Interaction of Bacteria and Fleas, Focusing on the Plague Bacterium—A Review
Abstract
1. Introduction
2. Rickettsia, Bartonella and Yersinia
3. Fleas
3.1. Occurrence, Morphology and Development
3.2. Host Preferences
3.3. Attraction and Blood Ingestion and Digestion
3.4. Conditions in the Intestinal Tract of Fleas
3.5. Against Pathogens: The Immune System of Fleas
4. The Microbiota of Fleas
4.1. General Aspects of Insect Microbiota
4.2. Microbiota of Fleas: Infection and Groups of Bacteria
4.2.1. Rickettsia sp.
4.2.2. Bartonella sp.
4.2.3. Wolbachia sp.
4.2.4. Interactions of Rickettsia sp., Bartonella sp., Yersinia pestis and Other Bacteria in the Flea
5. Interactions of Fleas with the Bacteria
5.1. Associations of Fleas and Bacteria
5.2. Effects of the Vector on the Bacteria—Development of Bacteria in the Fleas
5.2.1. Development of Rickettsia and Bartonella in Fleas
5.2.2. Development of Yersinia pestis in Fleas: General Aspects
5.2.3. The Course of the Development of Yersinia pestis in Fleas: Importance of Bacterial Factors
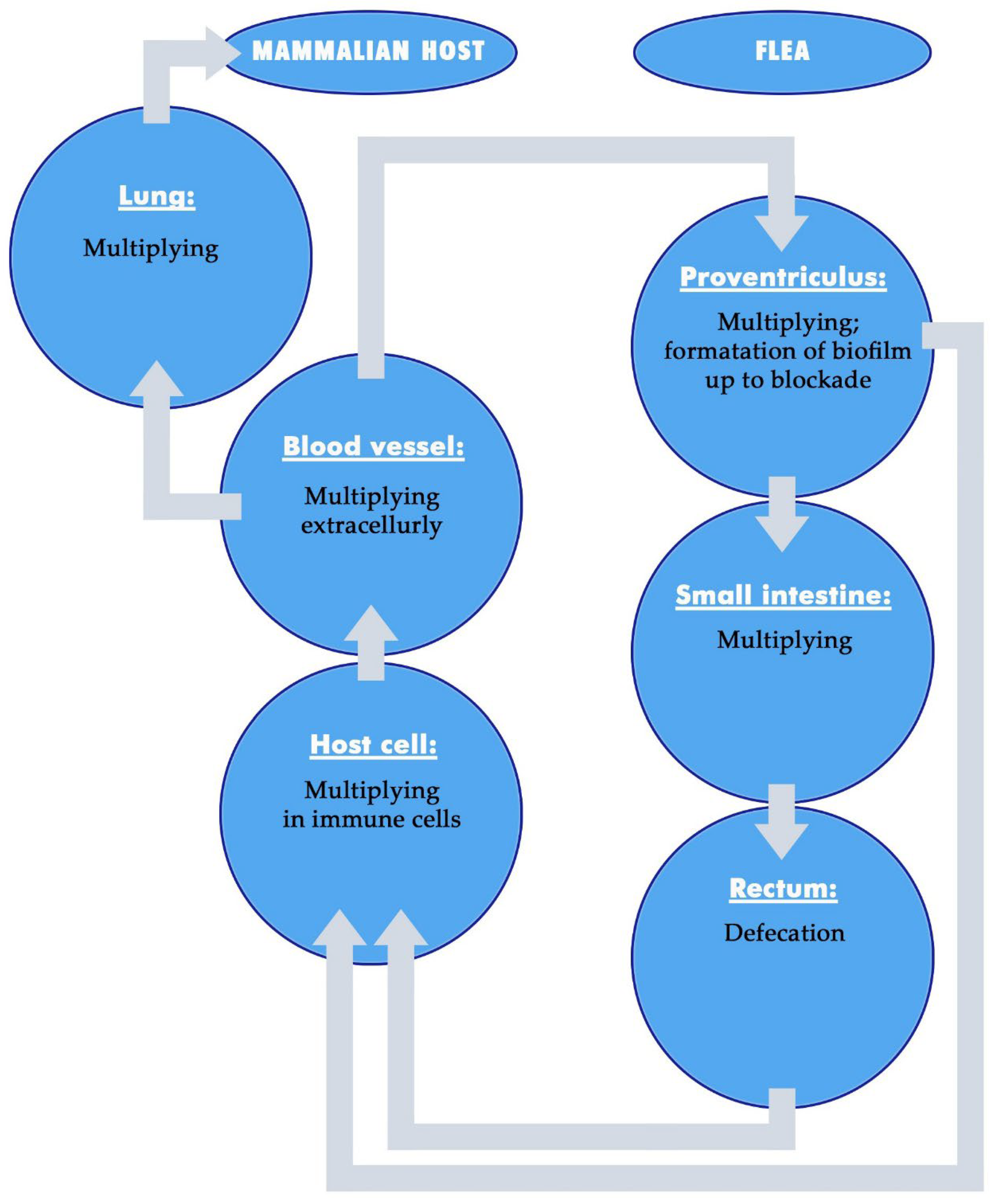
6. Transmission of Yersinia pestis by Fleas
6.1. Different Modes of Transmission of Yersinia pestis
6.2. Formation of Biofilms by Yersinia pestis
6.3. Early-Phase Transmission of Yersinia pestis
6.4. Foregut Blockage Transmission of Yersinia pestis
6.5. Comparing Efficiency of Transmission Modes
7. Effects of the Bacteria on the Fleas
7.1. Effects of Bacteria on the Immune Response of the Fleas
7.2. Effects of Bacteria on the Intestine, Behavior and Fitnes of the Fleas
8. Comments for Future Research
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McElroy, K.M.; Blagburn, B.L.; Breitschwerdt, E.B.; Mead, P.S.; McQuiston, J.H. Flea-associated zoonotic diseases of cats in the USA: Bartonellosis, flea-borne rickettsioses, and plague. Trends Parasitol. 2010, 26, 197–204. [Google Scholar] [CrossRef]
- Bitam, I.; Dittmar, K.; Parola, P.; Whiting, M.F.; Raoult, D. Fleas and flea-borne diseases. Int. J. Infect. Dis. 2010, 14, e667–e676. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.; Saleh, H.M.; Paterek, E. Flea bites. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK541118 (accessed on 17 June 2025).
- Thepparit, C.; Hirunkanokpun, S.; Popov, V.L.; Foil, L.D.; Macaluso, K.R. Dissemination of bloodmeal acquired Rickettsia felis in cat fleas, Ctenocephalides felis. Parasites Vectors 2013, 6, 149. [Google Scholar] [CrossRef]
- Brown, L.D.; Macaluso, K.R. Rickettsia felis, an emerging flea-borne rickettsiosis. Curr. Trop. Med. Rep. 2016, 3, 27–39. [Google Scholar] [CrossRef]
- Caravedo Martinez, M.A.; Ramírez-Hernández, A.; Blanton, L.S. Manifestations and management of flea-borne rickettsioses. Res. Rep. Trop. Med. 2021, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Minahan, N.T.; Wu, W.J.; Tsai, K.H. Rickettsia felis is an emerging human pathogen associated with cat fleas: A review of findings in Taiwan. J. Microbiol. Immunol. Infect. 2023, 56, 10–19. [Google Scholar] [CrossRef]
- Laukaitis, H.J.; Macaluso, K.R. Unpacking the intricacies of Rickettsia-vector interactions. Trends Parasitol. 2021, 37, 734–746. [Google Scholar] [CrossRef]
- Gillespie, J.J.; Ammerman, N.C.; Beier-Sexton, M.; Sobral, B.S.; Azad, A.F. Louse- and flea-borne rickettsioses: Biological and genomic analyses. Vet. Res. 2009, 40, 12. [Google Scholar] [CrossRef]
- Blanton, L.S.; Walker, D.H. Flea-borne rickettsioses and Rickettsiae. Am. J. Trop. Med. Hyg. 2017, 96, 53–56. [Google Scholar] [CrossRef]
- Legendre, K.P.; Macaluso, K.R. Rickettsia felis: A review of transmission mechanisms of an emerging pathogen. Trop. Med. Infect. Dis. 2017, 2, 64. [Google Scholar] [CrossRef]
- Gutiérrez, R.; Krasnov, B.; Morick, D.; Gottlieb, Y.; Khokhlova, I.S.; Harrus, S. Bartonella infection in rodents and their flea ectoparasites: An overview. Vector-Borne Zoonotic Dis. 2015, 15, 27–39. [Google Scholar] [CrossRef]
- Abdullah, S.; Helps, C.; Tasker, S.; Newbury, H.; Wall, R. Pathogens in fleas collected from cats and dogs: Distribution and prevalence in the UK. Parasites Vectors 2019, 12, 71. [Google Scholar] [CrossRef] [PubMed]
- Krügel, M.; Król, N.; Kempf, V.A.J.; Pfeffer, M.; Obiegala, A. Emerging rodent-associated Bartonella: A threat for human health? Parasites Vectors 2022, 15, 113. [Google Scholar] [CrossRef] [PubMed]
- Baranowski, K.; Huang, B. Cat Scratch Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK482139/ (accessed on 9 November 2025).
- Deng, H.; Le Rhun, D.; Buffet, J.P.; Cotté, V.; Read, A.; Birtles, R.J.; Vayssier-Taussat, M. Strategies of exploitation of mammalian reservoirs by Bartonella species. Vet. Res. 2012, 43, 15. [Google Scholar] [CrossRef] [PubMed]
- Rolain, J.M.; Franc, M.; Davoust, B.; Raoult, D. Molecular detection of Bartonella quintana, B. koehlerae, B. henselae, B. clarridgeiae, Rickettsia felis, and Wolbachia pipientis in cat fleas, France. Emerg. Infect. Dis. 2003, 9, 338–342. [Google Scholar] [CrossRef]
- Boodman, C.; Gupta, N.; van Griensven, J.; Van Bortel, W. Bartonella quintana detection among arthropods and their hosts: A systematic review and meta-analysis. Parasites Vectors 2024, 17, 328. [Google Scholar] [CrossRef]
- Bullard, R.L.; Olsen, E.L.; Cheslock, M.A.; Embers, M.E. Evaluation of the available animal models for Bartonella infections. One Health 2024, 18, 100665. [Google Scholar] [CrossRef]
- Kabeya, H.; Inoue, K.; Izumi, Y.; Morita, T.; Imai, S.; Maruyama, S. Bartonella species in wild rodents and fleas from them in Japan. J. Vet. Med. Sci. 2011, 73, 1561–1567. [Google Scholar] [CrossRef]
- Chomel, B.B.; Kasten, R.W.; Floyd-Hawkins, K.; Chi, B.; Yamamoto, K.; Roberts-Wilson, J.; Gurfield, A.N.; Abbott, R.C.; Pedersen, N.C.; Koehler, J.E. Experimental transmission of Bartonella henselae by the cat flea. J. Clin. Microbiol. 1996, 34, 1952–1956. [Google Scholar] [CrossRef]
- Mahmoudi, A.; Kryštufek, B.; Sludsky, A.; Schmid, B.V.; DE Almeida, A.M.P.; Lei, X.; Ramasindrazana, B.; Bertherat, E.; Yeszhanov, A.; Stenseth, N.C.; et al. Plague reservoir species throughout the world. Integr. Zool. 2021, 16, 820–833. [Google Scholar] [CrossRef]
- Bevins, S.N.; Chandler, J.C.; Barrett, N.; Schmit, B.S.; Wiscomb, G.W.; Shriner, S.A. Plague exposure in mammalian wildlife across the Western United States. Vector-Borne Zoonotic Dis. 2021, 21, 667–674. [Google Scholar] [CrossRef]
- Seersholm, F.V.; Sjögren, K.G.; Koelman, J.; Blank, M.; Svensson, E.M.; Staring, J.; Fraser, M.; Pinotti, T.; McColl, H.; Gaunitz, C.; et al. Repeated plague infections across six generations of neolithic farmers. Nature 2024, 632, 114–121. [Google Scholar] [CrossRef]
- Glatter, K.A.; Finkelman, P. History of the plague: An ancient pandemic for the age of COVID-19. Am. J. Med. 2021, 134, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Vogel, P.U.B.; Schaub, G.A. Seuchen, Alte und Neue Gefahren—Von der Pest bis COVID-19; Springer Spektrum: Wiesbaden, Germany, 2023. (In German) [Google Scholar] [CrossRef]
- Butler, T. Plague gives surprises in the first decade of the 21st century in the United States and worldwide. Am. J. Trop. Med. Hyg. 2013, 89, 788–793. [Google Scholar] [CrossRef] [PubMed]
- Kugeler, K.J.; Staples, J.E.; Hinckley, A.F.; Gage, K.L.; Mead, P.S. Epidemiology of human plague in the United States, 1900–2012. Emerg. Infect. Dis. 2015, 21, 16–22. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Wei, B.; Zhang, Y.; Liu, J.; Xi, J.; Ciren, D.; Qi, T.; Liang, J.; Duan, R.; Qin, S.; et al. Distribution and characteristics of human plague cases and Yersinia pestis isolates from 4 Marmota plague foci, China, 1950–2019. Emerg. Infect. Dis. 2021, 27, 2544–2553. [Google Scholar] [CrossRef]
- Han, H.; Liang, Y.; Song, Z.; He, Z.; Duan, R.; Chen, Y.; Gao, Z.; Qin, S.; Liang, J.; Tang, D.; et al. Epidemiological characteristics of human and animal plague in Yunnan province, China, 1950 to 2020. Microbiol. Spectr. 2022, 10, e0166222. [Google Scholar] [CrossRef]
- Dillard, R.L.; Juergens, A.L. Plague. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK549855/ (accessed on 17 June 2025).
