Supplementation with Mo, Co, and Ni Enhances the Effectiveness of Co-Inoculation with the Rhizobacteria Azospirillum brasilense and Bradyrhizobium diazoefficiens in Soybean
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material, Inoculants, and Experimental Conditions
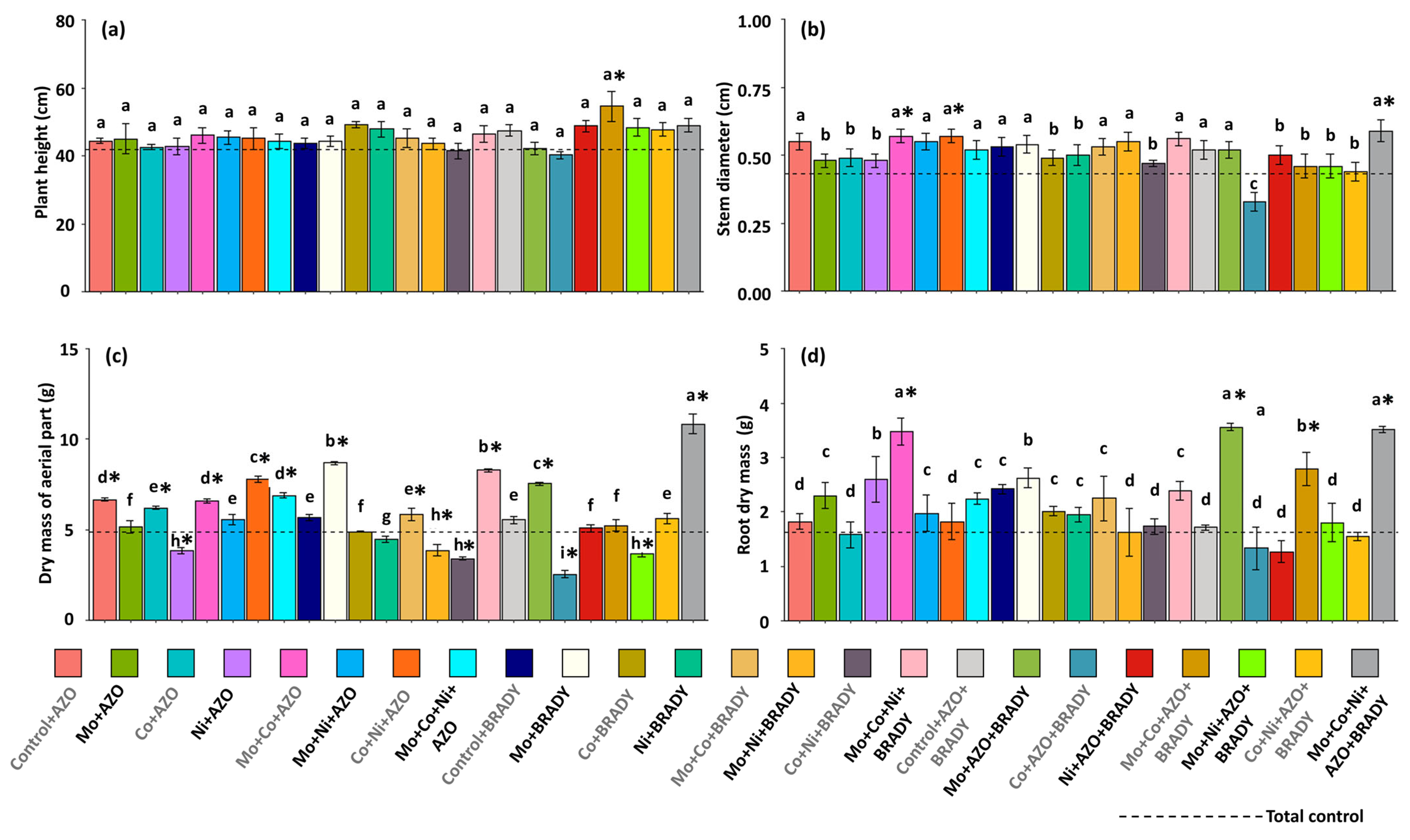
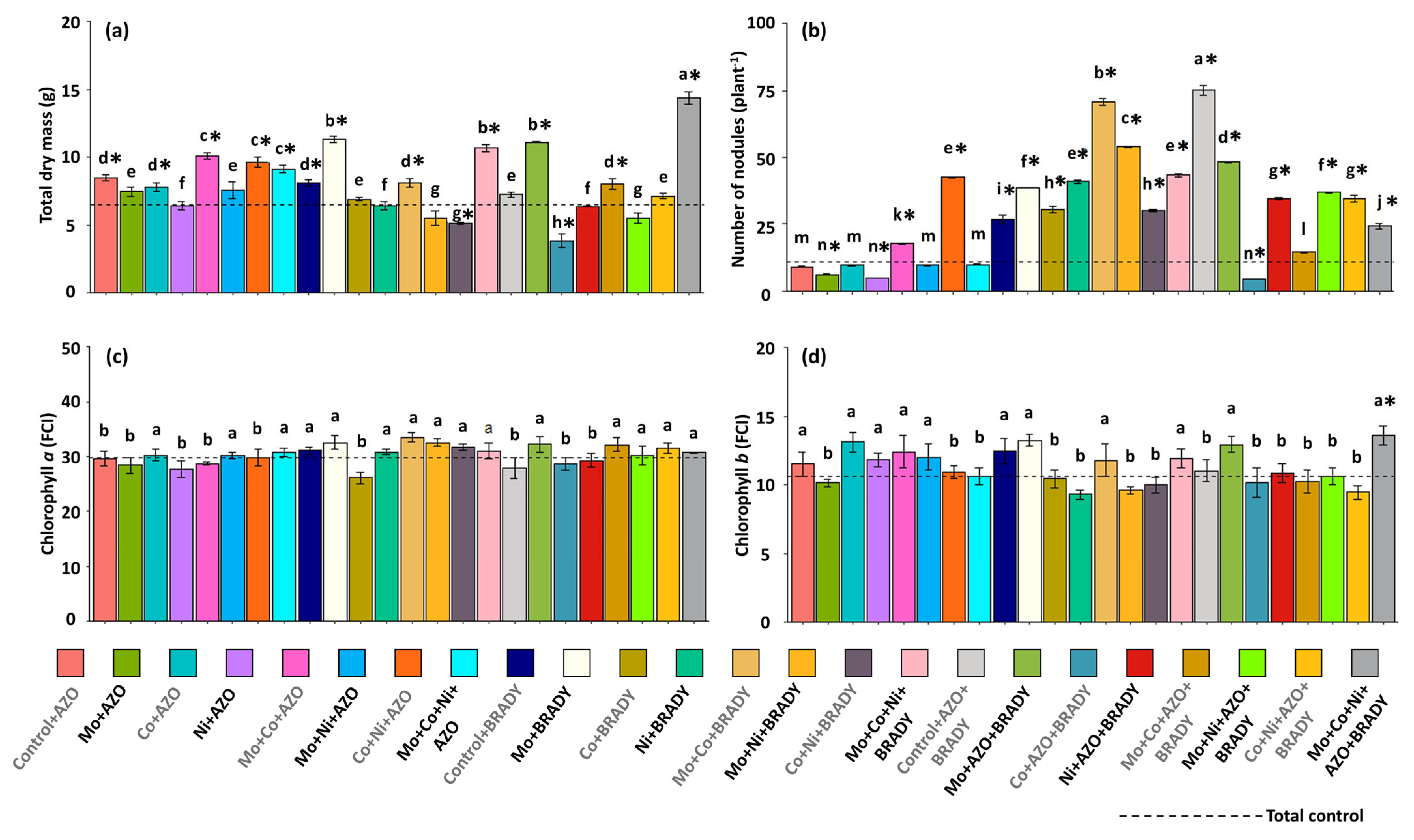
2.2. Biometric Evaluations and Nodulation
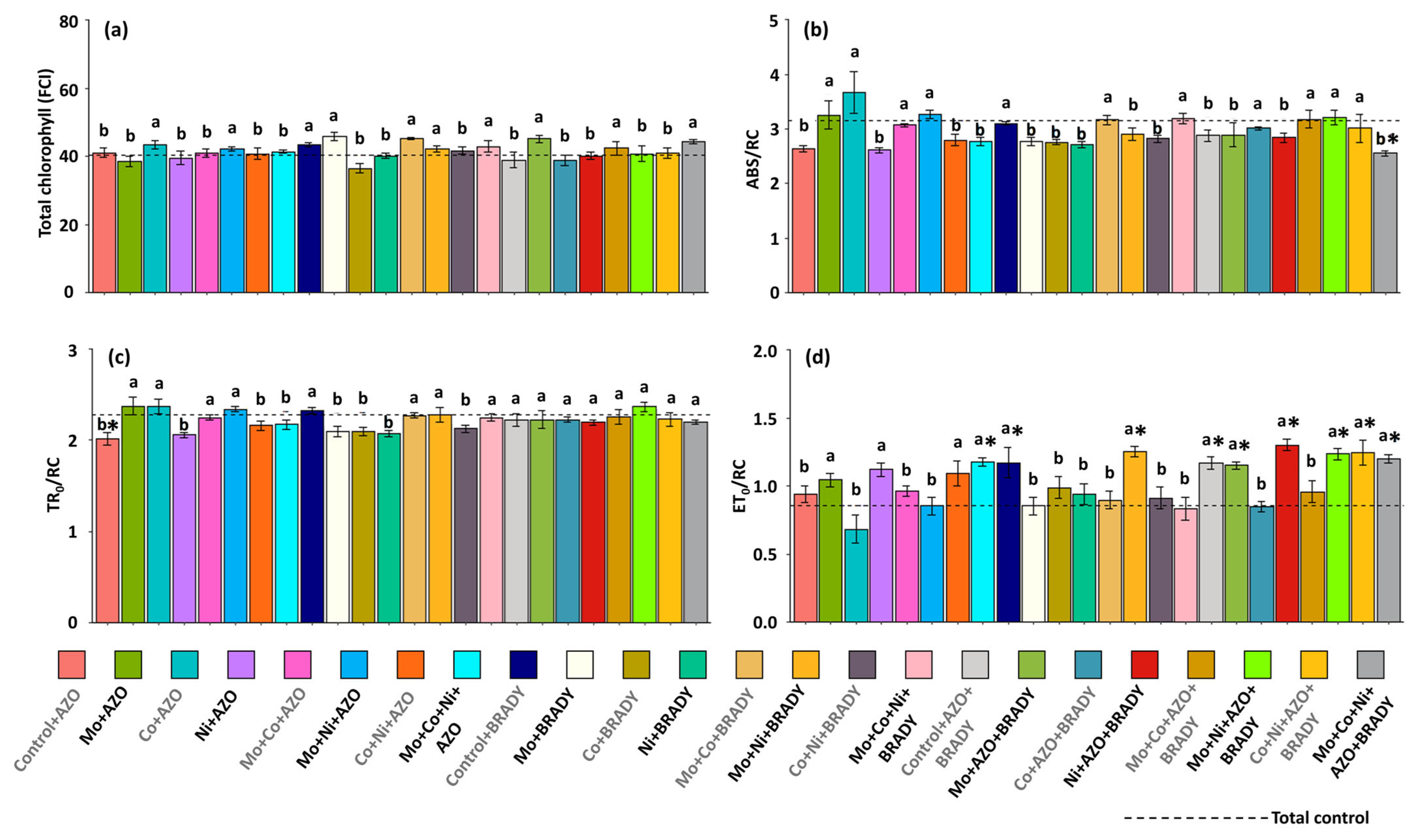
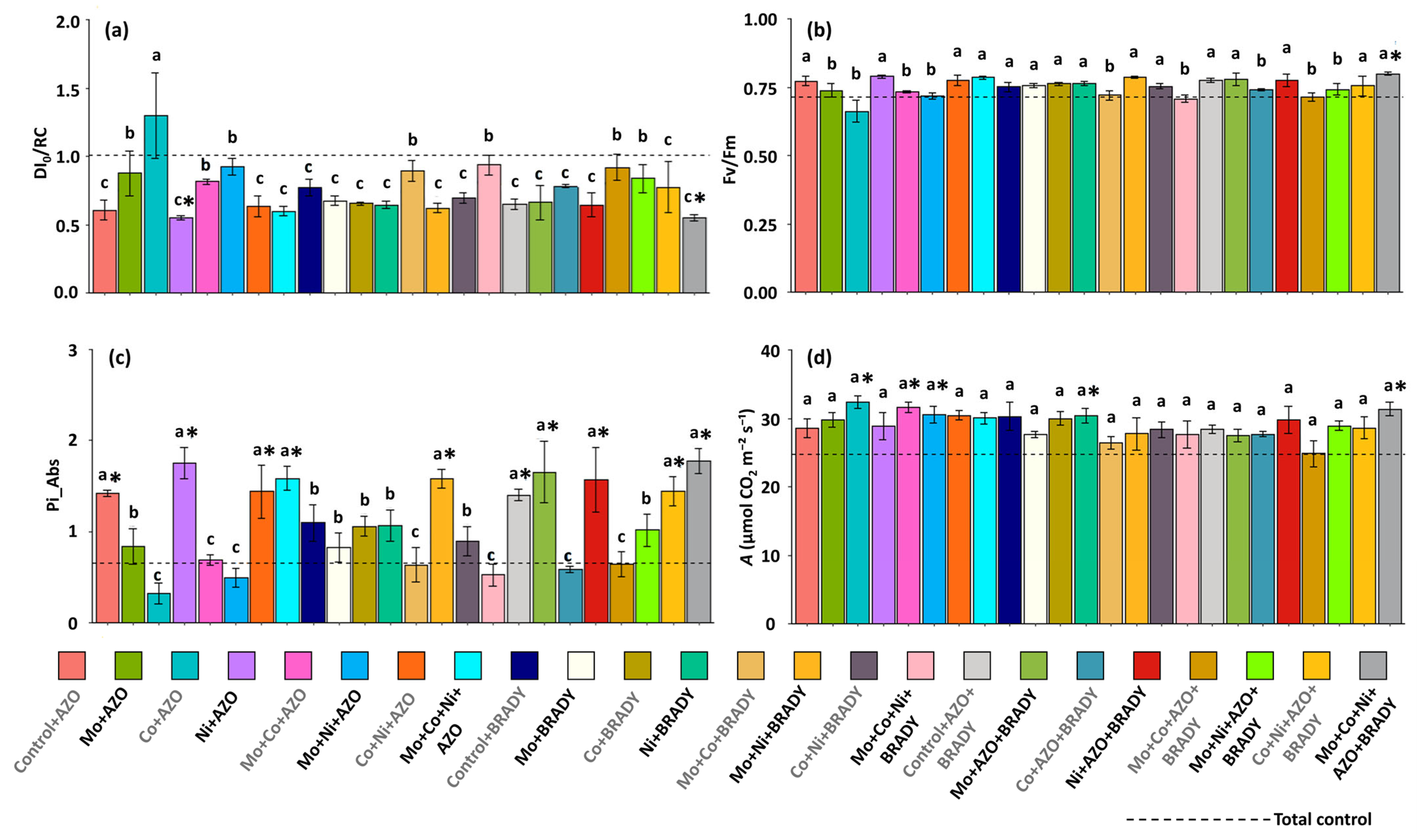
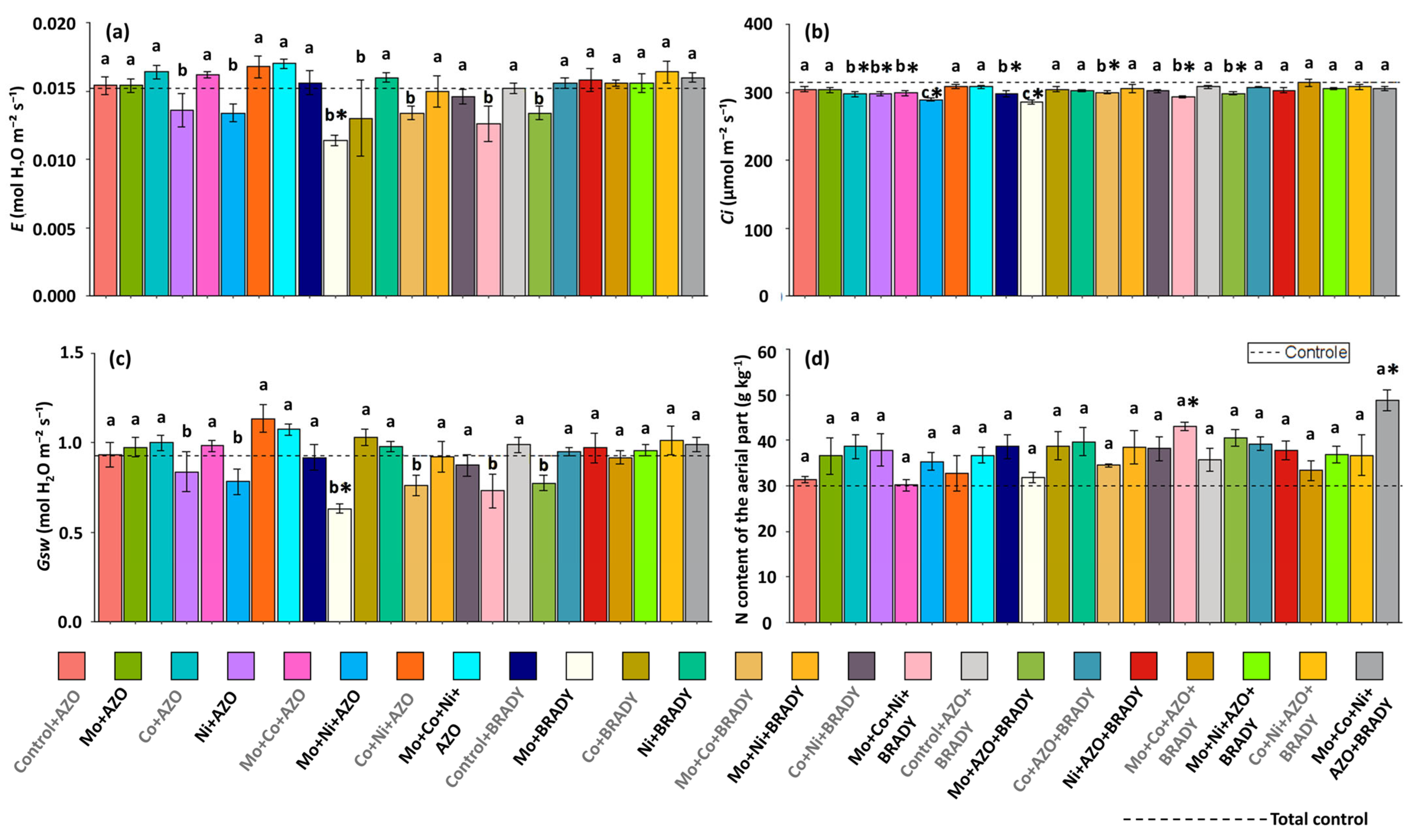
2.3. Physiological Assessments
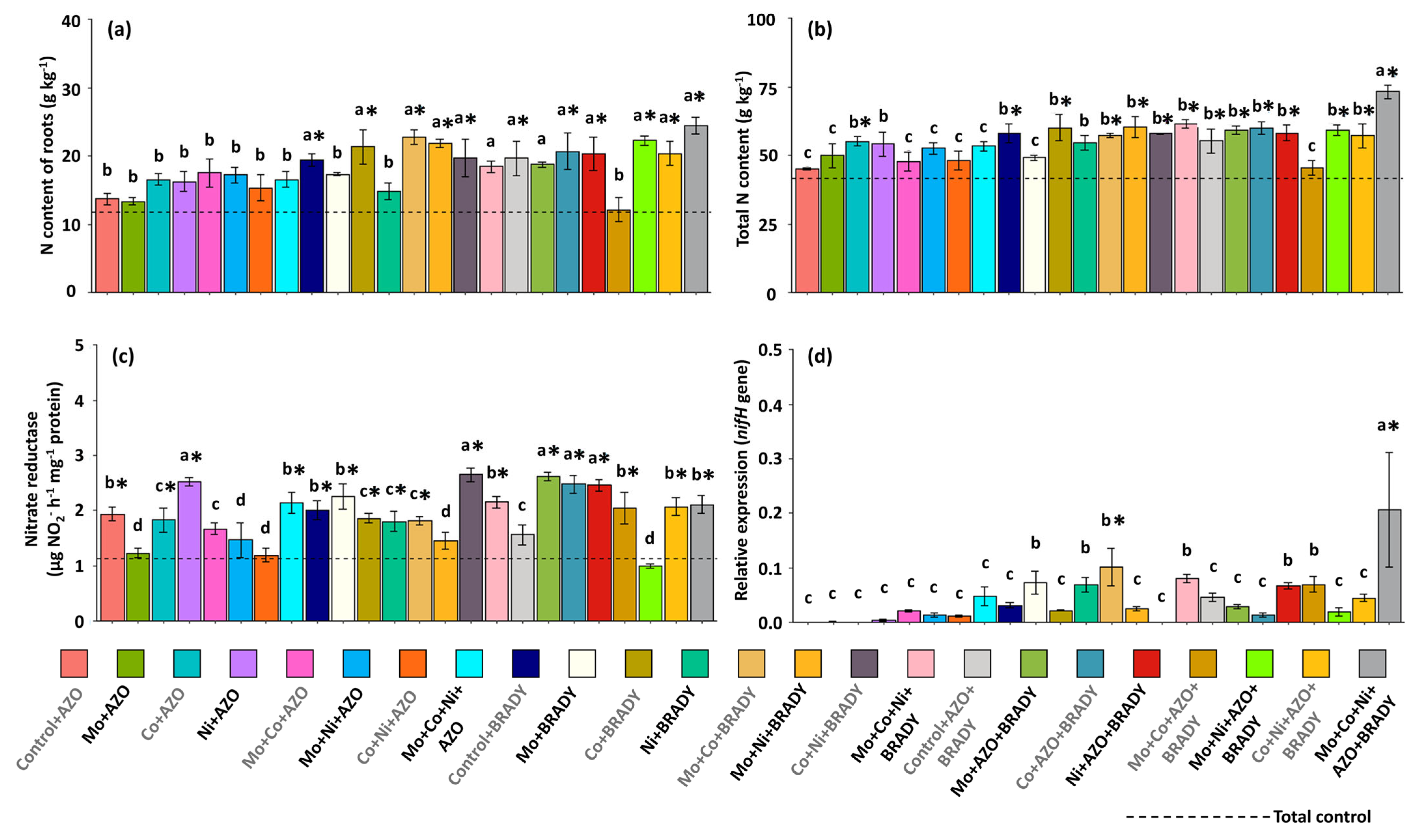
2.4. N Content and Nitrate Reductase (NR, EC 1.6.6.1) Activity
2.5. nifH Gene Expression Levels
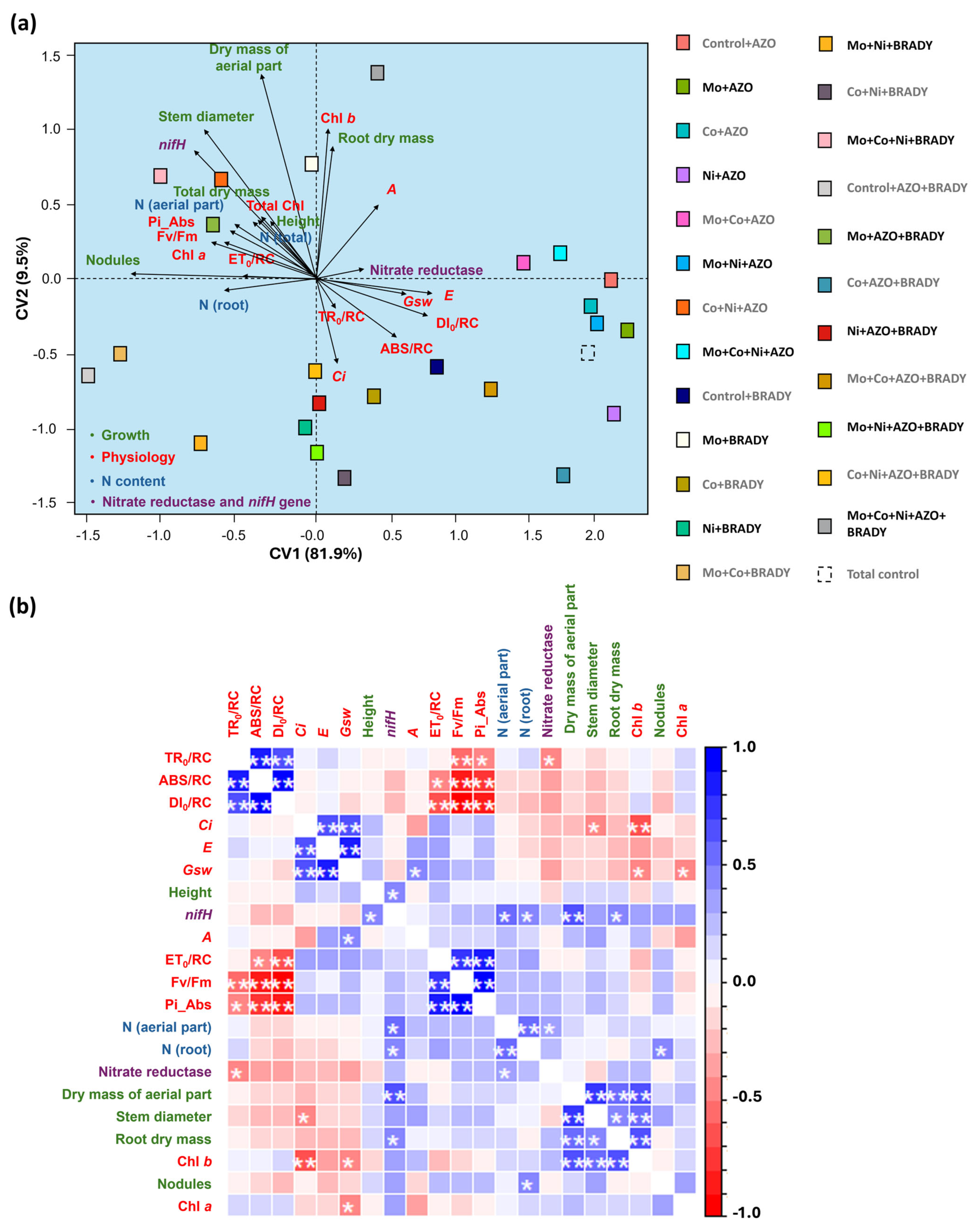
2.6. Statistical Analyses
3. Results
4. Discussion
4.1. The Combined Application of the Micronutrients Mo, Co, and Ni, Together with the Co-Inoculation of A. brasilense and B. diazoefficiens, Enhances the Vegetative Growth of G. max
4.2. The Combined Application of the Micronutrients Mo, Co, and Ni, Together with Co-Inoculation of A. brasilense and B. diazoefficiens, Enhances the Primary Photochemical Performance of G. max
4.3. Mo, Co, and Ni Optimize Co-Inoculation, Increasing the Expression of the Gene nifH and BNF in Soybean Nodules
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soumare, A.; Diedhiou, A.G.; Thuita, M.; Hafidi, M.; Ouhdouch, Y.; Gopalakrishnan, S.; Kouisni, L. Exploiting biological nitrogen fixation: A route towards a sustainable agriculture. Plants 2020, 9, 1011. [Google Scholar] [CrossRef]
- Zilli, J.É.; Alves, B.J.R.; Rouws, L.F.M.; Simões-Araujo, J.L.; de Barros Soares, L.H.; Cassán, F.; Castellanos, M.O.; O’Hara, G. The importance of denitrification performed by nitrogen-fixing bacteria used as inoculants in South America. Plant Soil 2020, 451, 5–24. [Google Scholar] [CrossRef]
- Hungria, M.; Nogueira, M.A.; Araujo, R.S. Soybean seed co-inoculation with Bradyrhizobium spp. and Azospirillum brasilense: A new biotechnological tool to improve yield and sustainability. Am. J. Plant Sci. 2015, 6, 811–817. [Google Scholar] [CrossRef]
- Garcia, M.V.C.; Nogueira, M.A.; Hungria, M. Combining microorganisms in inoculants is agronomically important but industrially challenging: Case study of a composite inoculant containing Bradyrhizobium and Azospirillum for the soybean crop. AMB Expr. 2021, 11, 71. [Google Scholar] [CrossRef]
