Antifungal Efficacy of Selected Plant Essential Oils Against Clinical Canine Isolates Malassezia pachydermatis
Abstract
1. Introduction
2. Material and Methods
2.1. Malassezia pachydermatis Strains
2.2. Plant Essential Oils Tested
2.3. Testing Quality Control
2.4. Determination of Minimum Inhibitory Concentration (MIC) of Tested Antifungal Agents
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Teramoto, H.; Kumeda, Y.; Yokoigawa, K.; Hosomi, K.; Kozaki, S.; Mukamoto, M.; Kohda, T. Genotyping and characterisation of the secretory lipolytic enzymes of Malassezia pachydermatis isolates collected from dogs. Vet. Rec. Open 2015, 21, e000124. [Google Scholar] [CrossRef]
- Buommino, E.; Nocera, F.P.; Parisi, A.; Rizzo, A.; Donnarumma, G.; Mallardo, K.; Fiorito, F.; Baroni, A.; De Martino, L. Correlation between genetic variability and virulence factors in clinical strains of Malassezia pachydermatis of animal origin. New Microbiol. 2016, 39, 216–223. [Google Scholar]
- Pistelli, L.; Mancianti, F.; Bertoli, A.; Cioni, P.L.; Leonardi, M.; Pisseri, F.; Mugnaini, L.; Nardoni, S. Antimycotic activity of some aromatic plants essential oils against canine isolates of Malassezia pachydermatis: An in vitro assay. Open Mycol. J. 2012, 6, 17–21. [Google Scholar] [CrossRef]
- Peano, A.; Johnson, E.; Chiavassa, E.; Tizzani, P.; Guillot, J.; Pasquetti, M. Antifungal resistance regarding Malassezia pachydermatis: Where are we now? J. Fungi 2020, 6, 93. [Google Scholar] [CrossRef] [PubMed]
- Daniel, A.K. Canine malasseziosis: An overview. Indian J. Anim. Health 2022, 61, 228–234. [Google Scholar] [CrossRef]
- Khosravi, A.R.; Shokri, H.; Fahimirad, S. Efficacy of medicinal essential oils against pathogenic Malassezia sp. isolates. J. Mycol. Med. 2016, 26, 28–34. [Google Scholar] [CrossRef]
- Donato, R.; Sacco, C.; Pini, G.; Bilia, A.R. Antifungal activity of different essential oils against Malassezia pathogenic species. J. Ethnopharmacol. 2020, 1, 112376. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; Feo, V. Essential Oils and Antifungal Activity. Pharmaceuticals 2017, 2, 86. [Google Scholar] [CrossRef]
- Blowman, K.; Magalhães, M.; Lemos, M.F.L.; Cabral, C.; Pires, I.M. Anticancer properties of essential oils and other natural products. Evid. Based Complement. Altern. Med. 2018, 25, 3149362. [Google Scholar] [CrossRef]
- Man, A.; Santacroce, L.; Jacob, R.; Mare, A.; Man, L. Antimicrobial activity of six essential oils against a group of human pathogens: A comparative study. Pathogens 2019, 8, 15, Erratum in Pathogens 2019, 8, 108. https://doi.org/10.3390/pathogens8030108. [Google Scholar] [CrossRef]
