Effects of Long-Term Heavy Metal Exposure on the Species Diversity, Functional Diversity, and Network Structure of Oral Mycobiome
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Soil Sample Collection
2.2. Soil Heavy Metal Analysis
2.3. The Collection of Oral Buccal Mucosa Sample
2.4. Oral Microbial DNA Extraction and Sequencing Data Processing
2.5. Statistical Analysis
3. Results
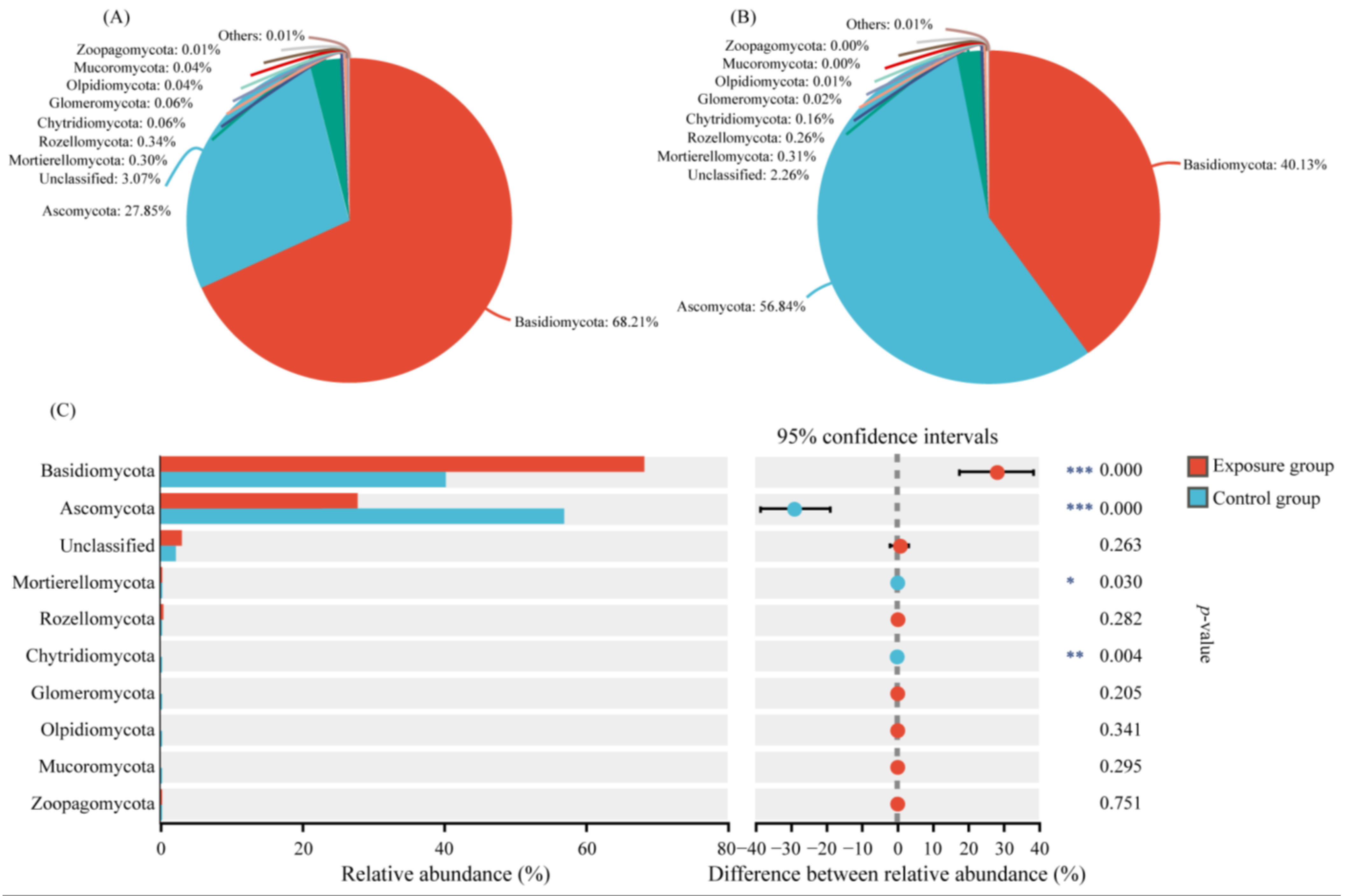
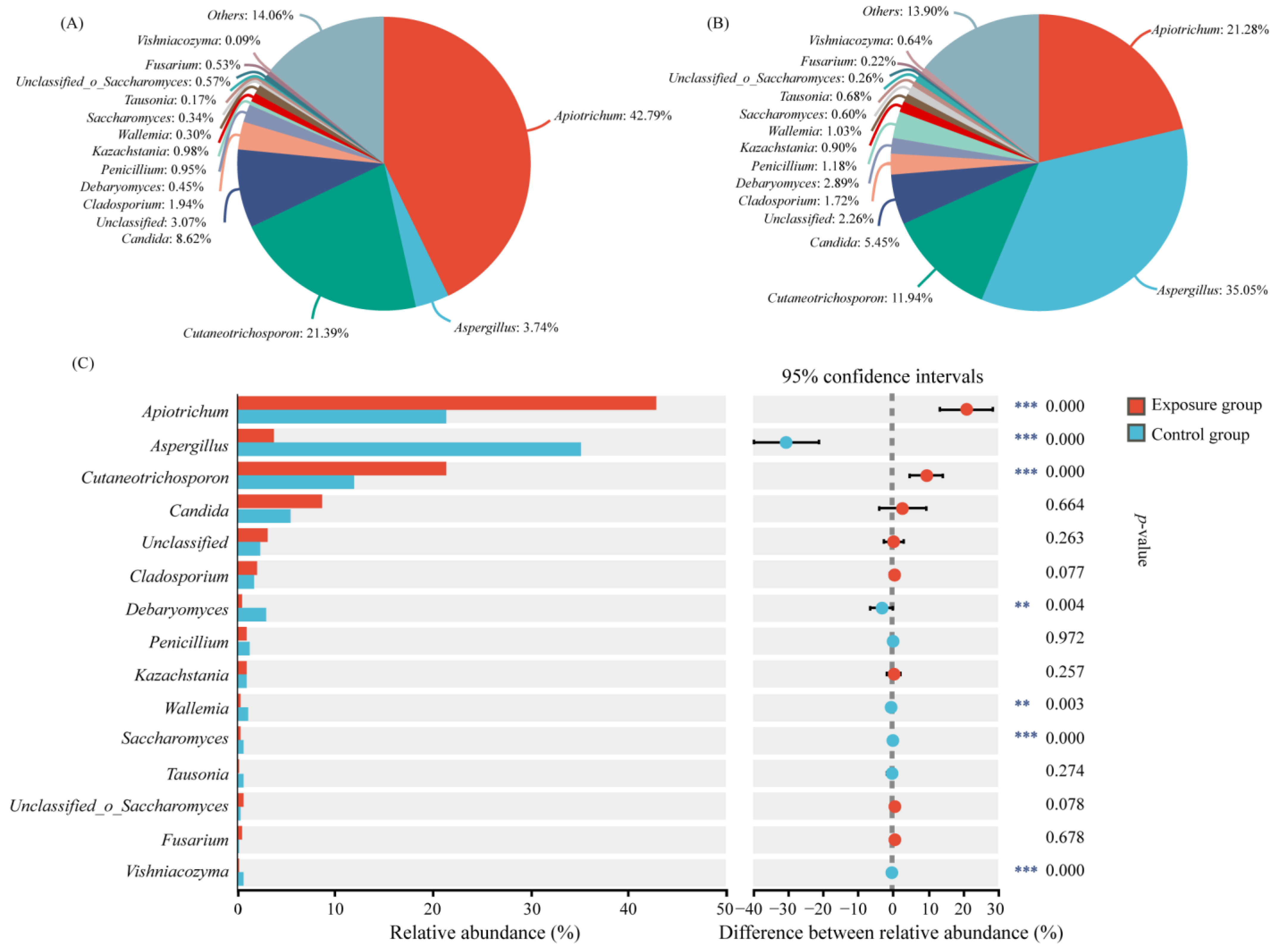
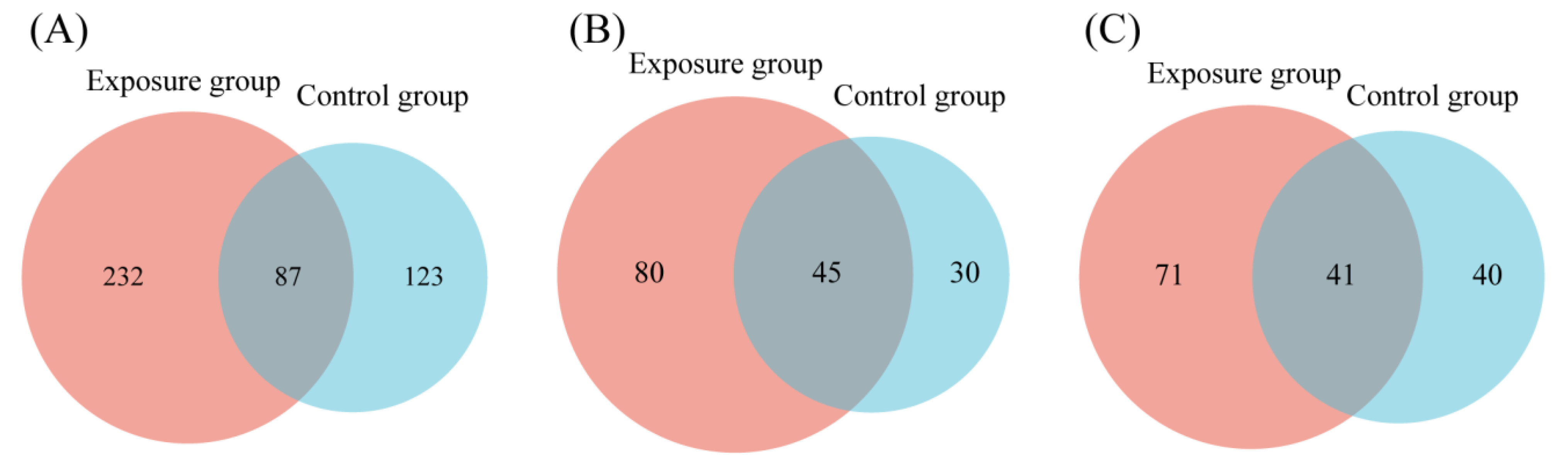
3.1. Fungal Community Composition
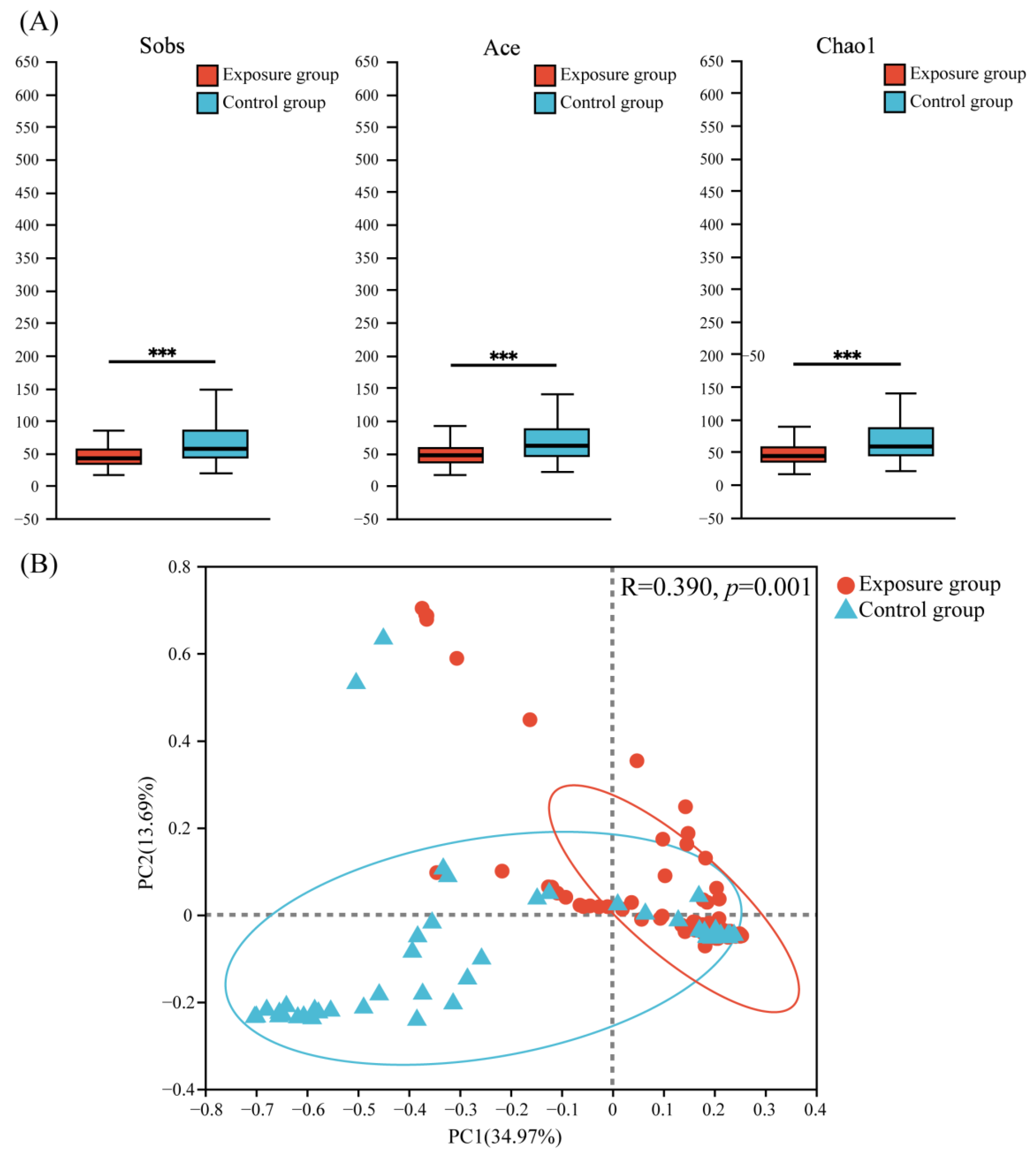
3.2. Diversity Analysis of Fungal Communities
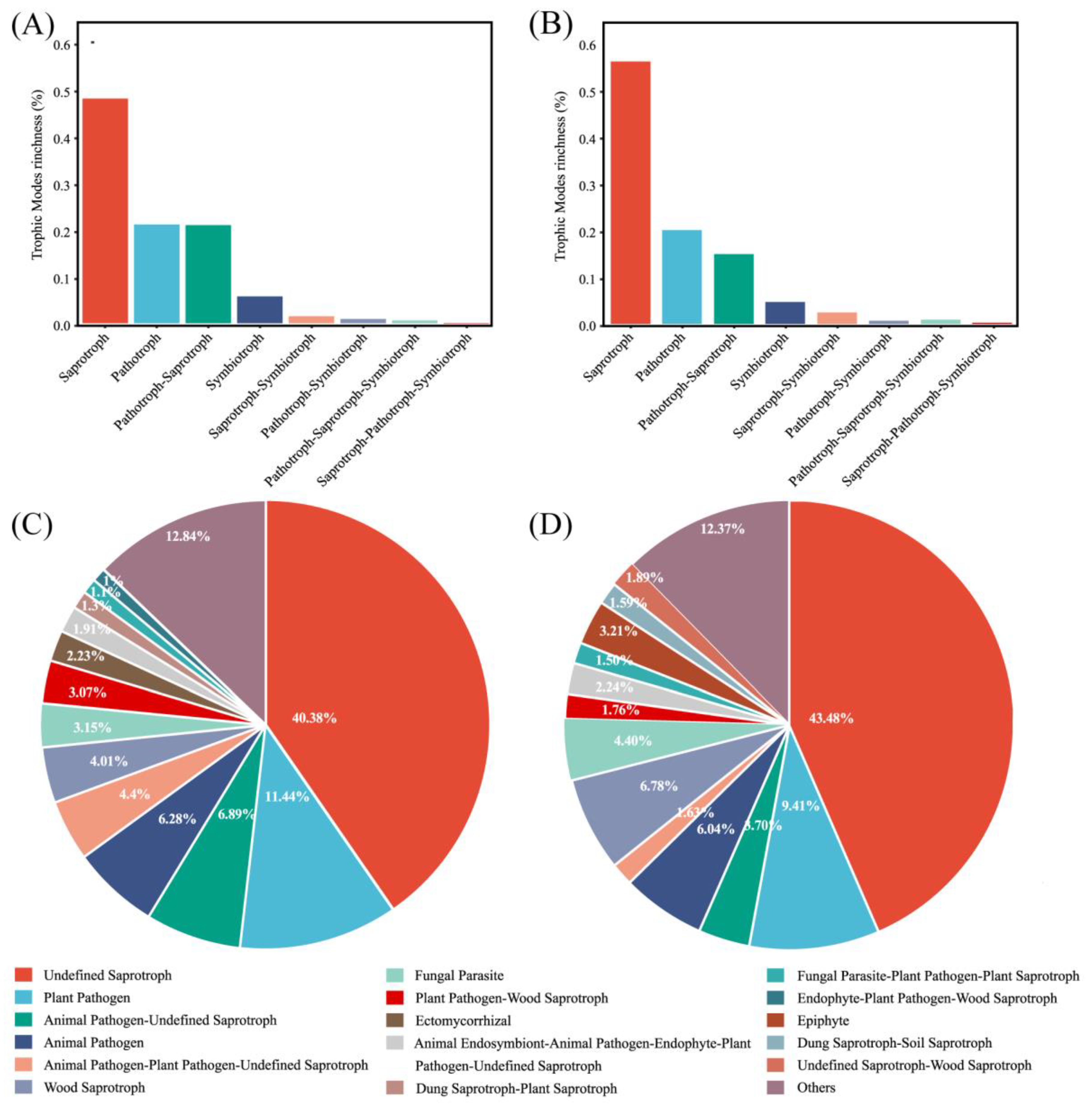
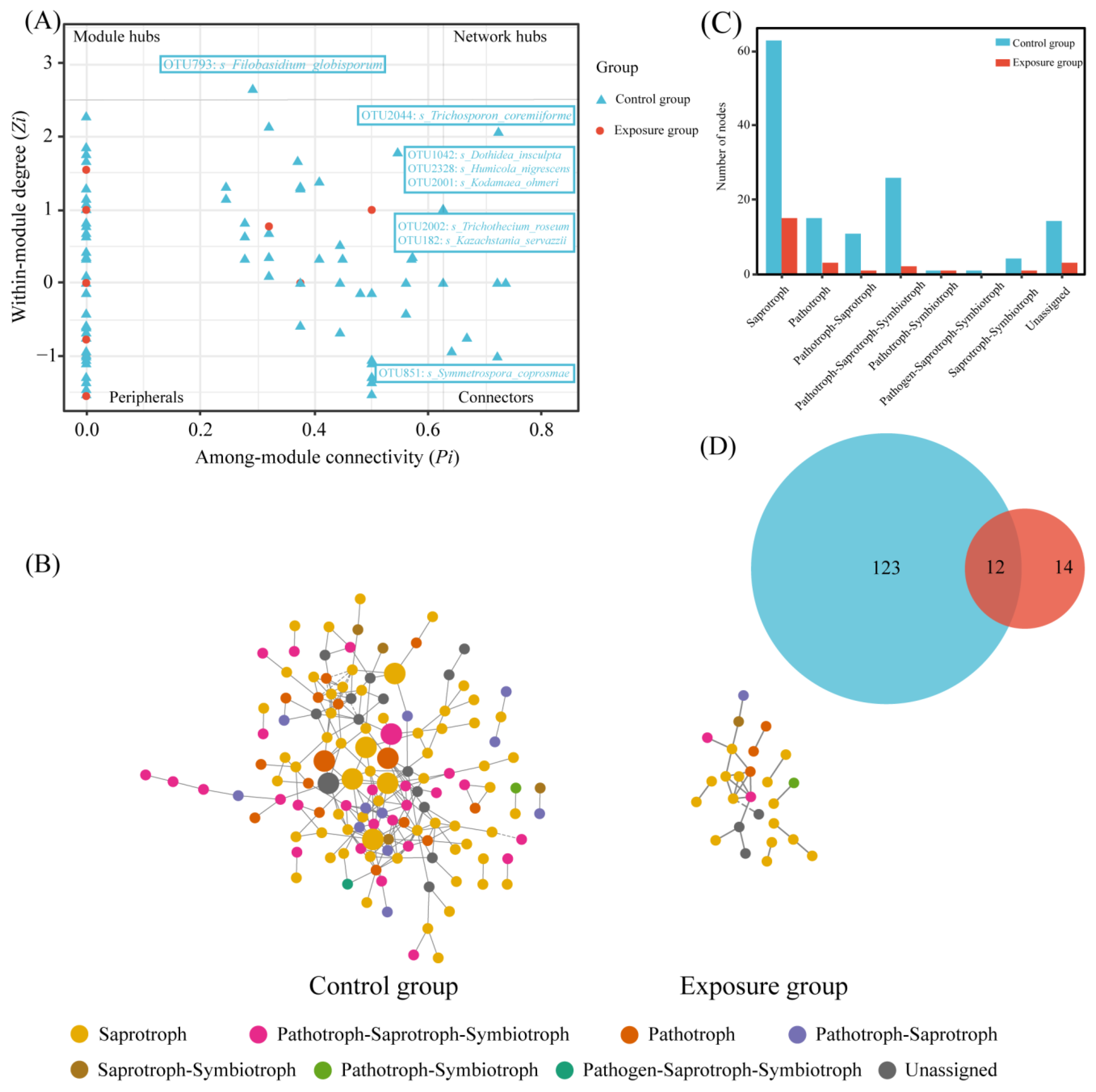
3.3. The Trophic Modes of Fungal Communities
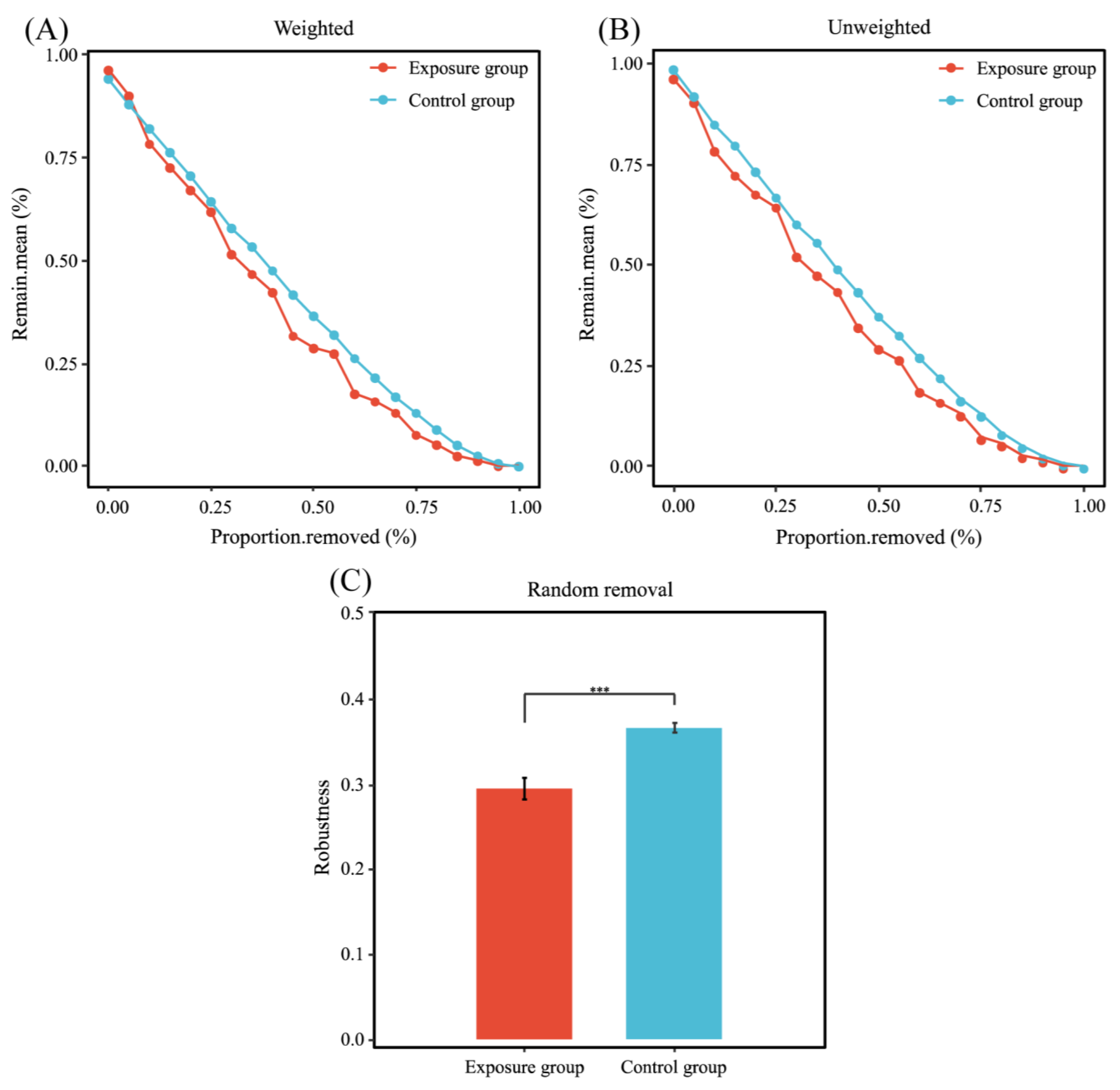
3.4. Patterns of Fungal Ecological Networks
4. Discussion
4.1. Effects of Heavy Metal Exposure on the Composition of Human Oral Fungal Communities
4.2. Trophic Strategies and Co-Occurrence Network Analysis of Human Oral Fungal Communities
