Induction of Immune Responses in Mice and Newborn Piglets by Oral Immunization with Recombinant Lactococcus lactis Expressing S1 and M Proteins of Porcine Epidemic Diarrhea Virus
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Experimental Animals
2.3. The Cultivation of Recombinant L. lactis
2.4. Immunization Protocol for Mice
2.5. Immunization Protocol for Piglets
2.6. Lymphocyte Proliferation Assay in Mice
2.7. Assay of Spleen Lymphocyte Subsets in Mice
2.8. Assay of Peripheral Blood Lymphocyte Subsets in Piglets
2.9. Assay of Cytokines IFN-γ and IL-4 in Piglets
2.10. Assay of Serum IgG Antibody in Piglets
2.11. Assay of Serum IgA Antibody in Piglets
2.12. Assay of SIgA Antibodies in the Intestinal Mucosa and Feces of Piglets
2.13. Statistical Analysis
3. Results
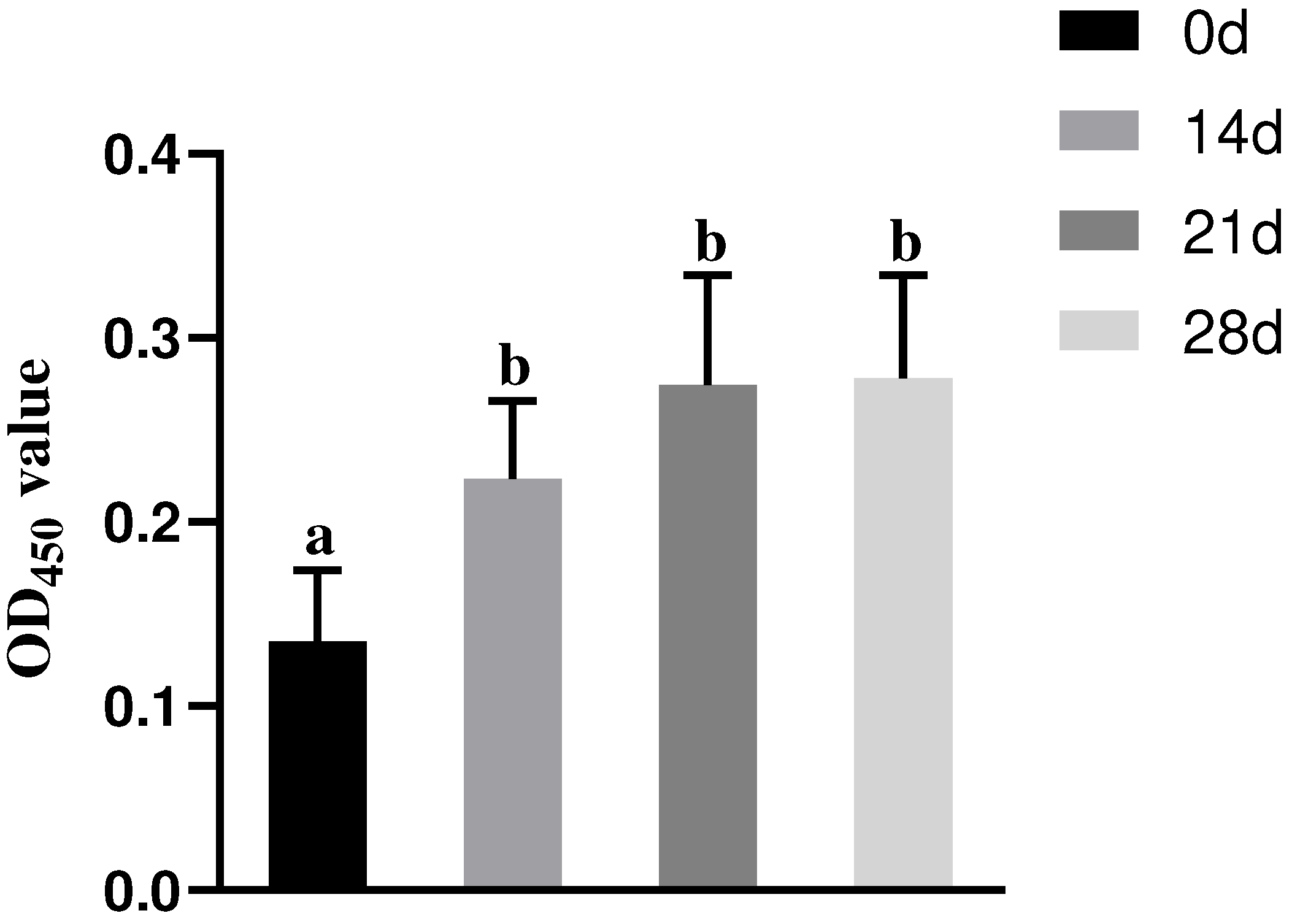
3.1. Results of Splenic Lymphocyte Proliferation Assay in Mice
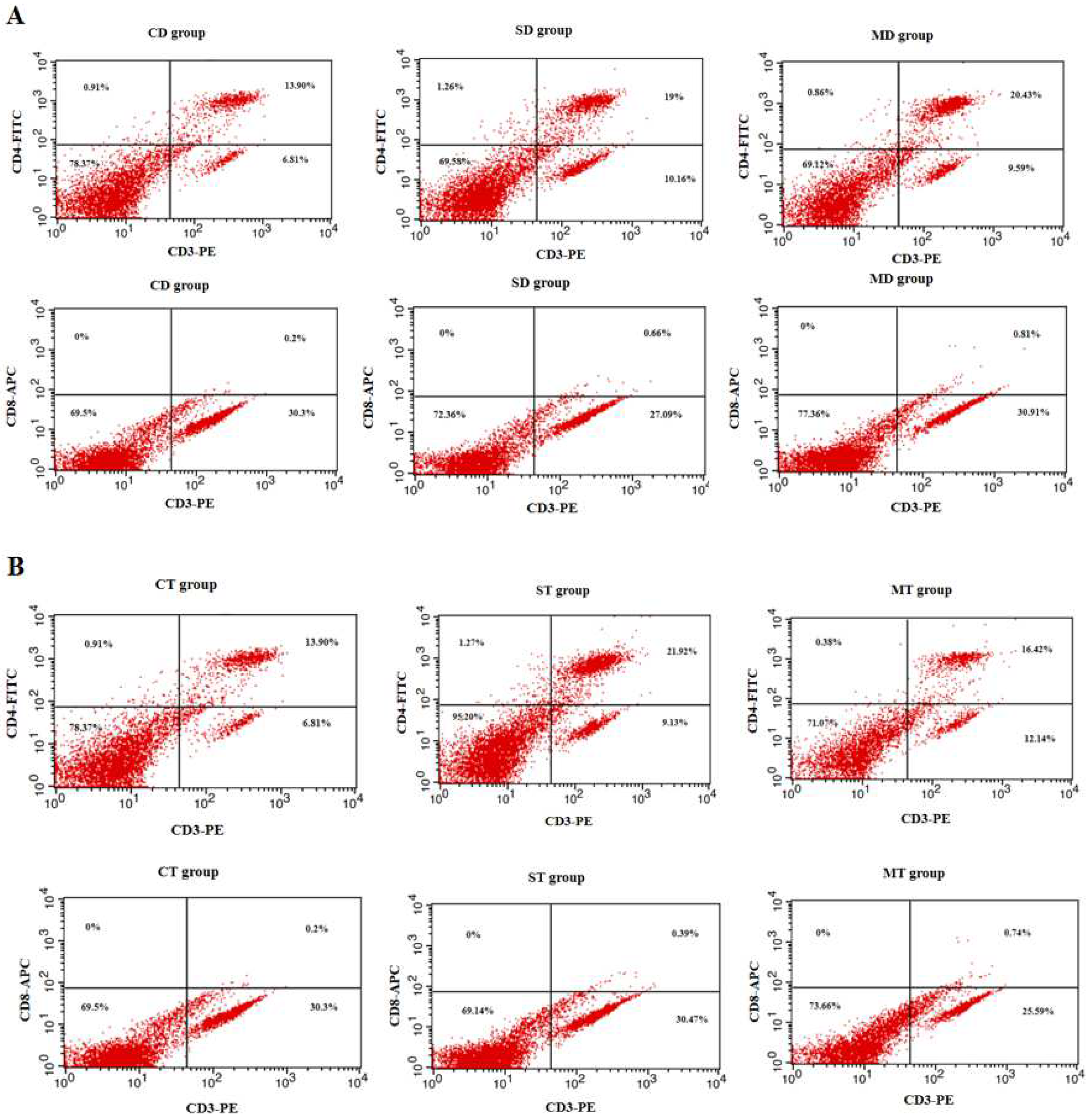
3.2. Results of Splenic Lymphocyte Subset Assay in Mice
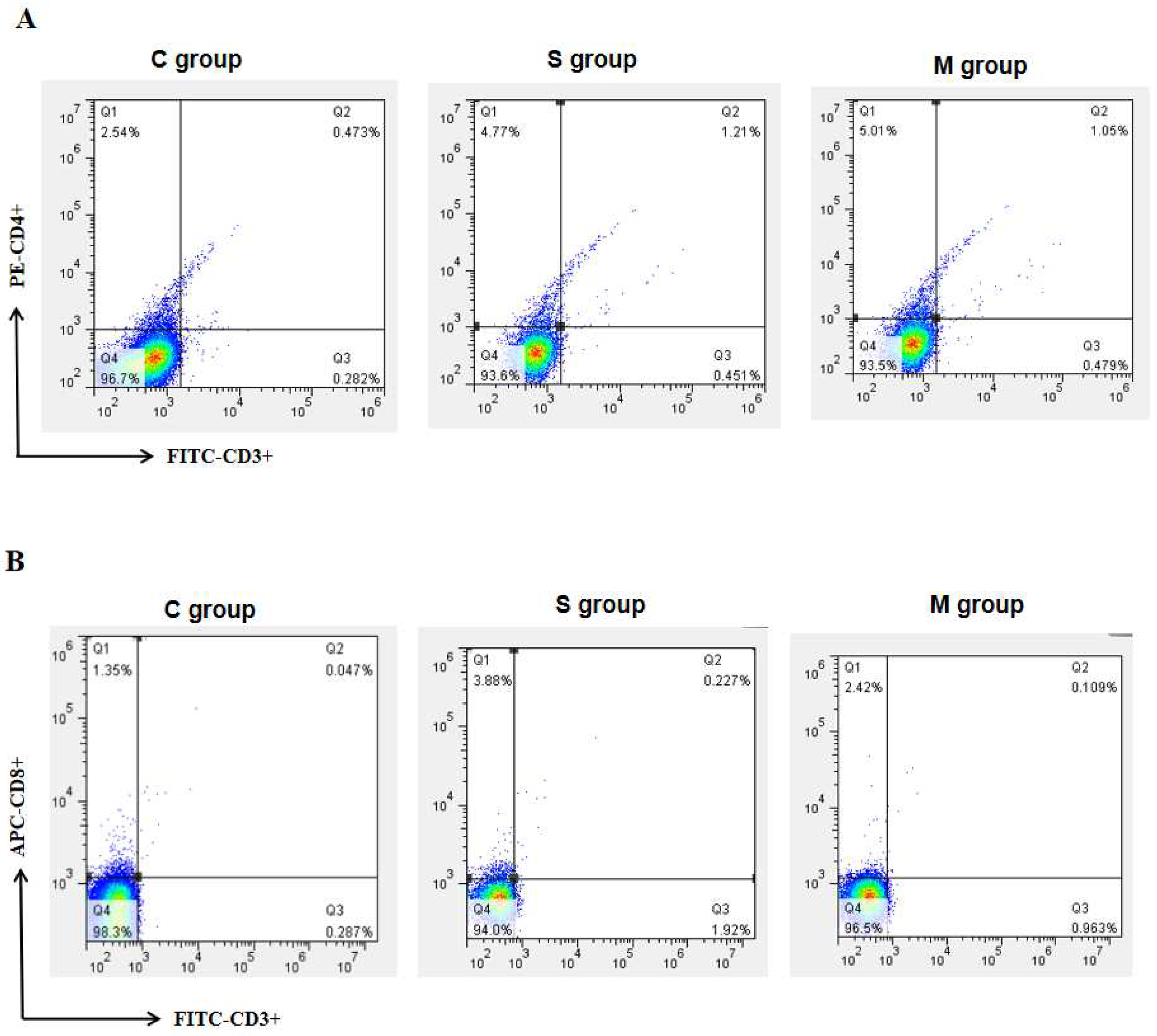
3.3. Results of Peripheral Blood Lymphocyte Subset Assay in Piglets
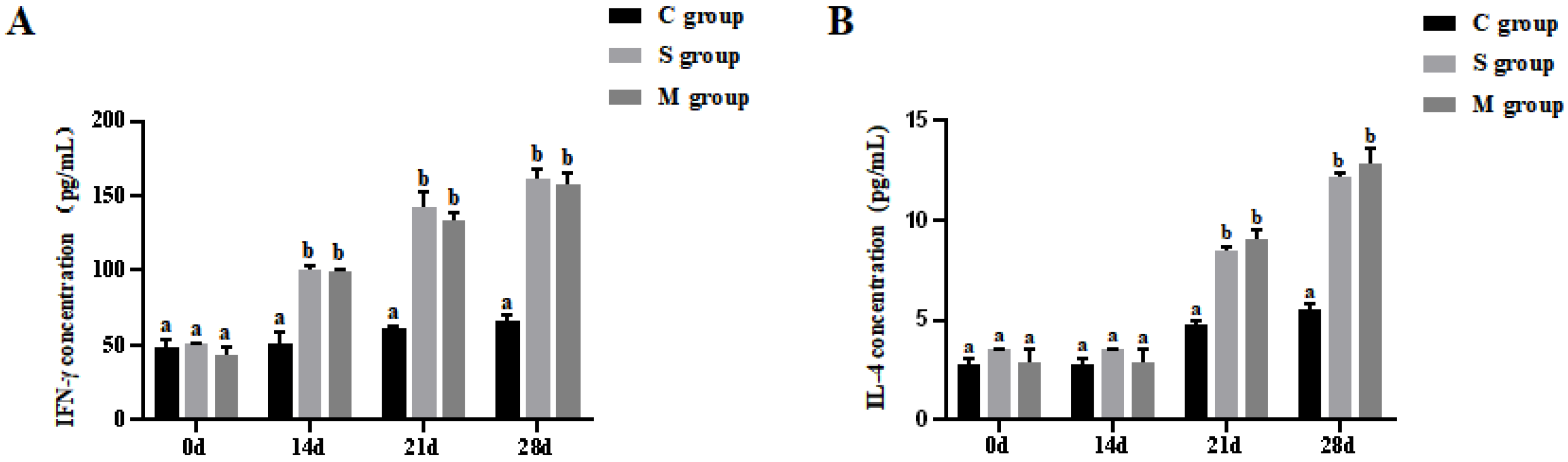
3.4. Results of Cytokines Assay in Piglets
3.5. Results of Serum IgG Antibody Assay in Piglets
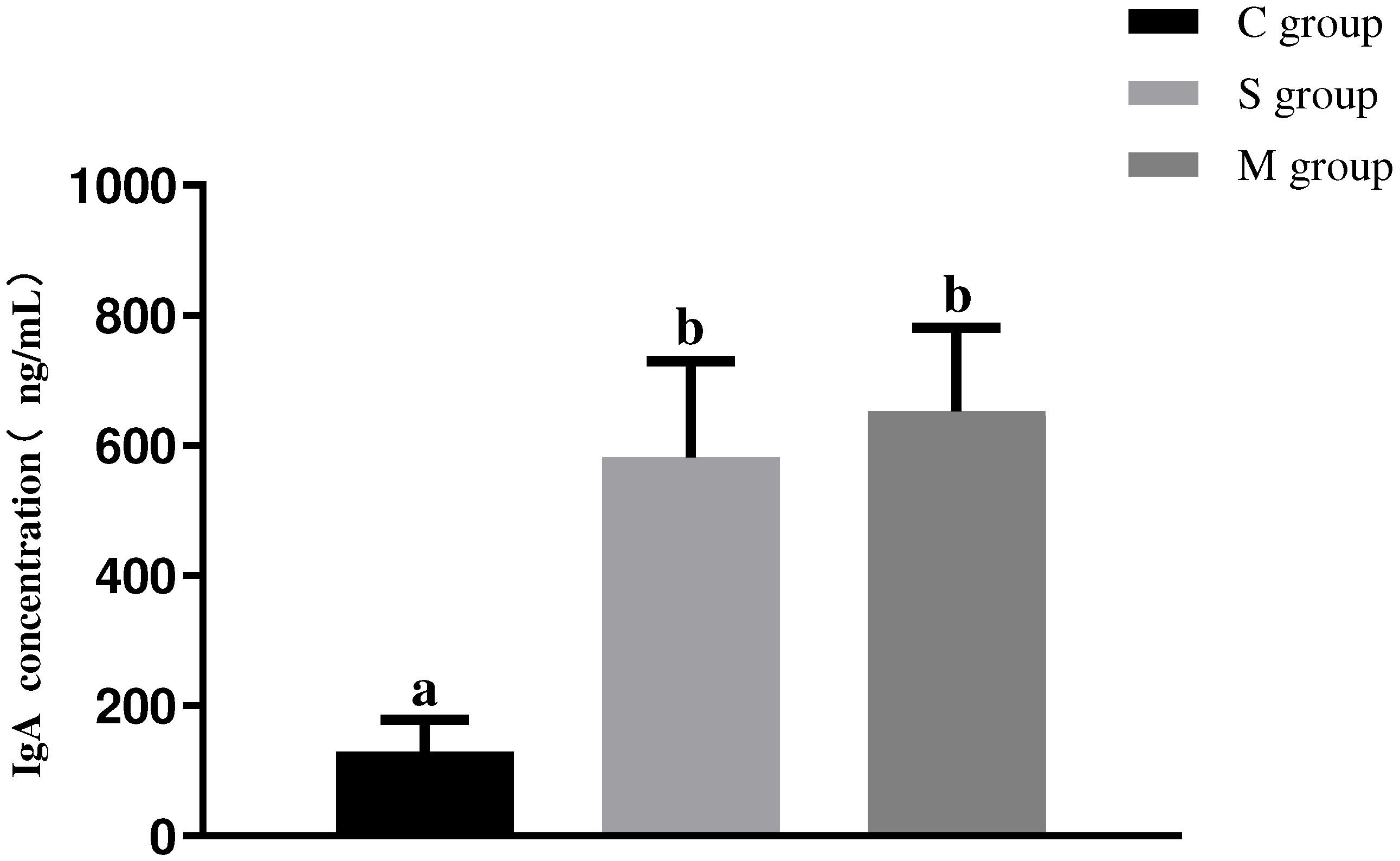
3.6. Results of Serum IgA Antibody Assay in Piglets
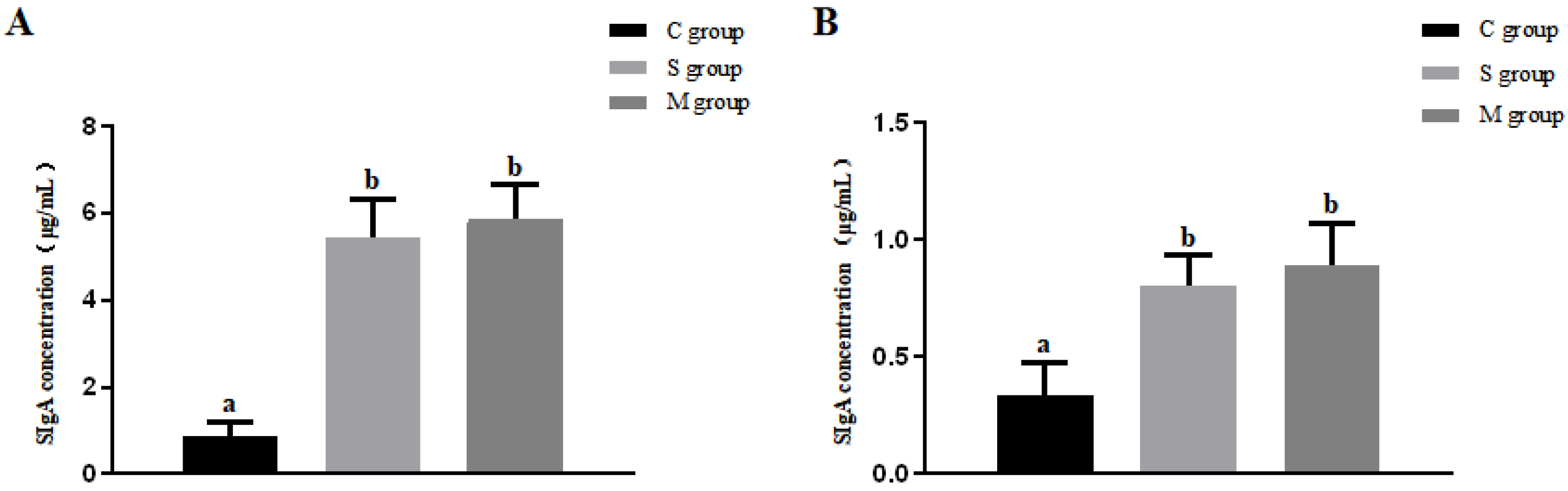
3.7. Results of SIgA Antibody Assay in Intestinal Mucosa and Feces of Piglets
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wood, E.N. An apparently new syndrome of porcine epidemic diarrhoea. Vet. Rec. 1977, 100, 243–244. [Google Scholar] [CrossRef]
- Chen, J.-F.; Sun, D.-B.; Wang, C.-B.; Shi, H.-Y.; Cui, X.-C.; Liu, S.-W.; Qiu, H.-J.; Feng, L. Molecular characterization and phylogenetic analysis of membrane protein genes of porcine epidemic diarrhea virus isolates in China. Virus Genes 2008, 36, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Debouck, P.; Pensaert, M. Experimental infection of pigs with a new porcine enteric coronavirus, CV 777. Am. J. Vet. Res. 1980, 41, 219–223. [Google Scholar] [CrossRef]