- Peixoto, L.R.; Rosalen, P.L.; Ferreira, G.L.S.; Freires, I.A.; de Carvalho, F.G.; Castellano, L.R.; de Castro, R.D. Antifungal activity, mode of action and anti-biofilm effects of Laurus nobilis Linnaeus essential oil against Candida spp. Arch. Oral Biol. 2017, 73, 179–185. [Google Scholar] [CrossRef]
- Bunse, M.; Daniels, R.; Gründemann, C.; Heilmann, J.; Kammerer, D.R.; Keusgen, M.; Lindequist, U.; Melzig, M.F.; Morlock, G.E.; Schulz, H.; et al. Essential oils as multicomponent mixtures and their potential for human health and well-being. Front. Pharmacol. 2022, 24, 956541. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, D.P.; Damasceno, R.O.S.; Amorati, R.; Elshabrawy, H.A.; de Castro, R.D.; Bezerra, D.P.; Nunes, V.R.V.; Gomes, R.C.; Lima, T.C. Essential oils: Chemistry and pharmacological activities. Biomolecules 2023, 18, 1144. [Google Scholar] [CrossRef] [PubMed]
- Ben Miri, Y. Essential oils: Chemical composition and diverse biological activities: A comprehensive review. Nat. Prod. Commun. 2025, 20, 1–29. [Google Scholar] [CrossRef]
- Pan, C.; Yang, K.; Erhunmwunsee, F.; Li, Y.X.; Liu, M.; Pan, S.; Yang, D.; Lu, G.; Ma, D.; Tian, J. Inhibitory effect of cinnamaldehyde on Fusarium solani and its application in postharvest preservation of sweet potato. Food Chem. 2023, 408, 135213. [Google Scholar] [CrossRef]
- Kaneko, T.; Makimura, K.; Abe, M.; Shiota, R.; Nakamura, Y.; Kano, R.; Hasegawa, A.; Sugita, T.; Shibuya, S.; Watanabe, S.; et al. Revised culture-based system for identification of Malassezia species. J. Clin. Microbiol. 2007, 45, 3737–3742. [Google Scholar] [CrossRef]
- Gaitanis, G.; Robert, V.; Velegraki, A. Verifiable single nucleotide polymorphisms of the internal transcribed spacer 2 region for the identification of 11 Malassezia species. J. Dermatol. Sci. 2006, 43, 214–217. [Google Scholar] [CrossRef]
- CLSI—Clinical and Laboratory Standard Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts Approved Standard, M27-A3 Guideline, 3rd ed.; CLSI: Wayne, PA, USA, 2008; 25p. [Google Scholar]
- Liu, M.; Seidel, V.; Katerere, D.R.; Gray, A.I. Colorimetric broth microdilution method for the antifungal screening of plant extracts against yeasts. Methods 2007, 42, 325–329. [Google Scholar] [CrossRef]
- Figueredo, L.A.; Cafarchia, C.; Otranto, D. Antifungal susceptibility of Malassezia pachydermatis biofilm. Med. Mycol. 2013, 51, 863–867. [Google Scholar] [CrossRef]
- Váczi, P.; Čonková, E.; Marcinčáková, D.; Sihelská, Z. Antifungal effect of selected essential oils on Malassezia pachydermatis growth. Folia Vet. 2018, 62, 67–72. [Google Scholar] [CrossRef]
- Bismarck, D.; Dusold, A.; Heusinger, A.; Müller, E. Antifungal in vitro activity of essential oils against clinical isolates of Malassezia pachydermatis from canine ears: A report from a practice laboratory. Complement. Med. Res. 2020, 27, 143–154. [Google Scholar] [CrossRef]