- Marks, B.B.; Megías, M.; Nogueira, M.A.; Hungria, M. Biotechnological potential of rhizobial metabolites to enhance the performance of Bradyrhizobium spp. and Azospirillum brasilense inoculants with soybean and maize. AMB Expr. 2013, 3, 21. [Google Scholar] [CrossRef] [PubMed]
- Rondina, A.B.L.; dos Santos Sanzovo, A.W.; Guimarães, G.S.; Wendling, J.R.; Nogueira, M.A.; Hungria, M. Changes in root morphological traits in soybean co-inoculated with Bradyrhizobium spp. and Azospirillum brasilense or treated with A. brasilense exudates. Biol. Fertil. Soils 2020, 56, 537–549. [Google Scholar] [CrossRef]
- Prando, A.M.; Barbosa, J.Z.; de Oliveira, A.B.; Nogueira, M.A.; Possamai, E.J.; Hungria, M. Benefits of soybean co-inoculation with Bradyrhizobium spp. and Azospirillum brasilense: Large-scale validation with farmers in Brazil. Eur. J. Agron. 2024, 155, 127112. [Google Scholar] [CrossRef]
- Figueiredo, M.V.B.; Mergulhão, A.C.E.S.; Sobral, J.K.; Lira Junior, M.A.; Araújo, A.S.F. Biological nitrogen fixation: Importance, associated diversity, and estimates. In Plant Microbe Symbiosis: Fundamentals and Advances; Arora, N., Ed.; Springer: New Delhi, India, 2013; pp. 267–289. [Google Scholar] [CrossRef]
- Saha, B.; Saha, S.; Das, A.; Bhattacharyya, P.K.; Basak, N.; Sinha, A.K.; Poddar, P. Biological nitrogen fixation for sustainable agriculture. In Agriculturally Important Microbes for Sustainable Agriculture: Volume 2: Applications in Crop Production and Protection; Meena, V., Mishra, P., Bisht, J., Pattanayak, A., Eds.; Springer: Singapore, 2017; pp. 81–128. [Google Scholar] [CrossRef]
- Fachinelli, R.; Ceccon, G. Bradyrhizobium and Azospirillum coinoculation in soybean in succession to safrinha corn in sandy and clay soil. Acta Iguazu 2020, 9, 99–108. [Google Scholar] [CrossRef]
- Barbosa, H.M.; Alvarez, R.D.C.F.; Lima, S.F.D.; Cordeiro, M.A.S.; Zanella, M.S.; Bernardo, V.F. Bradyrhizobium and Azospirillum co-inoculation associated with cobalt and molybdenum application in the soybean crop. Ciênc. Rural 2022, 53, e20210871. [Google Scholar] [CrossRef]
- Bonilla, I.; Bolaños, L. Mineral nutrition for legume–rhizobia symbiosis: B, Ca, N, P, S, K, Fe, Mo, Co, and Ni: A review. In Organic Farming, Pest Control and Remediation of Soil Pollutants. Sustainable Agriculture Reviews; Lichtfouse, E., Ed.; Springer: Dordrecht, The Netherlands, 2009; pp. 253–274. [Google Scholar] [CrossRef]