- Ojkic, D.; Hazlett, M.; Fairles, J.; Marom, A.; Slavic, D.; Maxie, G.; Alexandersen, S.; Pasick, J.; Alsop, J.; Burlatschenko, S. The first case of porcine epidemic diarrhea in Canada. Can. Vet. J. 2015, 56, 149–152. [Google Scholar] [PubMed]
- Takahashi, K.; Okada, K.; Ohshima, K. An outbreak of swine diarrhea of a new-type associated with coronavirus-like particles in Japan. Nihon Juigaku Zasshi 1983, 45, 829–832. [Google Scholar] [CrossRef]
- Vlasova, A.N.; Marthaler, D.; Wang, Q.; Culhane, M.R.; Rossow, K.D.; Rovira, A.; Collins, J.; Saif, L.J. Distinct characteristics and complex evolution of PEDV strains, North America, May 2013–February 2014. Emerg. Infect. Dis. 2014, 20, 1620–1628. [Google Scholar] [CrossRef]
- Pensaert, M.B.; de Bouck, P. A new coronavirus-like particle associated with diarrhea in swine. Arch. Virol. 1978, 58, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Weng, L.; Weersink, A.; Poljak, Z.; de Lange, K.; von Massow, M. An economic evaluation of intervention strategies for Porcine Epidemic Diarrhea (PED). Prev. Vet. Med. 2016, 134, 58–68. [Google Scholar] [CrossRef]
- Jung, K.; Annamalai, T.; Lu, Z.; Saif, L.J. Comparative pathogenesis of US porcine epidemic diarrhea virus (PEDV) strain PC21A in conventional 9-day-old nursing piglets vs. 26-day-old weaned pigs. Vet. Microbiol. 2015, 178, 31–40. [Google Scholar] [CrossRef]
- Yang, D.-Q.; Ge, F.-F.; Ju, H.-B.; Wang, J.; Liu, J.; Ning, K.; Liu, P.-H.; Zhou, J.-P.; Sun, Q.-Y. Whole-genome analysis of porcine epidemic diarrhea virus (PEDV) from eastern China. Arch. Virol. 2014, 159, 2777–2785. [Google Scholar] [CrossRef]
- Kocherhans, R.; Bridgen, A.; Ackermann, M.; Tobler, K. Completion of the porcine epidemic diarrhoea coronavirus (PEDV) genome sequence. Virus Genes 2001, 23, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Lee, C. Porcine epidemic diarrhea virus: An emerging and re-emerging epizootic swine virus. Virol. J. 2015, 12, 193. [Google Scholar] [CrossRef]