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gonzalez, K.; Watts, T.L. Understanding the link between the oral microbiome and the development and progression of head and neck squamous cell carcinoma. Curr. Opin. Physiol. 2021, 23, 100471. [Google Scholar] [CrossRef]
- Morrison, A.G.; Sarkar, S.; Umar, S.; Lee, S.T.M.; Thomas, S.M. The Contribution of the Human Oral Microbiome to Oral Disease: A Review. Microorganisms 2023, 11, 318. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, K.; Chen, T.; Paster, B.J. A practical guide to the oral microbiome and its relation to health and disease. Oral Dis. 2016, 23, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Yano, A.; Shimoyama, Y.; Sato, T.; Sugiyama, Y.; Kishi, M. Associations of streptococci and fungi amounts in the oral cavity with nutritional and oral health status in institutionalized elders: A cross sectional study. BMC Oral Health 2021, 21, 590. [Google Scholar] [CrossRef]
- Diaz, P.I.; Dongari-Bagtzoglou, A. Critically Appraising the Significance of the Oral Mycobiome. J. Dent. Res. 2021, 100, 133–140. [Google Scholar] [CrossRef]
- Cheung, M.K.; Chan, J.Y.K.; Wong, M.C.S.; Wong, P.Y.; Lei, P.; Cai, L.; Lan, L.; Ho, W.C.S.; Yeung, A.C.M.; Chan, P.K.S.; et al. Determinants and Interactions of Oral Bacterial and Fungal Microbiota in Healthy Chinese Adults. Microbiol. Spectr. 2022, 10, e0241021. [Google Scholar] [CrossRef]
- Cannon, R.D. Oral Fungal Infections: Past, Present, and Future. Front. Oral Health 2022, 3, 838639. [Google Scholar] [CrossRef]
- He, S.; Sun, Y.; Sun, W.; Tang, M.; Meng, B.; Liu, Y.; Kong, Q.; Li, Y.; Yu, J.; Li, J. Oral microbiota disorder in GC patients revealed by 2b-RAD-M. J. Transl. Med. 2023, 21, 831. [Google Scholar] [CrossRef]
- Sajid, M.; Sharma, P.; Srivastava, S.; Hariprasad, R.; Singh, H.; Bharadwaj, M. Smokeless tobacco consumption induces dysbiosis of oral mycobiome: A pilot study. Appl. Microbiol. Biotechnol. 2022, 106, 5643–5657. [Google Scholar] [CrossRef]
- Baker, J.L.; Bor, B.; Agnello, M.; Shi, W.; He, X. Ecology of the Oral Microbiome: Beyond Bacteria. Trends Microbiol. 2017, 25, 362–374. [Google Scholar] [CrossRef]
- Acosta-Pagán, K.; Bolaños-Rosero, B.; Pérez, C.; Ortíz, A.P.; Godoy-Vitorino, F. Ecological competition in the oral mycobiome of Hispanic adults living in Puerto Rico associates with periodontitis. J. Oral Microbiol. 