- Gage, K.L.; Kosoy, M.Y. Natural history of plague: Perspectives from more than a century of research. Annu. Rev. Entomol. 2005, 50, 505–528. [Google Scholar] [CrossRef]
- Schaub, G.A.; Vogel, P.U.B. Plague disease: From Asia to Europe and back along the silk road. In Infectious Diseases Along the Silk Roads—The Spread of Parasitoses and Culture Past and Today; Mehlhorn, H., Wu, X., Wu, Z., Eds.; Springer: Cham, Switzerland, 2023; pp. 83–112. [Google Scholar] [CrossRef]
- Yang, R.; Atkinson, S.; Chen, Z.; Cui, Y.; Du, Z.; Han, Y.; Sebbane, F.; Slavin, P.; Song, Y.; Yan, Y.; et al. Yersinia pestis and plague: Some knowns and unknowns. Zoonoses 2023, 3, 5. [Google Scholar] [CrossRef]
- Eisen, R.J.; Bearden, S.W.; Wilder, A.P.; Montenieri, J.A.; Antolin, M.F. Early-phase transmission of Yersinia pestis by unblocked fleas as a mechanism explaining rapidly spreading plague epizootics. Proc. Natl. Acad. Sci. USA 2006, 103, 15380–15385. [Google Scholar] [CrossRef]
- Eisen, R.J.; Gage, K.L. Adaptive strategies of Yersinia pestis to persist during inter-epizootic and epizootic periods. Vet. Res. 2009, 40, 1. [Google Scholar] [CrossRef] [PubMed]
- Buhnerkempe, M.G.; Eisen, R.J.; Goodell, B.; Gage, K.L.; Antolin, M.F.; Webb, C.T. Transmission shifts underlie variability in population responses to Yersinia pestis infection. PLoS ONE 2011, 6, e22498. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Biggins, D.E.; Godbey, J.L.; Eads, D.A. Epizootic plague in prairie dogs: Correlates and control with deltamethrin. Vector-Borne Zoonotic Dis. 2021, 21, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Duan, R.; Chen, Y.; Liang, J.; Zheng, X.; Qin, S.; Bukai, A.; Lu, X.; Xi, J.; Lv, D.; et al. Plague outbreak of a Marmota himalayana family emerging from hibernation. Vector-Borne Zoonotic Dis. 2022, 22, 410–418. [Google Scholar] [CrossRef]
- Karimova, T.Y.; Neronov, V.M.; Popov, V.P. Development of views on natural focality of plague. Biol. Bull. 2010, 37, 725–732. [Google Scholar] [CrossRef]
- Barbieri, R.; Signoli, M.; Chevé, D.; Costedoat, C.; Tzortzis, S.; Aboudharam, G.; Raoult, D.; Drancourt, M. Yersinia pestis: The natural history of plague. Clin. Microbiol. Rev. 2020, 34, e00044-19. [Google Scholar] [CrossRef]
- Williams, S.K.; Schotthoefer, A.M.; Montenieri, J.A.; Holmes, J.L.; Vetter, S.M.; Gage, K.L.; Bearden, S.W. Effects of low-temperature flea maintenance on the transmission of Yersinia pestis by Oropsylla montana. Vector-Borne Zoonotic Dis. 2013, 13, 468–478. [Google Scholar] [CrossRef]
- Easterday, W.R.; Kausrud, K.L.; Star, B.; Heier, L.; Haley, B.J.; Ageyev, V.; Colwell, R.R.; Stenseth, N.C. An additional step in the transmission of Yersinia pestis? ISME J. 2012, 6, 231–236. [Google Scholar] [CrossRef]
- Eisen, R.J.; Petersen, J.M.; Higgins, C.L.; Wong, D.; Levy, C.E.; Mead, P.S.; Schriefer, M.E.; Griffith, K.S.; Gage, K.L.; Beard, C.B. Persistence of Yersinia pestis in soil under natural conditions. Emerg. Infect. Dis. 2008, 14, 941–943. [Google Scholar] [CrossRef]
- Ayyadurai, S.; Houhamdi, L.; Lepidi, H.; Nappez, C.; Raoult, D.; Drancourt, M. Long-term persistence of virulent Yersinia pestis in soil. Microbiology 2008, 154, 2865–2871. [Google Scholar] [CrossRef]
- Malek, M.A.; Bitam, I.; Levasseur, A.; Terras, J.; Gaudart, J. Yersinia pestis halotolerance illuminates plague reservoirs. Sci. Rep. 2017, 7, 40022. [Google Scholar] [CrossRef] [PubMed]
- Andrianaivoarimanana, V.; Kreppel, K.; Elissa, N.; Duplantier, J.M.; Carniel, E.; Rajerison, M.; Jambou, R. Understanding the persistence of plague foci in Madagascar. PLoS Negl. Trop. Dis. 2013, 7, e2382. [Google Scholar] [CrossRef] [PubMed]
- Haikukutu, L.; Lyaku, J.R.; Lyimo, C.M.; Eiseb, S.J.; Makundi, R.H. Immunogenetics, sylvatic plague and its vectors: Insights from the pathogen reservoir Mastomys natalensis in Tanzania. Immunogenetics 2023, 75, 517–530. [Google Scholar] [CrossRef] [PubMed]
- Miarinjara, A.; Bland, D.M.; Belthoff, J.R.; Hinnebusch, B.J. Poor vector competence of the human flea, Pulex irritans, to transmit Yersinia pestis. Parasites Vectors 2021, 14, 317. [Google Scholar] [CrossRef]
- Pauling, C.D.; Beerntsen, B.T.; Song, Q.; Anderson, D.M. Transovarial transmission of Yersinia pestis in its flea vector Xenopsylla cheopis. Nat. Commun. 2024, 15, 7266. [Google Scholar] [CrossRef]
- Mitchell, C.L.; Schwarzer, A.R.; Miarinjara, A.; Jarrett, C.O.; Luis, A.D.; Hinnebusch, B.J. A role for early-phase transmission in the enzootic maintenance of plague. PLoS Pathog. 2022, 18, e1010996. [Google Scholar] [CrossRef]
- Alderson, J.; Quastel, M.; Wilson, E.; Bellamy, D. Factors influencing the re-emergence of plague in Madagascar. Emerg. Top. Life Sci. 2020, 4, 411–421. [Google Scholar] [CrossRef]
- Fell, H.G.; Jones, M.; Atkinson, S.; Stenseth, N.C.; Algar, A.C. The role of reservoir species in mediating plague’s dynamic response to climate. R. Soc. Open Sci. 2023, 10, 230021. [Google Scholar] [CrossRef]
- Macaluso, K.R.; Pornwiroon, W.; Popov, V.L.; Foil, L.D. Identification of Rickettsia felis in the salivary glands of cat fleas. Vector-Borne Zoonotic Dis. 2008, 8, 391–396. [Google Scholar] [CrossRef]
- Danchenko, M.; Laukaitis, H.J.; Macaluso, K.R. Dynamic gene expression in salivary glands of the cat flea during Rickettsia felis infection. Pathog. Dis. 2021, 79, ftab020. [Google Scholar] [CrossRef]
- Brown, L.D.; Banajee, K.H.; Foil, L.D.; Macaluso, K.R. Transmission mechanisms of an emerging insect-borne rickettsial pathogen. Parasites Vectors 2016, 9, 237. [Google Scholar] [CrossRef]
- Mediannikov, O.; Bechah, Y.; Amanzougaghene, N.; Lepidi, H.; Bassene, H.; Sambou, M.; Lienhard, C.; Benkacimi, L.; Dieme, C.; Sokhna, C.; et al. Booklice Liposcelis bostrychophila naturally infected by Rickettsia felis cause fever and experimental pneumonia in mammals. J. Infect. Dis. 2022, 226, 1075–1083. [Google Scholar] [CrossRef]
- Reif, K.E.; Kearney, M.T.; Foil, L.D.; Macaluso, K.R. Acquisition of Rickettsia felis by cat fleas during feeding. Vector-Borne Zoonotic Dis. 2011, 11, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Hinnebusch, B.J.; Erickson, D.L. Yersinia pestis biofilm in the flea vector and its role in the transmission of plague. Curr. Top. Microbiol. Immunol. 2008, 322, 229–248. [Google Scholar] [CrossRef] [PubMed]
- Eisen, R.J.; Gage, K.L. Transmission of flea-borne zoonotic agents. Annu. Rev. Entomol. 2012, 57, 61–82. [Google Scholar] [CrossRef] [PubMed]
- Hinnebusch, B.J.; Jarrett, C.O.; Bland, D.M. “Fleaing” the plague: Adaptations of Yersinia pestis to its insect vector that lead to transmission. Annu. Rev. Microbiol. 2017, 71, 215–232. [Google Scholar] [CrossRef] [PubMed]
- Hinnebusch, B.J.; Jarrett, C.O.; Bland, D.M. Molecular and genetic mechanisms that mediate transmission of Yersinia pestis by fleas. Biomolecules 2021, 11, 210. [Google Scholar] [CrossRef]
- Yin, J.X.; Cheng, X.O.; Luo, Y.Y.; Zhao, Q.F.; Wie, Z.F.; Xu, D.D.; Wang, M.D.; Zhou, Y.; Wang, X.F.; Liu, Z.X. The relationship between fleas and small mammals in households of the Western Yunnan Province, China. Sci. Rep. 2020, 10, 16705. [Google Scholar] [CrossRef]
- Laukaitis-Yousey, H.J.; Macaluso, K.R. Cat flea coinfection with Rickettsia felis and Rickettsia typhi. Vector-Borne Zoonotic Dis. 2024, 24, 201–213. [Google Scholar] [CrossRef]
- Pizzuti, M.; Bailey, P.; Derrick, C.; Albrecht, B.; Carr, A.L.; Covington, E.W.; Deri, C.R.; Green, S.B.; Hayes, J.; Hobbs, A.L.V.; et al. Epidemiology and treatment of invasive Bartonella spp. infections in the United States. Infection 2024, 52, 1307–1314. [Google Scholar] [CrossRef]
- Deng, H.; Pang, Q.; Zhao, B.; Vayssier-Taussat, M. Molecular mechanisms of Bartonella and mammalian erythrocyte interactions: A review. Front. Cell. Infect. Microbiol. 2018, 8, 431. [Google Scholar] [CrossRef]
- Perry, R.D.; Fetherston, J.D. Yersinia pestis—Etiologic agent of plague. Clin. Microbiol. Rev. 1997, 10, 35–66. [Google Scholar] [CrossRef]
- Ke, Y.; Chen, Z.; Yang, R. Yersinia pestis: Mechanisms of entry into and resistance to the host cell. Front. Cell. Infect. Microbiol. 2013, 3, 106. [Google Scholar] [CrossRef]
- Bennasar-Figueras, A. The natural and clinical history of plague: From the ancient pandemics to modern insights. Microorganisms 2024, 12, 146. [Google Scholar] [CrossRef]
- Fleck-Derderian, S.; Cooley, K.M.; Nelson, C.A. Plague in disguise: The discovery of occult buboes on surgical procedure or autopsy. Vector-Borne Zoonotic Dis. 2022, 22, 225–231. [Google Scholar] [CrossRef]
- Gonzalez, R.J.; Miller, V.L. A deadly path: Bacterial spread during bubonic plague. Trends Microbiol. 2016, 24, 239–241. [Google Scholar] [CrossRef]
- Demeure, C.E.; Dussurget, O.; Mas Fiol, G.; Le Guern, A.S.; Savin, C.; Pizarro-Cerdá, J. Yersinia pestis and plague: An updated view on evolution, virulence determinants, immune subversion, vaccination, and diagnostics. Genes Immun. 2019, 20, 357–370. [Google Scholar] [CrossRef]
- Hartley, L.; Harold, S.; Hawe, E. The efficacy, safety, and immunogenicity of plague vaccines: A systematic literature review. Curr. Res. Immunol. 2023, 4, 100072. [Google Scholar] [CrossRef] [PubMed]
- Hutton, S.M.; Miarinjara, A.; Stone, N.E.; Raharimalala, F.N.; Raveloson, A.O.; Rakotobe Harimanana, R.; Harimalala, M.; Rahelinirina, S.; McDonough, R.F.; Ames, A.D.; et al. Knockdown resistance mutations are common and widely distributed in Xenopsylla cheopis fleas that transmit plague in Madagascar. PLoS Negl. Trop. Dis. 2023, 17, e0011401. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Nian, Y.; Zeng, Z.; Han, T.; Liu, W.; Zheng, K.; Xiao, F. Epidemiological survey and genetic diversity of Bartonella in fleas collected from rodents in Fujian Province, Southeast China. Parasites Vectors 2024, 17, 264. [Google Scholar] [CrossRef] [PubMed]
