Complete Genome Sequencing of Erwinia phyllosphaerae ZX-13, a Novel Biocontrol Agent to Against the Stem Blight Pathogen Pseudocryphonectria elaeocarpicola in Elaeocarpus spp.
Abstract
1. Introduction
2. Materials and Methods
2.1. Evaluation of the Antibacterial Activity of Experimental Strain
2.2. Morphological and Physiological-Biochemical Analysis
2.3. The Biocontrol Effect of Strain ZX-13 Fermentation Broth on Elaeocarpus Species Stem Blight Under Laboratory Conditions
2.4. Genome Sequencing, Assembly and Annotation
2.5. Phylogenetic Analyses
2.6. ANI and GGDC Analyses
2.7. Discovery of Bioactive Compound
2.8. Photographs Editing and Statistical Analysis
3. Results
3.1. The Biocontrol Efficacy of Strain ZX-13
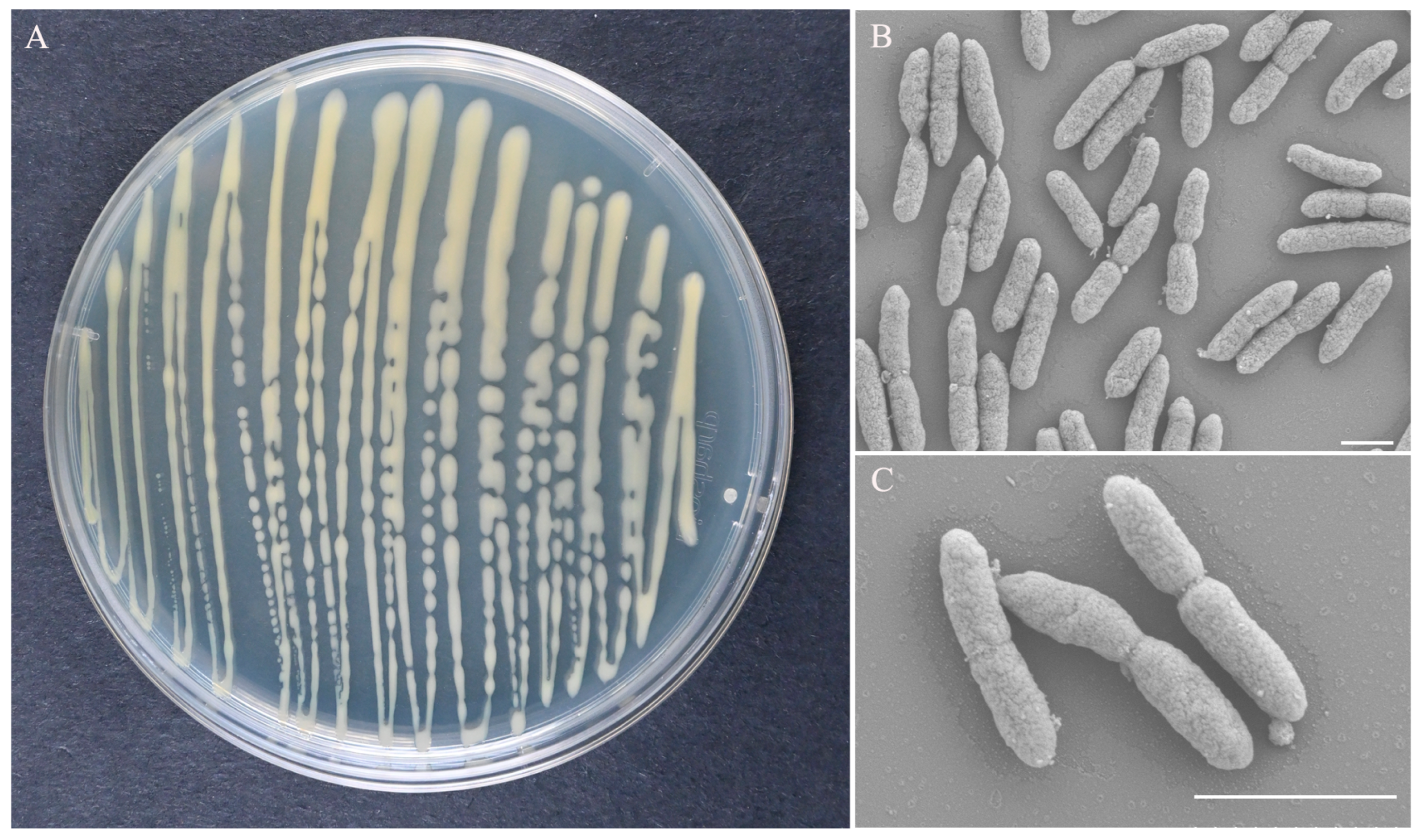
3.2. Morphological Characteristics and Physiological and Biochemical Properties of Strain ZX-13
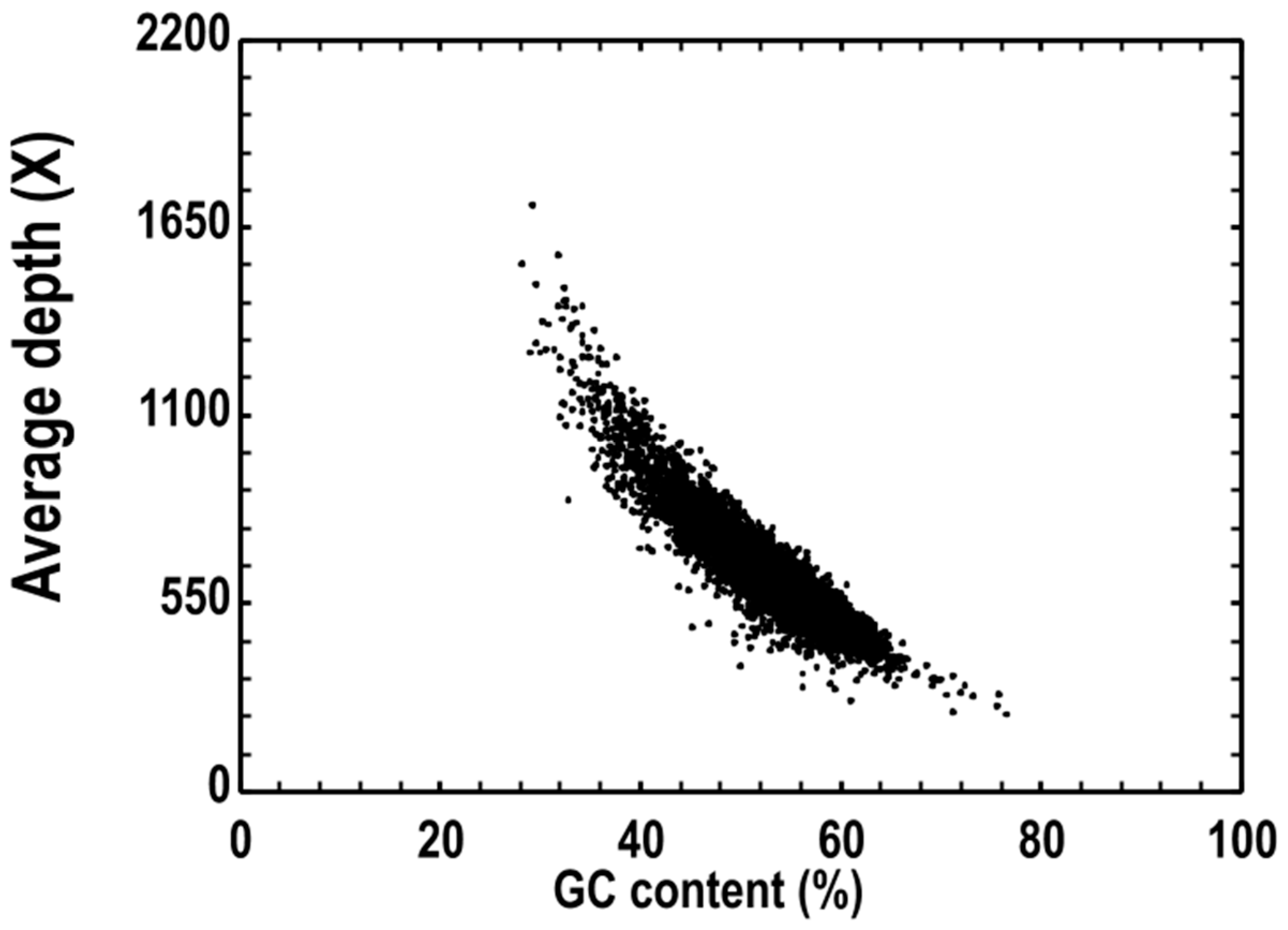
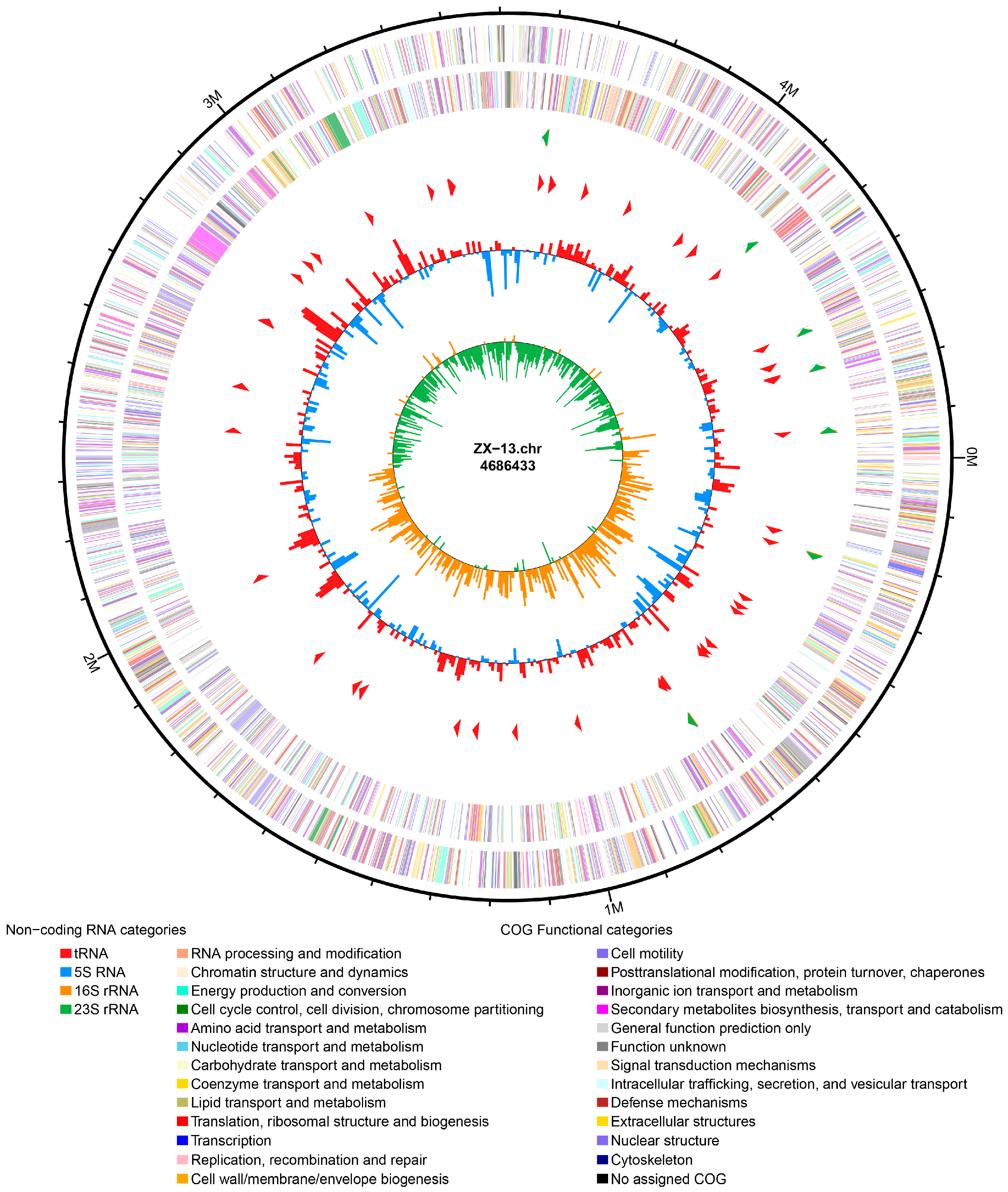
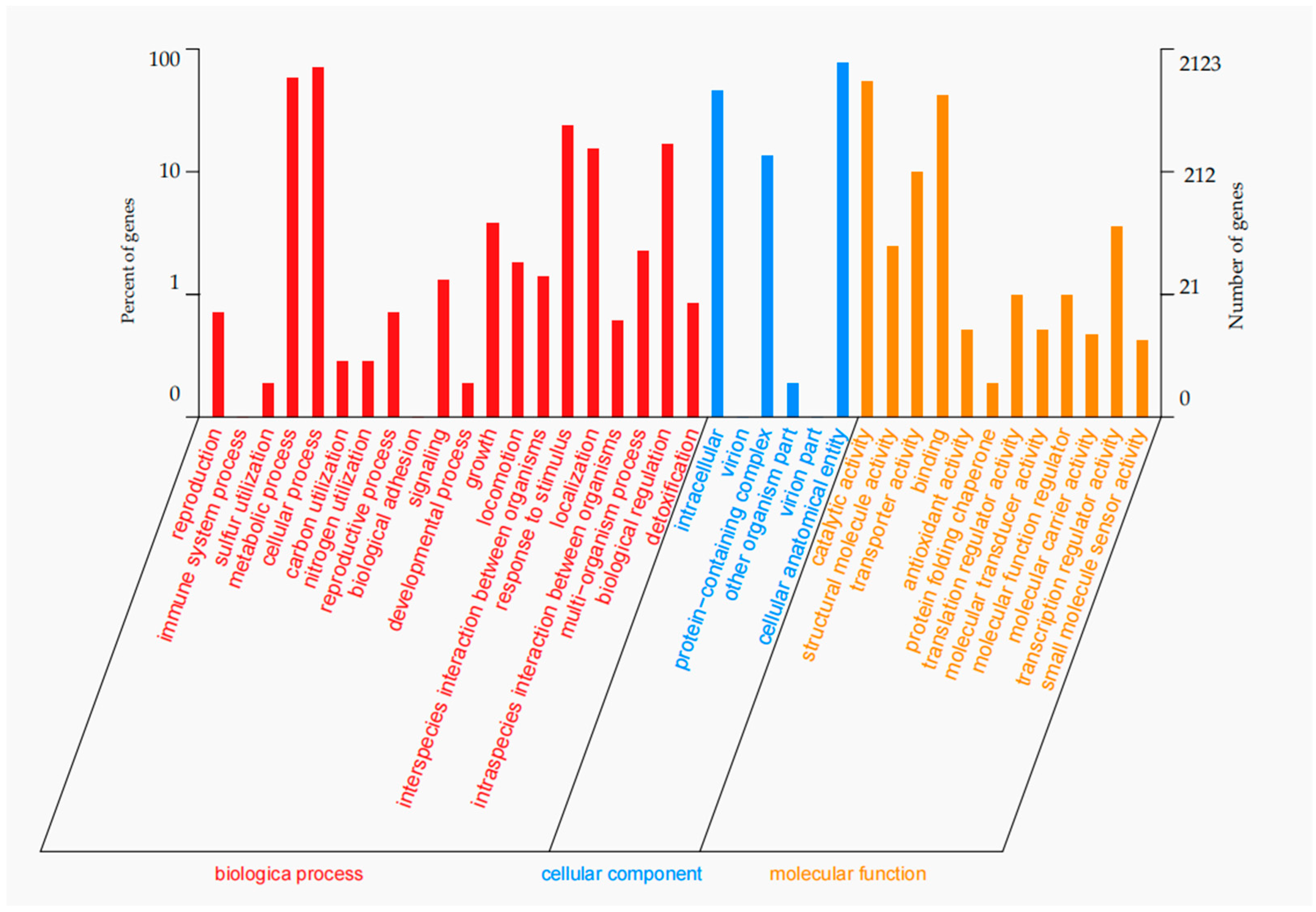
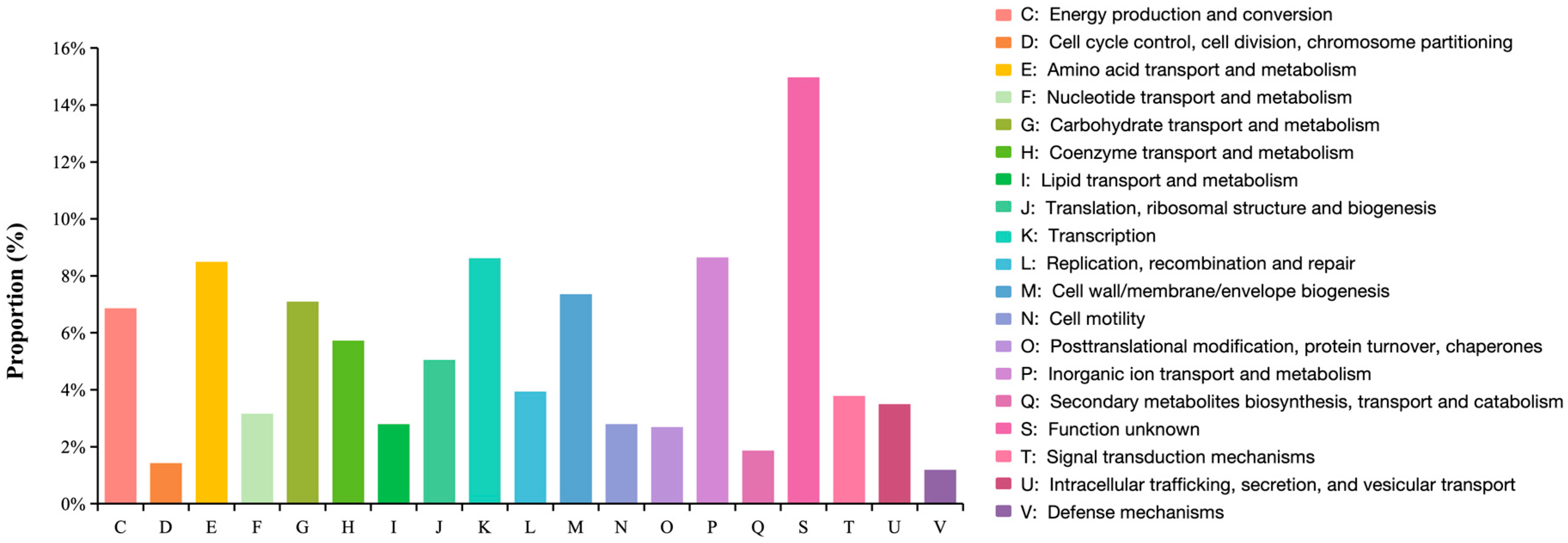
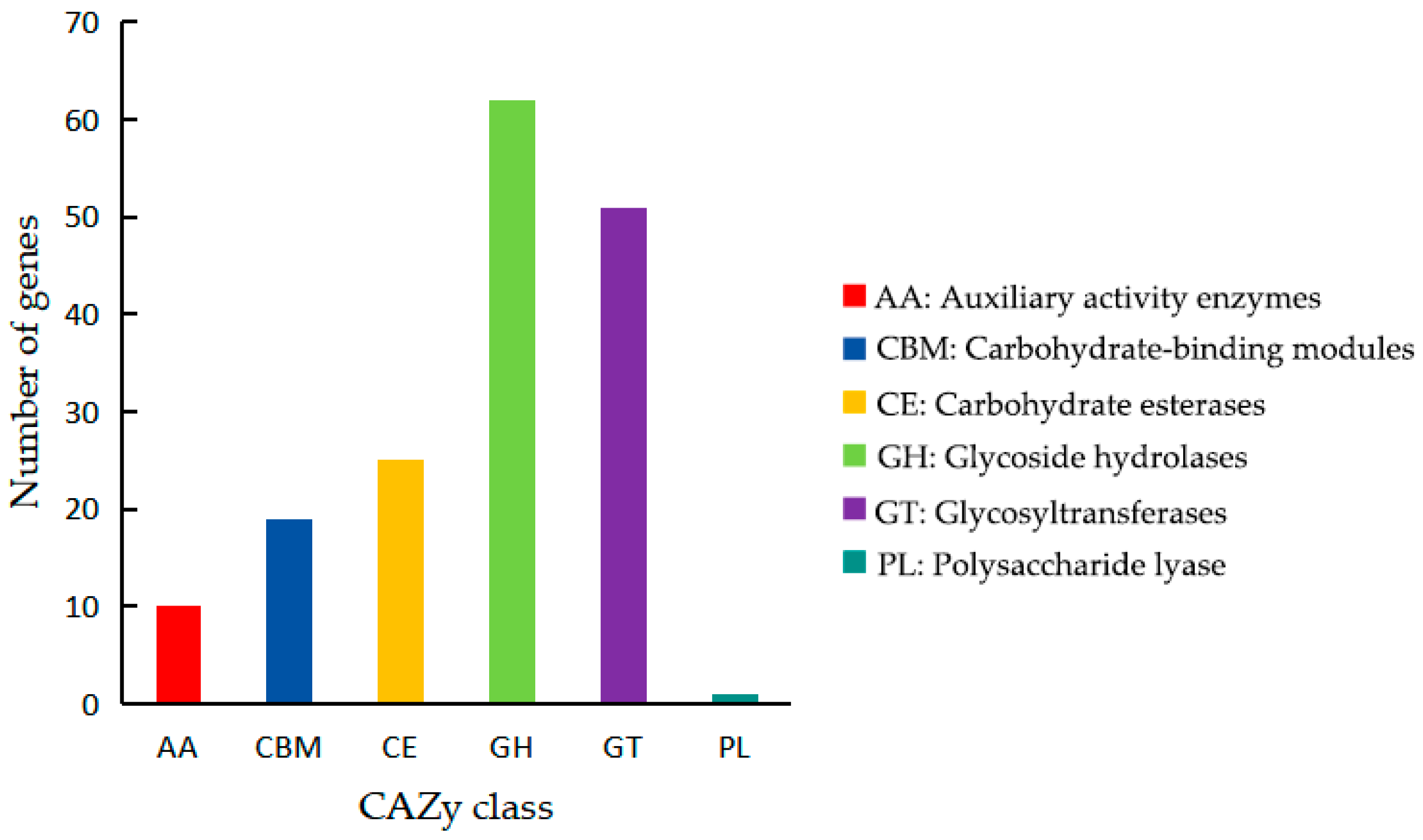
3.3. Genome Feature Analysis and Function Annotation
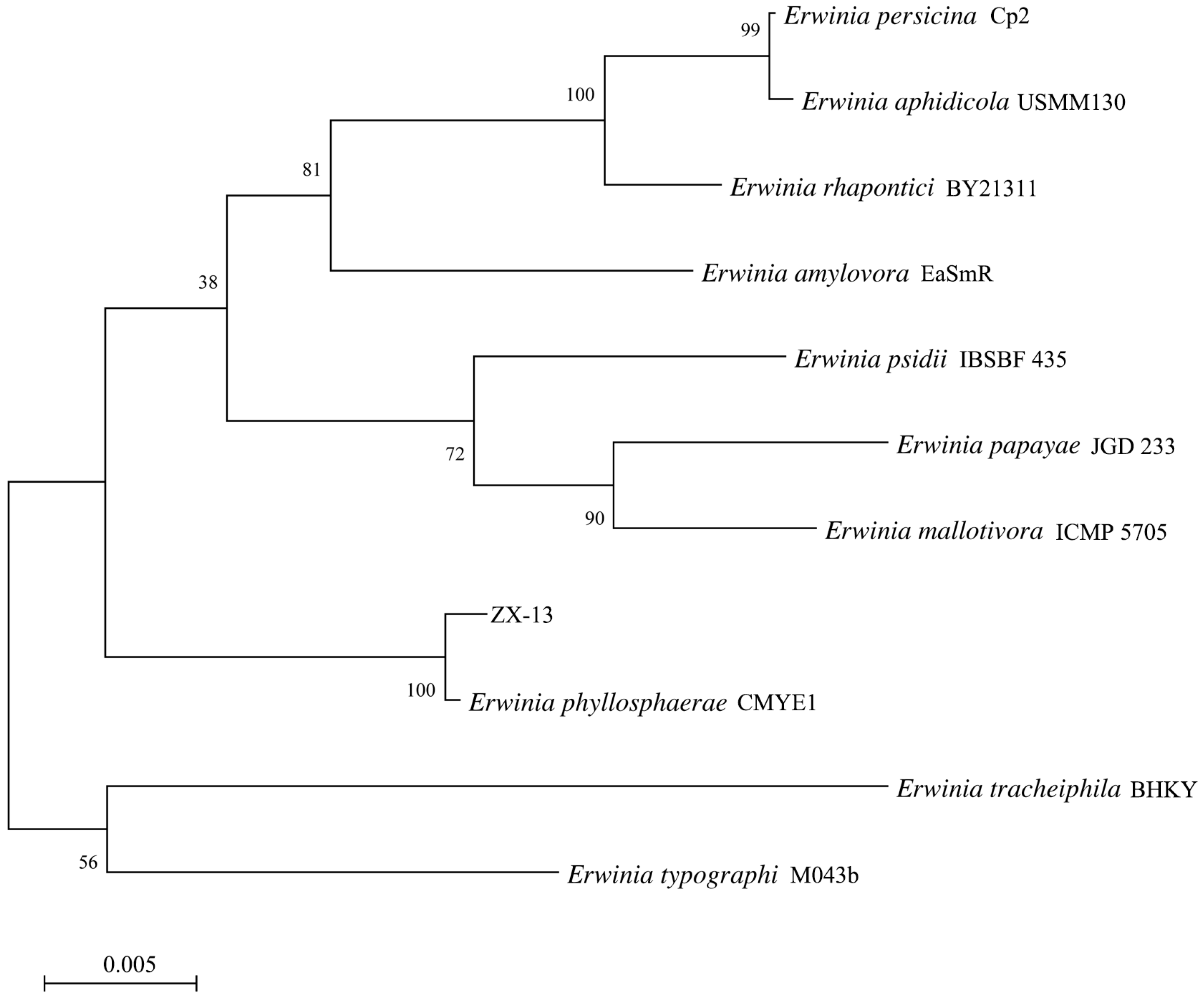
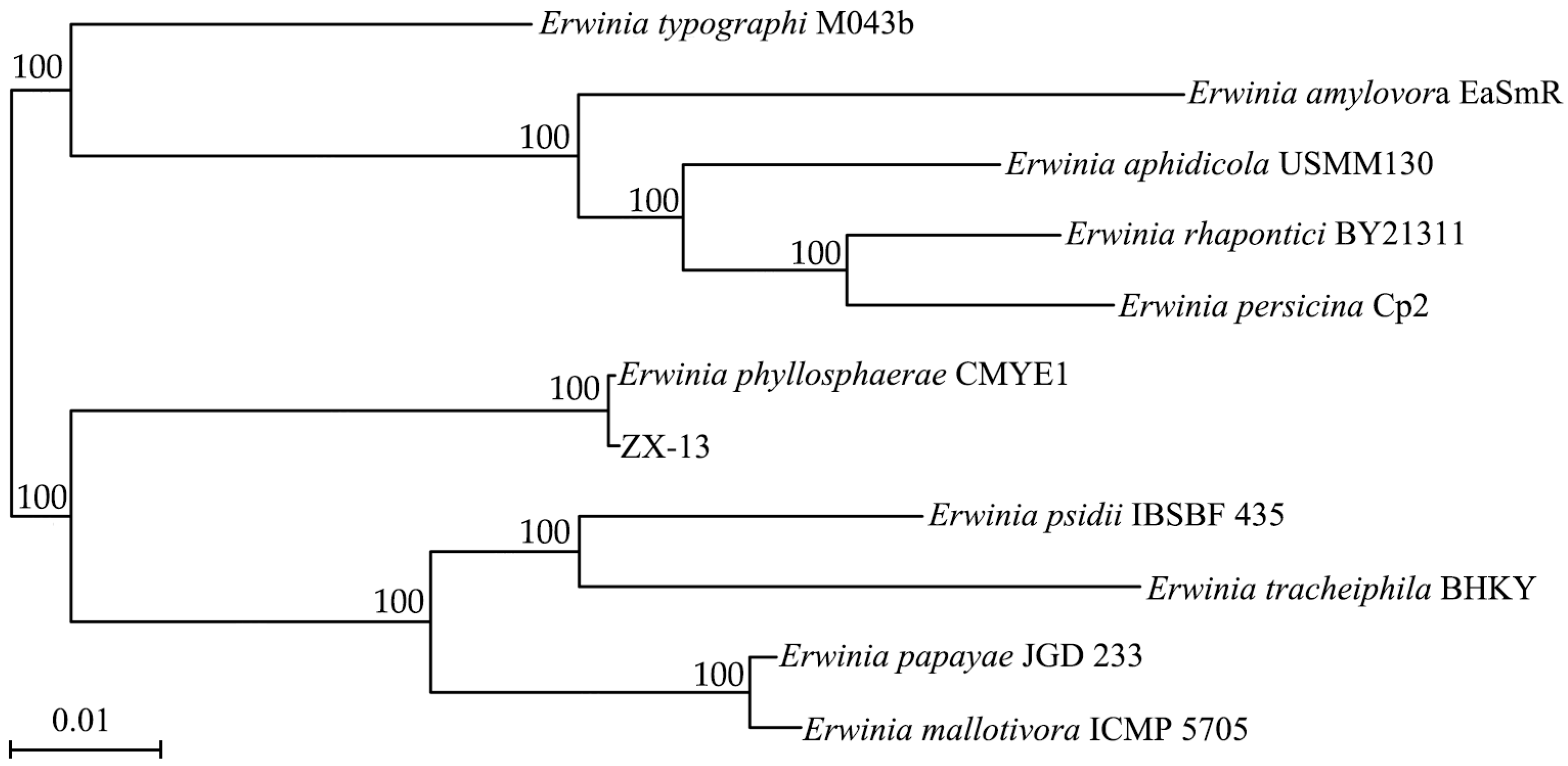
3.4. Identification of Strain ZX-13 as Erwinia phyllosphaerae Using 16S rRNA Gene and Whole-Genome Phylogeny
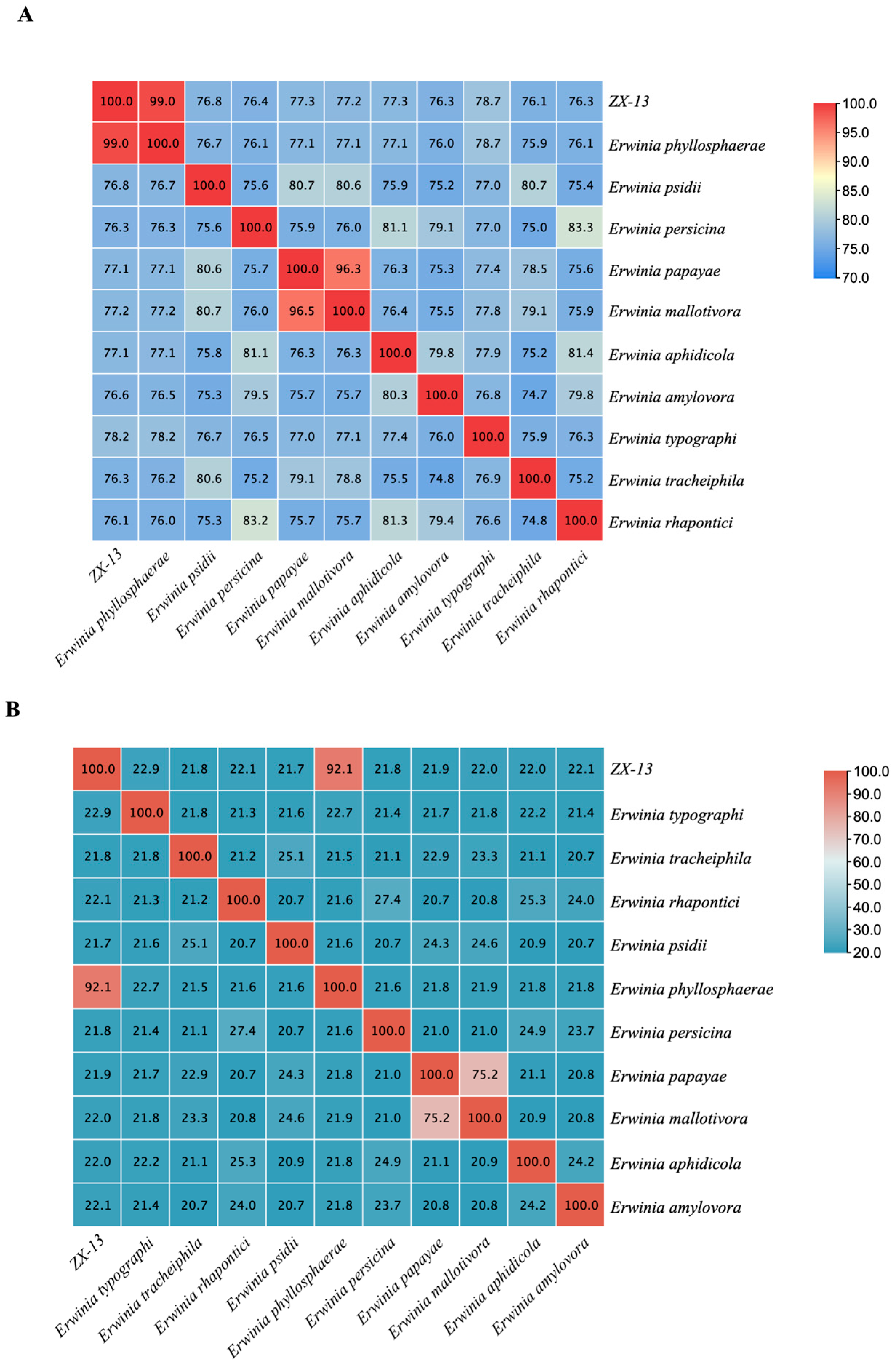
3.5. ANI and GGDC in Genomic Distance Estimation
3.6. Bioactive Compound Prediction
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prasannan, P.; Jeyaram, Y.; Pandian, A.; Raju, R.; Sekar, S. A review on taxonomy, phytochemistry, pharmacology, threats and conservation of Elaeocarpus L. (Elaeocarpaceae). Bot. Rev. 2020, 86, 298–328. [Google Scholar] [CrossRef]