- El Youssfi, C.; Dadou, S.; Loukili, E.H.; El Hammoudani, Y.; Soujaa, H.; Rejdali, M.; Aarab, S. Biological activities of essential oils: A mini-review. BIO Web Conf. 2024, 109, 01031. [Google Scholar] [CrossRef]
- Begnami, A.F.; Duarte, M.C.T.; Furletti, V.; Rehder, V.L.G. Antimicrobial potential of Coriandrum sativum L. against different Candida species in vitro. Food Chem. 2010, 118, 74–77. [Google Scholar] [CrossRef]
- Chahal, K.K.; Singh, R.; Kumar, A.; Bhardwaj, U. Chemical composition and biological activity of Coriandrum sativum L.: A review. Indian J. Nat. Prod. Resour. 2018, 8, 193–203. [Google Scholar]
- Laribi, B.; Kouki, K.; M’Hamdi, M.; Bettaieb, T. Coriander (Coriandrum sativum L.) and its bioactive constituents. Fitoterapia 2015, 103, 9–26. [Google Scholar] [CrossRef]
- Héral, B.; Stierlin, É.; Fernandez, X.; Michel, T. Phytochemicals from the genus Lavandula: A review. Phytochem. Rev. 2021, 20, 751–771. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Kumar, M.; Akram, M.; Amin, M.; Iqbal, M.; Koirala, N.; Sytar, O.; Kregiel, D.; Nicola, S.; et al. Hyssopus essential oil: An update of its phytochemistry, biological activities, and safety profile. Oxid. Med. Cell Longev. 2022, 2022, 8442734. [Google Scholar] [CrossRef]
- Hristova, Y.; Wanner, J.; Jirovetz, L.; Stappen, I.; Iliev, I.; Gochev, V. Chemical composition and antifungal activity of essential oil of Hyssopus officinalis L. from Bulgaria against clinical isolates of Candida species. Biotechnol. Biotechnol. Equip. 2015, 29, 592–601. [Google Scholar] [CrossRef]
- Benyaich, A.; Aksissou, M. The pharmacological and nutritional properties of Rosmarinus officinalis: A comprehensive review. Trop. J. Nat. Prod. Res. 2024, 8, 8945–8954. [Google Scholar] [CrossRef]
- Waller, S.B.; Ripoll, M.K.; Silva, A.L.; Serra, E.F.; Dias, T.P.; Neves, V.B.D.; Cleff, M.B. Activities and mechanisms of oregano, marjoram and rosemary essential oils against Malassezia pachydermatitis isolates from canine and feline otitis. Turk. J. Vet. Anim. Sci. 2022, 46, 549–558. [Google Scholar] [CrossRef]
- Porres-Martínez, M.; González-Burgos, E.; Carretero, M.E.; Gómez-Serranillos, M.P. Influence of phenological stage on chemical composition and antioxidant activity of Salvia lavandulifolia Vahl. essential oils. Ind. Crop. Prod. 2014, 53, 71–77. [Google Scholar] [CrossRef]