- Jarrett, C.O.; Deak, E.; Isherwood, K.E.; Oyston, P.C.; Fischer, E.R.; Whitney, A.R.; Kobayashi, S.D.; DeLeo, F.R.; Hinnebusch, B.J. Transmission of Yersinia pestis from an infectious biofilm in the flea vector. J. Infect. Dis. 2004, 190, 783–792. [Google Scholar] [CrossRef]
- Chouikha, I.; Hinnebusch, B.J. Yersinia-flea interactions and the evolution of the arthropod-borne transmission route of plague. Curr. Opin. Microbiol. 2012, 15, 239–246. [Google Scholar] [CrossRef]
- Spyrou, M.A.; Tukhbatova, R.I.; Wang, C.C.; Valtueña, A.A.; Lankapalli, A.K.; Kondrashin, V.V.; Tsybin, V.A.; Khokhlov, A.; Kühnert, D.; Herbig, A.; et al. Analysis of 3800-year-old Yersinia pestis genomes suggests Bronze Age origin for bubonic plague. Nat. Commun. 2018, 9, 2234. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Susat, J.; Lübke, H.; Immel, A.; Brinker, U.; Macāne, A.; Meadows, J.; Steer, B.; Tholey, A.; Zagorska, I.; Gerhards, G.; et al. A 5000-year-old hunter-gatherer already plagued by Yersinia pestis. Cell Rep. 2021, 35, 109278. [Google Scholar] [CrossRef] [PubMed]
- Bland, D.M.; Jarrett, C.O.; Bosio, C.F.; Hinnebusch, B.J. Infectious blood source alters early foregut infection and regurgitative transmission of Yersinia pestis by rodent fleas. PLoS Pathog. 2018, 14, e1006859. [Google Scholar] [CrossRef] [PubMed]
- Bosio, C.F.; Jarrett, C.O.; Scott, D.P.; Fintzi, J.; Hinnebusch, B.J. Comparison of the transmission efficiency and plague progression dynamics associated with two mechanisms by which fleas transmit Yersinia pestis. PLoS Pathog. 2020, 16, e1009092. [Google Scholar] [CrossRef]
- Dewitte, A.; Bouvenot, T.; Pierre, F.; Ricard, I.; Pradel, E.; Barois, N.; Hujeux, A.; Bontemps-Gallo, S.; Sebbane, F. A refined model of how Yersinia pestis produces a transmissible infection in its flea vector. PLoS Pathog. 2020, 16, e1008440. [Google Scholar] [CrossRef]
- Bland, D.M.; Martens, C.A.; Virtaneva, K.; Kanakabandi, K.; Long, D.; Rosenke, R.; Saturday, G.A.; Hoyt, F.H.; Bruno, D.P.; Ribeiro, J.M.; et al. Transcriptomic profiling of the digestive tract of the rat flea, Xenopsylla cheopis, following blood feeding and infection with Yersinia pestis. PLoS Negl. Trop. Dis. 2020, 14, e0008688. [Google Scholar] [CrossRef]
- Lemon, A.; Sagawa, J.; Gravelle, K.; Vadyvaloo, V. Biovar-related differences apparent in the flea foregut colonization phenotype of distinct Yersinia pestis strains do not impact transmission efficiency. Parasites Vectors 2020, 13, 335. [Google Scholar] [CrossRef]
- Bland, D.M.; Miarinjara, A.; Bosio, C.F.; Calarco, J.; Hinnebusch, B.J. Acquisition of yersinia murine toxin enabled Yersinia pestis to expand the range of mammalian hosts that sustain flea-borne plague. PLoS Pathog. 2021, 17, e1009995. [Google Scholar] [CrossRef]
- Dewitte, A.; Werkmeister, E.; Pierre, F.; Sebbane, F.; Bontemps-Gallo, S. A widefield light microscopy-based approach provides further insights into the colonization of the flea proventriculus by Yersinia pestis. Appl. Environ. Microbiol. 2023, 89, e0209122. [Google Scholar] [CrossRef]
- Coles, T.B.; Dryden, M.W. Insecticide/acaricide resistance in fleas and ticks infesting dogs and cats. Parasites Vectors 2014, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Boyer, S.; Miarinjara, A.; Elissa, N. Xenopsylla cheopis (Siphonaptera: Pulicidae) susceptibility to Deltamethrin in Madagascar. PLoS ONE 2014, 9, e111998. [Google Scholar] [CrossRef] [PubMed]
- Rust, M.K. Insecticide resistance in fleas. Insects 2016, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Grácio, A.J.D.S.; Grácio, M.A.A. Plague: A millenary infectious disease reemerging in the XXI century. BioMed Res. Int. 2017, 2017, 5696542. [Google Scholar] [CrossRef]
- Jacomo, V.; Kelly, P.J.; Raoult, D. Natural history of Bartonella infections (an exception to Koch’s postulate). Clin. Vaccine Immunol. 2002, 9, 8–18. [Google Scholar] [CrossRef]
- Reed, S.C.O.; Lamason, R.L.; Risca, V.I.; Abernathy, E.; Welch, M.D. Rickettsia actin-based motility occurs in distinct phases mediated by different actin nucleators. Curr. Biol. 2014, 24, 98–103. [Google Scholar] [CrossRef]
- Bowman, D.D. Introduction to the alpha-proteobacteria: Wolbachia and Bartonella, Rickettsia, Brucella, Ehrlichia, and Anaplasma. Top. Companion Anim. Med. 2011, 26, 173–177. [Google Scholar] [CrossRef]
- Xi, Y.; Li, X.; Liu, L.; Xiu, F.; Yi, X.; Chen, H.; You, X. Sneaky tactics: Ingenious immune evasion mechanisms of Bartonella. Virulence 2024, 15, 2322961. [Google Scholar] [CrossRef]
- Ditchburn, J.L.; Hodgkins, R. Yersinia pestis, a problem of the past and a re-emerging threat. Biosaf. Health 2019, 1, 65–70. [Google Scholar] [CrossRef]
- Prakash, A.; Yogeeshwari, S.; Sircar, S.; Agrawal, S. Protein domain of unknown function 3233 is a translocation domain of autotransporter secretory mechanism in gamma proteobacteria. PLoS ONE 2011, 6, e25570. [Google Scholar] [CrossRef]
- Seabaugh, J.A.; Anderson, D.M. Pathogenicity and virulence of Yersinia. Virulence 2024, 15, 2316439. [Google Scholar] [CrossRef]
- Dekker, J.P.; Frank, K.M. Salmonella, Shigella, and Yersinia. Clin. Lab. Med. 2015, 35, 225–246. [Google Scholar] [CrossRef]
- Aziz, M.; Yelamanchili, V.S. Yersinia enterocolitica. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK499837/ (accessed on 17 June 2025).
- Brady, M.F.; Yarrarapu, S.N.S.; Anjum, F. Yersinia pseudotuberculosis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK430717/ (accessed on 17 June 2025).
- Vogel, P.U.B.; Borrelli, J. Identifizierung von Bakterien—Grundlagen Sowie Stärken und Schwächen von Klassischen und Modernen Methoden; Springer: Berlin/Heidelberg, Germany, 2024. (In German) [Google Scholar] [CrossRef]
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union One Health 2021 Zoonoses Report. EFSA J. 2022, 20, e07666. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC). The European Union One Health 2022 Zoonoses Report. EFSA J. 2023, 21, e8442. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.C.; Jarrett, C.O.; Bosio, C.F.; Hinnebusch, B.J. Retracing the evolutionary path that led to flea-borne transmission of Yersinia pestis. Cell Host Microbe 2014, 15, 578–586. [Google Scholar] [CrossRef]
- Parkhill, J.; Wren, B.W.; Thomson, N.R.; Titball, R.W.; Holden, M.T.; Prentice, M.B.; Sebaihia, M.; James, K.D.; Churcher, C.; Mungall, K.L.; et al. Genome sequence of Yersinia pestis, the causative agent of plague. Nature 2001, 413, 523–527. [Google Scholar] [CrossRef]
- Achtman, M.; Zurth, K.; Morelli, G.; Torrea, G.; Guiyoule, A.; Carniel, E. Yersinia pestis, the cause of plague, is a recently emerged clone of Yersinia pseudotuberculosis. Proc. Natl. Acad. Sci. USA 1999, 96, 14043–14048, Erratum in Proc. Natl. Acad. Sci. USA 2000, 97, 8192. https://doi.org/10.1073/pnas.97.14.8192. [Google Scholar] [CrossRef]
- Zhou, D.; Tong, Z.; Song, Y.; Han, Y.; Pei, D.; Pang, X.; Zhai, J.; Li, M.; Cui, B.; Qi, Z.; et al. Genetics of metabolic variations between Yersinia pestis biovars and the proposal of a new biovar, microtus. J. Bacteriol. 2004, 186, 5147–5152. [Google Scholar] [CrossRef]
- Portnoy, D.A.; Falkow, S. Virulence-associated plasmids from Yersinia enterocolitica and Yersinia pestis. J. Bacteriol. 1981, 148, 877–883. [Google Scholar] [CrossRef]
- Chouikha, I.; Hinnebusch, B.J. Silencing urease: A key evolutionary step that facilitated the adaptation of Yersinia pestis to the flea-borne transmission route. Proc. Natl. Acad. Sci. USA 2014, 111, 18709–18714. [Google Scholar] [CrossRef]
- Hinnebusch, B.J.; Chouikha, I.; Sun, Y.C. Ecological opportunity, evolution, and the emergence of flea-borne plague. Infect. Immun. 2016, 84, 1932–1940. [Google Scholar] [CrossRef]
- Ferber, D.M.; Brubaker, R.R. Plasmids in Yersinia pestis. Infect. Immun. 1981, 31, 839–841. [Google Scholar] [CrossRef]
- Hinnebusch, B.J.; Rudolph, A.E.; Cherepanov, P.; Dixon, J.E.; Schwan, T.G.; Forsberg, A. Role of Yersinia murine toxin in survival of Yersinia pestis in the midgut of the flea vector. Science 2002, 296, 733–735. [Google Scholar] [CrossRef]
- Chain, P.S.; Carniel, E.; Larimer, F.W.; Lamerdin, J.; Stoutland, P.O.; Regala, W.M.; Georgescu, A.M.; Vergez, L.M.; Land, M.L.; Motin, V.L.; et al. Insights into the evolution of Yersinia pestis through whole-genome comparison with Yersinia pseudotuberculosis. Proc. Natl. Acad. Sci. USA 2004, 101, 13826–13831. [Google Scholar] [CrossRef]
- Rebeil, R.; Jarrett, C.O.; Driver, J.D.; Ernst, R.K.; Oyston, P.C. Induction of the Yersinia pestis PhoP-PhoQ regulatory system in the flea and its role in producing a transmissible infection. J. Bacteriol. 2013, 195, 1920–1930. [Google Scholar] [CrossRef]
- Fukuto, H.S.; Viboud, G.I.; Vadyvaloo, V. The diverse roles of the global transcriptional regulator PhoP in the lifecycle of Yersinia pestis. Pathogens 2020, 9, 1039. [Google Scholar] [CrossRef]
- Bontemps-Gallo, S.; Lacroix, J.M.; Sebbane, F. What do we know about osmoadaptation of Yersinia pestis? Arch. Microbiol. 2021, 204, 11. [Google Scholar] [CrossRef]
- Robin, B.; Dewitte, A.; Alaimo, V.; Lecoeur, C.; Pierre, F.; Billon, G.; Sebbane, F.; Bontemps-Gallo, S. The CpxAR signaling system confers a fitness advantage for flea gut colonization by the plague bacillus. J. Bacteriol. 2024, 206, e0017324. [Google Scholar] [CrossRef]
- Carniel, E. The Yersinia high-pathogenicity island. Int. Microbiol. 1999, 2, 161–167. [Google Scholar]
- Price, S.L.; Vadyvaloo, V.; DeMarco, J.K.; Brady, A.; Gray, P.A.; Kehl-Fie, T.E.; Garneau-Tsodikova, S.; Perry, R.D.; Lawrenz, M.B. Yersiniabactin contributes to overcoming zinc restriction during Yersinia pestis infection of mammalian and insect hosts. Proc. Natl. Acad. Sci. USA 2021, 118, e2104073118. [Google Scholar] [CrossRef]
