Influence of Sodium Chloride on the Behaviour of Pseudomonas fluorescens in Ripened Sheep Cheese
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Bacterial Strains and Culture Conditions
2.3. Cheese Production
2.4. Physicochemical Analyses
2.5. Microbiological Analyses
2.6. Statistical Analysis
3. Results
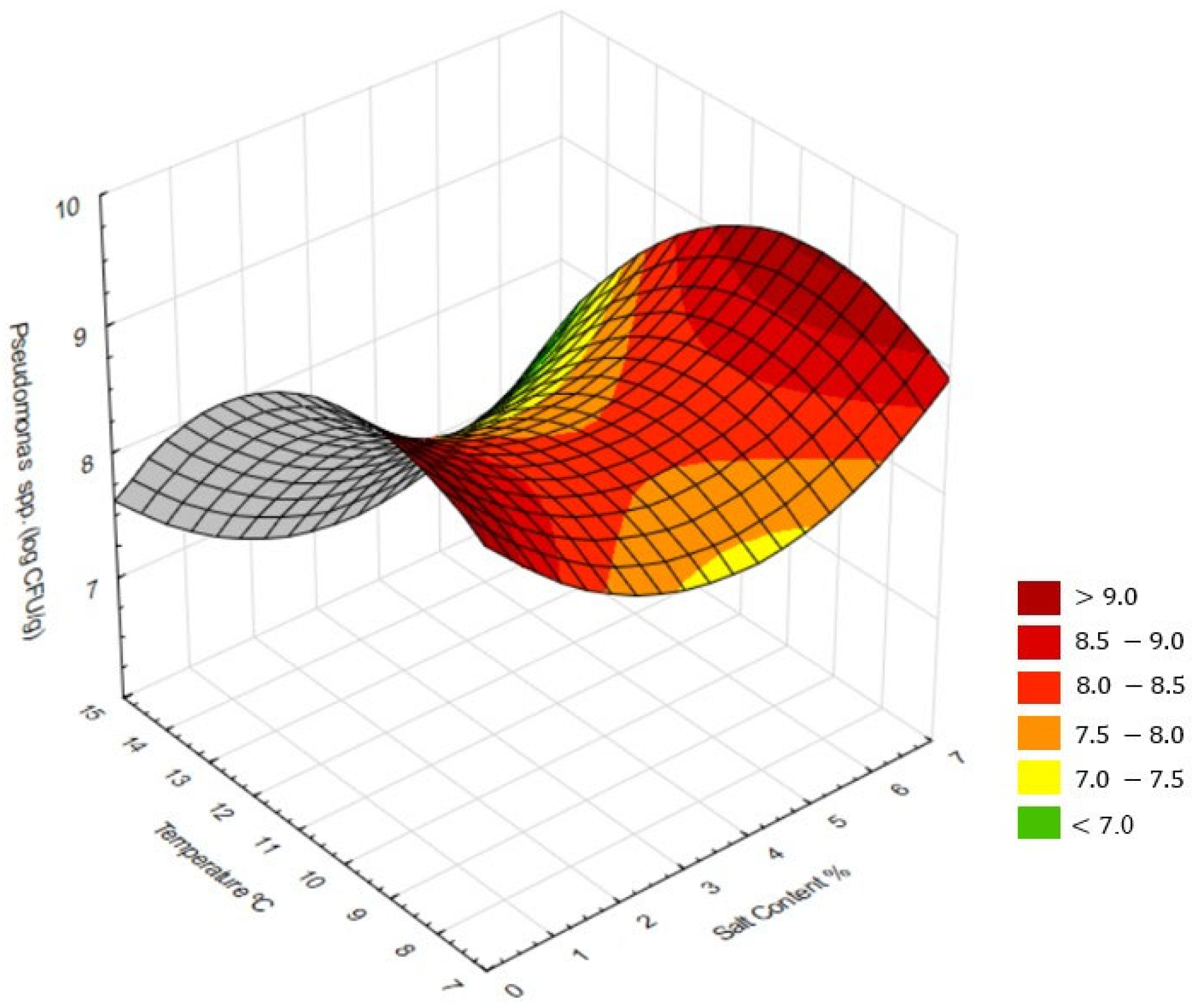
3.1. Response Surface Analysis (RSM)
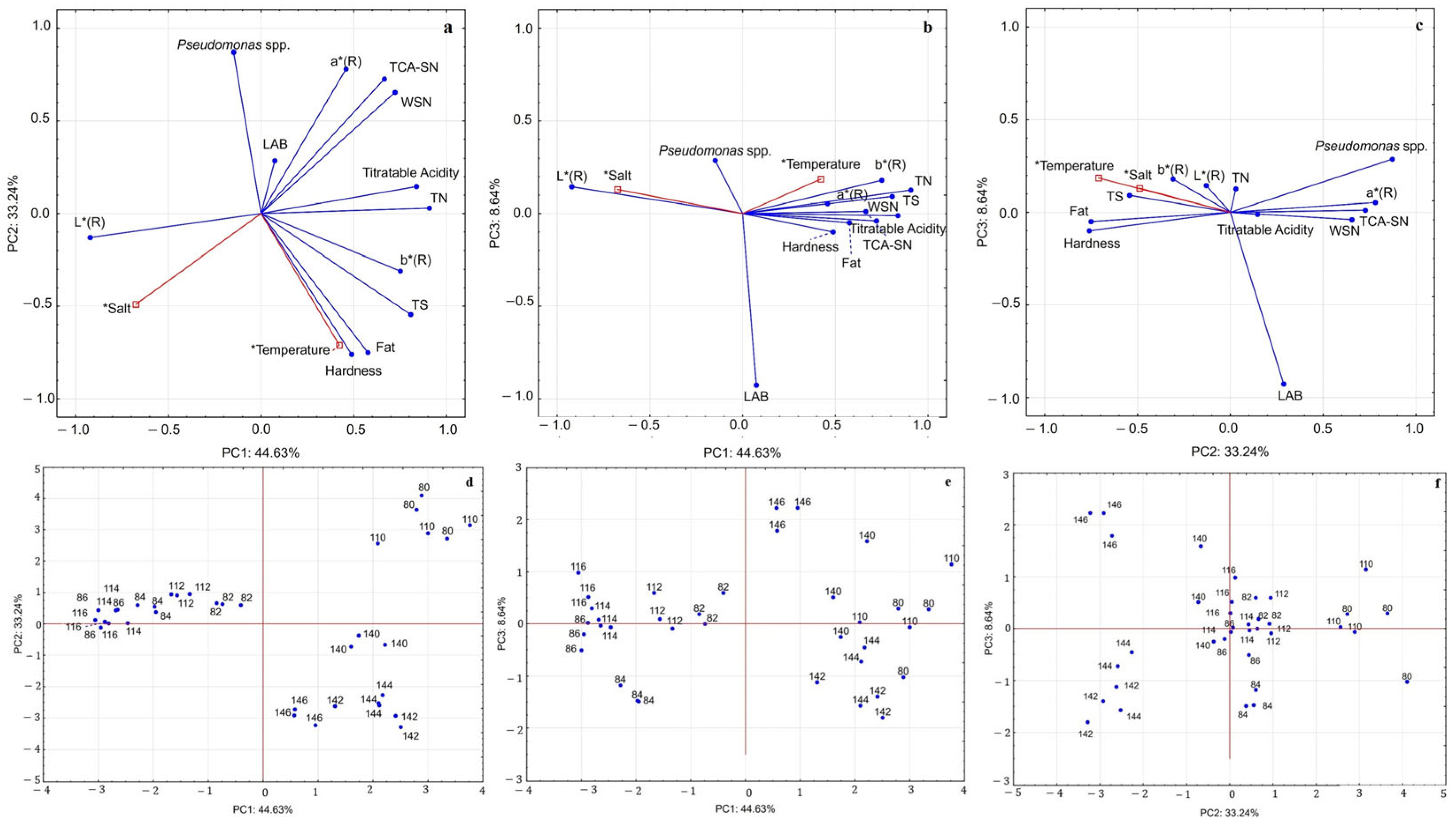
3.2. Principal Component Analysis (PCA)
4. Discussion
4.1. Microbiological Dynamics
4.2. Physicochemical and Proteolysis Composition
4.3. Texture Development and Colour Changes
4.4. Response Surface Analysis (RSM)
4.5. Principal Component Analysis (PCA)
5. Conclusions
- Low temperatures without salt enhance colour defects caused by the growth of undesirable microorganisms, particularly Pseudomonas spp.
- Very high salt levels, although contributing to partial control of Pseudomonas spp., delay ripening and result in cheeses with a pale, uncharacteristic appearance.
- Higher ripening temperatures accelerate both proteolysis and dehydration, leading to harder cheeses.
