One Health, Many Gaps: Rethinking Epidemic Intelligence in Resource-Limited Settings to Prepare for the Global Threat of Disease X
Abstract
1. Introduction
2. Materials and Methods
3. Surveillance Systems for WHO Priority Diseases: Current Landscape in Resource-Limited Settings
3.1. Fragmented Health Information Systems
3.2. Inadequate Laboratory and Diagnostic Capacity
3.3. Workforce and Infrastructure Constraints
3.4. Underutilization of Community-Based Surveillance
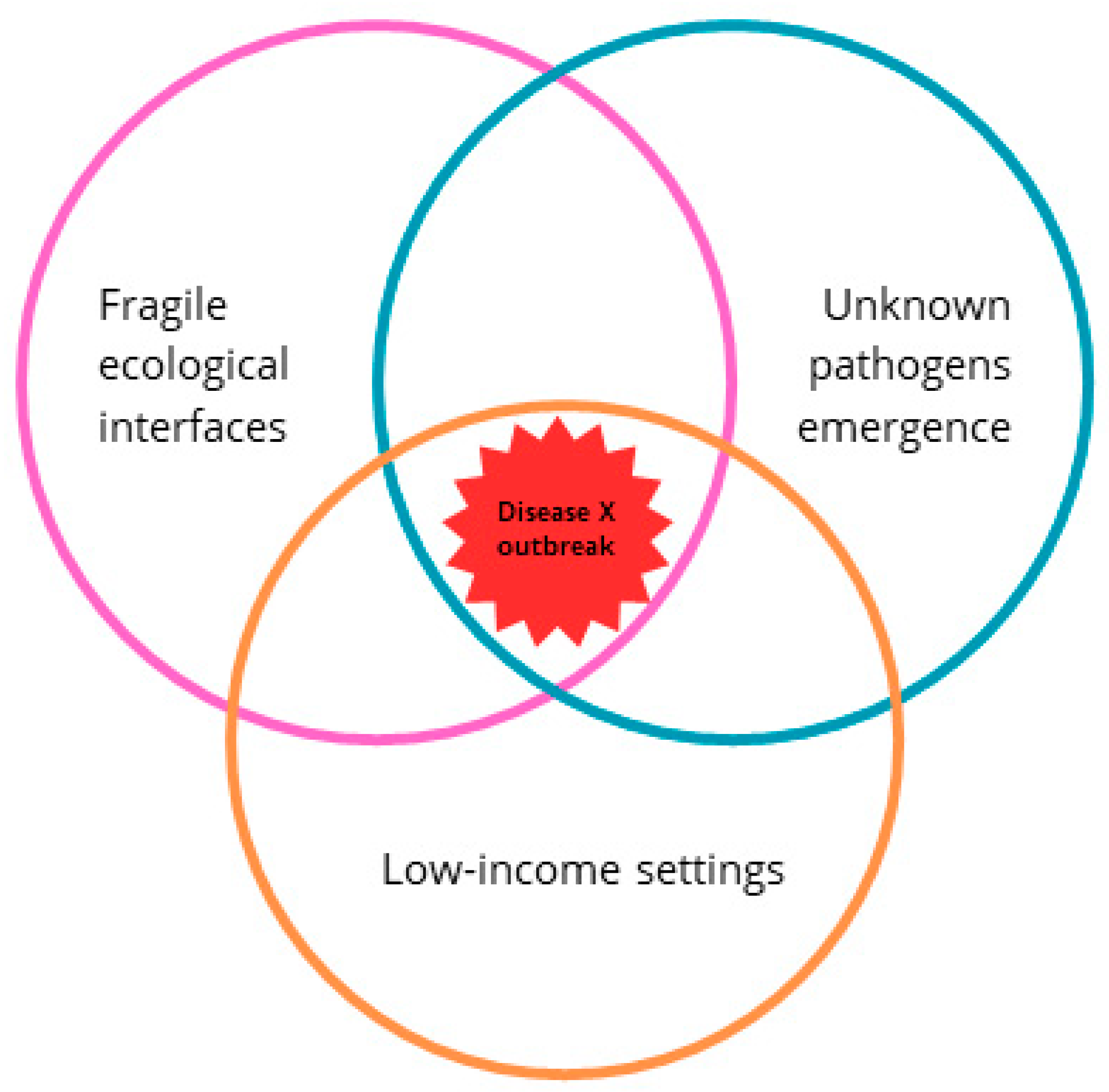
4. Implications for Future Emerging Threats: Disease X
4.1. LMICs as Potential Epicenters of Disease X
4.2. Peripheral Surveillance: The First Line of Defense
4.3. Genomic Surveillance and Global Equity
4.4. One Health Blind Spots
4.5. From Detection to Action: The Political Economy of Epidemic Intelligence
4.6. Lessons from Mpox and COVID-19
5. Rethinking Surveillance: Emerging Strategies for Resource-Limited Settings
5.1. Integrated One Health Surveillance
5.2. Genomic Surveillance and Digital Platforms
5.3. Community-Based and Participatory Approaches
5.4. Regional Collaboration and Data Sharing
5.5. Leveraging Non-Traditional Data Sources
6. Recommendations and Way Forward
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Africa CDC | Africa Centers for Diseases Control and prevention |
| CBS | Community-based surveillance |
| COVID-19 | Coronavirus disease 2019 |
| EVD | Ebola virus disease |
| IDSR | Integrated disease surveillance and response |
| LMICs | Low- and middle-income countries |
| PCR | Polymerase chain reaction |
| WHO | World Health Organization |
References
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global Trends in Emerging Infectious Diseases. Nature 2008, 451, 990–993. [Google Scholar] [CrossRef] [PubMed]
- Tambo, E.; Ugwu, E.C.; Ngogang, J.Y. Need of Surveillance Response Systems to Combat Ebola Outbreaks and Other Emerging Infectious Diseases in African Countries. Infect. Dis. Poverty 2014, 3, 29. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. An R&D Blueprint for Action to Prevent Epidemics—Update 2017; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Inzaule, S.C.; Tessema, S.K.; Kebede, Y.; Ogwell Ouma, A.E.; Nkengasong, J.N. Genomic-Informed Pathogen Surveillance in Africa: Opportunities and Challenges. Lancet Infect. Dis. 2021, 21, e281–e289. [Google Scholar] [CrossRef] [PubMed]
