Isolation and Characterization of a Duck Hepatitis A Virus Type 3 SH52 Strain in Ducklings
Abstract
1. Introduction
2. Materials and Methods
2.1. Eggs, Animals, and Reagents
2.2. Viral (RT)-PCR Detection
2.3. Virus Isolation, Purification, and Titration
2.4. Whole-Genome Sequencing and Analysis
2.5. Duck Experiments
2.6. Statistical Analysis
3. Results
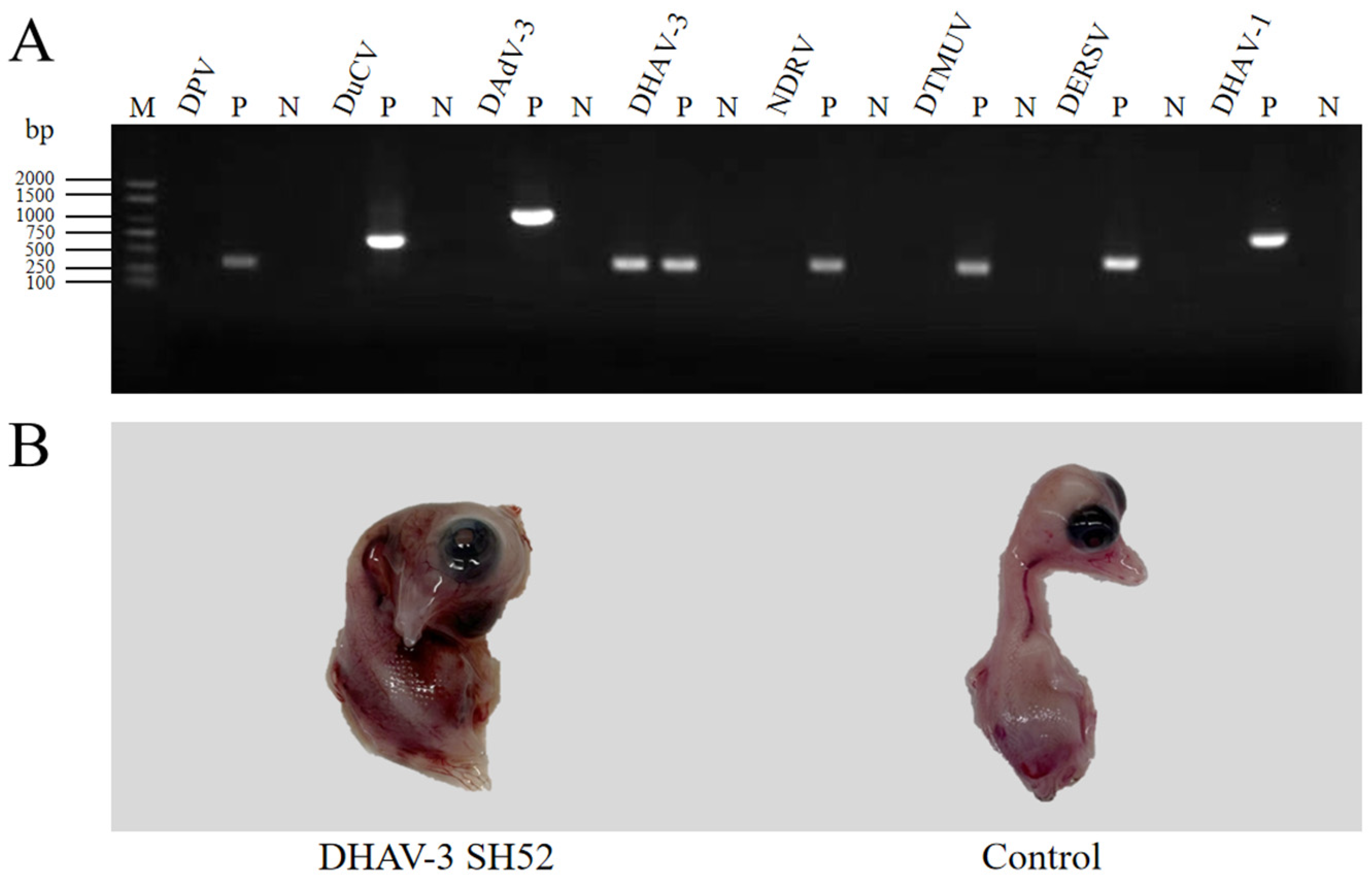
3.1. Viral RT-PCR Detection
3.2. Virus Isolation, Purification, and Titration
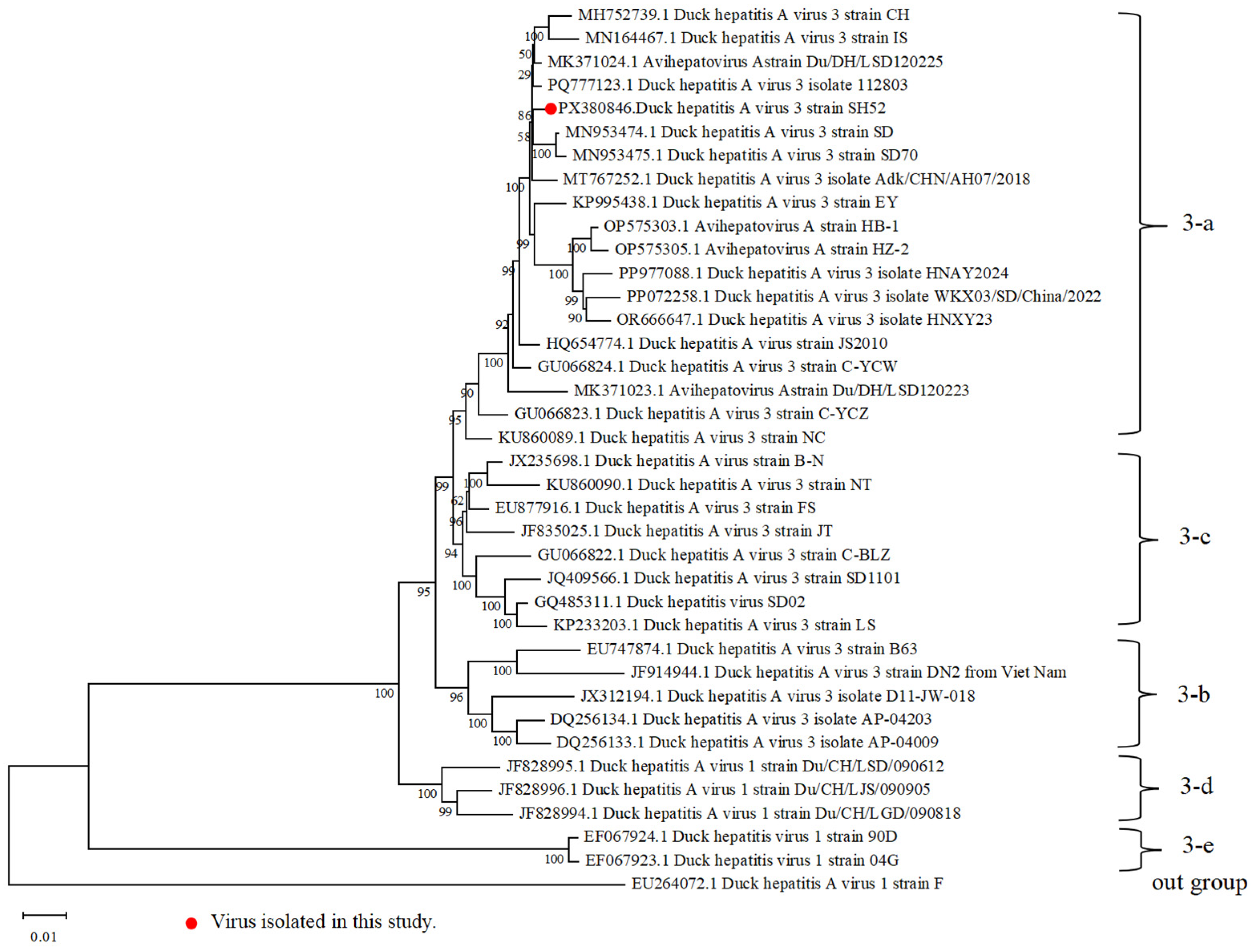
3.3. Whole-Genome Sequencing and Phylogenetic Analysis of the DHAV-3 SH52
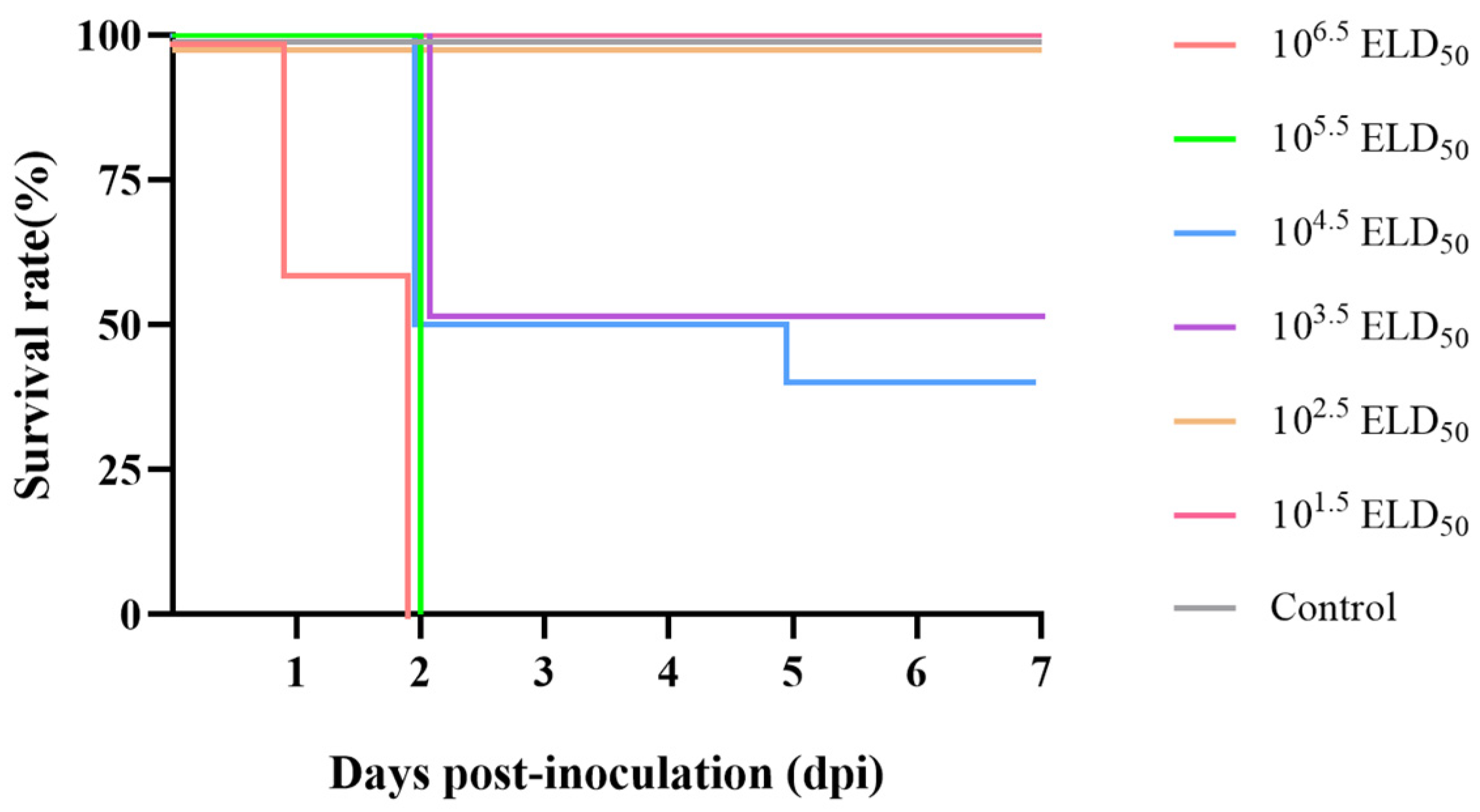
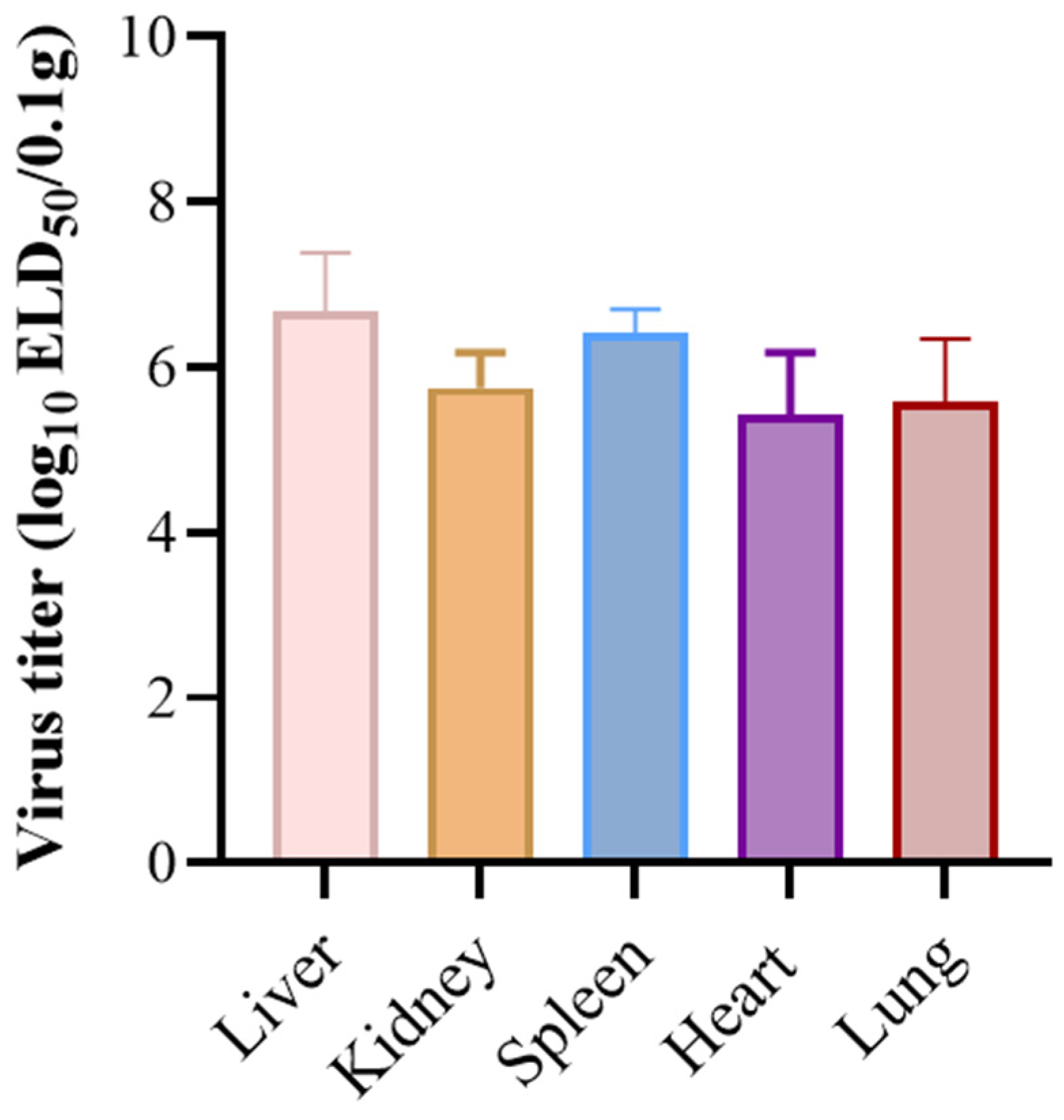
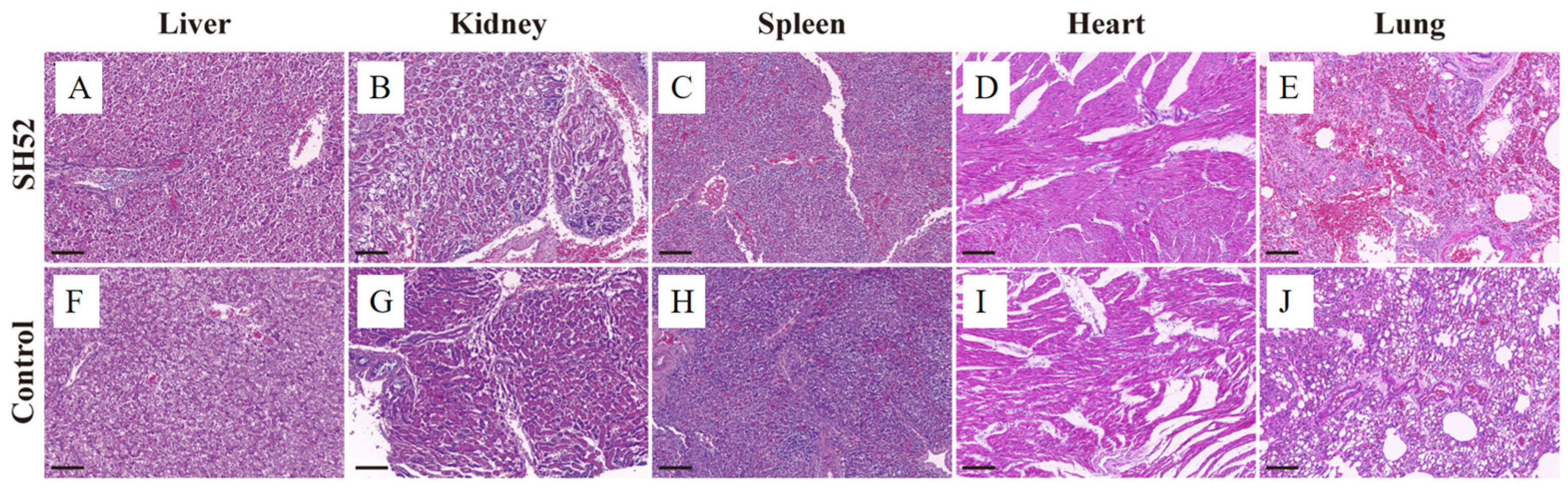
3.4. Pathogenicity in Ducklings
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, S.; Li, S.; Wang, Y.; Li, X.; Zhang, T. Duck hepatitis A virus prevalence in mainland China between 2009 and 2021: A systematic review and meta-analysis. Prev. Vet. Med. 2022, 208, 105730. [Google Scholar] [CrossRef]
- Niu, Y.; Ma, H.; Ding, Y.; Li, Z.; Sun, Y.; Li, M.; Shi, Y. The pathogenicity of duck hepatitis A virus types 1 and 3 on ducklings. Poult. Sci. 2019, 98, 6333–6339. [Google Scholar] [CrossRef]