- Weisany, W.; Raei, Y.; Allahverdipoor, K.H. Role of some mineral nutrients in biological nitrogen fixation. Bull. Environ. Pharmacol. Life Sci. 2013, 2, 77–84. [Google Scholar]
- Lima, E.G.; Zanuzo, M.R.; Vieira, C.V.; Pelissari, F.; de Andrade Coimbra, R. Effect of nickel seed treatment in soybeans. Sci. Electron. Arch. 2024, 17, 1. [Google Scholar] [CrossRef]
- Hu, X.; Wei, X.; Ling, J.; Chen, J. Cobalt in plant life: Responses and deficiency symptoms. In Beneficial Chemical Elements of Plants: Recent Developments and Future Prospects; Pandey, S., Tripathi, D.K., Singh, V.P., Sharma, S., Chauhan, D.K., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2023; pp. 182–204. [Google Scholar] [CrossRef]
- Fatima, P.; Mishra, A.; Om, H.; Saha, B.; Kumar, P. Free living nitrogen fixation and their response to agricultural crops. In Biofertilizers and Biopesticides in Sustainable Agriculture; Kaushik, B.D., Kumar, D., Shamim, M., Eds.; Apple Academic Press: Waretown, NJ, USA, 2019; pp. 173–200. [Google Scholar] [CrossRef]
- Verma, D.K.; Kaur, B.; Pandey, A.K.; Asthir, B. Nitrogenase: A key enzyme in microbial nitrogen fixation for soil health. In Microbiology for Sustainable Agriculture, Soil Health, and Environmental Protection; Verma, D.K., Ed.; Apple Academic Press: Boca Raton, FL, USA, 2019; pp. 261–294. [Google Scholar] [CrossRef]
- Fenice, M. The nitrogen cycle: An overview. In Nitrogen Cycle; Gonzalez-Lopez, J., Gonzalez-Martinez, A., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 1–21. [Google Scholar] [CrossRef]
- Lavres, J.; Castro Franco, G.; de Sousa Câmara, G.M. Soybean seed treatment with nickel improves biological nitrogen fixation and urease activity. Front. Environ. Sci. 2016, 4, 37. [Google Scholar] [CrossRef]
- Izaguirre-Mayoral, M.L.; Lazarovits, G.; Baral, B. Ureide metabolism in plant-associated bacteria: Purine plant-bacteria interactive scenarios under nitrogen deficiency. Plant Soil 2018, 428, 1–34. [Google Scholar] [CrossRef]
- Kumari, R.; Bhatnagar, S.; Kalra, C. Nitrogen assimilation in plants. In Advances in Plant Nitrogen Metabolism; Yousuf, P.Y., Shabir, P.A., Hakeem, K.R., Eds.; CRC Press: Boca Raton, FL, USA, 2022; pp. 38–54. [Google Scholar]
- Lal, M.A.; Bhatla, S.C. Nitrogen metabolism. In Plant Physiology, Development and Metabolism; Bhatla, S.C., Lal, M.A., Eds.; Springer Nature: Singapore, 2023; pp. 295–334. [Google Scholar] [CrossRef]