2024, 16, 2316485. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Gao, P.; Zong, D.-P.; Liu, J.-J.; Zhang, M.-Y.; Wang, C.-C.; Wang, Z.-X.; Wang, J.-M.; Niu, Y.-Y.; Xiang, P. The oral bioaccessibility and gingival cytotoxicity of metal(loid)s in wild vegetables from mining areas: Implication for human oral health. Front. Nutr. 2022, 9, 1042300. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.; Rabow, S.; Rousk, J. Can heavy metal pollution stress reduce microbial carbon-use efficiencies? Soil Biol. Biochem. 2024, 195, 109458. [Google Scholar] [CrossRef]
- Pei, S.; Feng, L.; Zhang, Y.; Liu, J.; Li, J.; Zheng, Q.; Liu, X.; Luo, B.; Ruan, Y.; Li, H.; et al. Effects of long-term metal exposure on the structure and co-occurrence patterns of the oral microbiota of residents around a mining area. Front. Microbiol. 2023, 14, 1264619. [Google Scholar] [CrossRef]
- Davis, E.; Bakulski, K.M.; Goodrich, J.M.; Peterson, K.E.; Marazita, M.L.; Foxman, B. Low levels of salivary metals, oral microbiome composition and dental decay. Sci. Rep. 2020, 10, 14640. [Google Scholar] [CrossRef]