- Cutillas, A.B.; Carrasco, A.; Martinez-Gutierrez, R.; Tomas, V.; Tudela, J. Composition and antioxidant, antienzymatic and antimicrobial activities of volatile molecules from Spanish Salvia lavandulifolia (Vahl) essential oils. Molecules 2017, 22, 1382. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Vioque, R.; Herraiz-Peñalver, D.; Melero Bravo, E.; Ortiz de Elguea-Culebras, G.; Herrero, B.; Santiago, Y.; Bueno, M.; Pérez-Magarino, S.; del Asensio, C.S.; Manzanera, M. Variability of the essential oil composition of cultivated populations of Salvia lavandulifolia Vahl. Crop Sci. 2022, 62, 744–752. [Google Scholar] [CrossRef]
- Valussi, M.; Donelli, D.; Firenzuoli, F.; Antonelli, M. Bergamot oil: Botany, production, pharmacology. Encyclopedia 2021, 1, 152–176. [Google Scholar] [CrossRef]
- Navarra, M.; Mannucci, C.; Delbò, M.; Calapai, G. Citrus bergamia essential oil: From basic research to clinical application. Front. Pharmacol. 2015, 6, 36. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, J.S. Inhibitory effect of plant essential oils on Malassezia pachydermatis. J. Appl. Biol. Chem. 2010, 53, 184–188. [Google Scholar] [CrossRef]
- Limtanyakul, P.; Smithrithee, R.; Wongwitthayakool, P.; Taweechotipatr, M.; Chottechathammanee, P.; Wattanasirichaigoon, S. Comparison of green-synthesized silver nanoparticle shampoo created by moringa and bergamot extraction versus 2% ketoconazole shampoo for scalp seborrheic dermatitis: A prospective, randomized, double-blinded, controlled trial. Dermatol. Ther. 2025, 1, 7166552. [Google Scholar] [CrossRef]
- Kairey, L.; Agnew, T.; Bowles, E.J.; Barkla, B.J.; Wardle, J.; Lauche, R. Efficacy and safety of Melaleuca alternifolia (tea tree) oil for human health—A systematic review of randomized controlled trials. Front. Pharmacol. 2023, 14, 1116077. [Google Scholar] [CrossRef]
- Weseler, A.; Geiss, H.K.; Saller, R.; Reichling, J. Antifungal effect of Australian tea tree oil on Malassezia pachydermatis isolated from canines suffering from cutaneous skin disease. Schweiz. Arch. Tierheilkd. 2002, 144, 215–221. [Google Scholar] [CrossRef]
- Vercelli, C.; Pasquetti, M.; Giovannetti, G.; Visioni, S.; Re, G.; Giorgi, M.; Gambino, G.; Peano, A. In vitro and in vivo evaluation of a new phytotherapic blend to treat acute externa otitis in dogs. J. Vet. Pharmacol. Ther. 2021, 44, 910–918. [Google Scholar] [CrossRef]
- Leyva-López, N.; Gutiérrez-Grijalva, E.P.; Vazquez-Olivo, G.; Heredia, J.B. Essential oils of oregano: Biological activity beyond their antimicrobial properties. Molecules 2017, 22, 989. [Google Scholar] [CrossRef] [PubMed]
- Veenstra, J.P.; Johnson, J.J. Oregano (Origanium Vulgare) Extract for food preservation and improving gastrointestinal health. Int. J. Nutr. 2019, 3, 43–52. [Google Scholar] [CrossRef]
- Ashokkumar, K.; Simal-Gandara, J.; Murugan, M.; Dhanya, M.K.; Pandian, A. Nutmeg (Myristica fragrans Houtt.) essential oil: A review on its composition, biological, and pharmacological activities. Phytother. Res. 2022, 36, 2839–2851. [Google Scholar] [CrossRef]