- Chaaban, T.; Mohsen, Y.; Ezzeddine, Z.; Ghssein, G. Overview of Yersinia pestis metallophores: Yersiniabactin and yersinopine. Biology 2023, 12, 598. [Google Scholar] [CrossRef]
- Han, Y.; Zhou, D.; Pang, X.; Song, Y.; Zhang, L.; Bao, J.; Tong, Z.; Wang, J.; Guo, Z.; Zhai, J.; et al. Microarray analysis of temperature-induced transcriptome of Yersinia pestis. Microbiol. Immunol. 2004, 48, 791–805. [Google Scholar] [CrossRef]
- Bosio, C.F.; Jarrett, C.O.; Hinnebusch, B.J. Evidence of a role for the F1 capsule of Yersinia pestis in enhancing transmission from mammals to fleas in a mouse model of bubonic plague. mBio 2025, 16, e0030125. [Google Scholar] [CrossRef]
- Prior, J.L.; Parkhill, J.; Hitchen, P.G.; Mungall, K.L.; Stevens, K. The failure of different strains of Yersinia pestis to produce lipopolysaccharide O-antigen under different growth conditions is due to mutations in the O-antigen gene cluster. FEMS Microbiol. Lett. 2001, 197, 229–233. [Google Scholar] [CrossRef]
- Chung, L.K.; Bliska, J.B. Yersinia versus host immunity: How a pathogen evades or triggers a protective response. Curr. Opin. Microbiol. 2016, 29, 56–62. [Google Scholar] [CrossRef]
- Makdasi, E.; Atiya-Nasagi, Y.; Gur, D.; Zauberman, A.; Schuster, O.; Glinert, I.; Shmaya, S.; Milrot, E.; Levy, H.; Weiss, S.; et al. An improvement in diagnostic blood culture conditions allows for the rapid detection and isolation of the slow growing pathogen Yersinia pestis. Pathogens 2022, 11, 255. [Google Scholar] [CrossRef]
- Johnson, T.L.; Hinnebusch, B.J.; Boegler, K.A.; Graham, C.B.; MacMillan, K.; Montenieri, J.A.; Bearden, S.W.; Gage, K.L.; Eisen, R.J. Yersinia murine toxin is not required for early-phase transmission of Yersinia pestis by Oropsylla montana (Siphonaptera: Ceratophyllidae) or Xenopsylla cheopis (Siphonaptera: Pulicidae). Microbiology 2014, 160, 2517–2525. [Google Scholar] [CrossRef]
- Earl, S.C.; Rogers, M.T.; Keen, J.; Bland, D.M.; Houppert, A.S. Resistance to innate immunity contributes to colonization of the insect gut by Yersinia pestis. PLoS ONE 2015, 10, e0133318. [Google Scholar] [CrossRef]
- Bland, D.M.; Hinnebusch, B.J. Feeding behavior modulates biofilm-mediated transmission of Yersinia pestis by the cat flea, Ctenocephalides felis. PLoS Negl. Trop. Dis. 2016, 10, e0004413. [Google Scholar] [CrossRef]
- Weber, K.; Karnik, D.; Brown, L.D. Transcriptional induction of the Imd signaling pathway and associated antibacterial activity in the digestive tract of cat fleas (Ctenocephalides felis). Parasites Vectors 2024, 17, 546. [Google Scholar] [CrossRef]
- Vanstreels, R.E.T.; Palma, R.L.; Sergey, V.; Mironov, S.V. Arthropod parasites of Antarctic and Subantarctic birds and pinnipeds: A review of host-parasite associations. Int. J. Parasitol. Parasites Wildl. 2020, 12, 275–290. [Google Scholar] [CrossRef]
- Gandon, S.; Heitzmann, L.; Sebbane, F. To block or not to block: The adaptive manipulation of plague transmission. Evol. Lett. 2019, 3, 152–161. [Google Scholar] [CrossRef]
- Maleki-Ravasan, N.; Solhjouy-Fard, S.; Beaucournu, J.C.; Laudisoit, A.; Mostafavi, E. The fleas (Siphonaptera) in Iran: Diversity, host range, and medical importance. PLoS Negl. Trop. Dis. 2017, 11, e0005260. [Google Scholar] [CrossRef]
- Hamzaoui, B.E.; Zurita, A.; Cutillas, C.; Parola, P. Fleas and flea-borne diseases of North Africa. Acta Trop. 2020, 211, 105627. [Google Scholar] [CrossRef]
- Schaub, G.A.; Mehlhorn, H. Fleas. In Encyclopedia of Parasitology, 4th ed.; Mehlhorn, H., Ed.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1037–1043. [Google Scholar]
- Schaub, G.A.; Mehlhorn, H. Insects. In Encyclopedia of Parasitology, 4th ed.; Mehlhorn, H., Ed.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1348–1357. [Google Scholar]
- Zurita, A.; Callejón, R.; García-Sánchez, Á.M.; Urdapilleta, M.; Lareschi, M. Origin; evolution; phylogeny and taxonomy of Pulex irritans. Med. Vet. Entomol. 2019, 33, 296–311. [Google Scholar] [CrossRef]
- Azarm, A.; Dalimi, A.; Pirestani, M.; Mohammadiha, A.; Zahraei-Ramazani, A.; Marvi-Moghaddam, N.; Amiri, E. Pulex irritans on dogs and cats: Morphological and molecular approach. J. Arthropod-Borne Dis. 2022, 16, 196–205. [Google Scholar] [CrossRef]
- Rothschild, M.; Schlein, Y.; Parker, K.; Sternberg, S. Jump of the oriental rat flea Xenopsylla cheopis (Roths.). Nature 1972, 239, 45–48. [Google Scholar] [CrossRef]
- Rust, M.K. The biology and ecology of cat fleas and advancements in their pest management: A review. Insects 2017, 8, 118. [Google Scholar] [CrossRef]
- Dryden, M. Host association, on-host longevity and egg production of Ctenocephalides felis felis. Vet. Parasitol. 1989, 34, 117–122. [Google Scholar] [CrossRef]
- Sousa, C.A. Fleas, flea allergy, and flea control: A review. Dermatol. Online J. 1997, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, A.L.; Hii, S.F.; Chong, R.; Webb, C.E.; Traub, R. Evaluation of the bacterial microbiome of two flea species using different DNA-isolation techniques provides insights into flea host ecology. FEMS Microbiol. Ecol. 2015, 91, fiv134. [Google Scholar] [CrossRef] [PubMed]
- Lehane, M.J. Managing the blood meal. In The Biology of Blood-Sucking in Insects, 2nd ed.; Lehane, M.J., Ed.; Cambridge University Press: Cambridge, UK, 2005; pp. 84–115. ISBN 9780511610493. [Google Scholar]
- Iannino, F.; Sulli, N.; Maitino, A.; Pascucci, I.; Pampiglione, G.; Salucci, S. Fleas of dog and cat: Species, biology and flea-borne diseases. Vet. Ital. 2017, 53, 277–288. [Google Scholar] [CrossRef]
- Franc, M.; Bouhsira, É.; Beugnet, F. Direct transmission of the cat flea (Ctenocephalides felis) between cats exhibiting social behaviour. Parasite 2013, 20, 49. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Zhang, T.; Su, J.; Huang, Z.; Wu, A.; Lin, G. Genetic differentiation of the oriental rat flea, Xenopsylla cheopis, from two sympatric host species. Parasites Vectors 2018, 11, 343. [Google Scholar] [CrossRef]
- Khokhlova, I.S.; Fielden, L.J.; Degen, A.A.; Krasnov, B.R. Feeding performance of fleas on different host species: Is phylogenetic distance between hosts important? Parasitology 2012, 139, 60–68. [Google Scholar] [CrossRef]
- Khokhlova, I.S.; Fielden, L.J.; Warburton, E.M.; van der Mescht, L.; Krasnov, B.R. Feeding performance on a novel host: No adaptation over generations and differential patterns in two flea species. Parasitology 2020, 147, 721–728. [Google Scholar] [CrossRef]
- Khokhlova, I.S.; van der Mescht, L.; Warburton, E.M.; Stavtseva, N.A.; Krasnov, B.R. Adaptation to a novel host and performance trade-off in host-generalist and host-specific insect ectoparasites. Insect Sci. 2022, 29, 567–580. [Google Scholar] [CrossRef]
- Lorange, E.A.; Race, B.L.; Sebbane, F.; Hinnebusch, B.J. Poor vector competence of fleas and the evolution of hypervirulence in Yersinia pestis. J. Infect. Dis. 2005, 191, 1907–1912. [Google Scholar] [CrossRef]
- Hinnebusch, B.J. The evolution of flea-borne transmission in Yersinia pestis. Curr. Issues Mol. Biol. 2005, 7, 197–212. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.M.C.; Francischetti, I.M.B. Role of arthropod saliva in blood feeding: Sialome and post-sialome perspectives. Annu. Rev. Entomol. 2003, 48, 73–88. [Google Scholar] [CrossRef]
- Schaub, G.A.; Vogel, P.; Balzcun, C. Parasite-vector interactions. In Molecular Parasitology—Protozoan Parasites and Their Molecules; Walochnik, J., Duchêne, M., Eds.; Springer-Verlag: Berlin/Heidelberg, Germany, 2016; pp. 431–489. [Google Scholar]
- Ribeiro, J.M.C.; Assumpção, T.C.; Ma, D.; Alvarenga, P.H.; Pham, V.M.; Andersen, J.F.; Francischetti, I.M.; Macaluso, K.R. An insight into the sialotranscriptome of the cat flea, Ctenocephalides felis. PLoS ONE 2012, 7, e44612. [Google Scholar] [CrossRef]
- Lu, S.; Tirloni, L.; Oliveira, M.B.; Bosio, C.F.; Nardone, G.A. Identification of a substrate-like cleavage-resistant thrombin inhibitor from the saliva of the flea Xenopsylla cheopis. J. Biol. Chem. 2021, 297, 101322. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Andersen, J.F.; Bosio, C.F.; Hinnebusch, B.J.; Ribeiro, J.M.C. Integrated analysis of the sialotranscriptome and sialoproteome of the rat flea Xenopsylla cheopis. J. Proteom. 2022, 254, 104476. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Danchenko, M.; Macaluso, K.R.; Ribeiro, J.M.C. Revisiting the sialome of the cat flea Ctenocephalides felis. PLoS ONE 2023, 18, e0279070. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, J.A.; Azad, A.F. Patterns of erythrocyte digestion by bloodsucking insects: Constraints on vector competence. J. Med. Entomol. 1993, 30, 214–216. [Google Scholar] [CrossRef]
- Azambuja, P.; Guimaraes, J.A.; Garcia, E.S. Haemolytic factor from the crop of Rhodnius prolixus: Evidence and partial characterization. J. Insect Physiol. 1983, 29, 833–837. [Google Scholar] [CrossRef]
- Khokhlova, I.S.; Serobyan, V.; Krasnov, B.R.; Degen, A.A. Effect of host gender on blood digestion in fleas: Mediating role of environment. Parasitol. Res. 2009, 105, 1667–1673. [Google Scholar] [CrossRef]
- Greene, W.K.; Macnish, M.G.; Rice, K.L.; Thompson, R.C. Identification of genes associated with blood feeding in the cat flea, Ctenocephalides felis. Parasites Vectors 2015, 8, 368. [Google Scholar] [CrossRef]
- Wigglesworth, V.B. The regulation of respiration in the flea, Xenopsylla cheopis, Roths. (Pulicidae). Proc. R. Soc. Ser. B Biol. Sci. 1935, 118, 397–419. [Google Scholar] [CrossRef]
- Peters, W. Peritrophic membranes. In Zoophysiology; Springer: Berlin/Heidelberg, Germany, 1992; Volume 30, ISBN 978-3-642-84416-4. [Google Scholar]