- Moderate salt levels (2%) combined with higher ripening temperatures promote more controlled maturation, with a uniform characteristic appearance and no structural defects.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Araújo-Rodrigues, H.; Tavaria, F.K.; dos Santos, M.T.P.G.; Alvarenga, N.; Pintado, M.M. A Review on Microbiological and Technological Aspects of Serpa PDO Cheese: An Ovine Raw Milk Cheese. Int. Dairy J. 2020, 100, 104561. [Google Scholar] [CrossRef]
- Dias, J.; Lage, P.; Garrido, A.; Machado, E.; Conceição, C.; Gomes, S.; Martins, A.; Paulino, A.; Duarte, M.F.; Alvarenga, N. Evaluation of Gas Holes in “Queijo de Nisa” PDO Cheese Using Computer Vision. J. Food Sci. Technol. 2021, 58, 1072–1080. [Google Scholar] [CrossRef]
- Montel, M.-C.; Buchin, S.; Mallet, A.; Delbes-Paus, C.; Vuitton, D.A.; Desmasures, N.; Berthier, F. Traditional Cheeses: Rich and Diverse Microbiota with Associated Benefits. Int. J. Food Microbiol. 2014, 177, 136–154. [Google Scholar] [CrossRef] [PubMed]
- Reis, P.J.M.; Malcata, F.X. Current State of Portuguese Dairy Products from Ovine and Caprine Milks. Small Rumin. Res. 2011, 101, 122–133. [Google Scholar] [CrossRef]
- Park, Y.W.; Juárez, M.; Ramos, M.; Haenlein, G.F.W. Physico-Chemical Characteristics of Goat and Sheep Milk. Small Rumin. Res. 2007, 68, 88–113. [Google Scholar] [CrossRef]
- Estrada, O.; Ariño, A.; Juan, T. Salt Distribution in Raw Sheep Milk Cheese during Ripening and the Effect on Proteolysis and Lipolysis. Foods 2019, 8, 100. [Google Scholar] [CrossRef]
- Zheng, X.; Shi, X.; Wang, B. A Review on the General Cheese Processing Technology, Flavor Biochemical Pathways and the Influence of Yeasts in Cheese. Front. Microbiol. 2021, 12, 703284. [Google Scholar] [CrossRef]
- Dugat-Bony, E.; Sarthou, A.-S.; Perello, M.-C.; de Revel, G.; Bonnarme, P.; Helinck, S. The Effect of Reduced Sodium Chloride Content on the Microbiological and Biochemical Properties of a Soft Surface-Ripened Cheese. J. Dairy Sci. 2016, 99, 2502–2511. [Google Scholar] [CrossRef]
- Guerreiro, O.; Velez, Z.; Alvarenga, N.; Matos, C.; Duarte, M. Molecular Screening of Ovine Mastitis in Different Breeds. J. Dairy Sci. 2013, 96, 752–760. [Google Scholar] [CrossRef]
- Rocha, R.; Pinto, R.P.; Vaz-Velho, M.; Fernandes, P.; Santos, J. Microbiological Characterization of Protected Designation of Origin Azeitão Cheese. Food Res. 2025, 9, 245–255. [Google Scholar] [CrossRef]
- Gonçalves, M.T.P.; Benito, M.J.; Córdoba, M.D.G.; Egas, C.; Merchán, A.V.; Galván, A.I.; Ruiz-Moyano, S. Bacterial Communities in Serpa Cheese by Culture Dependent Techniques, 16S RRNA Gene Sequencing and High-throughput Sequencing Analysis. J. Food Sci. 2018, 83, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
- Santamarina-García, G.; Yap, M.; Crispie, F.; Amores, G.; Lordan, C.; Virto, M.; Cotter, P.D. Shotgun Metagenomic Sequencing Reveals the Influence of Artisanal Dairy Environments on the Microbiomes, Quality, and Safety of Idiazabal, a Raw Ewe Milk PDO Cheese. Microbiome 2024, 12, 262. [Google Scholar] [CrossRef] [PubMed]
- Dugat-Bony, E.; Bonnarme, P.; Fraud, S.; Catellote, J.; Sarthou, A.-S.; Loux, V.; Rué, O.; Bel, N.; Chuzeville, S.; Helinck, S. Effect of Sodium Chloride Reduction or Partial Substitution with Potassium Chloride on the Microbiological, Biochemical and Sensory Characteristics of Semi-Hard and Soft Cheeses. Food Res. Int. 2019, 125, 108643. [Google Scholar] [CrossRef] [PubMed]
- Neviani, E.; Gatti, M.; Gardini, F.; Levante, A. Microbiota of Cheese Ecosystems: A Perspective on Cheesemaking. Foods 2025, 14, 830. [Google Scholar] [CrossRef]
- Rampanti, G.; Ferrocino, I.; Harasym, J.; Foligni, R.; Cardinali, F.; Orkusz, A.; Milanović, V.; Franciosa, I.; Garofalo, C.; Mannozzi, C.; et al. Queijo Serra Da Estrela PDO Cheese: Investigation into Its Morpho-Textural Traits, Microbiota, and Volatilome. Foods 2022, 12, 169. [Google Scholar] [CrossRef]
- Settanni, L.; Moschetti, G. Non-Starter Lactic Acid Bacteria Used to Improve Cheese Quality and Provide Health Benefits. Food Microbiol. 2010, 27, 691–697. [Google Scholar] [CrossRef]
- Quintieri, L.; Fanelli, F.; Caputo, L. Antibiotic Resistant Pseudomonas spp. Spoilers in Fresh Dairy Products: An Underestimated Risk and the Control Strategies. Foods 2019, 8, 372. [Google Scholar] [CrossRef]
- Meng, L.; Zhang, Y.; Liu, H.; Zhao, S.; Wang, J.; Zheng, N. Characterization of Pseudomonas spp. and Associated Proteolytic Properties in Raw Milk Stored at Low Temperatures. Front. Microbiol. 2017, 8, 2158. [Google Scholar] [CrossRef]