- Lefkowitz, E.J.; Dempsey, D.M.; Hendrickson, R.C.; Orton, R.J.; Siddell, S.G.; Smith, D.B. Virus taxonomy: The database of the International Committee on Taxonomy of Viruses (ICTV). Nucleic Acids Res. 2018, 46, D708–D717. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Kong, X. Isolation, identification and attenuation of a pathogenic duck hepatitis virus type 1 in China, and complete genomic sequence comparison between the embryo-passaged, attenuated derivatives and their parent. Pol. J. Vet. Sci. 2019, 22, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Liu, L.; Wang, Y.; Zhang, Y.; Qi, X.; Liu, C.; Gao, Y.; Wang, X.; Cui, H. The first whole genome sequence and pathogenicity characterization of a fowl adenovirus 4 isolated from ducks associated with inclusion body hepatitis and hydropericardium syndrome. Avian Pathol. 2017, 46, 571–578. [Google Scholar] [CrossRef]
- Kim, M.C.; Kwon, Y.K.; Joh, S.J.; Kim, S.-J.; Tolf, C.; Kim, J.-H.; Sung, H.-W.; Lindberg, A.M.; Kwon, J.-H. Recent Korean isolates of duck hepatitis virus reveal the presence of a new geno- and serotype when compared to duck hepatitis virus type 1 type strains. Arch. Virol. 2007, 152, 2059–2072. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Lu, F.; Fan, X.; Pan, Q.; Zhao, S.; Sun, H.; Zhang, J.; Liu, C.; Chao, J.; Zhang, X. Development of a live attenuated duck hepatitis A virus type 3 vaccine (strain SD70). Vaccine 2020, 38, 4695–4703. [Google Scholar] [CrossRef]