- Sun, R.; Leng, Z.; Zhai, S.L.; Chen, D.; Song, C. Genetic variability and phylogeny of current Chinese porcine epidemic diarrhea virus strains based on spike, ORF3, and membrane genes. Sci. World J. 2014, 2014, 208439. [Google Scholar] [CrossRef]
- Jung, K.; Saif, L.J. Porcine epidemic diarrhea virus infection: Etiology, epidemiology, pathogenesis and immunoprophylaxis. Vet. J. 2015, 204, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Park, B. Porcine epidemic diarrhoea virus: A comprehensive review of molecular epidemiology, diagnosis, and vaccines. Virus Genes 2012, 44, 167–175. [Google Scholar] [CrossRef]
- Lang, Q.; Huang, N.; Guo, J.; Ge, L.; Yang, X. High-affinity monoclonal antibodies against the porcine epidemic diarrhea virus S1 protein. BMC Vet. Res. 2024, 20, 239. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Suo, S.; Jang, Y.S. Development of a porcine epidemic diarrhea virus M protein-based ELISA for virus detection. Biotechnol. Lett. 2011, 33, 215–220. [Google Scholar] [CrossRef]
- Kong, F.; Jia, H.; Xiao, Q.; Fang, L.; Wang, Q. Prevention and Control of Swine Enteric Coronaviruses in China: A Review of Vaccine Development and Application. Vaccines 2023, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Nunnally, B.K.; Sitrin, R.D.; Turula, V.E. Vaccine Analysis: Strategies, Principles, and Control, 1st ed.; 2015 edition; Springer: Berlin/Heidelberg, Germany, 2015; p. 1. [Google Scholar]
- Hsueh, F.C.; Chang, Y.C.; Kao, C.F.; Hsu, C.W.; Chang, H.W. Intramuscular Immunization with Chemokine-Adjuvanted Inactive Porcine Epidemic Diarrhea Virus Induces Substantial Protection in Pigs. Vaccines 2020, 8, 102. [Google Scholar] [CrossRef]
- Zhong, K.; Chen, X.; Zhang, J.; Jiang, X.; Zhang, J.; Huang, M.; Bi, S.; Ju, C.; Luo, Y. Recent Advances in Oral Vaccines for Animals. Vet. Sci. 2024, 11, 353. [Google Scholar] [CrossRef]
