Isavuconazole for the Treatment of Invasive Fungal Disease in Hematology Patients: A Real-World Retrospective Study on Efficacy and Safety
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Definitions
2.3. Statistical Analysis
3. Results
3.1. Demographics and Clinical Characteristics
3.2. Invasive Fungal Disease
3.3. Outcomes
3.4. Treatment Related Adverse Events
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IFD | Invasive fungal disease |
| IFI | Invasive fungal infection |
| HSCT | Hematopoietic stem cell transplantation |
| FDA | Food and Drug Administration |
| IA | Invasive aspergillosis |
| ANC | Absolute neutrophil count |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| AA | Aplastic anemia |
| AL | Acute leukemia |
| CL | Chronic leukemia |
| HL | Hodgkin’s lymphoma |
| MDS | Myelodysplastic syndrome |
| MM | Multiple myeloma |
| NHL | Non-Hodgkin’s lymphoma |
| CR | Complete remission |
| PR | Partial remission |
| SD | Stable disease |
| PD | Progressive disease |
References
- Pagano, L.; Caira, M.; Candoni, A.; Offidani, M.; Fianchi, L.; Martino, B.; Pastore, D.; Picardi, M.; Bonini, A.; Chierichini, A.; et al. The epidemiology of fungal infections in patients with hematologic malignancies: The SEIFEM-2004 study. Haematologica 2006, 91, 1068–1075. [Google Scholar] [PubMed]
- Sun, Y.; Huang, H.; Chen, J.; Li, J.; Ma, J.; Li, J.; Liang, Y.; Wang, J.; Li, Y.; Yu, K.; et al. Invasive fungal infection in patients receiving chemotherapy for hematological malignancy: A multicenter, prospective, observational study in China. Tumor Biol. 2015, 36, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Tisi, M.C.; Hohaus, S.; Cuccaro, A.; Innocenti, I.; De Carolis, E.; Za, T.; D’Alò, F.; Laurenti, L.; Fianchi, L.; Sica, S.; et al. Invasive fungal infections in chronic lymphoproliferative disorders: A monocentric retrospective study. Haematologica 2017, 102, e108–e111. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Sheng, W.H.; Tien, F.M.; Lee, P.C.; Huang, S.Y.; Tang, J.L.; Tsay, W.; Tien, H.F.; Hsueh, P.R. Clinical characteristics and treatment outcomes of pulmonary invasive fungal infection among adult patients with hematological malignancy in a medical centre in Taiwan, 2008–2013. J. Microbiol. Immunol. Infect. 