Analysis of the Culturable Skin Microbiome of Horses from Southern Germany
Abstract
1. Introduction
2. Materials and Methods
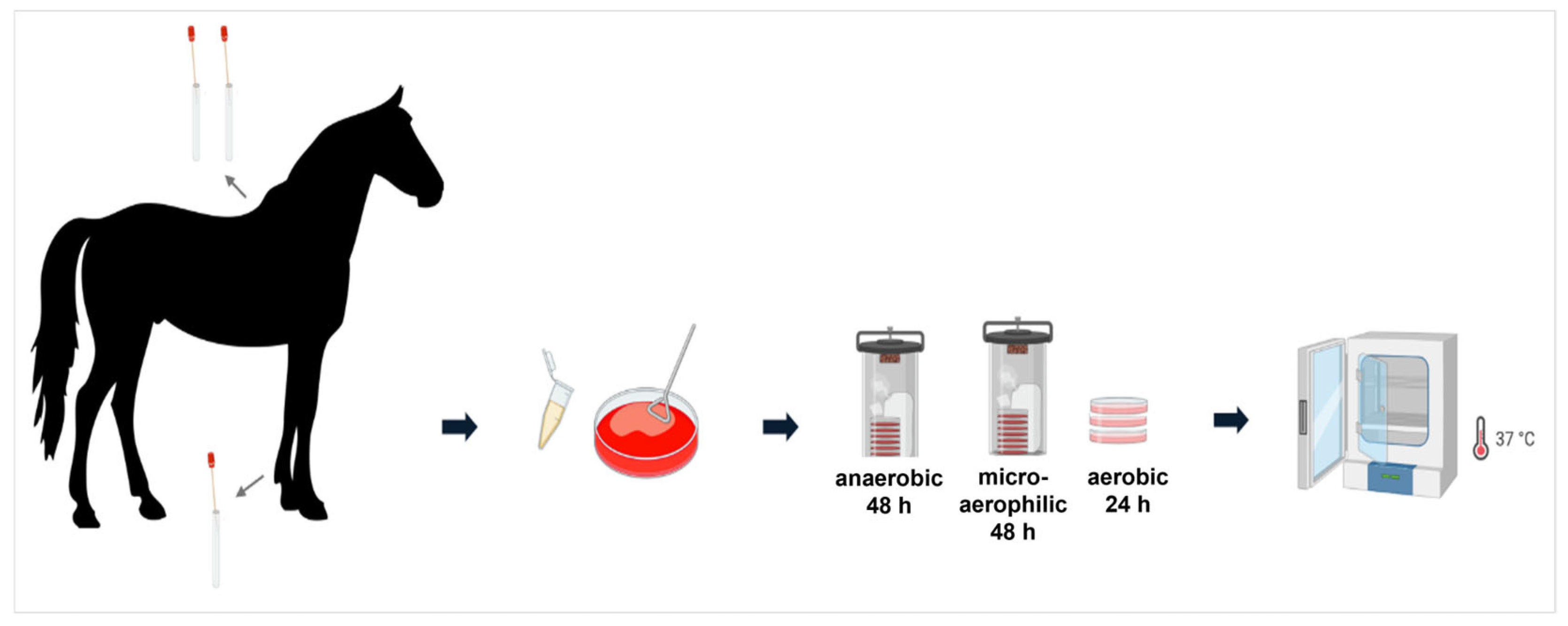
2.1. Sample Collection
2.2. Cultivation of Samples and Isolation of Pure Cultures
2.3. Identification of Bacteria
3. Results
3.1. Identification of Culturable Bacteria by MALDI-ToF MS
3.2. Identification of Dominant Culturable Bacterial Species on Equine Skin
3.3. Major Bacterial Species at Different Anatomical Sites
3.4. Comparison of Dominant Culturable Bacterial Species in Different Stables
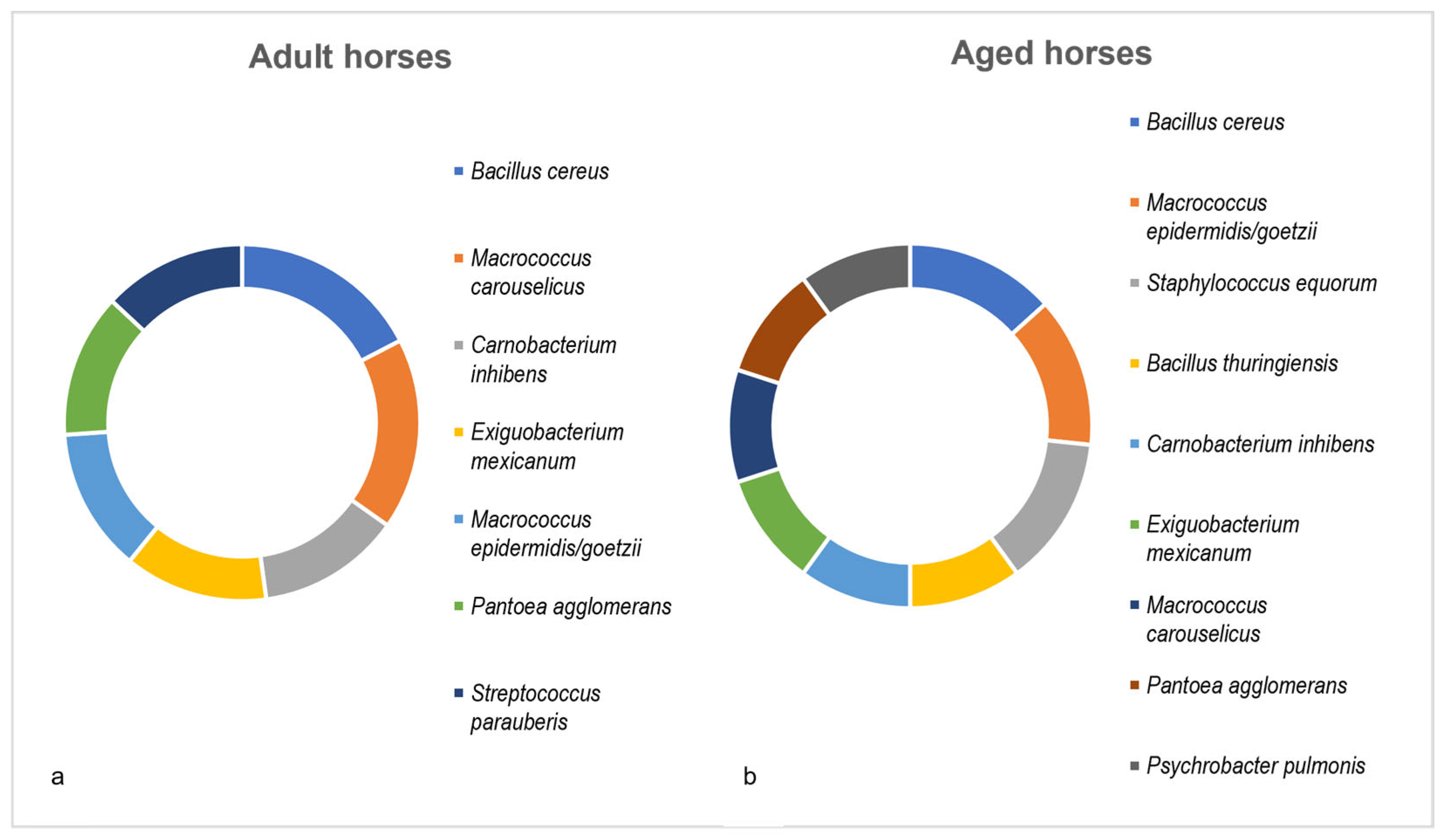
3.5. Age-Dependency of Dominant Culturable Bacterial Species
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kil, D.Y.; Swanson, K.S. Companion animals symposium: Role of microbes in canine and feline health. J. Anim. Sci. 2011, 89, 1498–1505. [Google Scholar] [CrossRef] [PubMed]
- Weese, J.S. The canine and feline skin microbiome in health and disease. Vet. Dermatol. 2013, 24, 137–145.e31. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues Hoffmann, A.; Patterson, A.P.; Diesel, A.; Lawhon, S.D.; Ly, H.J.; Elkins Stephenson, C.; Mansell, J.; Steiner, J.M.; Dowd, S.E.; Olivry, T.; et al. The skin microbiome in healthy and allergic dogs. PLoS ONE 2014, 9, e83197. [Google Scholar] [CrossRef]
- Noli, C. The microbiome of dogs and cats–what do we know in 2017. Rev. Vet. Clin. 2017, 52, 93–98. [Google Scholar] [CrossRef]
- Older, C.E.; Diesel, A.; Patterson, A.P.; Meason-Smith, C.; Johnson, T.J.; Mansell, J.; Rodrigues Hoffmann, A. The feline skin microbiota: The bacteria inhabiting the skin of healthy and allergic cats. PLoS ONE 2017, 12, e0178555. [Google Scholar] [CrossRef]
- Meason-Smith, C.; Diesel, A.; Patterson, A.P.; Older, C.E.; Johnson, T.J.; Mansell, J.M.; Suchodolski, J.S.; Rodrigues Hoffmann, A. Characterization of the cutaneous mycobiota in healthy and allergic cats using next generation sequencing. Vet. Dermatol. 2017, 28, 71-e17. [Google Scholar] [CrossRef]
- Hoffmann, A.R. The cutaneous ecosystem: The roles of the skin microbiome in health and its association with inflammatory skin conditions in humans and animals. Vet. Dermatol. 2017, 28, 60-e15. [Google Scholar] [CrossRef] [PubMed]
- Older, C.E.; Diesel, A.B.; Lawhon, S.D.; Queiroz, C.R.R.; Henker, L.C.; Rodrigues Hoffmann, A. The feline cutaneous and oral microbiota are influenced by breed and environment. PLoS ONE 2019, 14, e0220463. [Google Scholar] [CrossRef]