- Kandel, N.; Chungong, S.; Omaar, A.; Xing, J. Health Security Capacities in the Context of COVID-19 Outbreak: An Analysis of International Health Regulations Annual Report Data from 182 Countries. Lancet 2020, 395, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Report of the Review Committee on the Functioning of the International Health Regulations (2005) During the COVID-19 Response; WHO: Geneva, Switzerland, 2021; 79p. [Google Scholar]
- Tabish, S.A.; Nabil, S. An Age of Emerging and Reemerging Pandemic Threats. Health 2022, 14, 1021–1037. [Google Scholar] [CrossRef]
- Lal, A.; Ashworth, H.C.; Dada, S.; Hoemeke, L.; Tambo, E. Optimizing Pandemic Preparedness and Response Through Health Information Systems: Lessons Learned From Ebola to COVID-19. Disaster Med. Public Health Prep. 2020, 16, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.M.; Ellström, P.; Järhult, J.D.; Lundkvist, Å.; Olsen, B. Towards Pandemic Preparedness beyond COVID-19. Lancet Microbe 2020, 1, e185–e186. [Google Scholar] [CrossRef] [PubMed]
- Ihekweazu, C.; Agogo, E. Africa’s Response to COVID-19. BMC Med. 2020, 18, 151. [Google Scholar] [CrossRef] [PubMed]
- Makoni, M. COVID-19 in Africa: Half a Year Later. Lancet Infect. Dis. 2020, 20, 1127. [Google Scholar] [CrossRef] [PubMed]
- de Castañeda, R.R.; Villers, J.; Guzmán, C.A.F.; Eslanloo, T.; de Paula, N.; Machalaba, C.; Zinsstag, J.; Utzinger, J.; Flahault, A.; Bolon, I. One Health and Planetary Health Research: Leveraging Differences to Grow Together. Lancet Planet. Health 2023, 7, e109–e111. [Google Scholar] [CrossRef] [PubMed]
- Ghai, R.R.; Wallace, R.M.; Kile, J.C.; Shoemaker, T.R.; Vieira, A.R.; Negron, M.E.; Shadomy, S.V.; Sinclair, J.R.; Goryoka, G.W.; Salyer, S.J.; et al. A Generalizable One Health Framework for the Control of Zoonotic Diseases. Sci. Rep. 2022, 12, 8588. [Google Scholar] [CrossRef] [PubMed]