2020, 53, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Nicolle, M.C.; Bénet, T.; Thiebaut, A.; Bienvenu, A.L.; Voirin, N.; Duclos, A.; Sobh, M.; Cannas, G.; Thomas, X.; Nicolini, F.E.; et al. Invasive aspergillosis in patients with hematologic malignancies: Incidence and description of 127 cases enrolled in a single institution prospective survey from 2004 to 2009. Haematologica 2011, 96, 1685–1691. [Google Scholar] [CrossRef] [PubMed]
- Patterson, T.F.; Thompson, G.R., 3rd; Denning, D.W.; Fishman, J.A.; Hadley, S.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Nguyen, M.H.; et al. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 63, e1–e60. [Google Scholar] [CrossRef] [PubMed]
- Kontoyiannis, D.P.; Marr, K.A.; Park, B.J.; Alexander, B.D.; Anaissie, E.J.; Walsh, T.J.; Ito, J.; Andes, D.R.; Baddley, J.W.; Brown, J.M.; et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: Overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin. Infect. Dis. 2010, 50, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Dagher, H.; Hachem, R.; Chaftari, A.M.; Jiang, Y.; Ali, S.; Deeba, R.; Shah, S.; Raad, I. Real-World Use of Isavuconazole as Primary Therapy for Invasive Fungal Infections in High-Risk Patients with Hematologic Malignancy or Stem Cell Transplant. J. Fungi 2022, 8, 74. [Google Scholar] [CrossRef] [PubMed]
- Ellsworth, M.; Ostrosky-Zeichner, L. Isavuconazole: Mechanism of Action, Clinical Efficacy, and Resistance. J. Fungi 2020, 6, 324, Erratum in J. Fungi 2025, 11, 226. https://doi.org/10.3390/jof11030226. [Google Scholar] [CrossRef] [PubMed]
- Maertens, J.A.; Raad, I.I.; Marr, K.A.; Patterson, T.F.; Kontoyiannis, D.P.; Cornely, O.A.; Bow, E.J.; Rahav, G.; Neofytos, D.; Aoun, M.; et al. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): A phase 3, randomised-controlled, non-inferiority trial. Lancet 2016, 387, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Marty, F.M.; Ostrosky-Zeichner, L.; Cornely, O.A.; Mullane, K.M.; Perfect, J.R.; Thompson, G.R., 3rd; Alangaden, G.J.; Brown, J.M.; Fredricks, D.N.; Heinz, W.J.; et al. Isavuconazole treatment for mucormycosis: A single-arm open-label trial and case-control analysis. Lancet Infect. Dis. 2016, 16, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Donnelley, M.A.; Zhu, E.S.; Thompson, G.R., 3rd. Isavuconazole in the treatment of invasive aspergillosis and mucormycosis infections. Infect. Drug Resist. 2016, 9, 79–86. [Google Scholar] [CrossRef] [PubMed]