- Pereira, A.M.; Clemente, A. Dogs’ Microbiome from Tip to Toe. Top. Companion Anim. Med. 2021, 45, 100584. [Google Scholar] [CrossRef]
- Whittle, M.J.; Castillo-Fernandez, J.; Amos, G.C.A.; Watson, P. Metagenomic characterisation of canine skin reveals a core healthy skin microbiome. Sci. Rep. 2024, 14, 20104. [Google Scholar] [CrossRef]
- Strompfová, V.; Štempelová, L. Composition and diversity of 16S rRNA based skin bacterial microbiome in healthy horses. Vet. Res. Commun. 2024, 48, 2847–2855. [Google Scholar] [CrossRef]
- Kamus, L.J.; Theoret, C.; Costa, M.C. Use of next generation sequencing to investigate the microbiota of experimentally induced wounds and the effect of bandaging in horses. PLoS ONE 2018, 13, e0206989. [Google Scholar] [CrossRef]
- O’Shaughnessy-Hunter, L.C.; Yu, A.; Rousseau, J.D.; Foster, R.A.; Weese, J.S. Longitudinal study of the cutaneous microbiota of healthy horses. Vet. Dermatol. 2021, 32, 467-e128. [Google Scholar] [CrossRef] [PubMed]
- Bizzini, A.; Durussel, C.; Bille, J.; Greub, G.; Prod’Hom, G. Performance of matrix-assisted laser desorption ionization-time of flight mass spectrometry for identification of bacterial strains routinely isolated in a clinical microbiology laboratory. J. Clin. Microbiol. 2010, 48, 1549–1554. [Google Scholar] [CrossRef]
- Croxatto, A.; Prod’hom, G.; Greub, G. Applications of MALDI-TOF mass spectrometry in clinical diagnostic microbiology. FEMS Microbiol. Rev. 2012, 36, 380–407. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.E.; Kaleta, E.J.; Arora, A.; Wolk, D.M. Matrix assisted laser desorption ionization time of flight mass spectrometry: A fundamental shift in the routine practice of clinical microbiology. Clin. Microbiol. Rev. 2013, 26, 547–603. [Google Scholar] [CrossRef]
- Dietrich, R.; Jeßberger, N.; Ehling-Schulz, M.; Märtlbauer, E.; Granum, P.E. The food poisoning toxins of Bacillus cereus. Toxins 2021, 13, 98. [Google Scholar] [CrossRef] [PubMed]
- Dobrzyński, J.; Jakubowska, Z.; Dybek, B. Potential of Bacillus pumilus to directly promote plant growth. Front. Microbiol. 2022, 13, 1069053. [Google Scholar] [CrossRef]
- Mazhar, S.; Hill, C.; McAuliffe, O. The genus Macrococcus: An insight into its biology, evolution, and relationship with Staphylococcus. Adv. Appl. Microbiol. 2018, 105, 1–50. [Google Scholar]