- Nikolic, V.; Nikolic, L.; Dinic, A.; Gajic, I.; Urosevic, M.; Stanojevic, L.; Danilovic, B. Chemical composition, antioxidant and antimicrobial activity of nutmeg (Myristica fragrans Houtt.) seed essential oil. J. Essent. Oil-Bear. Plants 2021, 24, 218–227. [Google Scholar] [CrossRef]
- Cossetin, L.F.; Santi, E.M.T.; Garlet, Q.I.; Matos, A.F.I.M.; De Souza, T.P.; Loebens, L.; Heinzmann, B.M.; Monteiro, S.G. Comparing the efficacy of nutmeg essential oil and a chemical pesticide against Musca domestica and Chrysomya albiceps for selecting a new insecticide agent against synantropic vectors. Exp. Parasitol. 2021, 225, 108104. [Google Scholar] [CrossRef]
- Butzge, J.C.; Ferrão, S.K.; Mezzomo, L.; Calil, L.N.; Mezzari, A.; Limberger, R.P.; Apel, M.A. Antifungal activity of essential oils from Cinnamomum cassia, Myristica fragrans and Syzygium aromaticum against Rhodotorula mucilaginosa. Drug Anal. Res. 2020, 4, 3–11. [Google Scholar] [CrossRef]
- Khalid, A.; Hayee, S.; Nasir, N.; Habibullah. Biological activity of Citrus paradisi peel. Pak. BioMed. J. 2020, 3, 17–24. [Google Scholar] [CrossRef]
| Essential Oil | Botanical Name | Family | Plant Part | Major Components |
|---|---|---|---|---|
| Coriander | Coriandrum sativum L. | Apiaceae | Fruit | Linalool (64 ± 2%) |
| Hyssop | Hyssopus officinalis L. | Lamiaceae | Aerial part of the plant | Pinocamphone (50.0 ± 2%) Isopinocamphone (28.0 ± 1%) α-pinene (11.0 ± 1%) |
| Lavender | Lavandula angustifolia MILLER | Flower | Linalool (48 ± 2%) | |
| Rosemary | Rosmarinus officinalis L. | Leaf | 1,8-cineole (25.0 ± 1%) α-pinene (19.0 ± 1%) Camphor (19.0 ± 1%) | |
| Spanish sage | Salvia lavandulifolia Vahl | Aerial part of the plant | Camphor (27 ± 1) | |
| Oregano | Origanum vulgare L. | Aerial part of the plant | Carvacrol (57 ± 3%) | |
| Tea tree | Melaleuca alternifolia Cheef | Myrtaceae | Leaf | Terpinen-4-ol (33 ± 2) |
| Nutmeg | Myristica fragrans Houtt | Myristicaceae | Core | α-pinene (18.0 ± 1%) Sabinene (14.0 ± 1%) β-pinene (13.0 ± 1%) Myristicin (5 ± 0.2%) |
| Bergamot | Citrus aurantium L. subsp. bergamia | Rutaceae | Pericarp | Limonene (36 ± 1%) Linalyl acetate (23 ± 1%) Linalool (15 ± 1%) |
| Grapefruit | Citrus paradisi Macfad | Pericarp | Limonene (87 ± 3%) |
| Parameter | Bergamot | Grapefruit | Coriander | Hyssop | Lavender | Tea Tree | Nutmeg | Oregano | Rosemary | Sage |
|---|---|---|---|---|---|---|---|---|---|---|
| Clinical isolates | ||||||||||
| Minimum | 400 | 800 | 400 | 400 | 400 | 400 | 1600 | 400 | 400 | 400 |
| Maximum | 3125 | 100,000 | 400 | 1600 | 800 | 25,000 | 12,500 | 12,500 | 3125 | 3125 |
| 821.67 a | 15,015 a,b,c,d,e,f | 400 b | 666.67 c | 586.67 d | 2360 | 5838.33 | 2430 | 741.67 e | 816.67 f | |