- De Pauw, B.; Walsh, T.J.; Donnelly, J.P.; Stevens, D.A.; Edwards, J.E.; Calandra, T.; Pappas, P.G.; Maertens, J.; Lortholary, O.; Kauffman, C.A.; et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin. Infect. Dis. 2008, 46, 1813–1821. [Google Scholar] [CrossRef] [PubMed]
- Janssens, I.; Lambrecht, B.N.; Van Braeckel, E. Aspergillus and the Lung. Semin. Respir. Crit. Care Med. 2024, 45, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Herbrecht, R.; Denning, D.W.; Patterson, T.F.; Bennett, J.E.; Greene, R.E.; Oestmann, J.W.; Kern, W.V.; Marr, K.A.; Ribaud, P.; Lortholary, O.; et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N. Engl. J. Med. 2002, 347, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, R.N.; Mullane, K.; van Burik, J.A.; Raad, I.; Abzug, M.J.; Anstead, G.; Herbrecht, R.; Langston, A.; Marr, K.A.; Schiller, G.; et al. Posaconazole as salvage therapy for zygomycosis. Antimicrob. Agents Chemother. 2006, 50, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Lanternier, F.; Poiree, S.; Elie, C.; Garcia-Hermoso, D.; Bakouboula, P.; Sitbon, K.; Herbrecht, R.; Wolff, M.; Ribaud, P.; Lortholary, O.; et al. Prospective pilot study of high-dose (10 mg/kg/day) liposomal amphotericin B (L-AMB) for the initial treatment of mucormycosis. J. Antimicrob. Chemother. 2015, 70, 3116–3123. [Google Scholar] [CrossRef] [PubMed]
- Miceli, M.H.; Kauffman, C.A. Isavuconazole: A New Broad-Spectrum Triazole Antifungal Agent. Clin. Infect. Dis. 2015, 61, 1558–1565. [Google Scholar] [CrossRef] [PubMed]
- Maertens, J.A.; Rahav, G.; Lee, D.G.; Ponce-de-León, A.; Ramírez Sánchez, I.C.; Klimko, N.; Sonet, A.; Haider, S.; Diego Vélez, J.; Raad, I.; et al. Posaconazole versus voriconazole for primary treatment of invasive aspergillosis: A phase 3, randomised, controlled, non-inferiority trial. Lancet 2021, 397, 499–509, Erratum in Lancet 2021, 398, 490. https://doi.org/10.1016/S0140-6736(21)01745-1. [Google Scholar] [CrossRef] [PubMed]