- Bae, G.Y.; Lim, M.W.; Eom, S.W.; Lee, H.L.; Lee, D.Y.; Oh, Y.J. Exploring the potential of Elaeocarpus sylvestris as natural biomaterials: In vitro antimicrobial and antioxidant properties, chemical constituents, and its effect on skin fibroblasts. Plant Biotechnol. Rep. 2023, 17, 637–651. [Google Scholar] [CrossRef]
- Borase, B.S.; Surana, S.S. A Comprehensive review on Elaeocarpus floribundus Blume. Curr. Tradit. Med. 2024, 10, 32–36. [Google Scholar] [CrossRef]
- Habibah, N.A.; Nugrahaningsih, N.; Safitri, S.; Musafa, F.; Wijawati, N. Profile of flavonoid and antioxidant activity in cell suspension culture of Elaeocarpus grandiflorus. Biosaintifika 2021, 13, 328–335. [Google Scholar] [CrossRef]
- Huang, H.Y.; Huang, H.H.; Zhao, D.Y.; Shan, T.J.; Hu, L.L. Pseudocryphonectria elaeocarpicola gen. et sp. nov. (Cryphonectriaceae, Diaporthales) causing stem blight of Elaeocarpus spp. in China. MycoKeys 2022, 91, 67–84. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.L.; Huang, H.Y.; Dai, J.; Gao, C.L.; Zhao, Y.; Wang, Y.W. Biological characteristics of the pathogen for stem blight of Elaeocarpus. For. Environ. Sci. 2024, 40, 90–94. [Google Scholar] [CrossRef]
- Chen, T.T.; Zhang, Z.Z.; Li, W.Z.; Chen, J.; Chen, X.T.; Wang, B.C.; Dai, Y.Y.; Ma, J.L.; Ding, H.X.; Wang, W.Z.; et al. Biocontrol potential of Bacillus subtilis CTXW 7-6-2 against kiwifruit soft rot pathogens revealed by whole-genome sequencing and biochemical characterisation. Front. Microbiol. 2022, 13, 1069109. [Google Scholar] [CrossRef]
- Xie, L.Y.; Liu, L.F.; Luo, Y.J.; Rao, X.B.; Di, Y.N.; Liu, H.; Qian, Z.F.; Shen, Q.Q.; He, L.L.; Li, F.S. Complete genome sequence of biocontrol strain Bacillus velezensis YC89 and its biocontrol potential against sugarcane red rot. Front. Microbiol. 2023, 14, 1180474. [Google Scholar] [CrossRef]