- Maier, C.; Huptas, C.; von Neubeck, M.; Scherer, S.; Wenning, M.; Lücking, G. Genetic Organization of the AprX-LipA2 Operon Affects the Proteolytic Potential of Pseudomonas Species in Milk. Front. Microbiol. 2020, 11, 1190. [Google Scholar] [CrossRef]
- Savijoki, K.; Ingmer, H.; Varmanen, P. Proteolytic Systems of Lactic Acid Bacteria. Appl. Microbiol. Biotechnol. 2006, 71, 394–406. [Google Scholar] [CrossRef]
- Glück, C.; Rentschler, E.; Krewinkel, M.; Merz, M.; von Neubeck, M.; Wenning, M.; Scherer, S.; Stoeckel, M.; Hinrichs, J.; Stressler, T.; et al. Thermostability of Peptidases Secreted by Microorganisms Associated with Raw Milk. Int. Dairy J. 2016, 56, 186–197. [Google Scholar] [CrossRef]
- McPhee, J.B.; Lewenza, S.; Hancock, R.E.W. Cationic Antimicrobial Peptides Activate a Two-component Regulatory System, PmrA-PmrB, That Regulates Resistance to Polymyxin B and Cationic Antimicrobial Peptides in Pseudomonas aeruginosa. Mol. Microbiol. 2003, 50, 205–217. [Google Scholar] [CrossRef]
- Ivette Béjar-Lio, G.; Gutiérrez-Méndez, N.; Virginia Nevárez-Moorillón, G. Microbiological and Physicochemical Characteristics of Chihuahua Cheese Manufactured with Raw Milk. AIMS Agric. Food 2020, 5, 86–101. [Google Scholar] [CrossRef]
- Scatamburlo, T.M.; Yamazi, A.K.; Cavicchioli, V.Q.; Pieri, F.A.; Nero, L.A. Spoilage Potential of Pseudomonas Species Isolated from Goat Milk. J. Dairy Sci. 2015, 98, 759–764. [Google Scholar] [CrossRef]
- Martin, N.H.; Murphy, S.C.; Ralyea, R.D.; Wiedmann, M.; Boor, K.J. When Cheese Gets the Blues: Pseudomonas fluorescens as the Causative Agent of Cheese Spoilage. J. Dairy Sci. 2011, 94, 3176–3183. [Google Scholar] [CrossRef]
- Yoon, Y.; Lee, S.; Choi, K.H. Microbial Benefits and Risks of Raw Milk Cheese. Food Control 2016, 63, 201–215. [Google Scholar] [CrossRef]
- Araújo-Rodrigues, H.; Martins, A.P.L.; Tavaria, F.K.; Dias, J.; Santos, M.T.; Alvarenga, N.; Pintado, M.E. Impact of LAB from Serpa PDO Cheese in Cheese Models: Towards the Development of an Autochthonous Starter Culture. Foods 2023, 12, 701. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves Dos Santos, M.T.P.; Benito, M.J.; Córdoba, M.D.G.; Alvarenga, N.; Ruiz-Moyano Seco de Herrera, S. Yeast Community in Traditional Portuguese Serpa Cheese by Culture-Dependent and -Independent DNA Approaches. Int. J. Food Microbiol. 2017, 262, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Tabla, R.; Gómez, A.; Rebollo, J.E.; Roa, I. Salt Influence on Surface Microorganisms and Ripening of Soft Ewe Cheese. J. Dairy Res. 2015, 82, 215–221. [Google Scholar] [CrossRef]
- Salaberría, F.; Marzocchi, S.; Bortolazzo, E.; Carrín, M.E.; Caboni, M.F. Study of the Effect of NaCl on Lipolysis in Parmigiano Reggiano Cheese. ACS Food Sci. Technol. 2021, 1, 54–59. [Google Scholar] [CrossRef]
- Bae, I.; Park, J.H.; Choi, H.Y.; Jung, H.K. Emerging Innovations to Reduce the Salt Content in Cheese; Effects of Salt on Flavor, Texture, and Shelf Life of Cheese; And Current Salt Usage: A Review. Korean J. Food Sci. Anim. Resour. 2017, 37, 793–798. [Google Scholar] [PubMed]
- Tidona, F.; Zago, M.; Carminati, D.; Giraffa, G. The Reduction of Salt in Different Cheese Categories: Recent Advances and Future Challenges. Front. Nutr. 2022, 9, 859694. [Google Scholar] [CrossRef] [PubMed]
- Sakkas, L.; Moschopoulou, E.; Moatsou, G. Influence of Salting and Ripening Conditions on the Characteristics of a Reduced-Fat, Semi-Hard, Sheep Milk Cheese. Foods 2023, 12, 4501. [Google Scholar] [CrossRef] [PubMed]
- International Dairy Federation. The Importance of Salt in the Manufacturing and Ripening of Cheese; International Dairy Federation: Brussels, Belgium, 2017. [Google Scholar]
- Bansal, V.; Mishra, S.K. Reduced-sodium Cheeses: Implications of Reducing Sodium Chloride on Cheese Quality and Safety. Compr. Rev. Food Sci. Food Saf. 2020, 19, 733–758. [Google Scholar] [CrossRef]
- Cen, C.; Wang, X.; Li, H.; Chen, J.; Wang, Y. An Inhibitor of the Adaptability of Pseudomonas fluorescens in a High-Salt Environment. Phenomenon and Mechanism of Inhibition. Int. J. Food Microbiol. 2024, 412, 110553. [Google Scholar] [CrossRef]
- Girard, L.; Lood, C.; Rokni-Zadeh, H.; van Noort, V.; Lavigne, R.; De Mot, R. Reliable Identification of Environmental Pseudomonas Isolates Using the RpoD Gene. Microorganisms 2020, 8, 1166. [Google Scholar] [CrossRef]
- ISO 21543/IDF 201; Milk and Milk Products—Guidelines for the Application of Near Infrared Spectrometry. ISO: Geneva, Switzerland, 2020.