- Zhang, W.; Qi, T.; Yao, L.; Wang, W.; Yu, F.; Yan, Y.; Salama, E.-S.; Su, S.; Bai, M. Influence of Environmental Factors on Salivary Microbiota and Their Metabolic Pathway: Next-Generation Sequencing Approach. Microb. Ecol. 2022, 85, 317–329. [Google Scholar] [CrossRef]
- Adler, C.J.; Cao, K.A.L.; Hughes, T.; Kumar, P.; Austin, C. How does the early life environment influence the oral microbiome and determine oral health outcomes in childhood? BioEssays 2021, 43, e2000314. [Google Scholar] [CrossRef]
- Dong, K.; Wu, K.; Zheng, T.; Yue, J.; Wang, W.; Luo, R.; You, L.; He, X.; Li, J.; Hong, Z.; et al. Comparative Study of Oral Bacteria and Fungi Microbiota in Tibetan and Chinese Han Living at Different Altitude. Tohoku J. Exp. Med. 2021, 254, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.H.; Song, Z.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Bello, A.; Wang, B.; Zhao, Y.; Yang, W.; Ogundeji, A.; Deng, L.; Egbeagu, U.U.; Yu, S.; Zhao, L.; Li, D.; et al. Composted biochar affects structural dynamics, function and co-occurrence network patterns of fungi community. Sci. Total Environ. 2021, 775, 145672. [Google Scholar] [CrossRef]
- Amit, G.; Bashan, A. Top-down identification of keystone taxa in the microbiome. Nat. Commun. 2023, 14, 3951. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Gu, H.; Chen, S.; Ai, W.; Dang, Y.; Ai, S.; Li, Z. Assessment of heavy metal pollution and preschool children health risk in urban street dusts from different functional areas in a typical industrial and mining city, NW China. Environ. Geochem. Health 2023, 45, 7199–7214. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Xia, D.; Yu, Y.; Jia, J.; Nie, Y.; Wang, X. Detecting the sensitivity of magnetic response on different pollution sources—A case study from typical mining cities in northwestern China. Environ. Pollut. 2015, 207, 288–298. [Google Scholar] [CrossRef]
- Liu, B.; Ma, X.; Ai, S.; Zhu, S.; Zhang, W.; Zhang, Y. Spatial distribution and source identification of heavy metals in soils under different land uses in a sewage irrigation region, northwest China. J. Soils Sediments 2016, 16, 1547–1556. [Google Scholar] [CrossRef]
- HJ/ST166-2004; Technical Specification for Soil Environmental Monitoring. Ministry of Environmental Protection of the People’s Republic of China: Beijing, China, 2004.