- Samaras, A.; Nikolaidis, M.; Antequera-Gómez, M.L.; Cámara-Almirón, J.; Romero, D.; Moschakis, T.; Amoutzias, G.D.; Karaoglanidis, G.S. Whole genome sequencing and root colonization studies reveal novel insights in the biocontrol potential and growth promotion by Bacillus subtilis MBI 600 on cucumber. Front. Microbiol. 2021, 11, 600393. [Google Scholar] [CrossRef]
- Khalifa, M.W.; Rouag, N.; Bouhadida, M. Evaluation of the antagonistic effect of Pseudomonas rhizobacteria on Fusarium wilt of chickpea. Agriculture 2022, 12, 429. [Google Scholar] [CrossRef]
- Ganeshan, G.; Kumar, A.M. Pseudomonas fluorescens, a potential bacterial antagonist to control plant diseases. J. Plant Interact. 2005, 1, 123–134. [Google Scholar] [CrossRef]
- Al-Ghafri, H.M.; Velazhahan, R.; Shahid, M.S.; Al-Sadi, A.M. Antagonistic activity of Pseudomonas aeruginosa from compost against Pythium aphanidermatum and Fusarium solani. Biocontrol Sci. Technol. 2020, 30, 642–658. [Google Scholar] [CrossRef]
- Soenens, A.; Imperial, J. Biocontrol capabilities of the genus Serratia. Phytochem. Rev. 2020, 19, 577–587. [Google Scholar] [CrossRef]
- Dandurishvili, N.; Toklikishvili, N.; Ovadis, M.; Eliashvili, P.; Giorgobiani, N.; Keshelava, R.; Tediashvili, M.; Vainstein, A.; Khmel, I.; Szegedi, E.; et al. Broad-range antagonistic rhizobacteria Pseudomonas fluorescens and Serratia plymuthica suppress Agrobacterium crown gall tumours on tomato plants. J. Appl. Microbiol. 2011, 110, 341–352. [Google Scholar] [CrossRef]
- Sharma, A.; Gupta, A.K.; Khosla, K.; Mahajan, R.; Bharti; Mahajan, P.K. Antagonistic potential of native agrocin-producing non-pathogenic Agrobacterium tumefaciens strain UHFBA-218 to control crown gall in peach. Phytoprotection 2017, 97, 1–11. [Google Scholar] [CrossRef]
- Ciancio, A. Biocontrol potential of Pasteuria spp. for the management of plant parasitic nematodes. CABI Rev. 2018, 13, 1–13. [Google Scholar] [CrossRef]