- Zhu, T.; Qi, B.; Liu, R.; Jiang, X.; Lu, R.; Xiao, L.; Fu, G.; Fu, Q.; Shi, S.; Wan, C.; et al. Comparative pathogenicity of two subtypes (hepatitis-type and pancreatitis-type) of duck hepatitis A virus type 1 in experimentally infected Muscovy ducklings. Avian Pathol. 2019, 48, 352–361. [Google Scholar] [CrossRef]
- Ye, L.; Zhou, S.; Zhang, H.; Zhang, T.; Yang, D.; Hong, X. A meta-analysis for vaccine protection rate of duck hepatitis a virus in mainland China in 2009–2021. BMC Vet. Res. 2023, 19, 179. [Google Scholar] [CrossRef]
- Zhao, S.; Wu, B.; Wang, Q.; Wei, X.; Liu, X.; Tang, Y.; Diao, Y. Advances in the Duck Hepatitis A virus and lessons learned from those in recent years. Microb. Pathog. 2024, 197, 107018. [Google Scholar] [CrossRef]
- Hassan, T.I.R.; Eid, A.A.M.; Ghanem, I.A.I.; Shahin, A.M.; Adael, S.A.A.; Mohamed, F.F. First Report of Duck Hepatitis A Virus 3 from Duckling Flocks of Egypt. Avian Dis. 2020, 64, 269–276. [Google Scholar] [CrossRef]
- Mansour, S.M.G.; Mohamed, F.F.; ElBakrey, R.M.; Eid, A.A.M.; Mor, S.K.; Goyal, S.M. Outbreaks of Duck Hepatitis A Virus in Egyptian Duckling Flocks. Avian Dis. 2019, 63, 68–74. [Google Scholar] [CrossRef]