- NP 470; Leite—Determinação Da Acidez. Instituto Português da Qualidade: Lisboa, Portugal, 1983.
- NP 471; Leite—Determinação Do Teor de Cloretos Por Titulação Com Nitrato de Prata. Instituto Português da Qualidade: Lisboa, Portugal, 1985.
- Alvarenga, N.; Fernandes, J.; Gomes, S.; Baltazar, T.; Fiates, V.; Fidalgo, L.G.; Santos, T.; Conceição, C.; Dias, J. Impact of Different Cynara cardunculus L. Extracts on the Physicochemical, Microbial, and Sensory Properties of Serpa Cheese. Int. Dairy J. 2025, 162, 106159. [Google Scholar] [CrossRef]
- AOAC 920.124; Titratable Acidity of Milk and Dairy Products. AOAC: Washington, DC, USA, 1920.
- ISO 5943; Cheese and Processed Cheese Products—Determination of Chloride Content—Potentiometric Titration Method. ISO: Geneva, Switzerland, 2006.
- ISO 3433; Cheese—Determination of Fat Content—Van Gulik Method. ISO: Geneva, Switzerland, 2008.
- NP 3544; Queijos—Determinação Do Resíduo Seco. Instituto Português da Qualidade: Lisboa, Portugal, 1988.
- ISO 8968-1& IDF 20-1; Milk—Determination of Nitrogen Content—Part 1: Kjeldahl Method. ISO: Geneva, Switzerland, 2014.
- AOAC 920:123; Nitrogen in Cheese. AOAC: Washington, DC, USA, 1920.
- Maubois, J.-L.; Lorient, D. Dairy Proteins and Soy Proteins in Infant Foods Nitrogen-to-Protein Conversion Factors. Dairy Sci. Technol. 2016, 96, 15–25. [Google Scholar] [CrossRef]
- ISO 15214; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Mesophilic Lactic Acid Bacteria—Colony-Count Technique at 30 °C. ISO: Geneva, Switzerland, 1998.
- ISO 21527-1; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Yeasts and Moulds—Part 1: Colony Count Technique in Products with Water Activity Greater than 0.95. ISO: Geneva, Switzerland, 2008.
- ISO 13720; Meat and Meat Products—Enumeration of Pseudomonas spp. ISO: Geneva, Switzerland, 2010.
