M.; Robertson, B.; Daskalopoulos, G.; Dixon, M. A standardized mouse model of Helicobacter pylori infection: Introducing the Sydney strain. Gastroenterology 1997, 112, 1386–1397. [Google Scholar] [CrossRef] [PubMed]
- Karita, M.; Kouchiyama, T.; Okita, K.; Nakazawa, T. New small animal model for human gastric Helicobacter pylori infection: Success in both nude and euthymic mice. Am. J. Gastroenterol. 1991, 86, 1596–1603. [Google Scholar]
- Takeuchi, H.; Shirai, M.; Akada, J.K.; Tsuda, M.; Nakazawa, T. Nucleotide sequence and characterization of cdrA, a cell division-related gene of Helicobacter pylori. J. Bacteriol. 1998, 180, 5263–5268. [Google Scholar]
- Pasqua, M.; Zennaro, A.; Trirocco, R.; Fanelli, G.; Micheli, G.; Grossi, M.; Colonna, B.; Prosseda, G. Modulation of OMV Production by the Lysis Module of the DLP12 Defective Prophage of Escherichia coli K12. Microorganisms 2021, 9, 369. [Google Scholar] [CrossRef]
- Kosgodage, U.S.; Trindade, R.P.; Thompson, P.R.; Inal, J.M.; Lange, S. Chloramidine/Bisindolylmaleimide-I-Mediated Inhibition of Exosome and Microvesicle Release and Enhanced Efficacy of Cancer Chemotherapy. Int. J. Mol. Sci. 2017, 18, 1007. [Google Scholar] [CrossRef]
- Zhang, P.; Yeo, J.C.; Lim, C.T. Advances in Technologies for Purification and Enrichment of Extracellular Vesicles. SLAS Technol. 2019, 24, 477–488. [Google Scholar]
- Henriquez, T.; Santoro, F.; Medaglini, D.; Pallecchi, L.; Clemente, I.; Bonechi, C.; Magnani, A.; Paccagnini, E.; Gentile, M.; Lupetti, P.; et al. Analysis of the utility of a rapid vesicle isolation method for clinical strains of Pseudomonas aeruginosa. Microbiol. Spectr. 2024, 12, e0064924. [Google Scholar] [CrossRef]
- Palacios, E.; Lobos-González, L.; Guerrero, S.; Kogan, M.J.; Shao, B.; Heinecke, J.W.; Quest, A.F.; Leyton, L.; Valenzuela-Valderrama, M. Helicobacter pylori outer membrane vesicles induce astrocyte reactivity through nuclear factor-κappa B activation and cause neuronal damage in vivo in a murine model. J. Neuroinflammation 2023, 20, 66. [Google Scholar]
- Pernitzsch, S.R.; Alzheimer, M.; Bremer, B.U.; Robbe-Saule, M.; De Reuse, H.; Sharma, C.M. Small RNA mediated gradual control of lipopolysaccharide biosynthesis affects antibiotic resistance in Helicobacter pylori. Nat. Commun. 2021, 12, 4433. [Google Scholar]
- Jarque, I.; Andreu, R.; Llopis, I.; De la Rubia, J.; Gomis, F.; Senent, L.; Jiménez, C.; Martín, G.; Martínez, J.A.; Sanz, G.F.; et al. Absence of platelet response after eradication of Helicobacter pylori infection in patients with chronic idiopathic thrombocytopenic purpura. Br. J. Haematol. 2001, 115, 1002–1003. [Google Scholar] [PubMed]
- Michel, M.; Khellaf, M.; Desforges, L.; Lee, K.; Schaeffer, A.; Godeau, B.; Bierling, P. Autoimmune thrombocytopenic Purpura and Helicobacter pylori infection. Arch. Intern. Med. 2002, 162, 1033–1036. [Google Scholar]
- Michel, M.; Cooper, N.; Jean, C.; Frissora, C.; Bussel, J.B. Does Helicobater pylori initiate or perpetuate immune thrombocytopenic purpura? Blood 2004, 103, 890–896. [Google Scholar] [PubMed]
- Stasi, R.; Rossi, Z.; Stipa, E.; Amadori, S.; Newland, A.C.; Provan, D. Helicobacter pylori eradication in the management of patients with idiopathic thrombocytopenic purpura. Am. J. Med. 2005, 118, 414–419. [Google Scholar]