- Matiz, A.; Mioto, P.T.; Mercier, H. Urea in plants: Metabolic aspects and ecological implications. In Progress in Botany; Cánovas, F., Lüttge, U., Leuschner, C., Risueño, M.C., Eds.; Springer: Cham, Switzerland, 2019; Volume 81, pp. 157–187. [Google Scholar] [CrossRef]
- Ono, Y.; Fukasawa, M.; Sueyoshi, K.; Ohtake, N.; Sato, T.; Tanabata, S.; Toyota, R.; Higuchi, K.; Saito, A.; Ohyama, T. Application of nitrate, ammonium, or urea changes the concentrations of ureides, urea, amino acids and other metabolites in xylem sap and in the organs of soybean plants (Glycine max (L.) Merr.). Int. J. Mol. Sci. 2021, 22, 4573. [Google Scholar] [CrossRef]
- Rana, M.; Sankhyan, N.K.; Thakur, P.; Babal, B.; Anjali; Sharma, S.; Kumari, S.; Kumar, P. Molybdenum in soil-plant system: Bioavailability, dynamics and implications for sustainable crop production. Discov. Soil 2025, 2, 89. [Google Scholar] [CrossRef]
- Roychoudhury, A.; Chakraborty, S. Cobalt and molybdenum: Deficiency, toxicity, and nutritional role in plant growth and development. In Plant Nutrition and Food Security in the Era of Climate Change; Kumar, V., Srivastava, A.K., Suprasanna, P., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 255–270. [Google Scholar] [CrossRef]
- Huang, X.Y.; Hu, D.W.; Zhao, F.J. Molybdenum: More than an essential element. J. Exp. Bot. 2022, 73, 1766–1774. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wei, X.; Ling, J.; Chen, J. Cobalt: An essential micronutrient for plant growth? Front. Plant Sci. 2021, 12, 768523. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich, A.; Di, R.; Lindert, S.; Heckman, J. Nickel and soil fertility: Review of benefits to environment and food security. Environments 2024, 11, 177. [Google Scholar] [CrossRef]
- Dilawari, R.; Kaur, N.; Priyadarshi, N.; Prakash, I.; Patra, A.; Mehta, S.; Singh, B.; Jain, P.; Islam, M.A. Soybean: A key player for global food security. In Soybean Improvement: Physiological, Molecular and Genetic Perspectives; Wani, S.H., ul Rehman Sofi, N., Bhat, M.A., Lin, F., Eds.; Springer: Cham, Switzerland, 2022; pp. 1–46. [Google Scholar] [CrossRef]
- Islam, M.S.; Muhyidiyn, I.; Islam, M.R.; Hasan, M.K.; Hafeez, A.G.; Hosen, M.M.; Saneoka, H.; Ueda, A.; Liu, L.; Naz, M.; et al. Soybean and sustainable agriculture for food security. In Soybean—Recent Advances in Research and Applications; Ohyama, T., Takahashi, Y., Ohtake, N., Sato, T., Tanabata, S., Eds.; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Kumari, S.; Dambale, A.S.; Samantara, R.; Jincy, M.; Bains, G. Introduction, history, geographical distribution, importance, and uses of soybean (Glycine max L.). In Soybean Production Technology; Singh, K.P., Singh, N.K., Aravind, T., Eds.; Springer Nature: Singapore, 2025; pp. 1–17. [Google Scholar] [CrossRef]