| SD | 715.07 | 34,516.95 | 0 | 493.77 | 206.56 | 6281.13 | 2528.85 | 3057.90 | 736.53 | 947.44 |
| Mode | 400 | 3125 | 400 | 400 | 400 | 400 | 6250 | 3125 | 400 | 400 |
| Median | 800 | 1600 | 400 | 400 | 400 | 400 | 6250 | 1600 | 400 | 400 |
| MIC50 | 800 | 1600 | 400 | 400 | 400 | 400 | 6250 | 1600 | 400 | 400 |
| MIC90 | 1600 | 100,000 | 400 | 1600 | 800 | 1600 | 6250 | 3125 | 800 | 800 |
| Malassezia pachydermatis CBS 1879 | ||||||||||
| Minimum | 800 | 1600 | 400 | 400 | 400 | 400 | 1600 | 400 | 400 | 400 |
| Maximum | 800 | 1600 | 400 | 400 | 1600 | 400 | 3125 | 1600 | 400 | 400 |
| 800 h | 1600 | 400 g, | 400 i | 800 l | 400 j | 2616.67 g,h,i,j,k,l,m,n | 1200 k | 400 m | 400 n | |
| SD | 0 | 0 | 0 | 0 | 692.82 | 0 | 880.46 | 692.82 | 0 | 0 |
| Mode | 800 | 1600 | 400 | 400 | 400 | 400 | 3125 | 1600 | 400 | 400 |
| Median | 800 | 1600 | 400 | 400 | 400 | 400 | 3125 | 1600 | 400 | 400 |
| Parameter | Bergamot | Grapefruit | Coriander | Hyssop | Lavender | Tea Tree | Nutmeg | Oregano | Rosemary | Sage |
|---|---|---|---|---|---|---|---|---|---|---|
| Clinical isolates | ||||||||||
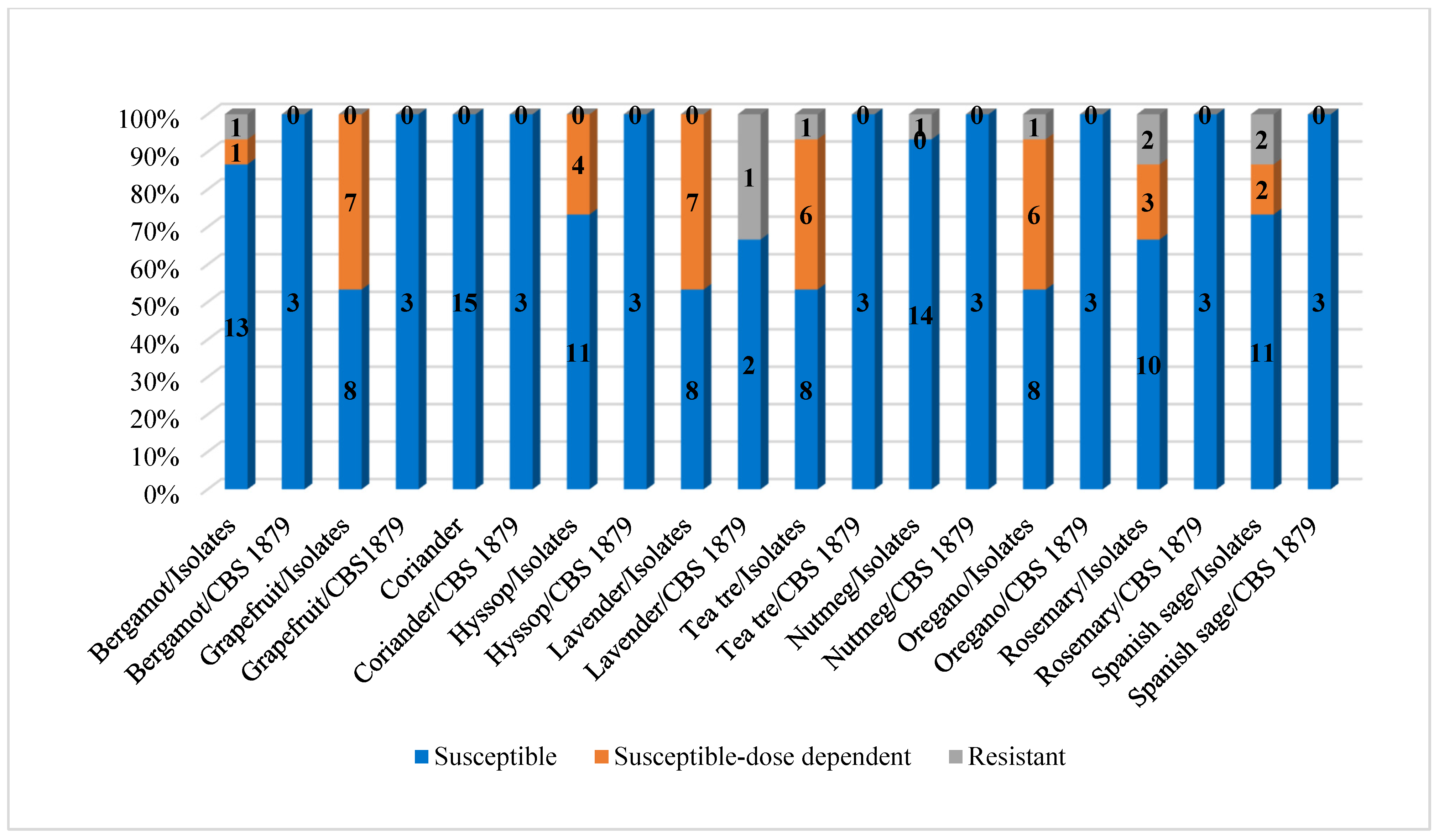
| S (n/%) | 13/86.66 | 8/53.33 | 15/100 | 11/73.33 | 8/53.33 | 8/53.33 | 14/93.33 | 8/53.33 | 10/66.67 | 11/73.34 |
| S-DD (n/%) | 1/6.67 | 7/46.67 | 0 | 4/26.67 | 7/46.67 | 6/40 | 0 | 6/40 | 3/20 | 2/13.33 |
| R (n/%) | 1/6.67 | 0 | 0 | 0 | 0 | 1/6.67 | 1/6.67 | 1/6.67 | 2/13.33 | 2/13.33 |
| Malassezia pachydermatis CBS 1879 | ||||||||||
| S-DD (n*/%) | 3/100 | 3/100 | 3/100 | 3/100 | 2/66.67 | 3/100 | 3/100 | 3/100 | 3/100 | 3/100 |
| Resistant (n*/%) | 0 | 0 | 0 | 0 | 1/33.33 | 0 | 0 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Čonková, E.; Váczi, P.; Malinovská, Z. Antifungal Efficacy of Selected Plant Essential Oils Against Clinical Canine Isolates Malassezia pachydermatis. Microorganisms 2025, 13, 2675. https://doi.org/10.3390/microorganisms13122675
Čonková E, Váczi P, Malinovská Z. Antifungal Efficacy of Selected Plant Essential Oils Against Clinical Canine Isolates Malassezia pachydermatis. Microorganisms. 2025; 13(12):2675. https://doi.org/10.3390/microorganisms13122675
Chicago/Turabian StyleČonková, Eva, Peter Váczi, and Zuzana Malinovská. 2025. "Antifungal Efficacy of Selected Plant Essential Oils Against Clinical Canine Isolates Malassezia pachydermatis" Microorganisms 13, no. 12: 2675. https://doi.org/10.3390/microorganisms13122675
APA StyleČonková, E., Váczi, P., & Malinovská, Z. (2025). Antifungal Efficacy of Selected Plant Essential Oils Against Clinical Canine Isolates Malassezia pachydermatis. Microorganisms, 13(12), 2675. https://doi.org/10.3390/microorganisms13122675