- Mumford, E.L.; Martinez, D.J.; Tyance-Hassell, K.; Cook, A.; Hansen, G.R.; Labonté, R.; Mazet, J.A.K.; Mumford, E.C.; Rizzo, D.M.; Togami, E.; et al. Evolution and Expansion of the One Health Approach to Promote Sustainable and Resilient Health and Well-Being: A Call to Action. Front. Public Health 2023, 10, 1056459. [Google Scholar] [CrossRef] [PubMed]
- Karamagi, H.C.; Tumusiime, P.; Titi-Ofei, R.; Droti, B.; Kipruto, H.; Nabyonga-Orem, J.; Seydi, A.B.-W.; Zawaira, F.; Schmets, G.; Cabore, J.W. Towards Universal Health Coverage in the WHO African Region: Assessing Health System Functionality, Incorporating Lessons from COVID-19. BMJ Glob. Health 2021, 6, e004618. [Google Scholar] [CrossRef] [PubMed]
- Fall, I.S.; Rajatonirina, S.; Yahaya, A.A.; Zabulon, Y.; Nsubuga, P.; Nanyunja, M.; Wamala, J.; Njuguna, C.; Lukoya, C.O.; Alemu, W.; et al. Integrated Disease Surveillance and Response (IDSR) Strategy: Current Status, Challenges and Perspectives for the Future in Africa. BMJ Glob. Health 2019, 4, e001427. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.; Sridhar, D.; Pate, M.A.; Jha, A.K.; Clinton, C.; Delaunay, S.; Edwin, V.; Fallah, M.; Fidler, D.P.; Garrett, L.; et al. Will Ebola Change the Game? Ten Essential Reforms before the next Pandemic. The Report of the Harvard-LSHTM Independent Panel on the Global Response to Ebola. Lancet 2015, 386, 2204–2221. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, E.; Giovanetti, M.; Tegally, H.; San, J.E.; Lessells, R.; Cuadros, D.; Martin, D.P.; Rasmussen, D.A.; Zekri, A.-R.N.; Sangare, A.K.; et al. A Year of Genomic Surveillance Reveals How the SARS-CoV-2 Pandemic Unfolded in Africa. Science 2021, 374, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Africa CDC Africa Pathogen Genomics Initiative|Africa PGI 2.0. Africa CDC 2022. Available online: https://africacdc.org/africa-pathogen-genomics-initiative-africa-pgi/ (accessed on 22 April 2025).
- Institut Pasteur. AFROSCREEN: A Key Network for Genomic Surveillance in Sub-Saharan Africa. Available online: https://www.pasteur.fr/en/press-area/press-documents/afroscreen-key-network-genomic-surveillance-sub-saharan-africa (accessed on 22 April 2025).