- Terra, W.R. The origin and functions of the insect peritrophic membrane and peritrophic gel. Arch. Insect Biochem. Physiol. 2001, 47, 47–61. [Google Scholar] [CrossRef]
- André, M.R.; Neupane, P.; Lappin, M.; Herrin, B.; Smith, V.; Williams, T.I.; Collins, L.; Bai, H.; Jorge, G.L.; Balbuena, T.S.; et al. Using proteomic approaches to unravel the response of Ctenocephalides felis felis to blood feeding and infection with Bartonella henselae. Front. Cell. Infect. Microbiol. 2022, 12, 828082. [Google Scholar] [CrossRef] [PubMed]
- Gaines, P.J.; Brandt, K.S.; Eisele, A.M.; Wagner, W.P.; Bozic, C.M.; Wisnewski, N. Analysis of expressed sequence tags from subtracted and unsubtracted Ctenocephalides felis hindgut and Malpighian tubule cDNA libraries. Insect Mol. Biol. 2002, 11, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Gaines, P.J.; Walmsley, S.J.; Wisnewski, N. Cloning and characterization of five cDNAs encoding peritrophin-A domains from the cat flea, Ctenocephalides felis. Insect Biochem. Mol. Biol. 2003, 33, 1061–1073. [Google Scholar] [CrossRef] [PubMed]
- Müller, U.; Vogel, P.; Alber, G.; Schaub, G.A. The innate immune system of mammals and insects. In Contributions to Microbiology; Egesten, A., Schmidt, A., Herwald, H., Eds.; Karger: Basel, Switzerland, 2008; Volume 15, pp. 21–44. [Google Scholar] [CrossRef]
- Ratcliffe, N.A.; Mello, C.B.; Castro, H.C.; Dyson, P.; Figueiredo, M. Immune reactions of vector insects to parasites and pathogens. Microorganisms 2024, 12, 568. [Google Scholar] [CrossRef]
- Matsuura, M. Structural modifications of bacterial lipopolysaccharide that facilitate Gram-negative bacteria evasion of host innate immunity. Front. Immunol. 2013, 4, 109. [Google Scholar] [CrossRef]
- Salcedo-Porras, N.; Noor, S.; Cai, C.; Oliveira, P.L.; Lowenberger, C. Rhodnius prolixus uses the peptidoglycan recognition receptor rpPGRP-LC/LA to detect Gram-negative bacteria and activate the Imd pathway. Curr. Res. Insect Sci. 2020, 1, 100006. [Google Scholar] [CrossRef]
- Alejandro, A.D.; Lilia, J.P.; Jesús, M.B.; Henry, R.M. The Imd and Toll canonical immune pathways of Triatoma pallidipennis are preferentially activated by Gram-negative and Gram-positive bacteria, respectively, but cross-activation also occurs. Parasites Vectors 2022, 15, 256. [Google Scholar] [CrossRef]
- Hixson, B.; Huot, L.; Morejon, B.; Yang, X.; Nagy, P.; Michel, K.; Buchon, N. The transcriptional response in mosquitoes distinguishes between fungi and bacteria but not Gram types. BMC Genom. 2024, 25, 353. [Google Scholar] [CrossRef]
- Rennoll, S.A.; Rennoll-Bankert, K.E.; Guillotte, M.L.; Lehman, S.S.; Driscoll, T.P.; Beier-Sexton, M.; Rahman, M.S.; Gillespie, J.J.; Azad, A.F. The cat flea (Ctenocephalides felis) immune deficiency signaling pathway regulates Rickettsia typhi infection. Infect. Immun. 2017, 86, e00562-17. [Google Scholar] [CrossRef]
- Kurtz, J. Specific memory within innate immune systems. Trends Immunol. 2005, 26, 186–192. [Google Scholar] [CrossRef]
- Brown, L.D. Immunity of fleas (Order Siphonaptera). Dev. Comp. Immunol. 2019, 98, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Ha, E.M.; Oh, C.T.; Ryu, J.H.; Bae, Y.S.; Kang, S.W.; Jang, I.H.; Brey, P.T.; Lee, W.J. An antioxidant system required for host protection against gut infection in Drosophila. Dev. Cell. 2005, 8, 125–132. [Google Scholar] [CrossRef]
- Schaub, G.A. Interaction of Trypanosoma cruzi, triatomines and the microbiota of the vectors—A review. Microorganisms 2024, 12, 855. [Google Scholar] [CrossRef]
- Mathew, B.; Aoyagi, K.L.; Fisher, M.A. Antibacterial activity of Xenopsylla cheopis attacins against Yersinia pestis. bioRxiv 2023. [Google Scholar] [CrossRef]
- Strand, M.R. Composition and functional roles of the gut microbiota in mosquitoes. Curr. Opin. Insect Sci. 2018, 28, 59–65. [Google Scholar] [CrossRef]
- Gavish, Y.; Kedem, H.; Messika, I.; Cohen, C.; Toh, E.; Munro, D.; Dong, Q.; Fuqua, C.; Clay, K.; Hawlena, H. Association of host and microbial species diversity across spatial scales in desert rodent communities. PLoS ONE 2014, 9, e109677. [Google Scholar] [CrossRef]
- Yun, J.H.; Roh, S.W.; Whon, T.W.; Jung, M.J.; Kim, M.S.; Park, D.S.; Yoon, C.; Nam, Y.D.; Kim, Y.J.; Choi, J.H.; et al. Insect gut bacterial diversity determined by environmental habitat, diet, developmental stage, and phylogeny of host. Appl. Environ. Microbiol. 2014, 80, 5254–5264. [Google Scholar] [CrossRef]
- Wang, J.; Gao, L.; Aksoy, S. Microbiota in disease-transmitting vectors. Nat. Rev. Microbiol. 2023, 21, 604–618. [Google Scholar] [CrossRef]
- Liu, H.; Yin, J.; Huang, X.; Zang, C.; Zhang, Y.; Cao, J.; Gong, M. Mosquito gut microbiota: A review. Pathogens 2024, 13, 691. [Google Scholar] [CrossRef]
- Jing, T.Z.; Qi, F.H.; Wang, Z.Y. Most dominant roles of insect gut bacteria: Digestion, detoxification, or essential nutrient provision? Microbiome 2020, 8, 38. [Google Scholar] [CrossRef]
- Jones, R.T.; Vetter, S.M.; Montenieiri, J.; Holmes, J.; Bernhardt, S.A.; Gage, K.L. Yersinia pestis infection and laboratory conditions alter flea-associated bacterial communities. ISME J. 2013, 7, 224–228. [Google Scholar] [CrossRef]
- Cohen, C.; Toh, E.; Munro, D.; Dong, Q.; Hawlena, H. Similarities and seasonal variations in bacterial communities from the blood of rodents and from their flea vectors. ISME J. 2015, 9, 1662–1676. [Google Scholar] [CrossRef]
- Wu, Y.L.; Hu, S.F.; Zhang, X.L.; Wang, H.M.; Pan, H.Y.; Liu, G.H.; Deng, Y.P. Complete bacterial profile and potential pathogens of cat fleas Ctenocephalides felis. Acta Trop. 2023, 243, 106923. [Google Scholar] [CrossRef] [PubMed]
- Manvell, C.; Berman, H.; Callahan, B.; Breitschwerdt, E.; Swain, W.; Ferris, K.; Maggi, R.; Lashnits, E. Identification of microbial taxa present in Ctenocephalides felis (cat flea) reveals widespread co-infection and associations with vector phylogeny. Parasites Vectors 2022, 15, 398. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.T.; Borchert, J.; Eisen, R.; MacMillan, K.; Boegler, K.; Gage, K.L. Flea-associated bacterial communities across an environmental transect in a plague-endemic region of Uganda. PLoS ONE 2015, 10, e0141057. [Google Scholar] [CrossRef] [PubMed]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Nageshwar Reddy, D. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef]
- Vemuri, R.; Gundamaraju, R.; Shastri, M.D.; Shukla, S.D.; Kalpurath, K.; Ball, M.; Tristram, S.; Shankar, E.M.; Ahuja, K.; Eri, R. Gut microbial changes, interactions, and their implications on human lifecycle: An ageing perspective. BioMed Res. Int. 2018, 2018, 4178607. [Google Scholar] [CrossRef]
- Malele, I.; Nyingilili, H.; Lyaruu, E.; Tauzin, M.; Bernard Ollivier, B.; Cayol, J.L.; Fardeau, M.L.; Geiger, A. Bacterial diversity obtained by culturable approaches in the gut of Glossina pallidipes population from a non sleeping sickness focus in Tanzania: Preliminary results. BMC Microbiol. 2018, 18, 164. [Google Scholar] [CrossRef]
- Orem, J.C.; Silva, W.M.C.; Raiol, T.; Magalhães, M.I.; Martins, P.H.; Cavalcante, D.A.; Kruger, R.H.; Brigido, M.M.; De-Souza, M.T. Phylogenetic diversity of aerobic spore-forming Bacillalles isolated from Brazilian soils. Int. Microbiol. 2019, 22, 511–520. [Google Scholar] [CrossRef]
- von Martels, J.Z.H.; Sadabad, M.S.; Bourgonje, A.R.; Blokzijl, T.; Dijkstra, G.; Faber, K.N.; Harmsen, H.J.M. The role of gut microbiota in health and disease: In vitro modeling of host-microbe interactions at the aerobe-anaerobe interphase of the human gut. Anaerobe 2017, 44, 3–12. [Google Scholar] [CrossRef]
- Hinnebusch, B.J.; Bland, D.M.; Bosio, C.F.; Jarrett, C.O. Comparative ability of Oropsylla montana and Xenopsylla cheopis fleas to transmit Yersinia pestis by two different mechanisms. PLoS Negl. Trop. Dis. 2017, 11, e0005276, Erratum in PLoS Negl. Trop. Dis. 2020, 14, e0008344. https://doi.org/10.1371/journal.pntd.0008344. [Google Scholar] [CrossRef]
- Jones, R.T.; Vetter, S.M.; Gage, K.L. Short report: Exposing laboratory-reared fleas to soil and wild flea feces increases transmission of Yersinia pestis. Am. J. Trop. Med. Hyg. 2013, 89, 784–787. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.; Lashnits, E.; Neupane, P.; Herrin, B.H.; Lappin, M.; André, M.R.; Breitschwerdt, E.B. Feeding on a Bartonella henselae infected host triggers temporary changes in the Ctenocephalides felis microbiome. Pathogens 2023, 12, 366. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Li, Y.; Yang, C.; Gong, J.; Zhu, W.; Huang, Y.; Kong, M.; Zhao, L.; Wang, F.; Lu, S.; et al. Species-level microbiota of ticks and fleas from Marmota himalayana in the Qinghai-Tibet plateau. Front. Microbiol. 2023, 14, 1188155. [Google Scholar] [CrossRef] [PubMed]
- Healy, S.P.; Brown, L.D.; Hagstrom, M.R.; Foil, L.D.; Macaluso, K.R. Effect of Rickettsia felis strain variation on infection, transmission, and fitness in the cat flea (Siphonaptera: Pulicidae). J. Med. Entomol. 2017, 54, 1037–1043. [Google Scholar] [CrossRef]
- Hirunkanokpun, S.; Thepparit, C.; Foil, L.D.; Macaluso, K.R. Horizontal transmission of Rickettsia felis between cat fleas, Ctenocephalides felis. Mol. Ecol. 2011, 20, 4577–4586. [Google Scholar] [CrossRef]
- Fongsaran, C.; Jirakanwisal, K.; Tongluan, N.; Latour, A.; Healy, S.; Christofferson, R.C.; Macaluso, K.R. The role of cofeeding arthropods in the transmission of Rickettsia felis. PLoS Negl. Trop. Dis. 2022, 16, e0010576. [Google Scholar] [CrossRef]
- Wang, X.R.; Burkhardt, N.Y.; Kurtti, T.J.; Oliver, J.D.; Price, L.D.; Cull, B.; Thorpe, C.J.; Thiel, M.S.; Munderloh, U.G. Mitochondrion-dependent apoptosis is essential for Rickettsia parkeri infection and replication in vector cells. mSystems 2021, 6, e01209-1220. [Google Scholar] [CrossRef]