- Annamalai, B.; Jaisree, S.; Thandavan Vembuvizhivendan, T. An outbreak of duck hepatitis A virus infection in nomadic ducklings. Vet. Ital. 2022, 58. [Google Scholar] [CrossRef]
- Shawki, M.M.; Abido, O.Y.; Saif, M.A.; Sobh, M.S.; Gado, A.R.; Elnaggar, A.; Nassif, S.A.; El-Shall, N.A. Comparative Pathogenicity of Duck Hepatitis A Virus Genotype 3 in Different Duck Breeds: Implications of the Diagnosis and Prevention of Duck Viral Hepatitis. Comp. Immunol. Microbiol. Infect. Dis. 2024, 114, 102256. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Han, X.; Zhu, C.; Jiao, W.; Liu, R.; Feng, Z.; Huang, Y.; Chen, Z.; Wan, C.; Lai, Z.; et al. Development of the First Officially Licensed Live Attenuated Duck Hepatitis A Virus Type 3 Vaccine Strain HB80 in China and Its Protective Efficacy against DHAV-3 Infection in Ducks. Poult. Sci. 2024, 103, 104087. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Shah, P.T.; Bahoussi, A.N.; Wu, C.; Wang, L.; Xing, L. Duck hepatitis a virus: Full-length genome-based phylogenetic and phylogeographic view during 1986–2020. Virus Res. 2023, 336, 199216. [Google Scholar] [CrossRef]