- Abimbola, S. The Foreign Gaze: Authorship in Academic Global Health. BMJ Glob. Health 2019, 4, e002068. [Google Scholar] [CrossRef] [PubMed]
- Kieny, M.-P.; Evans, D.B.; Schmets, G.; Kadandale, S. Health-System Resilience: Reflections on the Ebola Crisis in Western Africa. Bull. World Health Organ. 2014, 92, 850. [Google Scholar] [CrossRef] [PubMed]
- Debie, A.; Khatri, R.B.; Assefa, Y. Successes and Challenges of Health Systems Governance towards Universal Health Coverage and Global Health Security: A Narrative Review and Synthesis of the Literature. Health Res. Policy Syst. 2022, 20, 50. [Google Scholar] [CrossRef] [PubMed]
- Ratnayake, R.; Crowe, S.J.; Jasperse, J.; Privette, G.; Stone, E.; Miller, L.; Hertz, D.; Fu, C.; Maenner, M.J.; Jambai, A.; et al. Assessment of Community Event–Based Surveillance for Ebola Virus Disease, Sierra Leone, 2015. Emerg. Infect. Dis. 2016, 22, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- Maazou, A.A.; Oumarou, B.; Bienvenu, B.; Anya, B.-P.M.; Didier, T.; Ishagh, E.K.; Nsiari-muzeyi, B.J.; Katoto, P.; Wiysonge, C.S. Community-Based Surveillance Contribution to the Response of COVID-19 in Niger. Pan Afr. Med. J. 2021, 40, 88. Available online: https://www.panafrican-med-journal.com/content/article/40/88/full/ (accessed on 22 April 2025). [PubMed]
- World Health Organization. Pathogens Prioritization: A Scientific Framework for Epidemic and Pandemic Research Preparedness; WHO: Geneva, Switzerland, 2024; 38p. [Google Scholar]
- Allen, T.; Murray, K.A.; Zambrana-Torrelio, C.; Morse, S.S.; Rondinini, C.; Di Marco, M.; Breit, N.; Olival, K.J.; Daszak, P. Global Hotspots and Correlates of Emerging Zoonotic Diseases. Nat. Commun. 2017, 8, 1124. [Google Scholar] [CrossRef] [PubMed]
- Bonwitt, J.; Dawson, M.; Kandeh, M.; Ansumana, R.; Sahr, F.; Brown, H.; Kelly, A.H. Unintended Consequences of the ‘Bushmeat Ban’ in West Africa during the 2013–2016 Ebola Virus Disease Epidemic. Soc. Sci. Med. 2018, 200, 166–173. [Google Scholar] [CrossRef] [PubMed]
- UN-Habitat. World Cities Report 2020: The Value of Sustainable Urbanization; UN-Habitat: Nairobi, Kenya, 2020; 418p. [Google Scholar]
- Vega-Rodriguez, W.; Ly, H. Emergence of Deadly Viral Haemorrhagic Fever Disease Outbreaks in West Africa. Virulence 2023, 14, 2176980. [Google Scholar] [CrossRef] [PubMed]
- Adepoju, P. Challenges of SARS-CoV-2 Genomic Surveillance in Africa. Lancet Microbe 2021, 2, e139. [Google Scholar] [CrossRef] [PubMed]
- Bochner, A.F.; Makumbi, I.; Aderinola, O.; Abayneh, A.; Jetoh, R.; Yemanaberhan, R.L.; Danjuma, J.S.; Lazaro, F.T.; Mahmoud, H.J.; Yeabah, T.O.; et al. Implementation of the 7-1-7 Target for Detection, Notification, and Response to Public Health Threats in Five Countries: A Retrospective, Observational Study. Lancet Glob. Health 2023, 11, e871–e879. [Google Scholar] [CrossRef] [PubMed]
- GISAID. Global Sequencing Submissions by Region. 2012. Available online: https://gisaid.org/urls/ (accessed on 21 March 2025).