- Batista, M.V.; Ussetti, M.P.; Jiang, Y.; Neofytos, D.; Cortez, A.C.; Feriani, D.; Schmidt-Filho, J.; França-Silva, I.L.A.; Raad, I.; Hachem, R. Comparing the Real-World Use of Isavuconazole to Other Anti-Fungal Therapy for Invasive Fungal Infections in Patients with and without Underlying Disparities: A Multi-Center Retrospective Study. J. Fungi 2023, 9, 166. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Overall, N = 66 | Salvage Treatment, N = 42 | Primary Treatment, N = 24 | p-Value |
|---|---|---|---|---|
| Age (years), (median [IQR]) | 52.00 [41.00, 58.75] | 49.00 [34.75, 55.50] | 55.50 [48.75, 61.50] | 0.046 |
| Sex (%) | ||||
| Male | 40/66 (60.6) | 25/42 (59.5) | 15/24 (62.5) | 1 |
| Female | 26/66 (39.4) | 17/42 (40.5) | 9/24 (37.5) | |
| Primary disease (%) | 0.009 | |||
| AA | 6/66 (9.1) | 5/42 (11.9) | 1/24 (4.2) | |
| AL | 27/66 (40.9) | 24/42 (57.1) | 3/24 (12.5) | |
| CL | 2/66 (3.0) | 0/42 (0.0) | 2/24 (8.3) | |
| HL | 2/66 (3.0) | 1/42 (2.4) | 1/24 (4.2) | |
| MDS | 8/66 (12.1) | 4/42 (9.5) | 4/24 (16.7) | |
| MM | 5/66 (7.6) | 2/42 (4.8) | 3/24 (12.5) | |
| NHL | 10/66 (15.2) | 4/42 (9.5) | 6/24 (25.0) | |
| Others | 6/66 (9.1) | 2/42 (4.8) | 4/24 (16.7) | |
| Treatment for underlying hematological diseases (%) | 0.096 | |||
| Chemotherapy | 37/66 (56.1) | 20/42 (47.6) | 17/24 (70.8) | |
| HSCT | 25/66 (37.9) | 20/42 (47.6) | 5/24 (20.8) | |
| Untreated | 4/66 (6.1) | 2/42 (4.8) | 2/24 (8.3) | |
| Status of hematological disease at IFD (%) | 0.002 | |||
| CR/PR | 32/66 (48.5) | 27/42 (64.3) | 5/24 (20.8) | |
| NR | 29/66 (43.9) | 11/42 (26.2) | 18/24 (75.0) | |
| UT | 3/66 (4.5) | 2/42 (4.8) | 1/24 (4.2) | |
| UK | 2/66 (3.0) | 2/42 (4.8) | 0/24 (0.0) | |
| Classifications IFD (%) | 0.127 | |||
| Proven | 9/66 (13.6) | 8/42 (19.0) | 1/24 (4.2) | |
| Probable | 17/66 (25.8) | 12/42 (28.6) | 5/24 (20.8) | |
| Possible | 40/66 (60.6) | 22/42 (52.4) | 18/24 (75.0) | |
| Positive fungal culture (%) | 24/66 (36.4) | 20/42 (47.6) | 4/24 (16.7) | 0.025 |
| Species identified (%) | ||||
| Aspergillus | 11/66 (16.7) | 10/42 (23.8) | 1/24 (4.2) | |
| Candida | 4/66 (6.1) | 1/42 (2.4) | 3/24 (12.5) | |
| Fusarium | 1/66 (1.5) | 1/42 (2.4) | 0/24 (0.0) | |
| Mucor | 6/66 (9.1) | 5/42 (11.9) | 1/24 (4.2) | |
| Pneumocystis | 1/66 (1.5) | 1/42 (2.4) | 0/24 (0.0) | |