- Fehér, E.; Jakab, S.; Bali, K.; Kaszab, E.; Nagy, B.; Ihász, K.; Bálint, Á.; Palya, V.; Bányai, K. Genomic Epidemiology and Evolution of Duck Hepatitis A Virus. Viruses 2021, 13, 1592. [Google Scholar] [CrossRef]
- Wu, L.; Quan, W.; Zhang, Y.; Wang, M.; Ou, X.; Mao, S.; Sun, D.; Yang, Q.; Wu, Y.; Wei, Y.; et al. Attenuated Duck Hepatitis A Virus Infection Is Associated With High mRNA Maintenance in Duckling Liver via m6A Modification. Front. Immunol. 2022, 13, 839677. [Google Scholar] [CrossRef]
- Truong, T.N.; Cheng, L.T. Development of a Subunit Vaccine against Duck Hepatitis A Virus Serotype 3. Vaccines 2022, 10, 523. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, S.; Liu, W.; Hu, Z. Current status and future direction of duck hepatitis A virus vaccines. Avian Pathol. 2023, 52, 89–99. [Google Scholar] [CrossRef]
- Wen, X.; Zhu, D.; Cheng, A.; Wang, M.; Chen, S.; Jia, R.; Liu, M.; Sun, K.; Zhao, X.; Yang, Q.; et al. Molecular epidemiology of duck hepatitis a virus types 1 and 3 in China, 2010–2015. Transbound. Emerg. Dis. 2018, 65, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Yu, C.D.; Park, J.Y.; Ma, X.L.; Zhu, T.; Li, Y.F.; Cha, S.Y.; Jang, H.K.; Kang, M.; Wei, B. The Impact of Genetic Variation on Duck Hepatitis A Virus (DHAV) Vaccine Efficacy: A Comparative Study of DHAV-1 and DHAV-3 Against Emerging Variant Strains. Vaccines 2024, 12, 1416. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, H.; Zhang, W.; Fu, L.; Wang, Y. Molecular Detection and Genetic Characterization of Vertically Transmitted Viruses in Ducks. Animals 2023, 14, 6. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cao, C.; Qu, Z.; Zhang, W.; Liu, Y.; Qi, H.; Hao, C.; Zhang, W.; Gao, M.; Wang, J.; et al. Pathogenicity of duck hepatitis A virus type 3 and innate immune responses of the ducklings to virulent DHAV-3. Mol. Immunol. 2018, 95, 30–38. [Google Scholar] [CrossRef]
- Liang, S.; Hu, X.; Guo, Z.; Luo, D.; Tang, J.; Ji, Z.; Xie, M.; Hou, S. Comparative transcriptome reveals the effect of IFITM1 on differential resistance to duck hepatitis A virus genotype 3 in Pekin ducks. Virus Res. 2022, 322, 198930. [Google Scholar] [CrossRef]
- Mohamed, R.I.; Mosad, S.M.; Ali, H.S.; Albalawi, W.O.; Elsamadony, H.A.; Ramzy, N.M.; Saad, A.S.; Fallatah, D.; Abdel-Hafez, L.J.M.; Albrakati, A.; et al. A comprehensive pathological and molecular investigation of viral co-infections in ducks in Egypt. Front. Microbiol. 2025, 16, 1522669. [Google Scholar] [CrossRef]