- Fidler, D.P. Influenza Virus Samples, International Law, and Global Health Diplomacy. Emerg. Infect. Dis. 2008, 14, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, E.P.J. The Evolution of One Health: A Decade of Progress and Challenges for the Future. Vet. Rec. 2014, 174, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Carlson, C.J.; Albery, G.F.; Merow, C.; Trisos, C.H.; Zipfel, C.M.; Eskew, E.A.; Olival, K.J.; Ross, N.; Bansal, S. Climate Change Increases Cross-Species Viral Transmission Risk. Nature 2022, 607, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Heymann, D.L.; Rodier, G. Global Surveillance, National Surveillance, and SARS. Emerg. Infect. Dis. 2004, 10, 173–175. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, S.; Govindarajulu, M.; Parise, R.S.; Neel, L.; Shankar, T.; Patel, S.; Lowery, P.; Smith, F.; Dhanasekaran, M.; Moore, T. Emerging SARS-CoV-2 Variants: A Review of Its Mutations, Its Implications and Vaccine Efficacy. Vaccines 2021, 9, 1195. [Google Scholar] [CrossRef] [PubMed]
- Kelly, T.R.; Karesh, W.B.; Johnson, C.K.; Gilardi, K.V.K.; Anthony, S.J.; Goldstein, T.; Olson, S.H.; Machalaba, C.; Mazet, J.A.K. One Health Proof of Concept: Bringing a Transdisciplinary Approach to Surveillance for Zoonotic Viruses at the Human-Wild Animal Interface. Prev. Vet. Med. 2017, 137, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Falzon, L.C.; Alumasa, L.; Amanya, F.; Kang’ethe, E.; Kariuki, S.; Momanyi, K.; Muinde, P.; Murungi, M.K.; Njoroge, S.M.; Ogendo, A.; et al. One Health in Action: Operational Aspects of an Integrated Surveillance System for Zoonoses in Western Kenya. Front. Vet. Sci. 2019, 6, 252. [Google Scholar] [CrossRef] [PubMed]
- Buregyeya, E.; Atusingwize, E.; Nsamba, P.; Musoke, D.; Naigaga, I.; Kabasa, J.D.; Amuguni, H.; Bazeyo, W. Operationalizing the One Health Approach in Uganda: Challenges and Opportunities. J. Epidemiol. Glob. Health 2020, 10, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Rwego, I.B.; Babalobi, O.O.; Musotsi, P.; Nzietchueng, S.; Tiambo, C.K.; Kabasa, J.D.; Naigaga, I.; Kalema-Zikusoka, G.; Pelican, K. One Health Capacity Building in Sub-Saharan Africa. Infect. Ecol. Epidemiol. 2016, 6, 34032. [Google Scholar] [CrossRef] [PubMed]
- Hamzaoui, Z.; Ferjani, S.; Medini, I.; Charaa, L.; Landolsi, I.; Ben Ali, R.; Khaled, W.; Chammam, S.; Abid, S.; Kanzari, L.; et al. Genomic Surveillance of SARS-CoV-2 in North Africa: 4 Years of GISAID Data Sharing. IJID Reg. 2024, 11, 100356. [Google Scholar] [CrossRef] [PubMed]
- Kaburi, B.B.; Wyss, K.; Kenu, E.; Asiedu-Bekoe, F.; Hauri, A.M.; Laryea, D.O.; Klett-Tammen, C.J.; Leone, F.; Walter, C.; Krause, G. Facilitators and Barriers in the Implementation of a Digital Surveillance and Outbreak Response System in Ghana Before and During the COVID-19 Pandemic: Qualitative Analysis of Stakeholder Interviews. JMIR Form. Res. 2023, 7, e45715. [Google Scholar] [CrossRef] [PubMed]
- Otaigbe, I. Scaling up Artificial Intelligence to Curb Infectious Diseases in Africa. Front. Digit. Health 2022, 4, 1030427. [Google Scholar] [CrossRef] [PubMed]
- Silenou, B.C.; Nyirenda, J.L.Z.; Zaghloul, A.; Lange, B.; Doerrbecker, J.; Schenkel, K.; Krause, G. Availability and Suitability of Digital Health Tools in Africa for Pandemic Control: Scoping Review and Cluster Analysis. JMIR Public Health Surveill. 2021, 7, e30106. [Google Scholar] [CrossRef] [PubMed]
- Nsubuga, P.; Masiira, B.; Kihembo, C.; Byakika-Tusiime, J.; Ryan, C.; Nanyunja, M.; Kamadjeu, R.; Talisuna, A. Evaluation of the Ebola Virus Disease (EVD) Preparedness and Readiness Program in Uganda: 2018 to 2019. Pan Afr. Med. J. 2021, 38, 130. [Google Scholar] [CrossRef] [PubMed]
- Karimuribo, E.D.; Mutagahywa, E.; Sindato, C.; Mboera, L.; Mwabukusi, M.; Kariuki Njenga, M.; Teesdale, S.; Olsen, J.; Rweyemamu, M. A Smartphone App (AfyaData) for Innovative One Health Disease Surveillance from Community to National Levels in Africa: Intervention in Disease Surveillance. JMIR Public Health Surveill. 2017, 3, e94. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Defense Essence: Electronic Surveillance System—Early Notification Community Based Epidemics. Available online: www.med.navy.mil/Navy-and-Marine-Corps-Force-Health-Protection-Command/Preventive-Medicine/Program-and-Policy-Support/Disease-Surveillance/Essence-ESS-Early-Notification-Community-Based-Epidemics/ (accessed on 28 April 2025).