- Adams, R.I.; Miletto, M.; Taylor, J.W.; Bruns, T.D. Dispersal in microbes: Fungi in indoor air are dominated by outdoor air and show dispersal limitation at short distances. ISME J. 2013, 7, 1262–1273. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Gonzalez Peña, A.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Aliyu, H.; Gorte, O.; de Maayer, P.; Neumann, A.; Ochsenreither, K. Genomic insights into the lifestyles, functional capacities and oleagenicity of members of the fungal family Trichosporonaceae. Sci. Rep. 2020, 10, 2780. [Google Scholar] [CrossRef]
- Du, L.; Zhong, S.; Luo, K.; Yang, S.; Xia, J.; Chen, Q. Effect of metal pollution on the distribution and co-occurrence pattern of bacterial, archaeal and fungal communities throughout the soil profiles. Chemosphere 2023, 315, 137692. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Yang, X.; Li, C.; Song, Z. The Oral Microbiota: Community Composition, Influencing Factors, Pathogenesis, and Interventions. Front. Microbiol. 2022, 13, 895537. [Google Scholar] [CrossRef]
- Imabayashi, Y.; Moriyama, M.; Takeshita, T.; Ieda, S.; Hayashida, J.-N.; Tanaka, A.; Maehara, T.; Furukawa, S.; Ohta, M.; Kubota, K.; et al. Molecular analysis of fungal populations in patients with oral candidiasis using next-generation sequencing. Sci. Rep. 2016, 6, 28110. [Google Scholar] [CrossRef] [PubMed]
- McFarland, L.V. Systematic review and meta-analysis of Saccharomyces boulardiiin adult patients. World J. Gastroenterol. 2010, 16, 2202. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Parra, M.A.G.; Cruz-Neri, R.U.; Trujillo-Trujillo, X.A.R.; Dominguez-Mora, J.J.; Cruz-Neri, H.I.; Guzmán-Díaz, J.M.; Guzmán-Ruvalcaba, M.J.; Vega-Gastelum, J.O.; Ascencio-Díaz, K.V.; Zarate-Casas, M.F.; et al. Effectiveness of Saccharomyces boulardii CNCM I-745 probiotic in acute inflammatory viral diarrhoea in adults: Results from a single-centre randomized trial. BMC Gastroenterol. 2023, 23, 229. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Liu, J.; Wen, X.; Zhang, G.; Cai, J.; Qiao, Z.; An, Z.; Zheng, J.; Li, L. Unique Probiotic Properties and Bioactive Metabolites of Saccharomyces boulardii. Probiotics Antimicrob. Proteins 2022, 15, 967–982. [Google Scholar] [CrossRef]
- James, S.A.; Bond, C.J.; Stanley, R.; Ravella, S.R.; Péter, G.; Dlauchy, D.; Roberts, I.N. Apiotrichum terrigenum sp. nov., a soil-associated yeast found in both the UK and mainland Europe. Int. J. Syst. Evol. Microbiol. 2016, 66, 5046–5050. [Google Scholar] [CrossRef]
- Peng, L.; Jiang, G.M.; Ou, J.Y.; Zeng, L.T.; Zhang, H.H.; Chen, D.-Q.; Jiang, Y.-T. Molecular identification and biological characteristic analysis of an Apiotrichum mycotoxinivorans (formerly Trichosporon mycotoxinivorans) strain isolated from sputum specimens of a pediatric patient with pneumonia. J. Mycol. Médicale 2019, 29, 120–126. [Google Scholar] [CrossRef]
- Almeida, J.N.d., Jr.; Francisco, E.C.; Barberino, M.G.M.d.A.; Filho, L.V.R.F.d.S.; Brandão, O.M.; Colombo, A.L.; Padovan, A.C.B. Emergence of Trichosporon mycotoxinivorans (Apiotrichum mycotoxinivorans) invasive infections in Latin America. Memórias Inst. Oswaldo Cruz 2017, 112, 719–722. [Google Scholar] [CrossRef]
- Péter, G.; Mounier, J.; Garnier, L.; Soós, D.; Dlauchy, D. Cutaneotrichosporon suis sp. nov., a lipolytic yeast species from food and food-related environment. Int. J. Syst. Evol. Microbiol. 2019, 69, 2367–2371. [Google Scholar] [CrossRef]
- Martínez-Herrera, E.; Duarte-Escalante, E.; Reyes-Montes, M.d.R.; Arenas, R.; Acosta-Altamirano, G.; Moreno-Coutiño, G.; Vite-Garín, T.M.; Meza-Robles, A.; Frías-De-León, M.G. Molecular identification of yeasts from the order Trichosporonales causing superficial infections. Rev. Iberoam. Micol. 2021, 38, 119–124. [Google Scholar] [CrossRef]
- Priya, A.; Pandian, S.K. Piperine Impedes Biofilm Formation and Hyphal Morphogenesis of Candida albicans. Front. Microbiol. 2020, 11, 756. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y.; Du, W.; Yang, S.; He, Y.; Zhao, X.; Sun, W.; Chen, Q. Insights into the responses of fungal taxonomy and function to different metal(loid) contamination levels. Sci. Total Environ. 2023, 877, 162931. [Google Scholar] [CrossRef]
- Kang, P.; Pan, Y.; Ran, Y.; Li, W.; Shao, M.; Zhang, Y.; Ji, Q.; Ding, X. Soil saprophytic fungi could be used as an important ecological indicator for land management in desert steppe. Ecol. Indic. 2023, 150, 110224. [Google Scholar] [CrossRef]