- Zhang, R.; Zhou, G.; Xin, Y.; Chen, J.; Lin, S.; Tian, Y.; Xie, Z.; Jiang, S. Identification of a conserved neutralizing linear B-cell epitope in the VP1 proteins of duck hepatitis A virus type 1 and 3. Vet. Microbiol. 2015, 180, 196–204. [Google Scholar] [CrossRef]
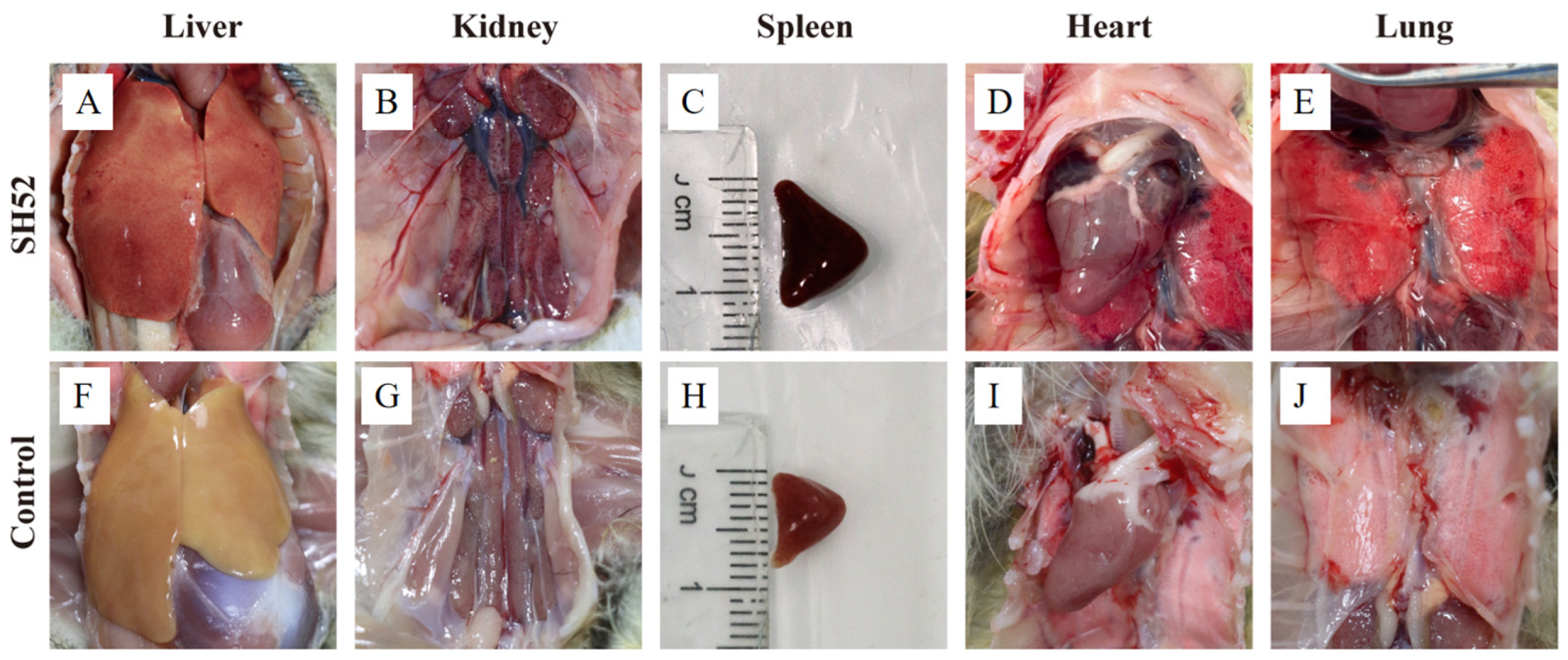
| Lesion | DHAV-3 SH52 | Controls |
|---|---|---|
| Hepatitis with Petechial hemorrhage | +++ | - |
| Mottled kidneys with hemorrhage | ++ | - |
| Splenomegaly with Subcapsular hemorrhage | ++ | - |
| Pericarditis | + | - |
| Pneumonia | + | - |
| Organ | Lesion | DHAV-3 SH52 | Controls |
|---|---|---|---|
| Liver | Degeneration | +++ | - |
| Necrosis | +++ | - | |
| Cellular atrophy | +++ | - | |
| Cellular cytolysis | ++ | - | |
| Kidney | Tubular epithelial degeneration | +++ | - |
| Necrosis | ++ | - | |
| Nuclear pyknosis | +++ | - | |
| Inflammatory infiltrate | + | - | |
| Spleen | Disrupted periarteriolar lymphoid | ++ | - |
| Lymphoid hyperplasia | + | - | |
| Necrosis | - | ||
| Heart | Degeneration | - | - |
| Necrosis | - | ||
| Dilated interstitial blood vessels | - | - | |
| Lung | Interstitial hemorrhage | ++ | - |
| Necrosis | - | ||
| Proliferation of fibrous connective tissue | ++ | - | |