- Moatsou, G.; Zoidou, E.; Choundala, E.; Koutsaris, K.; Kopsia, O.; Thergiaki, K.; Sakkas, L. Development of Reduced-Fat, Reduced-Sodium Semi-Hard Sheep Milk Cheese. Foods 2019, 8, 204. [Google Scholar] [CrossRef]
- Janštová, B.; Navrátilová, P.; Králová, M.; Vorlová, L. The Freezing Point of Raw and Heat-Treated Sheep Milk and Its Variation during Lactation. Acta Vet. Brno 2013, 82, 187–190. [Google Scholar] [CrossRef]
- Samelis, J.; Bosnea, L.; Kakouri, A. Microbiological Quality and Safety of Raw Sheep Milks from Native Epirus Breeds: Selective Effects of Thermization on the Microbiota Surviving in Resultant Thermized Milks Intended for Traditional Greek Hard Cheese Production. Appl. Microbiol. 2025, 5, 11. [Google Scholar] [CrossRef]
- Bruzaroski, S.R.; Correia, S.d.S.; Devara, L.F.d.S.; Poli-Frederico, R.C.; Fagnani, R.; de Santana, E.H.W. Influence of the Storage Temperature of Raw Sheep Milk on the Spoilage Potential of Pseudomonas spp. Small Rumin. Res. 2023, 224, 106998. [Google Scholar] [CrossRef]
- Araújo-Rodrigues, H.; Martins, A.P.L.; Tavaria, F.K.; Santos, M.T.G.; Carvalho, M.J.; Dias, J.; Alvarenga, N.B.; Pintado, M.E. Organoleptic Chemical Markers of Serpa PDO Cheese Specificity. Foods 2022, 11, 1898. [Google Scholar] [CrossRef] [PubMed]
- Pastorino, A.J.; Hansen, C.L.; McMahon, D.J. Effect of Salt on Structure-Function Relationships of Cheese. J. Dairy Sci. 2003, 86, 60–69. [Google Scholar] [CrossRef]
- Soltani, M. Changes in Texture Profile and Mineral Content of Ultrafiltered White Cheese Produced Using Different Salt Concentrations. Mljekarstvo 2024, 74, 64–74. [Google Scholar] [CrossRef]
- Alvarenga, N.; Canada, J.; Sousa, I. Effect of Freezing on the Rheological, Chemical and Colour Properties of Serpa Cheese. J. Dairy Res. 2011, 78, 80–87. [Google Scholar] [CrossRef]
- Alvarenga, N.; Silva, P.; Garcia, J.R.; Sousa, I. Estimation of Serpa Cheese Ripening Time Using Multiple Linear Regression (MLR) Considering Rheological, Physical and Chemical Data. J. Dairy Res. 2008, 75, 233–239. [Google Scholar] [CrossRef]
- López González, N.; Abarquero, D.; Combarros-Fuertes, P.; Prieto, B.; Fresno, J.M.; Tornadijo, M.E. Influence of Salting on Physicochemical and Sensory Parameters of Blue-Veined Cheeses. Dairy 2024, 5, 93–105. [Google Scholar] [CrossRef]
- Membré, J.M.; Burlot, P.M. Effects of Temperature, PH, and NaCl on Growth and Pectinolytic Activity of Pseudomonas marginalis. Appl. Environ. Microbiol. 1994, 60, 2017–2022. [Google Scholar] [CrossRef]
- Rattanachaikunsopon, P.; Phumkhachorn, P. Lactic Acid Bacteria: Their Antimicrobial Compounds and Their Uses in Food Production. Sch. Res. Libr. Ann. Biol. Res. 2010, 1, 218–228. [Google Scholar]
- Prior, B.A. The Effect of Water Activity on the Growth and Respiration of Pseudomonas fluorescens. J. Appl. Bacteriol. 1978, 44, 97–106. [Google Scholar] [CrossRef]
- Wasmuth, I.; Warinner, C.; Stallforth, P. Microbial Dynamics and Pseudomonas Natural Product Production in Milk and Dairy Products. Nat. Prod. Rep. 2025, 42, 842–855. [Google Scholar] [CrossRef]

| Raw Milk | |
|---|---|
| NaCl Content % (m/m) | 0.23 (0.01) |
| pH | 6.62 (0.03) |
| Titratable Acidity (mL NaOH 0.1 N/L) | 18.00 (0.00) |
| Protein % (m/m) | 2.52 (0.02) |
| Fat % (m/m) | 6.21 (0.04) |
| Lactose % (m/m) | 4.95 (0.01) |
| Cryoscopic Point (°C) | −0.516 (0.000) |
| Total Solids % (m/m) | 13.77 (0.04) |