- Nicholson, W.L.; Zhalnina, K.; de Oliveira, R.R.; Triplett, E.W. Proposal to rename Carnobacterium inhibens as Carnobacterium inhibens subsp. inhibens subsp. nov. and description of Carnobacterium inhibens subsp. gilichinskyi subsp. nov., a psychrotolerant bacterium isolated from Siberian permafrost. Int. J. Sys. Evol. Microbiol. 2015, 65, 556–561. [Google Scholar]
- Lo, C.K.L.; Sheth, P.M. Carnobacterium inhibens isolated in blood culture of an immunocompromised, metastatic cancer patient: A case report and literature review. BMC Infect. Dis. 2021, 21, 403. [Google Scholar] [CrossRef]
- Nováková, D.; Sedláček, I.; Pantůček, R.; Štětina, V.; Švec, P.; Petráš, P. Staphylococcus equorum and Staphylococcus succinus isolated from human clinical specimens. J. Med. Microbiol. 2006, 55, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.W.; Kim, H.R.; Han, S.; Jeon, C.O.; Lee, J.H. A proposal to unify two subspecies of Staphylococcus equorum: Staphylococcus equorum subsp. equorum and Staphylococcus equorum subsp. linens. Antonie Van Leeuwenhoek 2013, 104, 1049–1062. [Google Scholar] [CrossRef] [PubMed]
- López-Cortés, A.; Schumann, P.; Pukall, R.; Stackebrandt, E. Exiguobacterium mexicanum sp. nov. and Exiguobacterium artemiae sp. nov., isolated from the brine shrimp Artemia franciscana. Sys. Appl. Microbiol. 2006, 29, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, D.F.; Goris, J.; Vishnivetskaya, T.; Gilichinsky, D.; Thomashow, M.F.; Tiedje, J.M. Characterization of Exiguobacterium isolates from the Siberian permafrost. Description of Exiguobacterium sibiricum sp. nov. Extremophiles 2006, 10, 285–294. [Google Scholar] [CrossRef]
- Gavini, F.; Mergaert, J.; Beji, A.; Mielcarek, C.; Izard, D.; Kersters, K.; De Ley, J. Transfer of Enterobacter agglomerans (Beijerinck1888) Ewing & Fife 1972 to Pantoea gen. nov. as Pantoea agglomerans comb. nov. and description of Pantoea dispersa sp. nov. Int. J. Syst. Bacteriol. 1989, 39, 337–345. [Google Scholar]
- Grice, E.A.; Segre, J.A. The skin microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253. [Google Scholar] [CrossRef]
- Kaiser-Thom, S.; Hilty, M.; Axiak, S.; Gerber, V. The skin microbiota in equine pastern dermatitis: A case-control study of horses in Switzerland. Vet. Dermatol. 2021, 32, 646-e172. [Google Scholar] [CrossRef]