- Landete, J.M. A review of food-grade vectors in lactic acid bacteria: From the laboratory to their application. Crit. Rev. Biotechnol. 2017, 37, 296–308. [Google Scholar] [CrossRef]
- Pouwels, P.H.; Leer, R.J.; Shaw, M.; Bak-Glashouwer, M.-J.H.D.; Tielen, F.D.; Smit, E.; Martinez, B.; Jore, J.; Conway, P.L. Lactic acid bacteria as antigen delivery vehicles for oral immunization purposes. Int. J. Food Microbiol. 1998, 41, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, G.C.; Cazorla, S.I.; Lemme, D.J.; Velez, E.; Perdigon, G. Beneficial Effects of Probiotic Consumption on the Immune System. Ann. Nutr. Metab. 2019, 74, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, F.C.; Lin, C.N.; Chiou, H.-Y.; Chia, M.Y.; Chiou, M.T.; Haga, T.; Kao, C.F.; Chang, Y.C.; Chang, C.Y.; Jeng, C.R.; et al. Updated phylogenetic analysis of the spike gene and identification of a novel recombinant porcine epidemic diarrhoea virus strain in Taiwan. Transbound. Emerg. Dis. 2020, 67, 417–430. [Google Scholar] [CrossRef]
- Sun, R.Q.; Cai, R.J.; Chen, Y.Q.; Liang, P.S.; Chen, D.K.; Song, C.X. Outbreak of porcine epidemic diarrhea in suckling piglets, China. Emerg. Infect. Dis. 2012, 18, 161–163. [Google Scholar] [CrossRef]
- Wang, X.M.; Niu, B.B.; Yan, H.; Gao, D.S.; Yang, X.; Chen, L.; Chang, H.T.; Zhao, J.; Wang, C.Q. Genetic properties of endemic Chinese porcine epidemic diarrhea virus strains isolated since 2010. Arch. Virol. 2013, 158, 2487–2494. [Google Scholar] [CrossRef]
- Collin, E.A.; Anbalagan, S.; Okda, F.; Batman, R.; Nelson, E.; Hause, B.M. An inactivated vaccine made from a U.S. field isolate of porcine epidemic disease virus is immunogenic in pigs as demonstrated by a dose-titration. BMC Vet. Res. 2015, 11, 62. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Fang, L.; Xiao, S. Porcine epidemic diarrhea in China. Virus Res. 2016, 226, 7–13. [Google Scholar] [CrossRef]