- He, J.; Guo, Z.; Yang, P.; Cao, C.; Xu, J.; Zhou, X.; Li, S. Social Insights on the Implementation of One Health in Zoonosis Prevention and Control: A Scoping Review. Infect. Dis. Poverty 2022, 11, 48. [Google Scholar] [CrossRef] [PubMed]
- Nkengasong, J.N.; Maiyegun, O.; Moeti, M. Establishing the Africa Centres for Disease Control and Prevention: Responding to Africa’s Health Threats. Lancet Glob. Health 2017, 5, e246–e247. [Google Scholar] [CrossRef] [PubMed]
- Rahman-Shepherd, A.; Evaborhene, N.A.; Berman, A.; Amaya, A.B.; Boro, E.; Dar, O.; Ho, Z.J.M.; Jung, A.-S.; Khan, M.; Mohamed-Ahmed, O.; et al. Establishing the Value of Regional Cooperation and a Critical Role for Regional Organisations in Managing Future Health Emergencies. Lancet Glob. Health 2025, 13, e585–e592. [Google Scholar] [CrossRef] [PubMed]
- Kayembe-Mulumba, B. The WHO Pandemic Agreement: An Essential Catalyst for Global Health Equity and Preparedness. Speaking of Medicine and Health 2025. Available online: https://speakingofmedicine.plos.org/2025/06/17/the-who-pandemic-agreement-an-essential-catalyst-for-global-health-equity-and-preparedness/ (accessed on 18 June 2025).
- Al Wahaibi, A.; Al Maani, A.; Alyaquobi, F.; Al Manji, A.; Al Harthy, K.; Al Rawahi, B.; Alqayoudhi, A.; Al Khalili, S.; Al-Jardani, A.; Al-Abri, S. The Impact of Mobility Restriction Strategies in the Control of the COVID-19 Pandemic: Modelling the Relation between COVID-19 Health and Community Mobility Data. Int. J. Environ. Res. Public Health 2021, 18, 10560. [Google Scholar] [CrossRef] [PubMed]
- Barros, J.M.; Duggan, J.; Rebholz-Schuhmann, D. The Application of Internet-Based Sources for Public Health Surveillance (Infoveillance): Systematic Review. J. Med. Internet Res. 2020, 22, e13680. [Google Scholar] [CrossRef] [PubMed]
- Meseko, C.; Dzikwi-Emennaa, A. Influenza Surveillance Data from Africa to Inform Tailored Vaccination Programmes. Lancet Glob. Health 2023, 11, e640–e641. [Google Scholar] [CrossRef] [PubMed]
- Steffen, C.; Debellut, F.; Gessner, B.; Kasolo, F.; Yahaya, A.; Ayebazibwe, N.; Bassong, O.; Cardoso, Y.; Kebede, S.; Manoncourt, S.; et al. Improving Influenza Surveillance in Sub-Saharan Africa. Bull. World Health Organ. 2012, 90, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.S.; Koua, E.L.; Abdelmalik, P.; Kambale, F.; Kibangou, E.; Nguna, J.; Okot, C.; Akpan, G.; Moussana, F.; Kimenyi, J.P.; et al. Evaluation of the Epidemic Intelligence from Open Sources (EIOS) System for the Early Detection of Outbreaks and Health Emergencies in the African Region. BMC Public Health 2025, 25, 857. [Google Scholar] [CrossRef] [PubMed]
- Mennella, C.; Maniscalco, U.; De Pietro, G.; Esposito, M. Ethical and Regulatory Challenges of AI Technologies in Healthcare: A Narrative Review. Heliyon 2024, 10, e26297. [Google Scholar] [CrossRef] [PubMed]
- Kayembe-Mulumba, B.; Brunet, F.; Pomey, M.-P. What contributions could the metaverse bring to health systems? Santé Publique 2024, 36, 43–49. [Google Scholar] [CrossRef]