- Bhangu, R.; Virk, H.K. Nitrogen management in soybean: A review. Agric. Rev. 2019, 40, 129–135. [Google Scholar] [CrossRef]
- Almeida, L.H.C.; Nadur, M.A.; de Almeida, P.P.S.; Carrilho, E.M.; Ventura, M.U.; de Freitas Fregonezi, G.A. Cell protector, cobalt, and molybdenum in association with Bradyrhizobium and Azospirillum in soybean cultivation. Obs. Econ. Latinoam. 2025, 23, e9033. [Google Scholar] [CrossRef]
- Silveira, P.G.; da Silva, E.A.R.; Nakao, A.H.; de Carvalho, J.B. Efeito de doses de cobalto e molibdênio aplicadas no sulco de plantio da soja inoculada com Bradyrhizobium. Unifunec Científica Multidiscip. 2021, 10, 1–13. [Google Scholar] [CrossRef]
- The Sociedade Brasileira de Ciência do Solo (SBCS). Manual de Calagem e Adubação para os Estados do Rio Grande do Sul e de Santa Catarina; Comissão de Química e Fertilidade do Solo (CQFS): Porto Alegre, Brazil, 2016; 376p. [Google Scholar]
- Picazevicz, A.A.; Kusdra, J.F.; Moreno, A.D.L. Maize growth in response to Azospirillum brasilense, Rhizobium tropici, molybdenum and nitrogen. Rev. Bras. Eng. Agríc. Ambient. 2017, 21, 623–627. [Google Scholar] [CrossRef]
- Marcondes, J.A.P.; Caires, E.F. Aplicação de molibdênio e cobalto na semente para cultivo da soja. Bragantia 2005, 64, 687–694. [Google Scholar] [CrossRef]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The fluorescence transient as a tool to characterize and screen photosynthetic samples. In Probing Photosynthesis: Mechanisms, Regulation and Adaptation; Yunus, M., Pathre, U., Mohanty, P., Eds.; CRC Press: Boca Raton, FL, USA, 2000; pp. 445–483. [Google Scholar]
- Malavolta, E.; Vitti, G.C.; Oliveira, S.A. Avaliação do Estado Nutricional das Plantas: Princípios e Aplicações; Potafos: Piracicaba, Brazil, 1997; p. 319. [Google Scholar]
- Hageman, R.H.; Reed, A.J. Nitrate reductase from higher plants. In Methods in Enzymology; Olshansky, L., Ed.; Academic Press: New York, NY, USA, 1980; Volume 69, pp. 270–280. [Google Scholar] [CrossRef]
- Azevedo, A.M. Package ‘MultivariateAnalysis’. Available online: https://cran.r-project.org/web/packages/MultivariateAnalysis/index.html (accessed on 27 July 2025).