- Santarmaki, V.; Kourkoutas, Y.; Zoumpopoulou, G.; Mavrogonatou, E.; Kiourtzidis, M.; Chorianopoulos, N.; Tassou, C.; Tsakalidou, E.; Simopoulos, C.; Ypsilantis, P. Survival, Intestinal Mucosa Adhesion, and Immunomodulatory Potential of Lactobacillus plantarum Strains. Curr. Microbiol. 2017, 74, 1061–1067. [Google Scholar] [CrossRef]
- Nakamoto, N.; Amiya, T.; Aoki, R.; Taniki, N.; Koda, Y.; Miyamoto, K.; Teratani, T.; Suzuki, T.; Chiba, S.; Chu, P.-S.; et al. Commensal Lactobacillus Controls Immune Tolerance during Acute Liver Injury in Mice. Cell Rep. 2017, 21, 1215–1226. [Google Scholar] [CrossRef]
- Kanayama, M.; Kato, Y.; Tsuji, T.; Konoeda, Y.; Hashimoto, A.; Kanauchi, O.; Fujii, T.; Fujiwara, D. Enhancement of immunomodulative effect of lactic acid bacteria on plasmacytoid dendritic cells with sucrose palmitate. Sci. Rep. 2018, 8, 3147. [Google Scholar] [CrossRef]
- Gao, S.; Li, D.; Liu, Y.; Zha, E.; Zhou, T.; Yue, X. Oral immunization with recombinant hepatitis E virus antigen displayed on the Lactococcus lactis surface enhances ORF2-specific mucosal and systemic immune responses in mice. Int. Immunopharmacol. 2015, 24, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Song, Y.; Liu, L.; Zhang, Y.; Wang, T.; Zhang, W.; Li, K.; Qi, X.; Gao, Y.; Gao, L.; et al. Neutralizing-antibody-mediated protection of chickens against infectious bursal disease via one-time vaccination with inactivated recombinant Lactococcus lactis expressing a fusion protein constructed from the RCK protein of Salmonella enterica and VP2 of infectious bursal disease virus. Microb. Cell Factories 2019, 18, 21. [Google Scholar] [CrossRef]
- Wang, Z.-H.; Cao, X.-H.; Du, X.-G.; Feng, H.-B.; Wang, D.; Song, H.; Zeng, X.-Y. Mucosal and systemic immunity in mice after intranasal immunization with recombinant Lactococcus lactis expressing ORF6 of PRRSV. Cell. Immunol. 2014, 287, 69–73. [Google Scholar] [CrossRef]