- McElwain, T.F.; Thumbi, S.M. Animal pathogens and their impact on animal health, the economy, food security, food safety and public health. Rev. Sci. Tech. L’oie 2017, 36, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Shigyo, N.; Hirao, T. Saprotrophic and ectomycorrhizal fungi exhibit contrasting richness patterns along elevational gradients in cool-temperate montane forests. Fungal Ecol. 2021, 50, 101036. [Google Scholar] [CrossRef]
- Tian, L.; Chen, P.; Gao, Z.; Gao, X.; Feng, B. Deciphering the distinct mechanisms shaping the broomcorn millet rhizosphere bacterial and fungal communities in a typical agricultural ecosystem of Northern China. Plant Soil 2022, 474, 469–484. [Google Scholar] [CrossRef]
- Ren, G.; Ma, A.; Zhang, Y.; Deng, Y.; Zheng, G.; Zhuang, X.; Zhuang, G.; Fortin, D. Electron acceptors for anaerobic oxidation of methane drive microbial community structure and diversity in mud volcanoes. Environ. Microbiol. 2018, 20, 2370–2385. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.-Y.; Lin, W.-Z.; Li, Y.-L.; Bi, C.; Du, L.-J.; Liu, Y.; Zhou, L.-J.; Liu, T.; Xu, S.; Shi, C.-J.; et al. Characteristics and Correlations of the Oral and Gut Fungal Microbiome with Hypertension. Microbiol. Spectr. 2023, 11, e0195622. [Google Scholar] [CrossRef]
| Exposure Group | Control Group | p-Value | |
|---|---|---|---|
| Mo (ng/g) | 0.22 ± 0.16 | 0.08 ± 0.03 | 0.001 |
| Cd (ng/g) | 8.42 ± 14.09 | 0.10 ± 0.04 | 0.001 |
| Sb (ng/g) | 0.02 ± 0.01 | 0.00 ± 0.00 | 0.001 |
| Cu (ng/g) | 117.33 ± 53.13 | 17.38 ± 3.89 | 0.001 |
| Zn (ng/g) | 420.23 ± 709.09 | 47.47 ± 10.16 | 0.001 |
| Hg (ng/g) | 0.42 ± 0.38 | 0.02 ± 0.02 | 0.002 |
| Pb (ng/g) | 150.88 ± 146.13 | 12.15 ± 2.57 | 0.001 |
| Network Indices | Exposure | Control | Exposure | Control |
|---|---|---|---|---|
| Empirical | Random | |||
| Total nodes | 26 | 135 | n.a | n.a |
| Total links | 24 | 224 | n.a | n.a |
| RMT cut-off | 0.86 | 0.86 | n.a | n.a |
| R square of power-law (R2) | 0.905 | 0.817 | n.a | n.a |
| Average clustering coefficient (avgCC) | 0.127 | 0.202 | 0.007 +/− 0.019 | 0.015 +/− 0.011 |
| Average path distance (GD) | 2.558 | 4.634 | 3.017 +/− 0.358 | 3.927 +/− 0.094 |
| Geodesic efficiency (E) | 0.536 | 0.269 | 0.434 +/− 0.042 | 0.296 +/− 0.005 |
| Harmonic geodesic distance (HD) | 1.867 | 3.719 | 2.326 +/− 0.226 | 3.376 +/− 0.06 |
| Centralization of degree (CD) | 0.126 | 0.072 | 0.126 +/− 0 | 0.072 +/− 0 |
| Centralization of betweenness (CB) | 0.086 | 0.19 | 0.234 +/− 0.094 | 0.14 +/− 0.024 |
| Centralization of stress centrality (CS) | 0.162 | 0.426 | 0.017 +/− 0.007 | 0.056 +/− 0.031 |
| Centralization of eigenvector centrality (CE) | 0.828 | 0.894 | 0.778 +/− 0.032 | 0.823 +/− 0.019 |
| Centralization of closeness centrality (CCL) | 0.033 | 0.015 | 0.056 +/− 0.018 | 0.046 +/− 0.038 |
| Density (D) | 0.074 | 0.025 | 0.074 +/− 0 | 0.025 +/− 0 |
| Reciprocity | 1 | 1 | 1 +/− 0 | 1 +/− 0 |
| Transitivity (Trans) | 0.409 | 0.278 | 0.082 +/− 0.057 | 0.045 +/− 0.012 |
| Connectedness (Con) | 0.265 | 0.726 | 0.52 +/− 0.117 | 0.911 +/− 0.043 |
| Efficiency | 0.81 | 0.975 | 0.992 +/− 0.005 | 0.994 +/− 0 |
| Hierarchy | 0 | 0 | 0.074 +/− 0 | 0.025 +/− 0 |
| Lubness | 1 | 1 | 0.446 +/− 0.15 | 0.226 +/− 0.043 |
| Modularity | 0.629 | 0.662 | 0.602 +/− 0.028 | 0.537 +/− 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Pei, S.; Feng, L.; Liu, J.; Zheng, Q.; Liu, X.; Ruan, Y.; Hu, W.; Zhang, L.; Niu, J.; et al. Effects of Long-Term Heavy Metal Exposure on the Species Diversity, Functional Diversity, and Network Structure of Oral Mycobiome. Microorganisms 2025, 13, 622. https://doi.org/10.3390/microorganisms13030622
Li J, Pei S, Feng L, Liu J, Zheng Q, Liu X, Ruan Y, Hu W, Zhang L, Niu J, et al. Effects of Long-Term Heavy Metal Exposure on the Species Diversity, Functional Diversity, and Network Structure of Oral Mycobiome. Microorganisms. 2025; 13(3):622. https://doi.org/10.3390/microorganisms13030622
Chicago/Turabian StyleLi, Jia, Shuwei Pei, Lu Feng, Jiangyun Liu, Qiwen Zheng, Xingrong Liu, Ye Ruan, Weigang Hu, Li Zhang, Jingping Niu, and et al. 2025. "Effects of Long-Term Heavy Metal Exposure on the Species Diversity, Functional Diversity, and Network Structure of Oral Mycobiome" Microorganisms 13, no. 3: 622. https://doi.org/10.3390/microorganisms13030622
APA StyleLi, J., Pei, S., Feng, L., Liu, J., Zheng, Q., Liu, X., Ruan, Y., Hu, W., Zhang, L., Niu, J., & Tian, T. (2025). Effects of Long-Term Heavy Metal Exposure on the Species Diversity, Functional Diversity, and Network Structure of Oral Mycobiome. Microorganisms, 13(3), 622. https://doi.org/10.3390/microorganisms13030622