- Sangiorgio, D.B.; Hilty, M.; Kaiser-Thom, S.; Epper, P.G.; Ramseyer, A.A.; Overesch, G.; Gerber, V.M. The influence of clinical severity and topical antimicrobial treatment on bacteriological culture and the microbiota of equine pastern dermatitis. Vet. Dermatol. 2021, 32, 173-e41. [Google Scholar] [CrossRef]
- Kaiser-Thom, S.; Hilty, M.; Ramseyer, A.; Epper, P.; Gerber, V. The relationship between equine pastern dermatitis, meteorological factors, and the skin microbiota. Vet. Dermatol. 2022, 33, 165-e48. [Google Scholar] [CrossRef]
- Gerber, V.; Kaiser-Thom, S.; Oesch, S. Equine pastern dermatitis: A narrative review on clinical presentation, diagnosis, risk factors, prevention, and therapeutic approaches. J. Am. Vet. Med. Assoc. 2023, 261, S58–S65. [Google Scholar] [CrossRef] [PubMed]
- Jost, S.M.; Cardona, L.; Rohrbach, E.; Mathis, A.; Holliger, C.; Verhulst, N.O. Environment rather than breed or body site shapes the skin bacterial community of healthy sheep as revealed by metabarcoding. Vet. Dermatol. 2024, 35, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Yue, Y.; Kou, X.; Hou, W.; Wang, M.; Yang, X.; Wang, C. Dynamic Distribution of Skin Microorganisms in Donkeys at Different Ages and Various Sites of the Body. Animals 2023, 13, 1566. [Google Scholar] [CrossRef] [PubMed]


| No. | Stable | Breed | Sex | Age | Sterilized | Routine Riding |
|---|---|---|---|---|---|---|
| 1 | A | Irish Tinker | f | 3.5 | no | yes |
| 2 | A | Irish Tinker | m | 12 | yes | yes |
| 3 | A | Irish Tinker | m | 17 | yes | yes |
| 4 | B | Speed Racking | m | 1.5 | no | no |
| 5 | B | Curley | m | 7 | yes | yes |
| 6 | B | Shetland Pony | m | 10 | yes | no |
| 7 | B | Connemara Pony | m | 10 | yes | yes |
| 8 | C | Andalusier | m | 15 | yes | yes |
| 9 | C | American Paint | m | 8 | yes | yes |
| 10 | C | Moritzburger | m | 5 | yes | yes |
| 11 | D | Bayerisches Warmblut | f | 19 | no | yes |
| 12 | D | Pinto | f | 15 | yes | yes |
| 13 | D | Quarter | m | 31 | no | no |
| 14 | D | Quarter | m | 14 | yes | yes |
| Phylum | Genus | Species |
|---|---|---|
| Firmicutes | Aerococcus | Aerococcus viridans |
| Bacillus | Bacillus altitudinis | |
| Bacillus amyloliquefaciens | ||
| Bacillus cereus | ||
| Bacillus infantis | ||
| Bacillus licheniformis | ||
| Bacillus mycoides | ||
| Bacillus pumilus | ||
| Bacillus safensis | ||