- Seder, R.A.; Hill, A.V. Vaccines against intracellular infections requiring cellular immunity. Nature 2000, 406, 793–798. [Google Scholar] [CrossRef]
- Li, C.R.; Santoso, S.; Lo, D.D. Quantitative analysis of T cell homeostatic proliferation. Cell. Immunol. 2007, 250, 40–54. [Google Scholar] [CrossRef]
- Lin, J.; Mou, C.; Zhang, S.; Zhu, L.; Li, Y.; Yang, Q. Immune Responses Induced by Recombinant Bacillus subtilis Expressing the PEDV Spike Protein Targeted at Microfold Cells. Vet. Sci. 2022, 9, 211. [Google Scholar] [CrossRef]
- McNeela, E.A.; Mills, K.H. Manipulating the immune system: Humoral versus cell-mediated immunity. Adv. Drug Deliv. Rev. 2001, 51, 43–54. [Google Scholar] [CrossRef]
- Romagnani, S. T-cell subsets (Th1 versus Th2). Ann. Allergy Asthma Immunol. 2000, 85, 9–18, 21. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Huang, X.; Ma, S.; Yu, M.; Shi, W.; Qiao, X.; Tang, L.; Xu, Y.; Li, Y. Oral Delivery of Probiotics Expressing Dendritic Cell-Targeting Peptide Fused with Porcine Epidemic Diarrhea Virus COE Antigen: A Promising Vaccine Strategy against PEDV. Viruses 2017, 9, 312. [Google Scholar] [CrossRef]
- Wang, X.N.; Wang, L.; Zheng, D.Z.; Chen, S.; Shi, W.; Qiao, X.Y.; Jiang, Y.P.; Tang, L.J.; Xu, Y.G.; Li, Y.J. Oral immunization with a Lactobacillus casei-based anti-porcine epidemic diarrhoea virus (PEDV) vaccine expressing microfold cell-targeting peptide Co1 fused with the COE antigen of PEDV. J. Appl. Microbiol. 2018, 124, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Hoces, D.; Arnoldini, M.; Diard, M.; Loverdo, C.; Slack, E. Growing, evolving and sticking in a flowing environment: Understanding IgA interactions with bacteria in the gut. Immunology 2020, 159, 52–62. [Google Scholar] [CrossRef]
- Corthesy, B. Multi-faceted functions of secretory IgA at mucosal surfaces. Front. Immunol. 2013, 4, 185. [Google Scholar] [CrossRef]
- Mestecky, J.; Russell, M.W.; Elson, C.O. Intestinal IgA: Novel views on its function in the defence of the largest mucosal surface. Gut 1999, 44, 2–5. [Google Scholar] [CrossRef]