- Kiani, T.; Mehboob, F.; Hyder, M.Z.; Zainy, Z.; Xu, L.; Huang, L.; Farrakh, S. Control of stripe rust of wheat using indigenous endophytic bacteria at seedling and adult plant stage. Sci. Rep. 2021, 11, 14473. [Google Scholar] [CrossRef]
- Gardan, L.; Christen, R.; Achouak, W.; Prior, P. Erwinia papayae sp. nov., a pathogen of papaya (Carica papaya). Int. J. Syst. Evol. Microbiol. 2004, 54, 107–113. [Google Scholar] [CrossRef]
- López, M.M.; Rosello, M.; Llop, P.; Ferrer, S.; Christen, R.; Gardan, L. Erwinia piriflorinigrans sp. nov., a novel pathogen that causes necrosis of pear blossoms. Int. J. Syst. Evol. Microbiol. 2011, 61, 561–567. [Google Scholar] [CrossRef]
- Matsuura, T.; Mizuno, A.; Tsukamoto, T.; Shimizu, Y.; Saito, N.; Sato, S.; Kikuchi, S.; Uzuki, S.; Azegami, K.; Sawada, H. Erwinia uzenensis sp. nov., a novel pathogen that affects European pear trees (Pyrus communis L.). Int. J. Syst. Evol. Microbiol. 2012, 62, 1799–1803. [Google Scholar] [CrossRef]
- Rezzonico, F.; Smits, T.H.; Born, Y.; Blom, J.; Frey, J.E.; Goesmann, A.; Cleenwerck, I.; de Vos, P.; Bonaterra, A.; Duffy, B.; et al. Erwinia gerundensis sp. nov., a cosmopolitan epiphyte originally isolated from pome fruit trees. Int. J. Syst. Evol. Microbiol. 2016, 66, 1583–1592. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Wang, T.; Lv, C.; Yan, B.; Wan, X.; Wang, S.; Kang, C.Z.; Guo, L.P.; Huang, L. Whole Genome sequencing reveals novel insights about the biocontrol potential of Burkholderia ambifaria CF3 on Atractylodes lancea. Microorganisms 2024, 12, 1043. [Google Scholar] [CrossRef] [PubMed]
- Teber, R.; Asakawa, S. In silico screening of bacteriocin gene clusters within a set of marine Bacillota genomes. Int. J. Mol. Sci. 2024, 25, 2566. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.H.S.; Matthee, F.N.; Thomas, A.C. Biological control of Eutypa lata on grapevine by an antagonistic strain of Bacillus subtilis. Phytopathology 1991, 81, 283–287. [Google Scholar] [CrossRef]