- Wei, T.; Simko, V. R Package ‘Corrplot’: Visualization of a Correlation Matrix. Available online: https://github.com/taiyun/corrplot (accessed on 27 June 2025).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2025; Available online: https://www.R-project.org/ (accessed on 27 June 2025).
- Torres, D.; Donadio, F.; López, G.; Molina, R.; Obando, M.; Nievas, S.; Rosas, S.; Ćavar Zeljković, S.; Díaz-Zorita, M.; De Diego, N.; et al. Previous incubation of Bradyrhizobium japonicum E109 and Azospirillum argentinense Az39 (formerly A. brasilense Az39) improves the Bradyrhizobium–soybean symbiosis. J. Soil Sci. Plant Nutr. 2022, 22, 4669–4682. [Google Scholar] [CrossRef]
- Martin, T.N.; Vey, R.T.; Vieira, F.C.B.; Jacques, R.J.S.; Ferreira, M.M. How did the coinoculation of Bradyrhizobium and Azospirillum become indispensable for soybean production in Brazil? Symbiosis 2023, 91, 119–137. [Google Scholar] [CrossRef]
- Nicoud, Q.; Lamouche, F.; Chaumeret, A.; Balliau, T.; Le Bars, R.; Bourge, M.; Pierre, F.; Guérard, F.; Sallet, E.; Tuffigo, S.; et al. Bradyrhizobium diazoefficiens USDA110 nodulation of Aeschynomene afraspera is associated with atypical terminal bacteroid differentiation and suboptimal symbiotic efficiency. mSystems 2021, 6, e01237-20. [Google Scholar] [CrossRef]
- Coniglio, A.; Mora, V.; Puente, M.; Cassán, F. Azospirillum as biofertilizer for sustainable agriculture: Azospirillum brasilense AZ39 as a model of PGPR and field traceability. In Microbial Probiotics for Agricultural Systems: Advances in Agronomic Use; Zúñiga-Dávila, D., González-Andrés, F., Ormeño-Orrillo, E., Eds.; Springer: Cham, Switzerland, 2019; pp. 45–70. [Google Scholar] [CrossRef]
- Fukami, J.; Cerezini, P.; Hungria, M. Azospirillum: Benefits that go far beyond biological nitrogen fixation. AMB Expr. 2018, 8, 73. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.S.; Nogueira, M.A.; Hungria, M. Outstanding impact of Azospirillum brasilense strains Ab-V5 and Ab-V6 on the Brazilian agriculture: Lessons that farmers are receptive to adopt new microbial inoculants. Rev. Bras. Ciênc. Solo 2021, 45, e0200128. [Google Scholar] [CrossRef]
- Chibeba, A.M.; Guimarães, M.F.; Brito, O.R.; Nogueira, M.A.; Araújo, R.S.; Hungria, M. Co-inoculação de soja com Bradyrhizobium e Azospirillum promove nodulação precoce. Am. J. Plant Sci. 2015, 6, 1641–1649. [Google Scholar] [CrossRef]
- Bazzo, J.H.B.; Monteiro, J.; de Lucena Marinho, J. Inoculação e coinoculação de Azospirillum e Bradyrhizobium, via sementes e em cobertura, na qualidade fisiológica de sementes de soja. Rev. Cult. Agron. 2020, 29, 426–436. [Google Scholar] [CrossRef]
- Yuan, S.; Zhou, S.; Feng, Y.; Zhang, C.; Huang, Y.; Shan, Z.; Chen, S.; Guo, W.; Yang, H.; Yang, Z.; et al. Identification of the important genes of Bradyrhizobium diazoefficiens 113-2 involved in soybean nodule development and senescence. Front. Microbiol. 2021, 12, 754837. [Google Scholar] [CrossRef] [PubMed]
- Zaheer, M.S.; Ali, H.H.; Iqbal, M.A.; Erinle, K.O.; Javed, T.; Iqbal, J.; Makhdoom, I.U.H.; Mumtaz, M.Z.; Salama, E.A.A.; Kalaji, H.M.; et al. Cytokinin production by Azospirillum brasilense contributes to increase in growth, yield, antioxidant, and physiological systems of wheat (Triticum aestivum L.). Front. Microbiol. 2022, 13, 886041. [Google Scholar] [CrossRef]
- Ganusova, E.E.; Banerjee, I.; Seats, T.; Alexandre, G. Indole-3-acetic acid (IAA) protects Azospirillum brasilense from indole-induced stress. Appl. Environ. Microbiol. 2025, 91, e02384-24. [Google Scholar] [CrossRef]
- Fernandez-Göbel, T.F.; Deanna, R.; Muñoz, N.B.; Robert, G.; Asurmendi, S.; Lascano, R. Redox systemic signaling and induced tolerance responses during soybean–Bradyrhizobium japonicum interaction: Involvement of nod factor receptor and autoregulation of nodulation. Front. Plant Sci. 2019, 10, 141. [Google Scholar] [CrossRef]
- Zeffa, D.M.; Fantin, L.H.; Koltun, A.; de Oliveira, A.L.M.; Nunes, M.P.B.A.; Canteri, M.G.; Gonçalves, L.S.A. Effects of plant growth-promoting rhizobacteria on co-inoculation with Bradyrhizobium in soybean crop: A meta-analysis of studies from 1987 to 2018. PeerJ 2020, 8, e7905. [Google Scholar] [CrossRef] [PubMed]
- Chibeba, A.M.; Kyei-Boahen, S.; de Fátima Guimarães, M.; Nogueira, M.A.; Hungria, M. Towards sustainable yield improvement: Field inoculation of soybean with Bradyrhizobium and co-inoculation with Azospirillum in Mozambique. Arch. Microbiol. 2020, 202, 2579–2590. [Google Scholar] [CrossRef] [PubMed]
- Pahari, A.; Nayak, S.K.; Banik, A.; Lakra, P.B.; Mishra, B.B. Biological nitrogen fixation mechanism and applications. In Agriculturally Important Microorganisms; Mishra, B.B., Nayak, S.K., Pahari, A., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 137–151. [Google Scholar] [CrossRef]
- Devi, O.R.; Sarma, A.; Borah, K.; Prathibha, R.S.; Tamuly, G.; Maniratnam, K.; Laishram, B. Importance of zinc and molybdenum for sustainable pulse production in India. Environ. Ecol. 2023, 41, 1853–1859. [Google Scholar] [CrossRef]
- Kotthaus, J.; Kotthaus, J.; Schade, D.; Schwering, U.; Hungeling, H.; Müller-Fielitz, H.; Raasch, W.; Clement, B. New prodrugs of the antiprotozoal drug pentamidine. ChemMedChem 2011, 6, 2233–2242. [Google Scholar] [CrossRef]
- Clement, B.; Struwe, M.A. The history of mARC. Molecules 2023, 28, 4713. [Google Scholar] [CrossRef]
- Oliveira, M.S.; Santos, K.F.; de Paula, R.M.; Vitorino, L.C.; Bessa, L.A.; Greer, A.; Di Mascio, P.; De Souza, J.C.P.; Martin-Didonet, C.C. Nitric oxide detection using a chemical trap method for applications in bacterial systems. Microorganisms 2023, 11, 2210. [Google Scholar] [CrossRef]
- Puppo, A.; Pauly, N.; Boscari, A.; Mandon, K.; Brouquisse, R. Hydrogen peroxide and nitric oxide: Key regulators of the Legume-Rhizobium and mycorrhizal symbioses. Antioxid. Redox Signal. 2013, 18, 2202–2219. [Google Scholar] [CrossRef] [PubMed]