| Rhizopus | 3/66 (4.5) | 2/42 (4.8) | 1/24 (4.2) | |
| Yeast | 1/66 (1.5) | 1/42 (2.4) | 0/24 (0.0) | |
| Null | 39/66 (59.1) | 21/42 (50.0) | 18/24 (75.0) | |
| Site of IFD (%) | 0.408 | |||
| CNS | 1/66 (1.5) | 1/42 (2.4) | 0/24 (0.0) | |
| Lung | 63/66 (95.5) | 40/42 (95.2) | 23/24 (95.8) | |
| Lung + CNS | 1/66 (1.5) | 1/42 (2.4) | 0/24 (0.0) | |
| Maxillofacial | 1/66 (1.5) | 0/42 (0.0) | 1/24 (4.2) | |
| Neutropenia (%) | 16/66 (24.2) | 12/42 (28.6) | 4/24 (16.7) | 0.431 |
| Duration of neutropenia (%) | 0.209 | |||
| ≤7 d | 6/66 (9.1) | 6/42 (14.3) | 0/24 (0.0) | |
| 7–14 d | 1/66 (1.5) | 1/42 (2.4) | 0/24 (0.0) | |
| >14 d | 9/66 (13.6) | 5/42 (11.9) | 4/24 (16.7) | |
| Null | 50/66 (75.8) | 30/42 (71.4) | 20/24 (83.3) | |
| Combined bacterial infections (%) | 26/66 (39.4) | 15/42 (35.7) | 11/24 (45.8) | 0.584 |
| Combined viral infection (%) | 24/66 (36.4) | 18/42 (42.9) | 6/24 (25.0) | 0.236 |
| Laboratory findings | ||||
| WBC (×109/L), (median [IQR]) | 2.92 [1.26, 5.47] | 2.83 [0.88, 5.89] | 3.33 [1.64, 4.04] | 0.709 |
| NEUT (×109/L), (median [IQR]) | 2.24 [0.50, 4.38] | 2.28 [0.18, 4.54] | 1.94 [0.75, 2.68] | 0.668 |
| PLT (×109/L), (median [IQR]) | 28.00 [11.00, 116.00] | 17.00 [7.00, 73.00] | 81.50 [23.25, 164.50] | 0.013 |
| HB (g/L), (median [IQR]) | 75.00 [64.00, 99.00] | 76.00 [64.00, 99.00] | 72.50 [62.50, 91.75] | 0.946 |
| ALT (IU/L), (median [IQR]) | 22.00 [11.00, 42.00] | 22.00 [12.00, 44.25] | 22.00 [10.00, 28.05] | 0.479 |
| AST (IU/L), (median [IQR]) | 21.00 [15.00, 32.00] | 20.00 [15.00, 34.50] | 23.00 [13.00, 26.00] | 0.544 |
| TBIL (umol/L), (median [IQR]) | 9.00 [6.55, 13.70] | 9.70 [7.75, 13.95] | 7.90 [6.15, 12.75] | 0.328 |
| DBIL (umol/L), (median [IQR]) | 3.70 [2.30, 6.60] | 4.25 [2.48, 7.03] | 2.90 [1.95, 5.65] | 0.139 |
| IBIL (umol/L), (median [IQR]) | 5.90 [3.90, 8.60] | 6.05 [4.25, 8.53] | 5.60 [3.75, 9.05] | 0.534 |
| Scr (umol/L), (median [IQR]) | 70.00 [53.00, 101.75] | 84.00 [53.00, 107.00] | 61.00 [53.00, 77.00] | 0.284 |
| BUN (mmol/L), (median [IQR]) | 6.20 [4.32, 8.50] | 6.80 [4.90, 9.95] | 5.50 [4.15, 7.65] | 0.143 |
| Characteristic | Overall, N = 66 | Salvage Treatment, N = 42 | Primary Treatment, N = 24 | p-Value |
|---|---|---|---|---|