- Morick, D.; Krasnov, B.R.; Khokhlova, I.S.; Gutiérrez, R.; Fielden, L.J.; Gottlieb, Y.; Harrus, S. Effects of Bartonella spp. on flea feeding and reproductive performance. Appl. Environ. Microbiol. 2013, 79, 3438–3443. [Google Scholar] [CrossRef]
- Himsworth, C.G.; Byers, K.A.; Whelan, T.; Bai, Y.; Kosoy, M.Y. Flea presence and abundance are not predictors of Bartonella tribocorum carriage in Norway rats (Rattus norvegicus) from an underserved neighborhood of Vancouver, Canada. Vector-Borne Zoonotic Dis. 2021, 21, 121–124. [Google Scholar] [CrossRef]
- Moore, C.; Breitschwerdt, E.B.; Kim, L.; Li, Y.; Ferris, K. The association of host and vector characteristics with Ctenocephalides felis pathogen and endosymbiont infection. Front. Microbiol. 2023, 14, 1137059. [Google Scholar] [CrossRef] [PubMed]
- Welc-Falęciak, R.; Kowalec, M.; Karbowiak, G.; Bajer, A.; Behnke, J.M.; Siński, E. Rickettsiaceae and Anaplasmataceae infections in Ixodes ricinus ticks from urban and natural forested areas of Poland. Parasites Vectors 2014, 7, 121. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.C.; Stein, M.; Matías Hisgen, C.; Micieli, M.V. Abiotic factors affecting the prevalence of Wolbachia (Rickettsiaceae) in immature Aedes albopictus (Skuse) (Culicidae). J. Invertebr. Pathol. 2022, 189, 107730. [Google Scholar] [CrossRef]
- Flatau, R.; Segoli, M.; Hawlena, H. Wolbachia endosymbionts of fleas occur in all females but rarely in males and do not show evidence of obligatory relationships, fitness effects, or sex-distorting manipulations. Front. Microbiol. 2021, 12, 649248. [Google Scholar] [CrossRef]
- Tarabai, H.; Floriano, A.M.; Zima, J.; Filová, N.; Brown, J.J.; Roachell, W.; Smith, R.L.; Beatty, N.L.; Vogel, K.J.; Nováková, E. Microbiomes of blood-feeding triatomines in the context of their predatory relatives and the environment. Microbiol. Spectr. 2023, 11, e0168123. [Google Scholar] [CrossRef]
- Driscoll, T.P.; Verhoeve, V.I.; Brockway, C.; Shrewsberry, D.L.; Plumer, M.; Sevdalis, S.E.; Beckmann, J.F.; Krueger, L.M.; Macaluso, K.R.; Azad, A.F.; et al. Evolution of Wolbachia mutualism and reproductive parasitism: Insight from two novel strains that co-infect cat fleas. PeerJ 2020, 8, e10646. [Google Scholar] [CrossRef]
- Weyandt, N.; Aghdam, S.A.; Brown, A.M.V. Discovery of early-branching Wolbachia reveals functional enrichment on horizontally transferred genes. Front. Microbiol. 2022, 13, 867392. [Google Scholar] [CrossRef]
- Zurita, A.; Gutiérrez, S.G.; Cutillas, C. Infection rates of Wolbachia sp. and Bartonella sp. in different populations of fleas. Curr. Microbiol. 2016, 73, 704–713. [Google Scholar] [CrossRef]
- Jones, R.T.; McCormick, K.F.; Martin, A.P. Bacterial communities of Bartonella-positive fleas: Diversity and community assembly patterns. Appl. Environ. Microbiol. 2008, 74, 1667–1670. [Google Scholar] [CrossRef]
- Beliavskaia, A.; Tan, K.K.; Sinha, A.; Husin, N.A.; Lim, F.S.; Loong, S.K.; Bell-Sakyi, L.; Carlow, C.K.S.; AbuBakar, S.; Darby, A.C.; et al. Metagenomics of culture isolates and insect tissue illuminate the evolution of Wolbachia, Rickettsia and Bartonella symbionts in Ctenocephalides spp. fleas. Microb. Genom. 2023, 9, mgen001045. [Google Scholar] [CrossRef]
- Sharma, A.K.; Som, A. Assigning new supergroups V and W to the Wolbachia diversity. Bioinformation 2023, 19, 336–340. [Google Scholar] [CrossRef]
- Huynh, L.N.; Diarra, A.Z.; Pham, Q.L.; Berenger, J.M.; Ho, V.H.; Nguyen, X.Q.; Parola, P. Identification of Vietnamese flea species and their associated microorganisms using morphological, molecular, and protein profiling. Microorganisms 2023, 11, 716. [Google Scholar] [CrossRef]
- Li, T.P.; Zhou, C.Y.; Wang, M.K.; Zha, S.S.; Chen, J.; Bing, X.L.; Hoffmann, A.A.; Hong, X.Y. Endosymbionts reduce microbiome diversity and modify host metabolism and fecundity in the planthopper Sogatella furcifera. mSystems 2022, 7, e0151621. [Google Scholar] [CrossRef]
- Flores, G.A.M.; Lopez, R.P.; Cerrudo, C.S.; Perotti, M.A.; Consolo, V.F.; Berón, C.M. Wolbachia dominance influences the Culex quinquefasciatus microbiota. Sci. Rep. 2023, 13, 18980. [Google Scholar] [CrossRef] [PubMed]
- Pornwiroon, W.; Kearney, M.T.; Husseneder, C.; Foil, L.D.; Macaluso, K.R. Comparative microbiota of Rickettsia felis-uninfected and -infected colonized cat fleas, Ctenocephalides felis. ISME J. 2007, 1, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Okaro, U.; George, S.; Anderson, B. What Is in a cat scratch? Growth of Bartonella henselae in a biofilm. Microorganisms 2021, 9, 835. [Google Scholar] [CrossRef] [PubMed]
- Battisti, J.M.; Lawyer, P.G.; Minnick, M.F. Colonization of Lutzomyia verrucarum and Lutzomyia longipalpis sand flies (Diptera: Psychodidae) by Bartonella bacilliformis, the etiologic agent of Carrión’s disease. PLoS Negl. Trop. Dis. 2015, 9, e0004128. [Google Scholar] [CrossRef]
- Minnick, M.F.; Robinson, A.J.; Powell, R.D.; Rowland, T.E. Experimental colonization of sand flies (Lutzomyia longipalpis; Diptera: Psychodidae) by Bartonella ancashensis. Vector-Borne Zoonotic Dis. 2023, 23, 324–330. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Jubeh, B.; Karaman, R. Resistance of Gram-negative bacteria to current antibacterial agents and approaches to resolve it. Molecules 2020, 25, 1340. [Google Scholar] [CrossRef]
- Kelly, P.H.; Bahr, S.M.; Serafim, T.D.; Ajami, N.J.; Petrosino, J.F.; Meneses, C.; Kirby, J.R.; Valenzuela, J.G.; Kamhawi, S.; Wilson, M.E. The gut microbiome of the vector Lutzomyia longipalpis is essential for survival of Leishmania infantum. mBio 2017, 8, e01121-16. [Google Scholar] [CrossRef]
- Dubyanskiy, V.M.; Yeszhanov, A.B. Ecology of Yersinia pestis and the epidemiology of plague. Adv. Exp. Med. Biol. 2016, 918, 101–170. [Google Scholar] [CrossRef]
- Burroughs, A.L. Sylvatic plague studies: The vector efficiency of nine species of fleas compared with Xenopsylla cheopis. J. Hyg. 1947, 45, 371–396. [Google Scholar] [CrossRef]
- Miarinjara, A.; Eads, D.A.; Bland, D.M.; Matchett, M.R.; Biggins, D.E. Reevaluation of the role of blocked Oropsylla hirsuta prairie dog fleas (Siphonaptera: Ceratophyllidae) in Yersinia pestis (Enterobacterales: Enterobacteriaceae) transmission. J. Med. Entomol. 2022, 59, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Boyer, S.; Gillespie, T.R.; Miarinjara, A. Xenopsylla cheopis (rat flea). Trends Parasitol. 2022, 38, 607–608. [Google Scholar] [CrossRef] [PubMed]
- Eisen, R.J.; Borchert, J.N.; Holmes, J.L.; Amatre, G.; Van Wyk, K. Early-phase transmission of Yersinia pestis by cat fleas (Ctenocephalides felis) and their potential role as vectors in a plague-endemic region of Uganda. Am. J. Trop. Med. Hyg. 2008, 78, 949–956. [Google Scholar] [CrossRef]
- Ratovonjato, J.; Rajerison, M.; Rahelinirina, S.; Boyer, S. Yersinia pestis in Pulex irritans fleas during plague outbreak, Madagascar. Emerg. Infect. Dis. 2014, 20, 1414–1415. [Google Scholar] [CrossRef]
- Eisen, R.J.; Dennis, D.T.; Gage, K.L. The role of early-phase transmission in the spread of Yersinia pestis. J. Med. Entomol. 2015, 52, 1183–1192. [Google Scholar] [CrossRef]
- Bland, D.M.; Long, D.; Rosenke, R.; Hinnebusch, B.J. Yersinia pestis can infect the Pawlowsky glands of human body lice and be transmitted by louse bite. PLoS Biol. 2024, 22, e3002625. [Google Scholar] [CrossRef]
- Dean, K.R.; Krauer, F.; Walløe, L.; Lingjærde, O.C.; Bramanti, B.; Stenseth, N.C.; Schmid, B.V. Human ectoparasites and the spread of plague in Europe during the Second Pandemic. Proc. Natl. Acad. Sci. USA 2018, 115, 1304–1309. [Google Scholar] [CrossRef]
- Ayyadurai, S.; Sebbane, F.; Raoult, D.; Drancourt, M. Body lice, Yersinia pestis orientalis, and Black Death. Emerg. Infect. Dis. 2010, 16, 892–893. [Google Scholar] [CrossRef]
- Schaub, G.A.; Mehlhorn, H. Lice. In Encyclopedia of Parasitology, 4th ed.; Mehlhorn, H., Ed.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1472–1479. [Google Scholar]
- Barbieri, R.; Drancourt, M.; Raoult, D. The role of louse-transmitted diseases in historical plague pandemics. Lancet Infect. Dis. 2021, 21, e17–e25. [Google Scholar] [CrossRef] [PubMed]
- Hinnebusch, B.J.; Perry, R.D.; Schwan, T.G. Role of the Yersinia pestis hemin storage (hms) locus in the transmission of plague by fleas. Science 1996, 273, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Chouikha, I.; Sturdevant, D.E.; Jarrett, C.; Sun, Y.C.; Hinnebusch, B.J. Differential gene expression patterns of Yersinia pestis and Yersinia pseudotuberculosis during infection and biofilm formation in the flea digestive tract. mSystems 2019, 4, e00217-18. [Google Scholar] [CrossRef] [PubMed]
- Vadyvaloo, V.; Jarrett, C.; Sturdevant, D.E.; Sebbane, F.; Hinnebusch, B.J. Transit through the flea vector induces a pretransmission innate immunity resistance phenotype in Yersinia pestis. PLoS Pathog. 2010, 6, e1000783. [Google Scholar] [CrossRef]
- Solano, C.; Echeverz, M.; Lasa, I. Biofilm dispersion and quorum sensing. Curr. Opin. Microbiol. 2014, 18, 96–104. [Google Scholar] [CrossRef]
- Kirwan, J.P.; Gould, T.A.; Schweizer, H.P.; Bearden, S.W.; Murphy, R.C.; Churchill, M.E.A. Quorum-sensing signal synthesis by the Yersinia pestis acyl homoserine lactonesynthase YspI. J. Bacteriol. 2006, 188, 784–788. [Google Scholar] [CrossRef]
- Jarrett, C.O.; Leung, J.M.; Motoshi, S.; Sturdevant, D.E.; Zhang, Y.; Hoyt, F.H.; Hinnebusch, B.J. Role of the Yersinia pestis phospholipase D (Ymt) in the initial aggregation step of biofilm formation in the flea. mBio 2024, 15, e0012424. [Google Scholar] [CrossRef]
- Engelthaler, D.M.; Hinnebusch, B.J.; Rittner, C.M.; Gage, K.L. Quantitative competitive PCR as a technique for exploring flea-Yersina pestis dynamics. Am. J. Trop. Med. Hyg. 2000, 62, 552–560. [Google Scholar] [CrossRef]