- Signorelli, S.; Sainz, M.; Rosa, S.T.; Monza, J. The role of nitric oxide in nitrogen fixation by legumes. Front. Plant Sci. 2020, 11, 521. [Google Scholar] [CrossRef] [PubMed]
- Akeel, A.; Jahan, A. Role of cobalt in plants: Its stress and alleviation. In Contaminants in Agriculture: Sources, Impacts and Management; Naeem, M., Ansari, A., Gill, S., Eds.; Springer: Cham, Switzerland, 2020; pp. 339–357. [Google Scholar] [CrossRef]
- Marschner, P. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Elsevier: Amsterdam, The Netherlands; Academic Press: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Hopkins, W. Introduction to Plant Physiology; John Wiley & Sons: New York, NY, USA, 1995. [Google Scholar]
- Kolberg, M.; Strand, K.R.; Graff, P.; Andersson, K.K. Structure, function, and mechanism of ribonucleotide reductases. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2004, 1699, 1–34. [Google Scholar] [CrossRef]
- Gerendás, J.; Polacco, J.C.; Freyermuth, S.K.; Sattelmacher, B. Significance of nickel for plant growth and metabolism. J. Plant Nutr. Soil Sci. 1999, 162, 241–256. [Google Scholar] [CrossRef]
- Gupta, S.; Yildirim, S.; Andrikopoulos, B.; Wille, U.; Roessner, U. Deciphering the interactions in the root–soil nexus caused by urease and nitrification inhibitors: A review. Agronomy 2023, 13, 1603. [Google Scholar] [CrossRef]
- Salam, A.; Afridi, M.S.; Khan, A.R.; Azhar, W.; Shuaiqi, Y.; Ulhassan, Z.; Qi, J.; Xuo, N.; Chunyan, Y.; Chen, N.; et al. Cobalt induced toxicity and tolerance in plants: Insights from omics approaches. In Heavy Metal Toxicity and Tolerance in Plants: A Biological, Omics, and Genetic Engineering Approach; Hossain, M.A., Hossain, A.K.M.Z., Bourgerie, S., Fujita, M., Dhankher, O.P., Haris, P., Eds.; Wiley: Hoboken, NJ, USA, 2023; pp. 207–229. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Höfte, M.; Tzortzakis, N.; Petropoulos, S.A.; Di Gioia, F. Micronutrients: The borderline between their beneficial role and toxicity in plants. Front. Plant Sci. 2022, 13, 840624. [Google Scholar] [CrossRef]
- Teixeira, G.C.M.; Prado, R.D.M.; Rocha, A.M.S.; Silva, J.L.F.D.; Lata-Tenesaca, L.F.; Dias, M.A.N. The adequate dose of Mo required for soybean seed treatment is low when associated with Cu, Mn, and Zn compared to its association with Co and Ni, although increasing the risk of toxicity. J. Plant Nutr. 2023, 46, 1545–1559. [Google Scholar] [CrossRef]
- Yang, J.; Song, Z.; Ma, J.; Han, H. Toxicity of molybdenum-based nanomaterials on the soybean–rhizobia symbiotic system: Implications for nutrition. ACS Appl. Nano Mater. 2020, 3, 5773–5782. [Google Scholar] [CrossRef]
- Razzaque, M.S.; Wimalawansa, S.J. Minerals and human health: From deficiency to toxicity. Nutrients 2025, 17, 454. [Google Scholar] [CrossRef]
- Aljohani, A.R.; Alharbi, S.; Althobaitri, R.S.; Aljohani, R.S.; Alnemari, R.O.; Atida, H. Micronutrients toxicity from causes to adverse effects: A review. Int. J. Med. Dev. Ctries. 2023, 7, 711–715. [Google Scholar] [CrossRef]
- Oliveira, S.L.; Crusciol, C.A.C.; Rodrigues, V.A.; Galeriani, T.M.; Portugal, J.R.; Bossolani, J.W.; Moretti, L.G.; Calonego, J.C.; Cantarella, H. Molybdenum foliar fertilization improves photosynthetic metabolism and grain yields of field-grown soybean and maize. Front. Plant Sci. 2022, 13, 887682. [Google Scholar] [CrossRef]
- Ma, J.; Song, Z.; Yang, J.; Wang, Y.; Han, H. Cobalt ferrite nanozyme for efficient symbiotic nitrogen fixation via regulating reactive oxygen metabolism. Environ. Sci. Nano 2021, 8, 188–203. [Google Scholar] [CrossRef]
- Tiwari, S.; Mchanty, P. Cobalt induced changes in photosystem activity in Synechocystis PCC 6803: Alterations in energy distribution and stoichiometry. Photosynth. Res. 1996, 50, 243–256. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; El-Naggar, A.H.; Osman, M.E.H.; El-Mazaly, E. Effect of cobalt on growth, pigments and the photosynthetic electron transport in Monoraphidium minutum and Nitzchia perminuta. Braz. J. Plant Physiol. 2003, 15, 159–166. [Google Scholar] [CrossRef]
- Krieger-Liszkay, A.; Shimakawa, G. Regulation of the generation of reactive oxygen species during photosynthetic electron transport. Biochem. Soc. Trans. 2022, 50, 1025–1034. [Google Scholar] [CrossRef]
- Kume, A.; Akitsu, T.; Nasahara, K.N. Why is chlorophyll b only used in light-harvesting systems? J. Plant Res. 2018, 131, 961–972. [Google Scholar] [CrossRef]
- Zayed, O.; Hewedy, O.A.; Abdelmoteleb, A.; Ali, M.; Youssef, M.S.; Roumia, A.F.; Seymour, D.; Yuan, Z.C. Nitrogen journey in plants: From uptake to metabolism, stress response, and microbe interaction. Biomolecules 2023, 13, 1443. [Google Scholar] [CrossRef] [PubMed]
- Farhan, M.; Sathish, M.; Kiran, R.; Mushtaq, A.; Baazeem, A.; Hasnain, A.; Hakim, F.F.; Naqvi, S.A.H.; Mubeen, M.; Iftikhar, Y.; et al. Plant nitrogen metabolism: Balancing resilience to nutritional stress and abiotic challenges. Phyton 2024, 93, 3. [Google Scholar] [CrossRef]
- Biswal, A.K.; Pattanayak, G.K.; Pandey, S.S.; Leelavathi, S.; Reddy, V.S.; Govindjee; Tripathy, B.C. Light intensity-dependent modulation of chlorophyll b biosynthesis and photosynthesis by overexpression of chlorophyllide a oxygenase in tobacco. Plant Physiol. 2012, 159, 433–449. [Google Scholar] [CrossRef]
- Miglani, G.S.; Kaur, R.; Sharma, P.; Gupta, N. Leveraging photosynthetic efficiency toward improving crop yields. J. Crop Improv. 2021, 35, 361–402. [Google Scholar] [CrossRef]
- Koch, H.; Sessitsch, A. The microbial-driven nitrogen cycle and its relevance for plant nutrition. J. Exp. Bot. 2024, 75, 5547–5556. [Google Scholar] [CrossRef]
- Cadoux, C.; Maslać, N.; Di Luzio, L.; Ratcliff, D.; Gu, W.; Wagner, T.; Milton, R.D. The mononuclear metal-binding site of Mo-nitrogenase is not required for activity. JACS Au 2023, 3, 2993–2999. [Google Scholar] [CrossRef]