- Pan, M.K.; Feng, G.D.; Yao, Q.; Li, J.L.; Liu, C.J.; Zhu, H.H. Erwinia phyllosphaerae sp. nov., a novel bacterium isolated from phyllosphere of pomelo (Citrus maxima). Int. J. Syst. Evol. Microbiol. 2022, 72, 005316. [Google Scholar] [CrossRef]
- Dai, P.B.; Zong, Z.F.; Ma, Q.; Wang, Y. Isolation, evaluation and identification of rhizosphere actinomycetes with potential application for biocontrol of Valsa mali. Eur. J. Plant Pathol. 2019, 153, 119–130. [Google Scholar] [CrossRef]
- Emms, D.M.; Kelly, S. OrthoFinder: Solving fundamental biases in whole genome comparisons dramatically improves orthogroup inference accuracy. Genome Biol. 2015, 16, 157. [Google Scholar] [CrossRef]
- Chen, C.J.; Wu, Y.; Li, J.W.; Wang, X.; Zeng, Z.H.; Xu, J.; Liu, Y.L.; Feng, J.T.; Chen, H.; He, Y.H.; et al. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef]
- Vanneste, J.L.; Yu, J. Biological control of fire blight using Erwinia herbicola Eh252 and Pseudomonas fluorescens A506 separately or in combination. In VII International Workshop on Fire Blight 411; International Society for Horticultural Science: Leuven, Belgium, 1995; pp. 351–354. [Google Scholar]
- Kempf, H.J.; Wolf, G. Erwinia herbicola as a biocontrol agent of Fusarium culmorum and Puccinia recondita f. sp. tritici on wheat. Phytopathology 1989, 79, 990–994. [Google Scholar] [CrossRef]
- Bi, W.L.; Wang, R.; Yang, Y.Y.; Wang, Y.; Ma, Z.T.; Wang, Q.; Zhang, D.F. Pantoea vagans strain BWL1 controls blue mold in mandarin fruit by inhibiting ergosterol biosynthesis in Penicillium expansum. Biol. Control 2021, 161, 104639. [Google Scholar] [CrossRef]