| Bacillus subtilis | ||
| Bacillus subtilis/amyloliquefaciens | ||
| Bacillus thuringiensis | ||
| Bacillus xiamenensis | ||
| Butyrivibrio | Butyrivibrio MabrTax32 | |
| Carnobacterium | Carnobacterium gallinarum | |
| Carnobacterium inhibens | ||
| Carnobacterium jeotgali | ||
| Carnobacterium maltaromaticum | ||
| Carnobacterium viridans | ||
| Desemzia | Desemzia incerta | |
| Enterococcus | Enterococcus mundtii | |
| Lactococcus | Lactococcus raffinolactis | |
| Latilactobacillus | Latilactobacillus fuchuensis | |
| Lysinibacillus | Lysinibacillus sp. | |
| Macrococcus | Macrococcus bohemicus/epidermidis/goetzii | |
| Macrococcus bovicus/brunensis | ||
| Macrococcus canis | ||
| Macrococcus carouselicus | ||
| Macrococcus caseolyticus | ||
| Macrococcus epidermidis/goetzii | ||
| Macrococcus equipercicus | ||
| Macrococcus flavus | ||
| Macrococcus hajekii | ||
| Macrococcus spp. | ||
| Mammaliicoccus | Mammaliicoccus vitulinus | |
| Micrococcus | Micrococcus sp. | |
| Micrococcus flavus | ||
| Micrococcus luteus | ||
| Neobacillus | Neobacillus bataviensis | |
| Niallia | Niallia circulans | |
| Paenibacillus | Paenibacillus amylolyticus | |
| Paenibacillus beijingensis | ||
| Paenibacillus MabrTax5 | ||
| Paenibacillus illinoisensis | ||
| Paenibacillus lautus | ||
| Paenibacillus saccharophilum | ||
| Paraclostridium | Paraclostridium benzoelyticum/bifermentans | |
| Peribacillus | Peribacillus simplex | |
| Peribacillus simplex/butanolivorans/muralis/frigoritolerans | ||
| Priestia | Priestia aryabhattai/megaterium | |
| Priestia megaterium | ||
| Psychrobacillus | Psychrobacillus lasiicapitis | |
| Psychrobacillus MabrTax3 | ||
| Psychrobacillus psychrodurans | ||
| Psychrobacillus lasiicapitis | ||
| Solibacillus | Solibacillus silvestris | |
| Staphylococcus | Staphylococcus aureus | |
| Staphylococcus chromogenes | ||
| Staphylococcus delphini/intermedius/pseudintermedius | ||
| Staphylococcus equorum | ||
| Staphylococcus sciuri | ||
| Staphylococcus succinus | ||
| Staphylococcus vitulinus | ||
| Streptococcus | Streptococcus equinus | |
| Streptococcus equorum | ||
| Streptococcus gallolyticus | ||
| Streptococcus parauberis | ||
| Proteobacteria | Acinetobacter | Acinebacter gandavensis |
| Acinetobacter lwoffii | ||
| Acinetobacter radioresistens | ||
| Acinetobacter schindleri | ||
| Acinetobacter terrestris | ||