| History of previous fungal infection (%) | 0.049 | |||
| Yes | 19/66 (28.8) | 14/42 (33.3) | 5/24 (20.8) | |
| No | 41/66 (62.1) | 22/42 (52.4) | 19/24 (79.2) | |
| UK | 6/66 (9.1) | 6/42 (14.3) | 0/24 (0.0) | |
| Antifungal prophylaxis (%) | 0.009 | |||
| Yes | 28/66 (42.4) | 23/42 (54.8) | 5/24 (20.8) | |
| No | 36/66 (54.5) | 17/42 (40.5) | 19/24 (79.2) | |
| UK | 2/66 (3.0) | 2/42 (4.8) | 0/24 (0.0) | |
| Type of prophylaxis (%) | 0.098 | |||
| Echinocandins | 5/66 (7.6) | 4/42 (9.5) | 1/24 (4.2) | |
| Other Azoles | 19/66 (28.8) | 15/42 (35.7) | 4/24 (16.7) | |
| Echinocandins + Other Azoles | 2/66 (3.0) | 2/42 (4.8) | 0/24 (0.0) | |
| Echinocandins + Polyenes | 2/66 (3.0) | 2/42 (4.8) | 0/24 (0.0) | |
| Null | 38/66 (57.6) | 19/42 (45.2) | 19/24 (79.2) | |
| Contains Other Azoles (%) | 26/66 (39.4) | 26/42 (61.9) | ||
| Fungal treatment prior to Isavuconazole (%) | ||||
| Echinocandins | 1/42 (2.4) | |||
| Other Azoles | 17/42 (40.5) | |||
| Polyenes | 8/42 (19.0) | |||
| Other Azoles + Echinocandins | 1/42 (2.4) | |||
| Polyenes + Echinocandins | 7/42 (16.7) | |||
| Polyenes + Other Azoles | 8/42 (19.0) | |||
| Duration of fungal treatment before Isavuconazole (median [IQR]) | 8.00 [5.00, 20.00] | |||
| Reason for switching to Isavuconazole (%) | ||||
| Ineffectiveness | 24/42 (57.1) | |||
| Intolerance | 15/42 (35.7) | |||
| Poor compliance | 1/42 (2.4) | |||
| Sequential therapy | 2/42 (4.8) | |||
| Therapy (%) | 0.123 | |||
| Target treatment | 20/66 (30.3) | 16/42 (38.1) | 4/24 (16.7) | |
| Empirical/Diagnosis-driven treatment | 44/66 (69.7) | 26/42 (61.9) | 20/24 (83.3) | |
| Combined antifungal therapy (%) | 0.687 | |||
| Echinocandins | 11/66 (16.7) | 6/42 (14.3) | 5/24 (20.8) | |
| Polyenes | 17/66 (25.8) | 12/42 (28.6) | 5/24 (20.8) | |
| Null | 38/66 (57.6) | 24/42 (57.1) | 14/24 (58.3) | |
| Dosage form (%) | 0.051 | |||
| Intravenous administration | 8/66 (12.1) | 7/42 (16.7) | 1/24 (4.2) | |
| Oral | 53/66 (80.3) | 30/42 (71.4) | 23/24 (95.8) | |
| Oral + Intravenous administration | 5/66 (7.6) | 5/42 (11.9) | 0/24 (0.0) | |
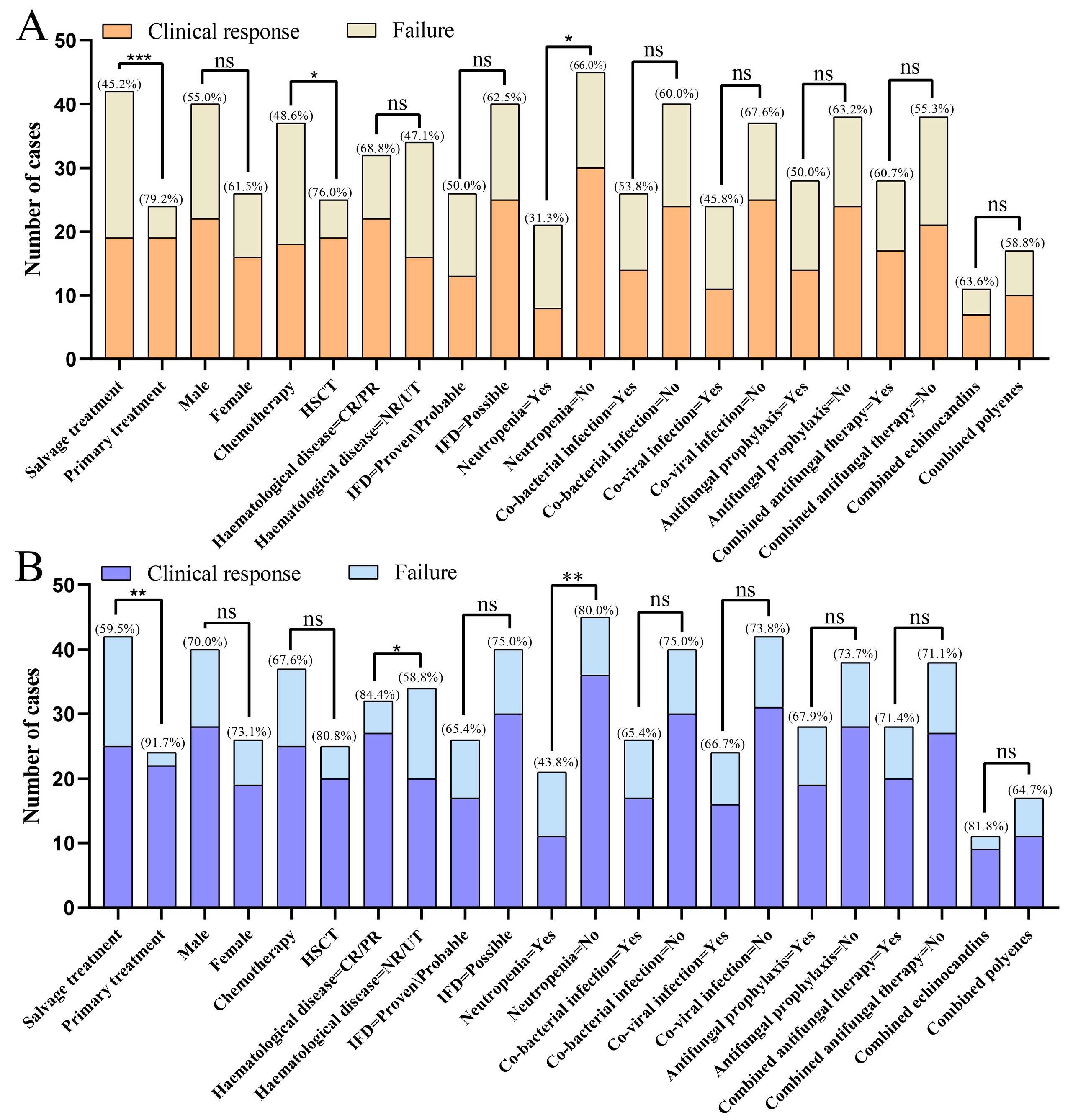
| Response to Isavuconazole therapy (%) | 0.136 | |||
| CR | 36/66 (54.5) | 19/42 (45.2) | 17/24 (70.8) | |
| PR | 12/66 (18.2) | 7/42 (16.7) | 5/24 (20.8) | |