- Kim, W.I.; Choi, S.Y.; Han, I.; Cho, S.K.; Lee, Y.; Kim, S.; Kang, B.; Choi, O.; Kim, J. Inhibition of Salmonella enterica growth by competitive exclusion during early alfalfa sprout development using a seed-dwelling Erwinia persicina strain EUS78. Int. J. Food Microbiol. 2020, 312, 108374. [Google Scholar] [CrossRef] [PubMed]
- Maslova, T.G.; Markovskaya, E.F.; Slemnev, N.N. Functions of carotenoids in leaves of higher plants. Biol. Bull. Rev. 2021, 11, 476–487. [Google Scholar] [CrossRef]
- Smits, T.H.; Rezzonico, F.; Kamber, T.; Goesmann, A.; Ishimaru, C.A.; Stockwell, V.O.; Frey, E.J.; Duffy, B. Genome sequence of the biocontrol agent Pantoea vagans strain C9-1. J. Bacteriol. 2010, 192, 6486–6487. [Google Scholar] [CrossRef] [PubMed]
- Knirel, Y.A.; Bystrova, O.V.; Kocharova, N.A.; Zähringer, U.; Pier, G.B. Conserved and variable structural features in the lipopolysaccharide of Pseudomonas aeruginosa. J. Endoxtin Res. 2006, 12, 324–336. [Google Scholar] [CrossRef]
- Humisto, A.; Jokela, J.; Teigen, K.; Wahlsten, M.; Permi, P.; Sivonen, K.; Herfindal, L. Characterization of the interaction of the antifungal and cytotoxic cyclic glycolipopeptide hassallidin with sterol-containing lipid membranes. Biochim. Biophys. Acta-Biomembr. 2019, 1861, 1510–1521. [Google Scholar] [CrossRef]
- Cai, L.; Seiple, I.B.; Li, Q. Modular chemical synthesis of streptogramin and lankacidin antibiotics. Acc. Chem. Res. 2021, 54, 1891–1908. [Google Scholar] [CrossRef]

| Strain | GenBank Accession Numbers |
|---|---|
| Erwinia amylovora EaSmR | GCA_043228865.1 |
| Erwinia aphidicola USMM130 | GCA_037149315.1 |
| Erwinia mallotivora ICMP 5705 | GCA_042432085.1 |
| Erwinia papayae JGD 233 | GCA_040741485.1 |
| Erwinia persicina Cp2 | GCA_019844095.1 |
| Erwinia phyllosphaerae CMYE1 | GCA_019132875.1 |
| Erwinia psidii IBSBF 435 | GCA_003846135.1 |
| Erwinia rhapontici BY21311 | GCA_020683125.1 |
| Erwinia tracheiphila BHKY | GCA_021365465.1 |