| Inflammatory cell infiltration | +++ | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, M.; Shuo, D.; Xiong, Y.; Tang, M.; Wang, Y.; Pan, X.; Yuan, C.; Liu, Q.; Zhang, Z.; Teng, Q.; et al. Isolation and Characterization of a Duck Hepatitis A Virus Type 3 SH52 Strain in Ducklings. Microorganisms 2025, 13, 2652. https://doi.org/10.3390/microorganisms13122652
Huang M, Shuo D, Xiong Y, Tang M, Wang Y, Pan X, Yuan C, Liu Q, Zhang Z, Teng Q, et al. Isolation and Characterization of a Duck Hepatitis A Virus Type 3 SH52 Strain in Ducklings. Microorganisms. 2025; 13(12):2652. https://doi.org/10.3390/microorganisms13122652
Chicago/Turabian StyleHuang, Minfan, Dun Shuo, Yifei Xiong, Mei Tang, Yufei Wang, Xue Pan, Chunxiu Yuan, Qinfang Liu, Zhifei Zhang, Qiaoyang Teng, and et al. 2025. "Isolation and Characterization of a Duck Hepatitis A Virus Type 3 SH52 Strain in Ducklings" Microorganisms 13, no. 12: 2652. https://doi.org/10.3390/microorganisms13122652
APA StyleHuang, M., Shuo, D., Xiong, Y., Tang, M., Wang, Y., Pan, X., Yuan, C., Liu, Q., Zhang, Z., Teng, Q., Xu, B., Shi, X., Yan, M., Jiao, P., Li, Z., & Yan, D. (2025). Isolation and Characterization of a Duck Hepatitis A Virus Type 3 SH52 Strain in Ducklings. Microorganisms, 13(12), 2652. https://doi.org/10.3390/microorganisms13122652