| Non-fat Solids % (m/m) | 9.05 (0.01) |
| LAB (log CFU/mL) | 4.38 (0.09) |
| Pseudomonas spp. (log CFU/mL) | 7.13 (0.02) |
| Ripening Temperature | Salt Concentration | Pseudomonas spp. | LAB | Yeasts and Molds |
|---|---|---|---|---|
| 8 °C | 0% | 9.30 (0.44) ab | 8.81 (0.23) ab | 8.56 (0.59) ab |
| 2% | 8.75 (0.25) abc | 8.60 (0.08) abc | 9.05 (0.20) a | |
| 4% | 8.70 (0.20) abc | 8.98 (0.04) a | 8.69 (0.06) ab | |
| 6% | 8.57 (0.17) abc | 8.65 (0.08) abc | 8.67 (0.16) ab | |
| 11 °C | 0% | 9.29 (0.25) ab | 8.62 (0.21) abc | 5.55 (0.18) c |
| 2% | 8.92 (0.09) ab | 8.60 (0.11) abc | 8.29 (0.57) ab | |
| 4% | 8.51 (0.20) abc | 8.60 (0.03) abc | 8.19 (0.06) ab | |
| 6% | 8.48 (0.11) bc | 8.41 (0.11) bc | 8.21 (0.13) ab | |
| 14 °C | 0% | 8.77 (0.15) abc | 8.51 (0.22) abc | 8.10 (0.27) ab |
| 2% | 6.58 (0.15) e | 8.74 (0.09) ab | 7.69 (0.25) b | |
| 4% | 7.33 (0.30) de | 8.82 (0.12) ab | 8.78 (0.15) ab | |
| 6% | 7.95 (0.20) cd | 8.09 (0.08) c | 8.61 (0.15) ab |
| Ripening Temperature | Salt Concentration | pH | Titratable Acidity (mL NaOH 0.1 N/100 g) | Fat % (m/m) | Total Solids % (m/m) |
|---|---|---|---|---|---|
| 8 °C | 0% | 5.43 (0.05) ab | 15.73 (0.46) a | 28.17 (1.26) e | 60.22 (1.45) b |
| 2% | 5.33 (0.01) b | 7.20 (0.80) e | 27.83 (0.29) e | 54.33 (0.90) c | |
| 4% | 5.20 (0.02) b | 9.07 (0.46) cde | 29.00 (1.00) de | 50.61 (1.25) cde | |
| 6% | 5.04 (0.05) b | 8.27 (0.46) de | 26.17 (0.76) e | 47.34 (0.95) e | |
| 11 °C | 0% | 5.43 (0.04) ab | 16.27 (2.44) a | 27.83 (1.04) e | 59.56 (1.20) b |
| 2% | 5.32 (0.05) b | 8.27 (0.46) de | 26.50 (1.00) e | 53.37 (1.83) cd | |
| 4% | 4.91 (0.02) b | 8.80 (0.80) de | 26.33 (0.58) e | 49.02 (0.76) de | |
| 6% | 5.13 (0.06) b | 10.40 (0.00) b–e | 25.50 (0.00) e | 49.01 (0.04) de | |
| 14 °C | 0% | 6.25 (0.70) a | 11.20 (0.80) bcd | 32.00 (0.50) cd | 67.99 (1.63) a |
| 2% | 5.05 (0.04) b | 13.60 (1.39) ab | 35.17 (1.61) bc | 69.49 (2.35) a | |
| 4% | 4.95 (0.04) b | 12.80 (0.00) abc | 39.33 (0.76) a | 71.53 (0.30) a | |
| 6% | 5.03 (0.03) b | 11.73 (0.46) bcd | 37.00 (0.87) ab | 71.29 (0.78) a |
| Ripening Temperature | Salt Concentration | TN % (m/m) | WSN/TN % | TCA-SN/TN % |
|---|---|---|---|---|
| 8 °C | 0% | 4.64 (0.09) ab | 53.99 (0.46) a | 21.85 (2.03) a |
| 2% | 3.84 (0.06) d | 39.17 (1.78) b | 10.93 (1.92) b | |
| 4% | 3.02 (0.14) e | 35.76 (1.57) bcd | 8.70 (0.37) bc | |
| 6% | 2.74 (0.06) e | 28.83 (2.57) cde | 3.05 (0.23) d | |
| 11 °C | 0% | 4.55 (0.14) abc | 51.27 (4.23) a | 21.73 (2.59) a |
| 2% | 3.87 (0.09) d | 34.17 (0.66) bcd | 8.69 (0.87) bc | |
| 4% | 2.76 (0.09) e | 37.75 (1.45) bc | 6.55 (1.14) bcd | |
| 6% | 2.70 (0.11) e | 27.97 (2.59) de | 3.48 (0.72) d | |
| 14 °C | 0% | 5.13 (0.15) a | 32.17 (2.10) bcd | 9.95 (0.79) b |
| 2% | 4.25 (0.30) bcd | 31.83 (2.49) bcd | 6.35 (0.24) bcd | |
| 4% | 4.15 (0.26) bcd | 27.58 (3.75) de | 4.68 (0.81) cd | |
| 6% | 4.02 (0.02) cd | 20.22 (1.39) e | 2.24 (0.26) d |
| Ripening °C | Salt % | Mechanical Properties | CIELab System | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Rind | Paste | ||||||||
| Hardness (N) | Adhesiveness (−Ns) | L* | a* | b* | L* | a* | b* | ||
| 8 °C | 0% | 3.05 c (0.29) | 12.99 c (1.01) | 67.97 d (2.69) | 5.21 a (1.21) | 16.55 ab (1.74) | 98.82 a (2.84) | −2.37 bcd (0.21) | 13.40 abc (0.62) |
| 2% | 4.43 c (0.10) | 14.26 c (3.54) | 75.54 bc (1.26) | −0.69 c (0.11) | 15.94 ab (2.09) | 80.29 bcd (0.99) | −2.29 a–d (0.06) | 13.53 abc (0.57) | |