- Holmgren, J.; Czerkinsky, C. Mucosal immunity and vaccines. Nat. Med. 2005, 11, S45–S53. [Google Scholar] [CrossRef]
- Stabel, T.J.; Mayfield, J.E.; Tabatabai, L.B.; Wannemuehler, M.J. Oral immunization of mice with attenuated Salmonella typhimurium containing a recombinant plasmid which codes for production of a 31-kilodalton protein of Brucella abortus. Infect. Immun. 1990, 58, 2048–2055. [Google Scholar] [CrossRef]
- Villena, J.; Medina, M.; Raya, R.; Alvarez, S. Oral immunization with recombinant Lactococcus lactis confers protection against respiratory pneumococcal infection. Can. J. Microbiol. 2008, 54, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Stone, S.; Drebes, D.; Greiner, L.L.; Dvorak, C.; Murtaugh, M.P. Characterization of anti-porcine epidemic diarrhea virus neutralizing activity in mammary secretions. Virus Res. 2016, 226, 85–92. [Google Scholar] [CrossRef]
- Hu, Z.; Li, Y.; Zhang, B.; Zhao, Y.; Guan, R.; Zhou, Y.; Du, J.; Zhang, Z.; Li, X. Serum IgA antibody level against porcine epidemic diarrhea virus is a potential pre-evaluation indicator of immunization effects in sows during parturition under field conditions. Porc. Health Manag. 2024, 10, 32. [Google Scholar] [CrossRef]
- Santaolalla, A.; Sollie, S.; Rislan, A.; Josephs, D.H.; Hammar, N.; Walldius, G.; Garmo, H.; Karagiannis, S.N.; Van Hemelrijck, M. Association between serum markers of the humoral immune system and inflammation in the Swedish AMORIS study. BMC Immunol. 2021, 22, 61. [Google Scholar] [CrossRef]
- Wang, J.; Huang, L.; Mou, C.; Zhang, E.; Wang, Y.; Cao, Y.; Yang, Q. Mucosal immune responses induced by oral administration recombinant Bacillus subtilis expressing the COE antigen of PEDV in newborn piglets. Biosci. Rep. 2019, 39, BSR20182028. [Google Scholar] [CrossRef] [PubMed]

| Group | Recombinant L. lactis Strains | Dose | Number of Immunizations |
|---|---|---|---|
| CD | - | 300 µL of PBS | 2 |