- Islam, J.M.; Yano, Y.; Okamoto, A.; Matsuda, R.; Shiraishi, M.; Hashimoto, Y.; Morita, N.; Takeuchi, H.; Suganuma, N.; Takeuchi, H. Evidence of Helicobacter pylori heterogeneity in human stomachs by susceptibility testing and characterization of mutations in drug-resistant isolates. Sci. Rep. 2024, 14, 12066. [Google Scholar]
- Chitcholtan, K.; Hampton, M.B.; Keenan, J.I. Outer membrane vesicles enhance the carcinogenic potential of Helicobacter pylori. Carcinogenesis 2008, 29, 2400–2405. [Google Scholar]
- Xia, X.; Zhang, L.; Chi, J.; Li, H.; Liu, X.; Hu, T.; Li, R.; Guo, Y.; Zhang, X.; Wang, H.; et al. Helicobacter pylori Infection Impairs Endothelial Function Through an Exosome-Mediated Mechanism. J. Am. Heart Assoc. 2020, 9, e014120. [Google Scholar]



| Bacterial Strain or Plasmid | Genotype or Characteristics α | Region | Reference |
|---|---|---|---|
| H. pylori | |||
| 26695 | Wild type | the United Kingdom | [31] |
| J99 | Wild type | the United States | [32] |
| SS1 | Wild type | Australia | [33] |
| HPK5 | Wild type | Japan | [34] |
| JSHR3 | Wild type | Japan | [30] |
| JSHR6 | Wild type | Japan | [30] |
| JSHR31 | Wild type | Japan | [30] |
| 26695ΔLpp20 | 26695 derivative; kan in lpp20; Kmr | This study | |
| HPK5ΔLpp20 | HPK5 derivative; kan in lpp20; Kmr | This study | |
| E. coli | |||
| DH5α | F− φ80dlacZΔM15 Δ(argF-lac)U169 deoR recA1 endA1 hsdR17(rK− mK+) supE44 thi-1 gyrA96 relA1 | GIBCO-BRL | |
| Plasmids | |||
| pGEM-Teasy | 3.0 kb cloning vector; Apr | Promega | |
| plpp20E-1 | lpp20 and its flanking ORFs (1.3 kb) of 26695 in pGEM-Teasy; Apr | This study | |
| plpp20E-2 | lpp20 and its flanking ORFs (1.3 kb) of HPK5 in pGEM-Teasy; Apr | This study | |
| plpp20E-km-1 | 1.3 kb kan in lpp20 of plpp20E-1; Apr, Kmr | This study | |
| plpp20E-km-2 | 1.3 kb kan in lpp20 of plpp20E-2; Apr, Kmr | This study |
| Target Genes | Primer Name | Sequence (5′-3′) |
|---|---|---|
| HP1455-HP1457 | 1457F-231 | TTCAGATGTGATTAACGACACC |
| 1455R-390 | CTCATTCATTAAAGCGACATGC | |
| lpp20 | 1456F-241Bam | TTGGATCCCTACTAACCAAGCTACAGCG |
| 1456R-224 | ATTAGTGATCAAATCTTCAGCC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okamoto, A.; Shibuta, T.; Morita, N.; Fujinuma, R.; Shiraishi, M.; Matsuda, R.; Okada, M.; Watanabe, S.; Umemura, T.; Takeuchi, H. Identification of Released Bacterial Extracellular Vesicles Containing Lpp20 from Helicobacter pylori. Microorganisms 2025, 13, 753. https://doi.org/10.3390/microorganisms13040753
Okamoto A, Shibuta T, Morita N, Fujinuma R, Shiraishi M, Matsuda R, Okada M, Watanabe S, Umemura T, Takeuchi H. Identification of Released Bacterial Extracellular Vesicles Containing Lpp20 from Helicobacter pylori. Microorganisms. 2025; 13(4):753. https://doi.org/10.3390/microorganisms13040753
Chicago/Turabian StyleOkamoto, Aoi, Tatsuki Shibuta, Nanaka Morita, Ryota Fujinuma, Masaya Shiraishi, Reimi Matsuda, Mayu Okada, Satoe Watanabe, Tsukuru Umemura, and Hiroaki Takeuchi. 2025. "Identification of Released Bacterial Extracellular Vesicles Containing Lpp20 from Helicobacter pylori" Microorganisms 13, no. 4: 753. https://doi.org/10.3390/microorganisms13040753
APA StyleOkamoto, A., Shibuta, T., Morita, N., Fujinuma, R., Shiraishi, M., Matsuda, R., Okada, M., Watanabe, S., Umemura, T., & Takeuchi, H. (2025). Identification of Released Bacterial Extracellular Vesicles Containing Lpp20 from Helicobacter pylori. Microorganisms, 13(4), 753. https://doi.org/10.3390/microorganisms13040753