| 4% | 1.85 c (0.13) | 9.14 c (0.74) | 83.49 a (0.90) | −1.34 c (0.34) | 12.33 b (1.40) | 87.43 bc (0.69) | −1.85 a–d (0.09) | 11.83 a–e (0.17) | |
| 6% | 3.19 c (0.15) | 11.80 c (1.12) | 84.85 a (0.63) | −1.28 c (0.34) | 12.66 b (0.40) | 88.35 ab (0.62) | −1.31 a (0.16) | 8.93 de (0.30) | |
| 11 °C | 0% | 3.13 c (0.73) | 10.68 c (1.64) | 60.39 e (2.40) | 2.58 b (1.64) | 16.61 ab (2.51) | 71.62 d (3.76) | −1.70 a–d (0.10) | 7.79 e (2.12) |
| 2% | 2.95 c (0.31) | 16.44 c (2.09) | 80.76 ab (0.64) | −0.52 c (0.20) | 11.45 b (0.53) | 81.55 bcd (0.48) | −1.99 a–d (0.37) | 12.31 a–d (1.04) | |
| 4% | 2.23 c (0.19) | 10.13 c (1.77) | 85.75 a (1.49) | −1.15 c (0.35) | 12.82 b (0.48) | 86.98 bc (1.85) | −1.53 abc (0.05) | 10.30 b–e (0.48) | |
| 6% | 3.17 c (0.45) | 11.04 c (0.54) | 85.83 a (0.44) | −1.21 c (0.08) | 11.12 b (1.13) | 86.62 bc (5.40) | −1.45 ab (0.22) | 9.67 cde (1.00) | |
| 14 °C | 0% | 26.33 b (6.60) | 38.62 c (7.70) | 69.74 cd (1.29) | −1.83 c (0.11) | 16.30 ab (1.61) | 72.83 d (1.58) | −2.06 a–d (0.37) | 14.56 ab (1.31) |
| 2% | 48.71 a (2.10) | 123.96 ab (8.52) | 66.48 de (3.79) | −2.24 c (0.43) | 15.13 ab (2.03) | 72.90 d (4.73) | −2.47 cd (0.38) | 13.80 abc (1.55) | |
| 4% | 20.39 b (2.92) | 97.79 b (15.54) | 69.43 cd (0.50) | −1.03 c (0.43) | 20.58 a (0.51) | 76.94 cd (2.05) | −2.56 d (0.26) | 15.46 a (0.20) | |
| 6% | 26.76 b (4.32) | 158.60 a (25.87) | 81.25 ab (1.11) | −1.58 c (0.33) | 18.81 a (1.03) | 84.28 bc (1.31) | −1.51 abc (0.28) | 12.67 a–d (1.33) | |
| Component | PC1 | PC2 | PC3 |
|---|---|---|---|
| Titratable Acidity | 0.84 * | 0.15 | −0.01 |
| Fat | 0.58 | −0.75 * | −0.05 |
| TS | 0.81 * | −0.54 | 0.09 |
| TN | 0.91 * | 0.03 | 0.13 |
| WSN | 0.72 * | 0.66 | −0.04 |
| TCA-SN | 0.66 | 0.73 * | 0.01 |
| Hardness | 0.49 | −0.76* | −0.1 |
| L* (R) | −0.92 * | −0.13 | 0.15 |
| a* (R) | 0.46 | 0.78 * | 0.05 |
| b* (R) | 0.75 * | −0.31 | 0.18 |
| Pseudomonas spp. count | −0.15 | 0.87 * | 0.29 |
| LAB count | 0.07 | 0.29 | −0.93 * |
| Eigenvalue | 5.36 | 3.99 | 1.04 |
| % Variance | 44.63 | 33.24 | 8.64 |
| % Cumulative variance | 44.63 | 44.63 | 86.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, S.; Vida, M.; Correia, C.; Fernandes, J.; Gomes, S.; Fernando, A.; Tabla, R.; Alvarenga, N. Influence of Sodium Chloride on the Behaviour of Pseudomonas fluorescens in Ripened Sheep Cheese. Microorganisms 2025, 13, 2693. https://doi.org/10.3390/microorganisms13122693
Lopes S, Vida M, Correia C, Fernandes J, Gomes S, Fernando A, Tabla R, Alvarenga N. Influence of Sodium Chloride on the Behaviour of Pseudomonas fluorescens in Ripened Sheep Cheese. Microorganisms. 2025; 13(12):2693. https://doi.org/10.3390/microorganisms13122693
Chicago/Turabian StyleLopes, Simone, Manuela Vida, Cláudia Correia, Jaime Fernandes, Sandra Gomes, Ana Fernando, Rafael Tabla, and Nuno Alvarenga. 2025. "Influence of Sodium Chloride on the Behaviour of Pseudomonas fluorescens in Ripened Sheep Cheese" Microorganisms 13, no. 12: 2693. https://doi.org/10.3390/microorganisms13122693
APA StyleLopes, S., Vida, M., Correia, C., Fernandes, J., Gomes, S., Fernando, A., Tabla, R., & Alvarenga, N. (2025). Influence of Sodium Chloride on the Behaviour of Pseudomonas fluorescens in Ripened Sheep Cheese. Microorganisms, 13(12), 2693. https://doi.org/10.3390/microorganisms13122693