| SD | MG1363-pMG36e-S1 | 300 µL of 1 × 109 CFU mL−1 | 2 |
| MD | MG1363-pMG36e-M | 300 µL of 1 × 109 CFU mL−1 | 2 |
| Group | Recombinant L. lactis Strains | Dose | Number of Immunizations |
|---|---|---|---|
| CT | - | 300 µL of PBS | 3 |
| ST | MG1363-pMG36e-S1 | 300 µL of 1 × 109 CFU mL−1 | 3 |
| MT | MG1363-pMG36e-M | 300 µL of 1 × 109 CFU mL−1 | 3 |
| Group | Recombinant L. lactis Strains | Dose | Number of Immunizations |
|---|---|---|---|
| C | - | 3 mL of PBS | 2 |
| S | MG1363-pMG36e-S1 | 3 mL of 1 × 109 CFU mL−1 | 2 |
| M | MG1363-pMG36e-M | 3 mL of 1 × 109 CFU mL−1 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, X.; Wang, Z.; Yan, X.; Wang, X.; Yue, X.; Zhang, H. Induction of Immune Responses in Mice and Newborn Piglets by Oral Immunization with Recombinant Lactococcus lactis Expressing S1 and M Proteins of Porcine Epidemic Diarrhea Virus. Microorganisms 2025, 13, 714. https://doi.org/10.3390/microorganisms13040714
Cai X, Wang Z, Yan X, Wang X, Yue X, Zhang H. Induction of Immune Responses in Mice and Newborn Piglets by Oral Immunization with Recombinant Lactococcus lactis Expressing S1 and M Proteins of Porcine Epidemic Diarrhea Virus. Microorganisms. 2025; 13(4):714. https://doi.org/10.3390/microorganisms13040714
Chicago/Turabian StyleCai, Xiulei, Zhikui Wang, Xinping Yan, Xu Wang, Xiaoxue Yue, and Hongliang Zhang. 2025. "Induction of Immune Responses in Mice and Newborn Piglets by Oral Immunization with Recombinant Lactococcus lactis Expressing S1 and M Proteins of Porcine Epidemic Diarrhea Virus" Microorganisms 13, no. 4: 714. https://doi.org/10.3390/microorganisms13040714
APA StyleCai, X., Wang, Z., Yan, X., Wang, X., Yue, X., & Zhang, H. (2025). Induction of Immune Responses in Mice and Newborn Piglets by Oral Immunization with Recombinant Lactococcus lactis Expressing S1 and M Proteins of Porcine Epidemic Diarrhea Virus. Microorganisms, 13(4), 714. https://doi.org/10.3390/microorganisms13040714