| Erwinia typographi M043b | GCA_000773975.1 |
| Characteristic | ZX-13 | CMYE1T |
|---|---|---|
| Growth at 4 °C | + | + |
| Hydrolysis of skimmed milk | + | + |
| Esterase lipase | − | − |
| Catalase test | + | / |
| Oxidase test | − | / |
| Production of hydrogen sulfide | − | / |
| Voges–Proskauer reaction | + | / |
| Phenylalanine deaminase test | − | / |
| β-Galactosidase | + | + |
| β-Glucosidase | + | + |
| Arginine dihydrolase | − | + |
| Assimilation of citrate | − | + |
| Indole production | − | + |
| Fermentation of: | ||
| D-xylose | + | + |
| Inositol | + | + |
| Arbutin | + | + |
| Salicin | + | + |
| Maltose | − | − |
| Xylitol | − | − |
| Gentiobiose | + | + |
| Lyxose | − | − |
| D-Arabitol | + | + |
| Gluconate | − | − |
| 2-Keto-gluconate | − | − |
| D-Glucose | + | − |
| Sucrose | + | + |
| Melibiose | + | + |
| Amygdalin | + | + |
| Fatty Acid | ZX-13 | CMYE1T |
|---|---|---|
| C12:0 | 3.47 | 4.2 |
| C14:0 | 5.66 | 5.4 |
| C16:0 | 37.46 | 32 |
| C17:0 | 0.32 | 0.7 |
| C17:0 cyclo | 7.84 | 13.5 |
| C19:0 cyclo ω8c | 0.67 | 1.8 |
| Summed feature 2 | 7.49 | 9 |
| Summed feature 3 | 27.86 | 19.5 |
| Summed feature 8 | 6.75 | 10.9 |
| Characteristics | Value |
|---|---|
| Sequence number | 1 |
| Total length | 4,686,433 |
| N50 length | 4,686,433 |
| Read coverage | 1767.19 |
| G + C content (%) | 53.85 |
| N rate (%) | 0 |
| Feature | Value |
|---|---|
| Genome size (bp) | 4,686,433 |
| G + C content (%) | 53.85 |
| tRNA | 86 |
| 5S rRNA | 8 |
| 16S rRNA | 7 |
| 23S rRNA | 7 |
| ncRNA | 110 |
| Total number of genes | 4189 |
| Genes assigned to NR | 4030 |
| Genes assigned to GO | 2127 |
| Genes assigned to KEGG | 2719 |
| Genes assigned to COG | 3538 |
| Genes assigned to Swiss-Prot | 3212 |
| Type | Start | End | Similar Cluster | Similarity |
|---|---|---|---|---|