- Panteli, D.; Adib, K.; Buttigieg, S.; Goiana-da-Silva, F.; Ladewig, K.; Azzopardi-Muscat, N.; Figueras, J.; Novillo-Ortiz, D.; McKee, M. Artificial Intelligence in Public Health: Promises, Challenges, and an Agenda for Policy Makers and Public Health Institutions. Lancet Public Health 2025, 10, e428–e432. [Google Scholar] [CrossRef] [PubMed]
| Surveillance Domains | Common Gaps | Implications for Disease X Detection | Past Examples |
|---|---|---|---|
| Community-based surveillance | Lack of digital tools, undertrained health workers | Spillover may go undetected at the local level | Ebola in Guinea (2014) |
| Zoonotic/veterinary surveillance | Poor lab infrastructure, low funding, weak reporting | Missed early warning of animal–human transmission | Rift Valley fever in Sudan |
| Genomic sequencing | Low lab throughput, shortage of trained bioinformaticians | Inability to track pathogen mutations | COVID-19 variants in Africa |
| Environmental health surveillance | Weak coordination with health ministries | Poor outbreak prediction (e.g., vector-borne or climate-linked diseases) | Dengue in Southeast Asia |
| Event-based surveillance (EBS) | Informal, poorly institutionalized systems | Delayed outbreak recognition | Cholera in Haiti (2010) |
| Cross-sectoral data integration | Legal, bureaucratic, and IT system silos | Delayed coordinated response | Mpox in Africa |
| African Regions | Countries with Functional NGS Capacity * | CoE † | Notable Initiatives/Networks |
|---|---|---|---|
| West Africa | 10 | 3 | AFROSCREEN project enhancing sequencing platforms in West and Central Africa ‡ |
| East Africa | 6 | 2 | Africa PGI supporting regional sequencing hubs § |
| Central Africa | 5 | 1 | AFROSCREEN project bolstering genomic surveillance |
| North Africa | 4 | 1 | Morocco’s established sequencing infrastructure |
| Southern Africa | 5 | 2 | South Africa’s multiple institutions and training centers |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kayembe-Mulumba, B.; N’gattia, A.K.; Belizaire, M.R.D. One Health, Many Gaps: Rethinking Epidemic Intelligence in Resource-Limited Settings to Prepare for the Global Threat of Disease X. Microorganisms 2025, 13, 2615. https://doi.org/10.3390/microorganisms13112615
Kayembe-Mulumba B, N’gattia AK, Belizaire MRD. One Health, Many Gaps: Rethinking Epidemic Intelligence in Resource-Limited Settings to Prepare for the Global Threat of Disease X. Microorganisms. 2025; 13(11):2615. https://doi.org/10.3390/microorganisms13112615
Chicago/Turabian StyleKayembe-Mulumba, Blondy, Anderson Kouabenan N’gattia, and Marie Roseline Darnycka Belizaire. 2025. "One Health, Many Gaps: Rethinking Epidemic Intelligence in Resource-Limited Settings to Prepare for the Global Threat of Disease X" Microorganisms 13, no. 11: 2615. https://doi.org/10.3390/microorganisms13112615
APA StyleKayembe-Mulumba, B., N’gattia, A. K., & Belizaire, M. R. D. (2025). One Health, Many Gaps: Rethinking Epidemic Intelligence in Resource-Limited Settings to Prepare for the Global Threat of Disease X. Microorganisms, 13(11), 2615. https://doi.org/10.3390/microorganisms13112615