- Rodríguez-Jiménez, T.D.J.; Ojeda-Barrios, D.L.; Blanco-Macías, F.; Valdez-Cepeda, R.D.; Parra-Quezada, R. Urease and nickel in plant physiology. Rev. Chapingo Ser. Hortic. 2016, 22, 69–82. [Google Scholar] [CrossRef]
- Abreu, I.; Reguera, M.; Bonilla, A.; Bolaños, L.; Bonilla, I. Mineral nutrition in the legume–rhizobia nitrogen fixing symbiosis. In Beneficial Plant-Microbial Interactions: Ecology and Applications; Rodelas González, M.B., Gonzalez-Lopez, J., Eds.; CRC Press: Boca Raton, FL, USA, 2016; pp. 123–140. [Google Scholar] [CrossRef]
- Barbosa, J.Z.; Hungria, M.; da Silva Sena, J.V.; Poggere, G.; dos Reis, A.R.; Corrêa, R.S. Meta-analysis reveals benefits of co-inoculation of soybean with Azospirillum brasilense and Bradyrhizobium spp. in Brazil. Appl. Soil Ecol. 2021, 163, 103913. [Google Scholar] [CrossRef]
- Reis, A.F.B.; Rosso, L.H.M.; Adee, E.; Davidson, D.; Kovács, P.; Purcell, L.C.; Below, F.E.; Casteel, S.N.; Knott, C.; Kandel, H.; et al. Seed inoculation with Azospirillum brasilense in the U.S. soybean systems. Field Crops Res. 2022, 283, 108537. [Google Scholar] [CrossRef]
- Naorem, A.; Tilgam, J.; Priyadarshini, P.; Tak, Y.; Bharati, A.; Patel, A. Nitrogenase enzyme complex: Functions, regulation, and biotechnological applications. In Advances in Plant Nitrogen Metabolism; Yousuf, P.Y., Shabir, P.A., Hakeem, K.R., Eds.; CRC Press: Boca Raton, FL, USA, 2022; pp. 142–154. [Google Scholar] [CrossRef]
- Nichio, B.T.D.L.; Chaves, R.B.R.; Pedrosa, F.D.O.; Raittz, R.T. Exploring diazotrophic diversity: Unveiling Nif core distribution and evolutionary patterns in nitrogen-fixing organisms. BMC Genom. 2025, 26, 81. [Google Scholar] [CrossRef] [PubMed]
| Ca | Mg | Ca+Mg | Al | H+Al | K | K | S | P | CaCl2 |
|---|---|---|---|---|---|---|---|---|---|
| cmolc dm−3 | mg dm−3 | pH | |||||||
| 1.57 | 0.90 | 2.47 | 0.06 | 2.22 | 0.34 | 133 | 11.4 | 23.81 | 5.0 |
| Na | Fe | Mn | Cu | Zn | B | CEC | SB | V | m |
| mg dm−3 | cmolc dm−3 | % | |||||||
| 0.0 | 31.3 | 154.5 | 4.00 | 1.18 | 0.10 | 5.03 | 2.81 | 55 | 2.1 |
| Texture (g kg−1) | OM | Ca/Mg | Ca/K | Mg/K | Ca/CEC | Mg/CEC | K/CEC | ||
| Clay | Silt | Sand | g dm−3 | Relationship between bases | |||||
| 48 | 8 | 44 | 36.1 | 1.7 | 4.6 | 2.7 | 31.21 | 17.89 | 6.76 |
| Treatment | Formulations |
|---|---|
| Control + AZO | A. brasilense |
| Mo + AZO | 30 g ha−1 ammonium molybdate + A. brasilense |
| Co + AZO | 3 g ha−1 cobalt sulfate + A. brasilense |
| Ni + AZO | 30 g ha−1 nickel sulfate + A. brasilense |
| Mo + Co + AZO | 30 g ha−1 ammonium molybdate + 3 g ha−1 cobalt sulfate + A. brasilense |
| Mo + Ni + AZO | 30 g ha−1 ammonium molybdate + 30 g ha−1 nickel sulfate + A. brasilense |
| Co + Ni + AZO | 3 g ha−1 cobalt sulfate + 30 g ha−1 nickel sulfate + A. brasilense |
| Mo + Co + Ni + AZO | 30 g ha−1 ammonium molybdate + 3 g ha−1 cobalt sulfate + 30 g ha−1 nickel sulfate + A. brasilense |
| Control + BRADY | B. diazoefficiens |
| Mo + BRADY | 30 g ha−1 ammonium molybdate + B. diazoefficiens |
| Co + BRADY | 3 g ha−1 cobalt sulfate + B. diazoefficiens |
| Ni + BRADY | 30 g ha−1 nickel sulfate + B. diazoefficiens |
| Mo + Co + BRADY | 30 g ha−1 ammonium molybdate + 3 g ha−1 cobalt sulfate + B. diazoefficiens |
| Mo + Ni + BRADY | 30 g ha−1 ammonium molybdate + 30 g ha−1 nickel sulfate + B. diazoefficiens |
| Co + Ni + BRADY | 3 g ha−1 cobalt sulfate + 30 g ha−1 nickel sulfate + B. diazoefficiens |
| Mo + Co + Ni + BRADY | 30 g ha−1 ammonium molybdate + 3 g ha−1 cobalt sulfate + 30 g ha−1 nickel sulfate + B. diazoefficiens |
| Control + AZO + BRADY | A. brasilense + B. diazoefficiens |
| Mo + AZO + BRADY | 30 g ha−1 ammonium molybdate + A. brasilense + B. diazoefficiens |
| Co + AZO + BRADY | 3 g ha−1 cobalt sulfate + A. brasilense + B. diazoefficiens |
| Ni + AZO + BRADY | 30 g ha−1 nickel sulfate + A. brasilense + B. diazoefficiens |
| Mo + Co + AZO + BRADY | 30 g ha−1 ammonium molybdate + 3 g ha−1 cobalt sulfate + A. brasilense + B. diazoefficiens |
| Mo + Ni + AZO + BRADY | 30 g ha−1 ammonium molybdate + 30 g ha−1 nickel sulfate + A. brasilense + B. diazoefficiens |
| Co + Ni + AZO + BRADY | 3 g ha−1 cobalt sulfate + 30 g ha−1 nickel sulfate + A. brasilense + B. diazoefficiens |
| Mo + Co + Ni + AZO + BRADY | 30 g ha−1 ammonium molybdate + 3 g ha−1 cobalt sulfate + 30 g ha−1 nickel sulfate + A. brasilense + B. diazoefficiens |
| Total control | Untreated seeds (lack of micronutrients and rhizobacteria) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reis, M.N.O.; Vitorino, L.C.; Moreira, M.A.; Macedo, A.S.; de Sousa, L.F.; Lourenço, L.L.; Bessa, L.A. Supplementation with Mo, Co, and Ni Enhances the Effectiveness of Co-Inoculation with the Rhizobacteria Azospirillum brasilense and Bradyrhizobium diazoefficiens in Soybean. Microorganisms 2025, 13, 2680. https://doi.org/10.3390/microorganisms13122680
Reis MNO, Vitorino LC, Moreira MA, Macedo AS, de Sousa LF, Lourenço LL, Bessa LA. Supplementation with Mo, Co, and Ni Enhances the Effectiveness of Co-Inoculation with the Rhizobacteria Azospirillum brasilense and Bradyrhizobium diazoefficiens in Soybean. Microorganisms. 2025; 13(12):2680. https://doi.org/10.3390/microorganisms13122680
Chicago/Turabian StyleReis, Mateus Neri Oliveira, Luciana Cristina Vitorino, Marialva Alvarenga Moreira, Alex Santos Macedo, Letícia Ferreira de Sousa, Lucas Loram Lourenço, and Layara Alexandre Bessa. 2025. "Supplementation with Mo, Co, and Ni Enhances the Effectiveness of Co-Inoculation with the Rhizobacteria Azospirillum brasilense and Bradyrhizobium diazoefficiens in Soybean" Microorganisms 13, no. 12: 2680. https://doi.org/10.3390/microorganisms13122680
APA StyleReis, M. N. O., Vitorino, L. C., Moreira, M. A., Macedo, A. S., de Sousa, L. F., Lourenço, L. L., & Bessa, L. A. (2025). Supplementation with Mo, Co, and Ni Enhances the Effectiveness of Co-Inoculation with the Rhizobacteria Azospirillum brasilense and Bradyrhizobium diazoefficiens in Soybean. Microorganisms, 13(12), 2680. https://doi.org/10.3390/microorganisms13122680