| Acinetobacter towneri | ||
| Acetobacter | Acetobacter ascendens | |
| Aeromonas | Aeromonas aquatica/encheleia | |
| Aeromonas bestiarum/salmonicida | ||
| Aeromonas encheleia | ||
| Aeromonas media/rivipollensis | ||
| Aeromonas spp. | ||
| Alcaligenes | Alcaligenes faecalis | |
| Aliidiomarina | Aliidiomarina haloalkalitolerans | |
| Bowmanella | Bowmanella yangjiangensis | |
| Colwellia | Colwellia MabrTax21 | |
| Dyella | Dyella amyloliquefaciens | |
| Erwinia | Erwinia billingiae | |
| Escherichia | Escherichia vulneris | |
| Haemophilus | Haemophilus parahaemolyticus | |
| Kosakonia | Kosakonia sacchari | |
| Leclercia | Leclercia adecarboxylata | |
| Lelliottia | Lelliottia amnigena | |
| Marinomonas | Marinomonas arctica/shanghaiensis | |
| Massilia | Massilia aurea | |
| Mycobacterium | Mycobacterium MabrTax33 | |
| Neisseria | Neisseria musculi | |
| Pannonibacter | Pannonibacter indicus | |
| Pantoea | Pantoea agglomerans | |
| Pantoea sp. | ||
| Pantoea vagans | ||
| Petrocella | Petrocella atlantisensis | |
| Photorhabdus | Photorhabdus heterorhabditis | |
| Planococcus | Planococcus glaciei | |
| Pseudomonas | Pseudomonas alloputida/capeferrum/hunanensis | |
| Pseudomonas azotoformans/carnis/lactis/paralactis | ||
| Pseudomonas frederiksbergensis | ||
| Pseudomonas koreensis | ||
| Pseudomonas lundensis | ||
| Pseudomonas MabrTax108 | ||
| Pseudomonas MabrTax304 | ||
| Pseudomonas MabrTax342 | ||
| Pseudomonas proteolytica | ||
| Pseudomonas spp. | ||
| Pseudomonas tolaasii | ||
| Pseudomonas umsongensis | ||
| Psychrobacter | Psychrobacter pulmonis | |
| Rahnella | Rahnella aquatilis complex | |
| Rhizobium | Rhizobium MabrTax114 | |
| Serratia | Serratia myotis/quinivorans | |
| Shewanella | Shewanella sp. | |
| Stenotrophomonas | Stenotrophomonas rhizophila | |
| Testudinibacter | Testudinibacter aquarius | |
| Thauera | Thauera sedimentorum | |
| Thioalkalivibrio | Thioalkalivibrio versutus | |
| Actinobacteria | Agrococcus | Agrococcus citreus |
| Arthrobacter | Arthrobacter citreus | |
| Corynebacterium | Corynebacterium gallinarum | |
| Corynebacterium kalinowskii | ||
| Curtobacterium | Curtobacterium flaccumfaciens | |
| Exiguobacterium | Exiguobacterium acetylicum | |
| Exiguobacterium artemiae/sibiricum | ||
| Exiguobacterium mexicanum | ||
| Exiguobacterium oxidotolerans | ||