- Butler, T.; Levin, J.; Linh, N.N.; Chau, D.M.; Adickman, M.; Arnold, K. Yersinia pestis infection in Vietnam. II. Quantitative blood cultures and detection of endotoxin in the cerebrospinal fluid of patients with meningitis. J. Infect. Dis. 1976, 133, 493–499. [Google Scholar] [CrossRef]
- Sebbane, F.; Gardner, D.; Long, D.; Gowen, B.B.; Hinnebusch, B.J. Kinetics of disease progression and host response in a rat model of bubonic plague. Am. J. Pathol. 2005, 166, 1427–1439. [Google Scholar] [CrossRef]
- Wu, G.; Zhou, Y.; Cao, S.; Wu, Y.; Wang, T.; Zhang, Y.; Wang, X.; Song, Y.; Yang, R.; Du, Z. Unveiling the dance of evolution: Pla-mediated cleavage of Ymt modulates the virulence dynamics of Yersinia pestis. mBio 2024, 15, e0107524. [Google Scholar] [CrossRef]
- Ames, C.T.; Quan, S.F.; Ryckman, R.E. Triatominae in experimental transmission of plague. Am. J. Trop. Med. Hyg. 1954, 3, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Bosio, C.F.; Viall, A.K.; Jarrett, C.O.; Gardner, D.; Rood, M.P.; Hinnebusch, B.J. Evaluation of the murine immune response to Xenopsylla cheopis flea saliva and its effect on transmission of Yersinia pestis. PLoS Negl. Trop. Dis. 2014, 8, e3196. [Google Scholar] [CrossRef] [PubMed]
- Hinnebusch, B.J. Biofilm-dependent and biofilm-independent mechanisms of transmission of Yersinia pestis by fleas. Adv. Exp. Med. Biol. 2012, 954, 237–243. [Google Scholar] [CrossRef]
- Bacot, A.W.; Martin, C.J. Observations on the mechanism of the transmission of plague by fleas. J. Hyg. 1914, 14, 423–429. [Google Scholar]
- Schaub, G.A. Parasitogenic alterations of vector behaviour. Int. J. Med. Microbiol. 2006, 296 (Suppl. S1), 37–40. [Google Scholar] [CrossRef]
- Dostálová, A.; Volf, P. Leishmania development in sand flies: Parasite-vector interactions overview. Parasites Vectors 2012, 5, 276. [Google Scholar] [CrossRef]
- Yan, J.; Bassler, B.L. Surviving as a community: Antibiotic tolerance and persistence in bacterial biofilms. Cell Host Microbe 2019, 26, 15–21. [Google Scholar] [CrossRef]
- Sauer, K.; Stoodley, P.; Goeres, D.M.; Hall-Stoodley, L.; Burmølle, M.; Stewart, P.S.; Bjarnsholt, T. The biofilm life cycle: Expanding the conceptual model of biofilm formation. Nat. Rev. Microbiol. 2022, 20, 608–620. [Google Scholar] [CrossRef]
- Alhede, M.; Kragh, K.N.; Qvortrup, K.; Allesen-Holm, M.; van Gennip, M.; Christensen, L.D.; Jensen, P.Ø.; Nielsen, A.K.; Parsek, M.; Wozniak, D.; et al. Phenotypes of non-attached Pseudomonas aeruginosa aggregates resemble surface attached biofilm. PLoS ONE 2011, 6, e27943. [Google Scholar] [CrossRef] [PubMed]
- Melaugh, G.; Martinez, V.A.; Baker, P.; Hill, P.J.; Howell, P.L.; Wozniak, D.J.; Allen, R.J. Distinct types of multicellular aggregates in Pseudomonas aeruginosa liquid cultures. npj Biofilms Microbiomes 2023, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, X.; Liu, H.; Zhang, L.; Guo, Y.; Yu, S.; Wozniak, D.J.; Ma, L.Z. The exopolysaccharide Psl-eDNA interaction enables the formation of a biofilm skeleton in Pseudomonas aeruginosa. Environ. Microbiol. Rep. 2015, 7, 330–340. [Google Scholar] [CrossRef]
- Armbruster, C.R.; Lee, C.K.; Parker-Gilham, J.; de Anda, J.; Xia, A.; Zhao, K.; Murakami, K.; Tseng, B.S.; Hoffman, L.R.; Jin, F.; et al. Heterogeneity in surface sensing suggests a division of labor in Pseudomonas aeruginosa populations. eLife 2019, 8, e45084, Erratum in eLife 2020, 9, e59154. https://doi.org/10.7554/eLife.59154. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, M.; Kaminska, D.; Dlugaszewska, J.; Gajecka, M. Antibiotic resistance, biofilm formation, and presence of genes encoding virulence factors in strains isolated from the pharmaceutical production environment. Pathogens 2021, 10, 130. [Google Scholar] [CrossRef]
- Valentini, M.; Filloux, A. Biofilms and cyclic di-GMP (c-di-GMP) signaling: Lessons from Pseudomonas aeruginosa and other bacteria. J. Biol. Chem. 2016, 291, 12547–12555. [Google Scholar] [CrossRef]
- Tam, C.; Demke, O.; Hermanas, T.; Mitchell, A.; Hendrickx, A.P.; Schneewind, O. YfbA, a Yersinia pestis regulator required for colonization and biofilm formation in the gut of cat fleas. J. Bacteriol. 2014, 196, 1165–1173. [Google Scholar] [CrossRef]
- Bobrov, A.G.; Kirillina, O.; Ryjenkov, D.A.; Waters, C.M.; Price, P.A.; Fetherston, J.D.; Mack, D.; Goldman, W.E.; Gomelsky, M.; Perry, R.D. Systematic analysis of cyclic di-GMP signalling enzymes and their role in biofilm formation and virulence in Yersinia pestis. Mol. Microbiol. 2011, 79, 533–551. [Google Scholar] [CrossRef]
- Erickson, D.L.; Jarrett, C.O.; Wren, B.W.; Hinnebusch, B.J. Serotype differences and lack of biofilm formation characterize Yersinia pseudotuberculosis infection of the Xenopsylla cheopis flea vector of Yersinia pestis. J. Bacteriol. 2006, 188, 1113–1119. [Google Scholar] [CrossRef]
- Randall, T.E.; Eckartt, K.; Kakumanu, S.; Price-Whelan, A.; Dietrich, L.E.P.; Harrison, J.J. Sensory perception in bacterial cyclic diguanylate signal transduction. J. Bacteriol. 2022, 204, e0043321. [Google Scholar] [CrossRef]
- Katharios-Lanwermeyer, S.; Koval, S.A.; Barrack, K.E.; O’Toole, G.A. The diguanylate cyclase YfiN of Pseudomonas aeruginosa regulates biofilm maintenance in response to peroxide. J. Bacteriol. 2022, 204, e0039621. [Google Scholar] [CrossRef]
- Sun, Y.C.; Koumoutsi, A.; Jarrett, C.; Lawrence, K.; Gherardini, F.C. Differential control of Yersinia pestis biofilm formation in vitro and in the flea vector by two c-di-GMP diguanylate cyclases. PLoS ONE 2011, 6, e19267. [Google Scholar] [CrossRef]
- Zhao, J.; Sun, Y. Regulation of c-di-GMP metabolism and biofilm formation in Yersinia pestis. Sheng Wu Gong Cheng Xue Bao 2017, 33, 1513–1524, (In Chinese with English Summary). [Google Scholar] [CrossRef] [PubMed]
- Ren, G.X.; Yan, H.Q.; Zhu, H.; Guo, X.P.; Sun, Y.C. HmsC, a periplasmic protein, controls biofilm formation via repression of HmsD, a diguanylate cyclase in Yersinia pestis. Environ. Microbiol. 2014, 16, 1202–1216. [Google Scholar] [CrossRef] [PubMed]
- Bobrov, A.G.; Kirillina, O.; Vadyvaloo, V.; Koestler, B.J.; Hinz, A.K.; Mack, D.; Waters, C.M.; Perry, R.D. The Yersinia pestis HmsCDE regulatory system is essential for blockage of the oriental rat flea (Xenopsylla cheopis), a classic plague vector. Environ. Microbiol. 2015, 17, 947–959. [Google Scholar] [CrossRef] [PubMed]
- Silva-Rohwer, A.R.; Held, K.; Yakhnin, H.; Babitzke, P.; Vadyvaloo, V. CsrA-mediated translational activation of the hmsE mRNA enhances HmsD-dependent c-di-GMP-enabled biofilm production in Yersinia pestis. J. Bacteriol. 2023, 205, e0010523. [Google Scholar] [CrossRef] [PubMed]
- Bellows, L.E.; Koestler, B.J.; Karaba, S.M.; Waters, C.M.; Lathem, W.W. Hfq-dependent, co-ordinate control of cyclic diguanylate synthesis and catabolism in the plague pathogen Yersinia pestis. Mol. Microbiol. 2012, 86, 661–674. [Google Scholar] [CrossRef]
- Rempe, K.A.; Hinz, A.K.; Vadyvaloo, V. Hfq regulates biofilm gut blockage that facilitates flea-borne transmission of Yersinia pestis. J. Bacteriol. 2012, 194, 2036–2040. [Google Scholar] [CrossRef]
- Silva-Rohwer, A.R.; Held, K.; Sagawa, J.; Fernandez, N.L.; Waters, C.M.; Vadyvaloo, V. CsrA enhances cyclic-di-GMP biosynthesis and Yersinia pestis biofilm blockage of the flea foregut by alleviating Hfq-dependent repression of the hmsT mRNA. mBio 2021, 12, e0135821. [Google Scholar] [CrossRef]
- Liu, L.; Liu, W.; He, Y.; Liu, Y.; Wu, H.; Zhang, Y.; Zhang, Q. Transcriptional regulation of hmsB, a temperature-dependent small RNA, by RovM in Yersinia pestis biovar microtus. Curr. Microbiol. 2023, 80, 182. [Google Scholar] [CrossRef]
- Fang, H.; Liu, L.; Zhang, Y.; Yang, H.; Yan, Y.; Ding, X.; Han, Y.; Zhou, D.; Yang, R. BfvR, an AraC-family regulator, controls biofilm formation and pH6 antigen production in opposite ways in Yersinia pestis biovar Microtus. Front. Cell. Infect. Microbiol. 2018, 8, 347. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Gao, X.; Wang, H.; Fang, H.; Yan, Y.; Liu, L.; Chen, R.; Zhou, D.; Yang, R.; Han, Y. Plasmid pPCP1-derived sRNA HmsA promotes biofilm formation of Yersinia pestis. BMC Microbiol. 2016, 16, 176. [Google Scholar] [CrossRef] [PubMed]
- Bontemps-Gallo, S.; Fernandez, M.; Dewitte, A.; Raphaël, E.; Gherardini, F.C.; Elizabeth, P.; Koch, L.; Biot, F.; Reboul, A.; Sebbane, F. Nutrient depletion may trigger the Yersinia pestis OmpR-EnvZ regulatory system to promote flea-borne plague transmission. Mol. Microbiol. 2019, 112, 1471–1482. [Google Scholar] [CrossRef] [PubMed]
- Vadyvaloo, V.; Viall, A.K.; Jarrett, C.O.; Hinz, A.K.; Sturdevant, D.E.; Hinnebusch, B.J. Role of the PhoP-PhoQ gene regulatory system in adaptation of Yersinia pestis to environmental stress in the flea digestive tract. Microbiology 2015, 161, 1198–1210. [Google Scholar] [CrossRef]
- Liu, L.; Fang, H.; Yang, H.; Zhang, Y.; Han, Y.; Zhou, D.; Yang, R. Reciprocal regulation of Yersinia pestis biofilm formation and virulence by RovM and RovA. Open Biol. 2016, 6, 150198. [Google Scholar] [CrossRef]
- Vadyvaloo, V.; Hinz, A.K. A LysR-type transcriptional regulator, RovM, senses nutritional cues suggesting that it is involved in metabolic adaptation of Yersinia pestis to the flea gut. PLoS ONE 2015, 10, e0137508. [Google Scholar] [CrossRef]
- Heroven, A.K.; Dersch, P. RovM, a novel LysR-type regulator of the virulence activator gene rovA, controls cell invasion, virulence and motility of Yersinia pseudotuberculosis. Mol. Microbiol. 2006, 62, 1469–1483. [Google Scholar] [CrossRef]