| NRPS | 641,502 | 685,410 | unknown | – |
| hserlactone | 1,127,742 | 1,148,401 | unknown | – |
| thiopeptide | 1,489,613 | 1,515,804 | O-antigen | 14% |
| RiPP-like | 1,907,458 | 1,917,655 | unknown | – |
| terpene | 2,335,363 | 2,358,975 | carotenoid | 100% |
| NRPS | 2,765,675 | 2,843,718 | hassallidin C | 6% |
| NRP-metallophore | 2,940,825 | 2,990,609 | trichrysobactin/cyclic trichrysobactin/chrysobactin/dichrysobactin | 53% |
| RRE-containing | 3,882,783 | 3,903,067 | lankacidin C | 13% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H.; Zhao, Y.; Hu, L.; Gao, C.; Chen, S.; Zhao, D. Complete Genome Sequencing of Erwinia phyllosphaerae ZX-13, a Novel Biocontrol Agent to Against the Stem Blight Pathogen Pseudocryphonectria elaeocarpicola in Elaeocarpus spp. Microorganisms 2025, 13, 2678. https://doi.org/10.3390/microorganisms13122678
Huang H, Zhao Y, Hu L, Gao C, Chen S, Zhao D. Complete Genome Sequencing of Erwinia phyllosphaerae ZX-13, a Novel Biocontrol Agent to Against the Stem Blight Pathogen Pseudocryphonectria elaeocarpicola in Elaeocarpus spp. Microorganisms. 2025; 13(12):2678. https://doi.org/10.3390/microorganisms13122678
Chicago/Turabian StyleHuang, Huayi, Yi Zhao, Lili Hu, Chenglong Gao, Shiying Chen, and Danyang Zhao. 2025. "Complete Genome Sequencing of Erwinia phyllosphaerae ZX-13, a Novel Biocontrol Agent to Against the Stem Blight Pathogen Pseudocryphonectria elaeocarpicola in Elaeocarpus spp." Microorganisms 13, no. 12: 2678. https://doi.org/10.3390/microorganisms13122678
APA StyleHuang, H., Zhao, Y., Hu, L., Gao, C., Chen, S., & Zhao, D. (2025). Complete Genome Sequencing of Erwinia phyllosphaerae ZX-13, a Novel Biocontrol Agent to Against the Stem Blight Pathogen Pseudocryphonectria elaeocarpicola in Elaeocarpus spp. Microorganisms, 13(12), 2678. https://doi.org/10.3390/microorganisms13122678