| SD | 9/66 (13.6) | 8/42 (19.0) | 1/24 (4.2) | |
| PD | 1/66 (1.5) | 1/42 (2.4) | 0/24 (0.0) | |
| UK | 8/66 (12.1) | 7/42 (16.7) | 1/24 (4.2) | |
| 6th week response (%) | 0.02 | |||
| Positive | 38/66 (57.6) | 19/42 (45.2) | 19/24 (79.2) | |
| Negative | 17/66 (25.8) | 13/42 (31.0) | 4/24 (16.7) | |
| UK | 11/66 (16.7) | 10/42 (23.8) | 1/24 (4.2) | |
| 12th week response (%) | 0.021 | |||
| Positive | 47/66 (71.2) | 25/42 (59.5) | 22/24 (91.7) | |
| Negative | 11/66 (16.7) | 10/42 (23.8) | 1/24 (4.2) | |
| UK | 8/66 (12.1) | 7/42 (16.7) | 1/24 (4.2) | |
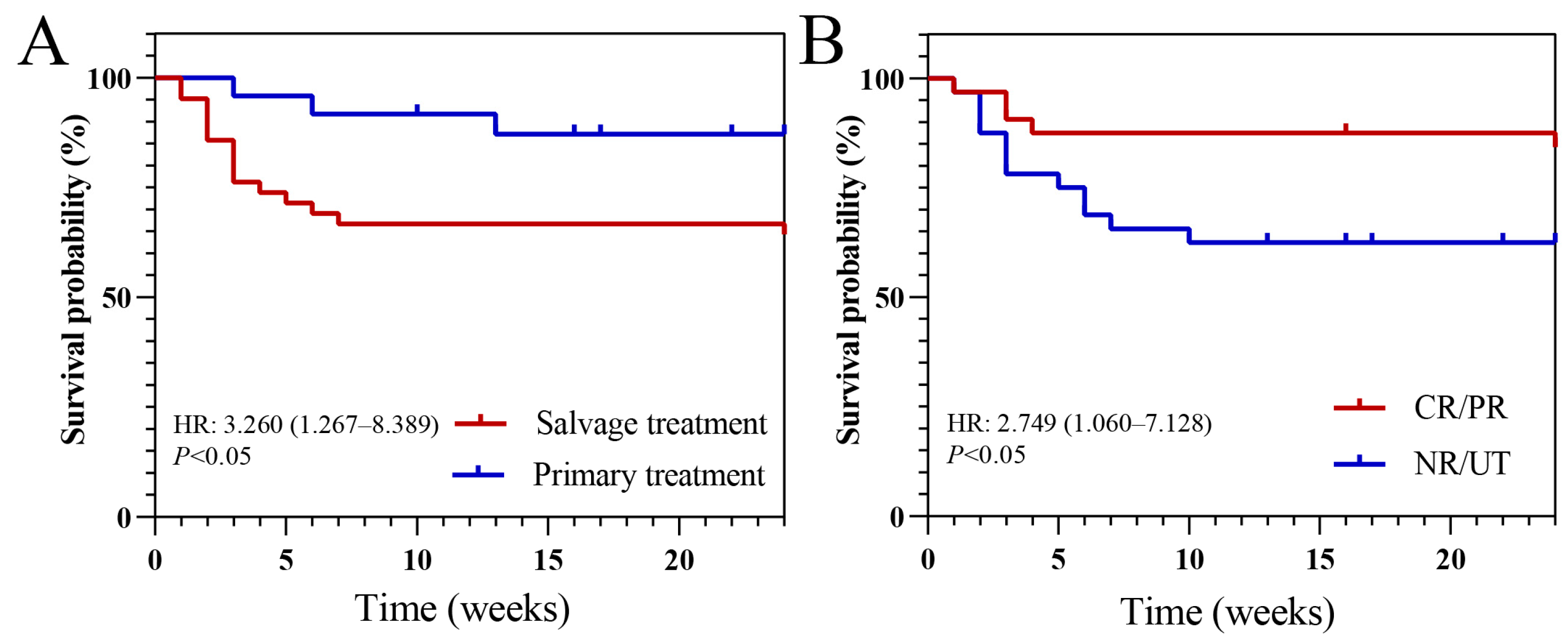
| 6th week death (%) | 18/66 (27.3) | 14/42 (33.3) | 4/24 (16.7) | 0.24 |
| 12th week death (%) | 19/66 (28.8) | 15/42 (35.7) | 4/24 (16.7) | 0.173 |
| Name | CR (N = 36) | PR/SD/PD (N = 30) | OR (Univariable) | OR (Multivariable) | OR (Final) | |
|---|---|---|---|---|---|---|
| The purpose of using Isavuconazole | Primary treatment | 17/36 (47.2%) | 7/30 (23.3%) | |||
| Salvage treatment | 19/36 (52.8%) | 23/30 (76.7%) | 0.34 (0.12–0.99, p = 0.048) | 0.10 (0.02–0.61, p = 0.013) | 0.10 (0.02–0.56, p = 0.008) | |
| Sex | Male | 20/36 (55.6%) | 20/30 (66.7%) | |||
| Female | 16/36 (44.4%) | 10/30 (33.3%) | 1.60 (0.59–4.37, p = 0.359) | |||
| Treatment | Non-HSCT | 21/36 (58.3%) | 20/30 (66.7%) | |||
| HSCT | 15/36 (41.7%) | 10/30 (33.3%) | 1.43 (0.52–3.91, p = 0.488) | |||
| Status of hematological disease at IFD | CR/PR | 22/36 (61.1%) | 10/30 (33.3%) | |||
| NR/UT | 14/36 (38.9%) | 20/30 (66.7%) | 0.32 (0.12–0.88, p = 0.027) | 0.06 (0.01–0.44, p = 0.005) | 0.07 (0.01–0.36, p = 0.002) | |
| Classifications IFD | Proven\Probable | 11/36 (30.6%) | 15/30 (50%) | |||
| Possible | 25/36 (69.4%) | 15/30 (50%) | 2.27 (0.83–6.22, p = 0.110) | 1.12 (0.23–5.47, p = 0.893) | ||
| Positive fungal culture | No | 27/36 (75%) | 15/30 (50%) | |||
| Yes | 9/36 (25%) | 15/30 (50%) | 0.33 (0.12–0.94, p = 0.038) | 0.33 (0.06–1.80, p = 0.201) | 0.31 (0.08–1.14, p = 0.078) | |
| Neutropenia | No | 31/36 (86.1%) | 19/30 (63.3%) | |||
| Yes | 5/36 (13.9%) | 11/30 (36.7%) | 0.28 (0.08–0.93, p = 0.037) | 1.10 (0.21–5.74, p = 0.906) | ||
| Combined bacterial infections | No | 24/36 (66.7%) | 16/30 (53.3%) | |||