- Zhao, R.; Song, Y.; Dai, Q.; Kang, Y.; Pan, J.; Zhu, L.; Zhang, L.; Wang, Y.; Shen, X. A starvation-induced regulator, RovM, acts as a switch for planktonic/biofilm state transition in Yersinia pseudotuberculosis. Sci. Rep. 2017, 7, 639. [Google Scholar] [CrossRef]
- Ren, G.X.; Fan, S.; Guo, X.P.; Chen, S.; Sun, Y.C. Differential regulation of c-di-GMP metabolic enzymes by environmental signals modulates biofilm formation in Yersinia pestis. Front. Microbiol. 2016, 7, 821. [Google Scholar] [CrossRef]
- Ren, G.X.; Guo, X.P.; Sun, Y.C. HmsC controls Yersinia pestis biofilm formation in response to redox environment. Front. Cell Infect. Microbiol. 2017, 7, 355, Erratum in Front. Cell Infect. Microbiol. 2018, 7, 525. https://doi.org/10.3389/fcimb.2017.00525. [Google Scholar] [CrossRef]
- Eisen, R.J.; Wilder, A.P.; Bearden, S.W.; Montenieri, J.A.; Gage, K.L. Early-phase transmission of Yersinia pestis by unblocked Xenopsylla cheopis (Siphonaptera: Pulicidae) is as efficient as transmission by blocked fleas. J. Med. Entomol. 2007, 44, 678–682. [Google Scholar] [CrossRef]
- Wilder, A.P.; Eisen, R.J.; Bearden, S.W.; Montenieri, J.A.; Tripp, D.W.; Brinkerhoff, R.J.; Gage, K.L.; Antolin, M.F. Transmission efficiency of two flea species (Oropsylla tuberculata cynomuris and Oropsylla hirsuta) involved in plague epizootics among prairie dogs. EcoHealth 2008, 5, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Schotthoefer, A.M.; Bearden, S.W.; Holmes, J.L.; Vetter, S.M.; Montenieri, J.A. Effects of temperature on the transmission of Yersinia pestis by the flea, Xenopsylla cheopis, in the late phase period. Parasites Vectors 2011, 4, 191. [Google Scholar] [CrossRef] [PubMed]
- Schotthoefer, A.M.; Bearden, S.W.; Vetter, S.M.; Holmes, J.; Montenieri, J.A. Effects of temperature on early-phase transmission of Yersinia pestis by the flea, Xenopsylla cheopis. J. Med. Entomol. 2011, 48, 411–417. [Google Scholar] [CrossRef][Green Version]
- Eisen, R.J.; Lowell, J.L.; Montenieri, J.A.; Bearden, S.W.; Gage, K.L. Temporal dynamics of early-phase transmission of Yersinia pestis by unblocked fleas: Secondary infectious feeds prolong efficient transmission by Oropsylla montana (Siphonaptera: Ceratophyllidae). J. Med. Entomol. 2007, 44, 672–677. [Google Scholar] [CrossRef]
- Wilder, A.P.; Eisen, R.J.; Bearden, S.W.; Montenieri, J.A.; Gage, K.L.; Antolin, M.F. Oropsylla hirsuta (Siphonaptera: Ceratophyllidae) can support plague epizootics in black-tailed prairie dogs (Cynomys ludovicianus) by early-phase transmission of Yersinia pestis. Vector-Borne Zoonotic Dis. 2008, 8, 359–367. [Google Scholar] [CrossRef]
- Eisen, R.J.; Vetter, S.M.; Holmes, J.L.; Bearden, S.W.; Montenieri, J.A.; Gage, K.L. Source of host blood affects prevalence of infection and bacterial loads of Yersinia pestis in fleas. J. Med. Entomol. 2008, 45, 933–938. [Google Scholar] [CrossRef]
- Vetter, S.M.; Eisen, R.J.; Schotthoefer, A.M.; Montenieri, J.A.; Holmes, J.L.; Bobrov, A.G.; Bearden, S.W.; Perry, R.D.; Gage, K.L. Biofilm formation is not required for early-phase transmission of Yersinia pestis. Microbiology 2010, 56 Pt 7, 2216–2225. [Google Scholar] [CrossRef]
- Boegler, K.A.; Graham, C.B.; Johnson, T.L.; Montenieri, J.A.; Eisen, R.J. Infection prevalence, bacterial loads, and transmission efficiency in Oropsylla montana (Siphonaptera: Ceratophyllidae) one day after exposure to varying concentrations of Yersinia pestis in blood. J. Med. Entomol. 2016, 53, 674–680. [Google Scholar] [CrossRef]
- Zhang, Y.; Dai, X.; Wang, Q.; Chen, H.; Meng, W.; Wu, K.; Luo, T.; Wang, X.; Rehemu, A.; Guo, R.; et al. Transmission efficiency of the plague pathogen (Y. pestis) by the flea, Xenopsylla skrjabini, to mice and great gerbils. Parasites Vectors 2015, 8, 256. [Google Scholar] [CrossRef]
- Bouvenot, T.; Dewitte, A.; Bennaceur, N.; Pradel, E.; Pierre, F.; Bontemps-Gallo, S.; Sebbane, F. Interplay between Yersinia pestis and its flea vector in lipoate metabolism. ISME J. 2021, 15, 1136–1149. [Google Scholar] [CrossRef]
- Hinnebusch, B.J.; Gage, K.L.; Schwan, T.G. Estimation of vector infectivity rates for plague by means of a standard curve-based competitive polymerase chain reaction method to quantify Yersinia pestis in fleas. Am. J. Trop. Med. Hyg. 1998, 58, 562–569. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dreher-Lesnick, S.M.; Ceraul, S.M.; Lesnick, S.C.; Gillespie, J.J.; Anderson, J.M.; Jochim, R.C.; Valenzuela, J.G.; Azad, A.F. Analysis of Rickettsia typhi-infected and uninfected cat flea (Ctenocephalides felis) midgut cDNA libraries: Deciphering molecular pathways involved in host response to R. typhi infection. Insect Mol. Biol. 2010, 19, 229–241. [Google Scholar] [CrossRef]
- Zhou, W.; Russell, C.W.; Johnson, K.L.; Mortensen, R.D.; Erickson, D.L. Gene expression analysis of Xenopsylla cheopis (Siphonaptera: Pulicidae) suggests a role for reactive oxygen species in response to Yersinia pestis infection. J. Med. Entomol. 2012, 49, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Z.; Huang, X.; Yin, Y. Beyond immunity: The Imd pathway as a coordinator of host defense, organismal physiology and behavior. Dev. Comp. Immunol. 2018, 83, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Aoyagi, K.L.; Brooks, B.D.; Bearden, S.W.; Montenieri, J.A.; Gage, K.L.; Fisher, M.A. LPS promotes maintenance of Yersinia pestis in fleas. Microbiology 2015, 161, 628–638. [Google Scholar] [CrossRef]
- Fukuto, H.S.; Vadyvaloo, V.; McPhee, J.B.; Poinar, H.N.; Holmes, E.C.; Bliska, J.B. A single amino acid change in the response regulator PhoP, acquired during Yersinia pestis evolution, affects PhoP target gene transcription and polymyxin B susceptibility. J. Bacteriol. 2018, 200, e00050-18. [Google Scholar] [CrossRef]
- Mathew, B.; Aoyagi, K.L.; Fisher, M.A. Yersinia pestis lipopolysaccharide remodeling confers resistance to a Xenopsylla cheopis cecropin. ACS Infect. Dis. 2021, 7, 2536–2545. [Google Scholar] [CrossRef]
- Brown, L.D.; Maness, R.; Hall, C.; Gibson, J.D. Reactive oxygen species-mediated immunity against bacterial infection in the gut of cat fleas (Ctenocephalides felis). Insect Biochem. Mol. Biol. 2021, 136, 103620. [Google Scholar] [CrossRef]
- Coutinho-Abreu, I.V.; Serafim, T.D.; Meneses, C.; Kamhawi, S.; Oliveira, F.; Valenzuela, J.G. Leishmania infection induces a limited differential gene expression in the sand fly midgut. BMC Genom. 2020, 21, 608. [Google Scholar] [CrossRef]
- Geiger, A.; Hamidou Soumana, I.; Tchicaya, B.; Rofidal, V.; Decourcelle, M.; Santoni, V.; Hem, S. Differential expression of midgut proteins in Trypanosoma brucei gambiense-stimulated vs. non-stimulated Glossina palpalis gambiensis flies. Front. Microbiol. 2015, 6, 444. [Google Scholar] [CrossRef] [PubMed]
- Schaub, G.A. Auswirkungen von Parasiten auf das Verhalten ihrer Wirte. Biol. Unserer Zeit 1989, 19, 196–202. (In German) [Google Scholar] [CrossRef]
- Schaub, G.A. Auswirkungen der Parasiten auf ihre Vektoren. Nova Acta Leopold. NF 1996, 71, 115–126. (In German) [Google Scholar]
- Bibikova, V.A. Contemporary views on the interrelationships between fleas and the pathogens of human and animal diseases. Annu. Rev. Entomol. 1977, 22, 23–32. [Google Scholar] [CrossRef]
- Wade, S.E.; Georgi, J.R. Survival and reproduction of artificially fed cat fleas, Ctenocephalides felis Bouché (Siphonaptera: Pulicidae). J. Med. Entomol. 1988, 25, 186–190. [Google Scholar] [CrossRef]
- Kyme, P.; Dillon, B.; Iredell, J. Phase variation in Bartonella henselae. Microbiology 2003, 149, 621–629. [Google Scholar] [CrossRef][Green Version]
- Duangurai, T.; Reamtong, O.; Rungruengkitkun, A.; Srinon, V.; Boonyuen, U.; Limmathurotsakul, D.; Chantratita, N.; Pumirat, P. In vitro passage alters virulence, immune activation and proteomic profiles of Burkholderia pseudomallei. Sci. Rep. 2020, 10, 8320. [Google Scholar] [CrossRef]
- Vogel, P.U.B.; Rebeski, D.E. Die Großen Tierseuchen; Springer Spektrum: Berlin/Heidelberg, Germany, 2023. (In German) [Google Scholar] [CrossRef]
- Rosenberg, R.; Beard, C.B. Vector-borne infections. Emerg. Infect. Dis. 2011, 17, 769–770. [Google Scholar] [CrossRef]
- Jones, S.D.; Atshabar, B.; Schmid, B.V.; Zuk, M.; Amramina, A.; Stenseth, N.C. Living with plague: Lessons from the Soviet Union’s antiplague system. Proc. Natl. Acad. Sci. USA 2019, 116, 9155–9163. [Google Scholar] [CrossRef]
- McLean, B.S.; Cook, J.A.; Durden, L.A.; Hoberg, E.P.; Guralnick, R.P. The next chapter of human-plague science. Proc. Natl. Acad. Sci. USA 2019, 116, 14411–14412. [Google Scholar] [CrossRef]
- Wells, L.E.; Elston, D.M. What’s eating you? Oriental rat flea (Xenopsylla cheopis). Cutis 2020, 106, 124–126. [Google Scholar] [CrossRef]
- Holcomb, K.M.; Biggerstaff, B.J.; Johansson, M.A.; Mead, P.S.; Kugeler, K.J.; Eisen, R.J. Revisiting the relationship between weather and interannual variation in human plague cases in the Southwestern United States. Am. J. Trop. Med. Hyg. 2025, 112, 840–844. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vogel, P.U.B.; Schaub, G.A. Interaction of Bacteria and Fleas, Focusing on the Plague Bacterium—A Review. Microorganisms 2025, 13, 2619. https://doi.org/10.3390/microorganisms13112619
Vogel PUB, Schaub GA. Interaction of Bacteria and Fleas, Focusing on the Plague Bacterium—A Review. Microorganisms. 2025; 13(11):2619. https://doi.org/10.3390/microorganisms13112619
Chicago/Turabian StyleVogel, Patric U. B., and Günter A. Schaub. 2025. "Interaction of Bacteria and Fleas, Focusing on the Plague Bacterium—A Review" Microorganisms 13, no. 11: 2619. https://doi.org/10.3390/microorganisms13112619
APA StyleVogel, P. U. B., & Schaub, G. A. (2025). Interaction of Bacteria and Fleas, Focusing on the Plague Bacterium—A Review. Microorganisms, 13(11), 2619. https://doi.org/10.3390/microorganisms13112619