| Exiguobacterium soli | ||
| Exiguobacterium sp. | ||
| Glutamicibacter | Glutamicibacter spp. | |
| Kocuria | Kocuria carniphila | |
| Luteococcus | Luteococcus japonicus | |
| Microbacterium | Microbacterium sp. | |
| Paenarthrobacter | Paenarthrobacter aurescens | |
| Pseudarthrobacter | Pseudarthrobacter chlorophenolicus | |
| Sanguibacter | Sanguibacter inulinus | |
| Bacteroidetes | Chryseobacterium | Chryseobacterium indoltheticum |
| Myroides | Myroides odoratimimus | |
| Deinococcota | Deinococcus | Deinococcus budaensis/piscis |
| Pseudomonadota | Buttiauxella | Buttiauxella agrestis |
| Buttiauxella MabrTax2 | ||
| Verrucomicrobiota | Faecalibacterium | Faecalibacter rhinopitheci |
| Stable | Type | Dominant Species |
|---|---|---|
| A | Small private stable | Bacillus cereus |
| Exiguobacterium acetylicum | ||
| Macrococcus carouselicus | ||
| Staphylococcus aureus | ||
| B | Large riding school | Bacillus cereus |
| Carnobacterium inhibens | ||
| Exiguobacterium artemiae/sibiricum | ||
| Macrococcus carouselicus | ||
| C | Large riding school | Bacillus cereus |
| Bacillus pumilus | ||
| Carnobacterium inhibens | ||
| Exiguobacterium mexicanum | ||
| Macrococcus carouselicus | ||
| Pantoea agglomerans | ||
| Streptococcus parauberis | ||
| D | Large riding school | Bacillus cereus |
| Carnobacterium inhibens | ||
| Staphylococcus equorum |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matinpour, M.; Zettner, N.; Neumann, K.; Bäumer, L.; Burkovski, A. Analysis of the Culturable Skin Microbiome of Horses from Southern Germany. Microorganisms 2025, 13, 623. https://doi.org/10.3390/microorganisms13030623
Matinpour M, Zettner N, Neumann K, Bäumer L, Burkovski A. Analysis of the Culturable Skin Microbiome of Horses from Southern Germany. Microorganisms. 2025; 13(3):623. https://doi.org/10.3390/microorganisms13030623
Chicago/Turabian StyleMatinpour, Mahdis, Nadine Zettner, Kristin Neumann, Lisa Bäumer, and Andreas Burkovski. 2025. "Analysis of the Culturable Skin Microbiome of Horses from Southern Germany" Microorganisms 13, no. 3: 623. https://doi.org/10.3390/microorganisms13030623
APA StyleMatinpour, M., Zettner, N., Neumann, K., Bäumer, L., & Burkovski, A. (2025). Analysis of the Culturable Skin Microbiome of Horses from Southern Germany. Microorganisms, 13(3), 623. https://doi.org/10.3390/microorganisms13030623