| Yes | 12/36 (33.3%) | 14/30 (46.7%) | 0.57 (0.21–1.55, p = 0.271) | |||
| Combined viral infection | No | 24/36 (66.7%) | 18/30 (60%) | |||
| Yes | 12/36 (33.3%) | 12/30 (40%) | 0.75 (0.27–2.05, p = 0.576) | |||
| Antifungal prophylaxis | No | 22/36 (61.1%) | 16/30 (53.3%) | |||
| Yes | 14/36 (38.9%) | 14/30 (46.7%) | 0.73 (0.27–1.94, p = 0.525) | |||
| Combined antifungal therapy | No | 19/36 (52.8%) | 19/30 (63.3%) | |||
| Yes | 17/36 (47.2%) | 11/30 (36.7%) | 1.55 (0.57–4.16, p = 0.389) | |||
| Age, years (Mean ± SD) | 48.4 ± 15.3 | 49.4 ± 17.0 | 1.00 (0.97–1.03, p = 0.797) |
| AE Type | Salvage Treatment, n (%) | Primary Treatment, n (%) |
|---|---|---|
| Elevated Liver Enzymes 1 | ||
| Grade 1–2 | 12/42 (28.6%) | 5/24 (20.8%) |
| Grade 3–4 | 0/42 (0.0%) | 0/24 (0.0%) |
| Nausea/Vomiting | ||
| Grade 1–2 | 3/42 (7.1%) | 0/24 (0.0%) |
| Grade 3–4 | 0/42 (0.0%) | 0/42 (0.0%) |
| Thrombocytopenia | ||
| Grade 1–2 | 0/42 (0.0%) | 0/24 (0.0%) |
| Grade 3–4 | 1/42 (2.4%) | 0/24 (0.0%) |
| Back Pain | 0/42 (0.0%) | 1/24 (4.2%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuohuti, P.; Chen, Y.; Zhao, A.; Yang, J.; Li, H.; Niu, T. Isavuconazole for the Treatment of Invasive Fungal Disease in Hematology Patients: A Real-World Retrospective Study on Efficacy and Safety. Microorganisms 2025, 13, 2677. https://doi.org/10.3390/microorganisms13122677
Tuohuti P, Chen Y, Zhao A, Yang J, Li H, Niu T. Isavuconazole for the Treatment of Invasive Fungal Disease in Hematology Patients: A Real-World Retrospective Study on Efficacy and Safety. Microorganisms. 2025; 13(12):2677. https://doi.org/10.3390/microorganisms13122677
Chicago/Turabian StyleTuohuti, Pazilaiti, Yuhui Chen, Ailin Zhao, Jinrong Yang, He Li, and Ting Niu. 2025. "Isavuconazole for the Treatment of Invasive Fungal Disease in Hematology Patients: A Real-World Retrospective Study on Efficacy and Safety" Microorganisms 13, no. 12: 2677. https://doi.org/10.3390/microorganisms13122677
APA StyleTuohuti, P., Chen, Y., Zhao, A., Yang, J., Li, H., & Niu, T. (2025). Isavuconazole for the Treatment of Invasive Fungal Disease in Hematology Patients: A Real-World Retrospective Study on Efficacy and Safety. Microorganisms, 13(12), 2677. https://doi.org/10.3390/microorganisms13122677







