Identification of a Linear B-Cell Epitope in the African Swine Fever Virus pE248R Protein Targeted by Monoclonal Antibodies
Abstract
1. Introduction
2. Materials and Methods
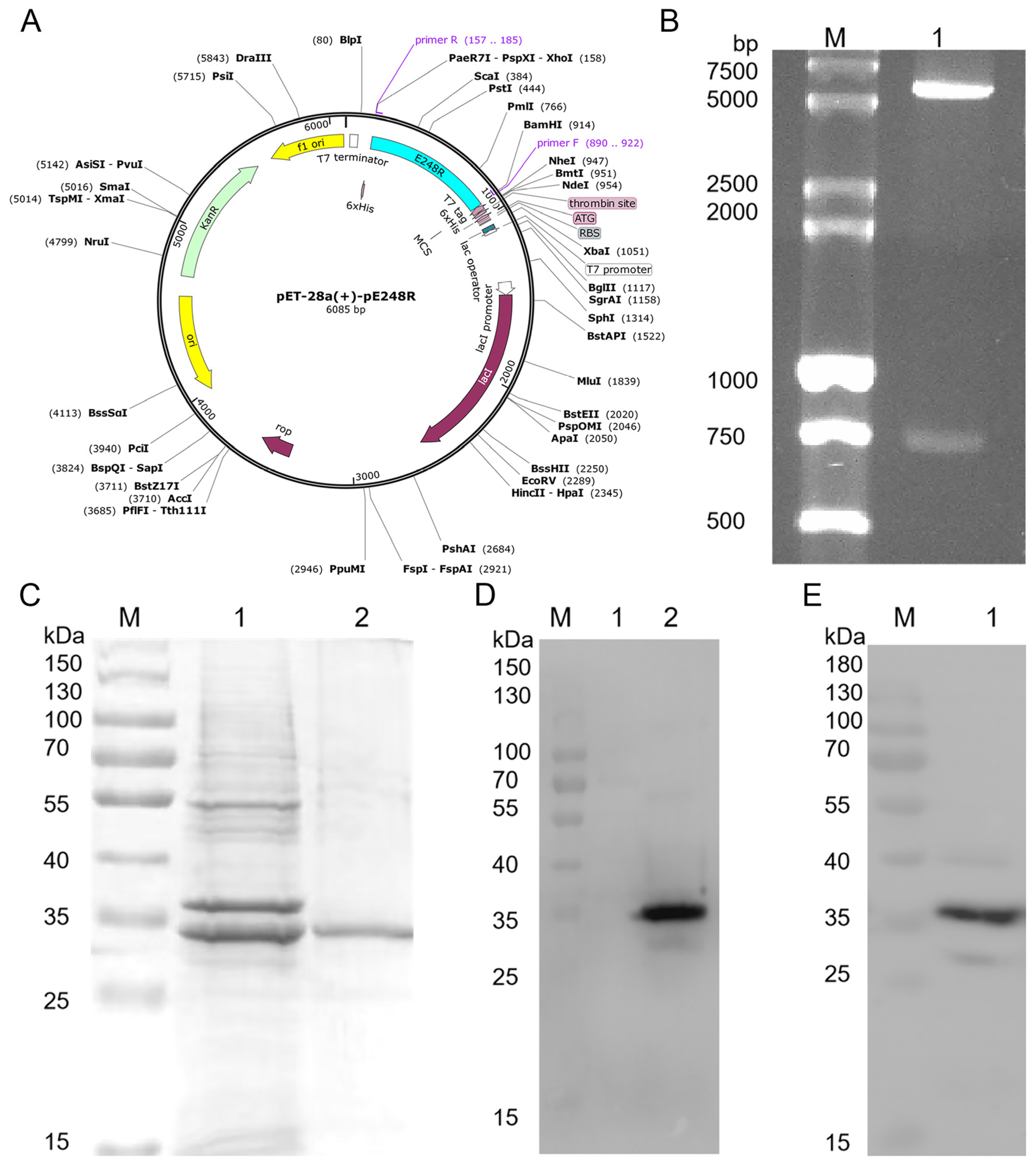
2.1. Plasmids
2.2. Purification of pE248R Protein
2.3. SDS-PAGE and Western Blot
2.4. Cell Culture and Animals
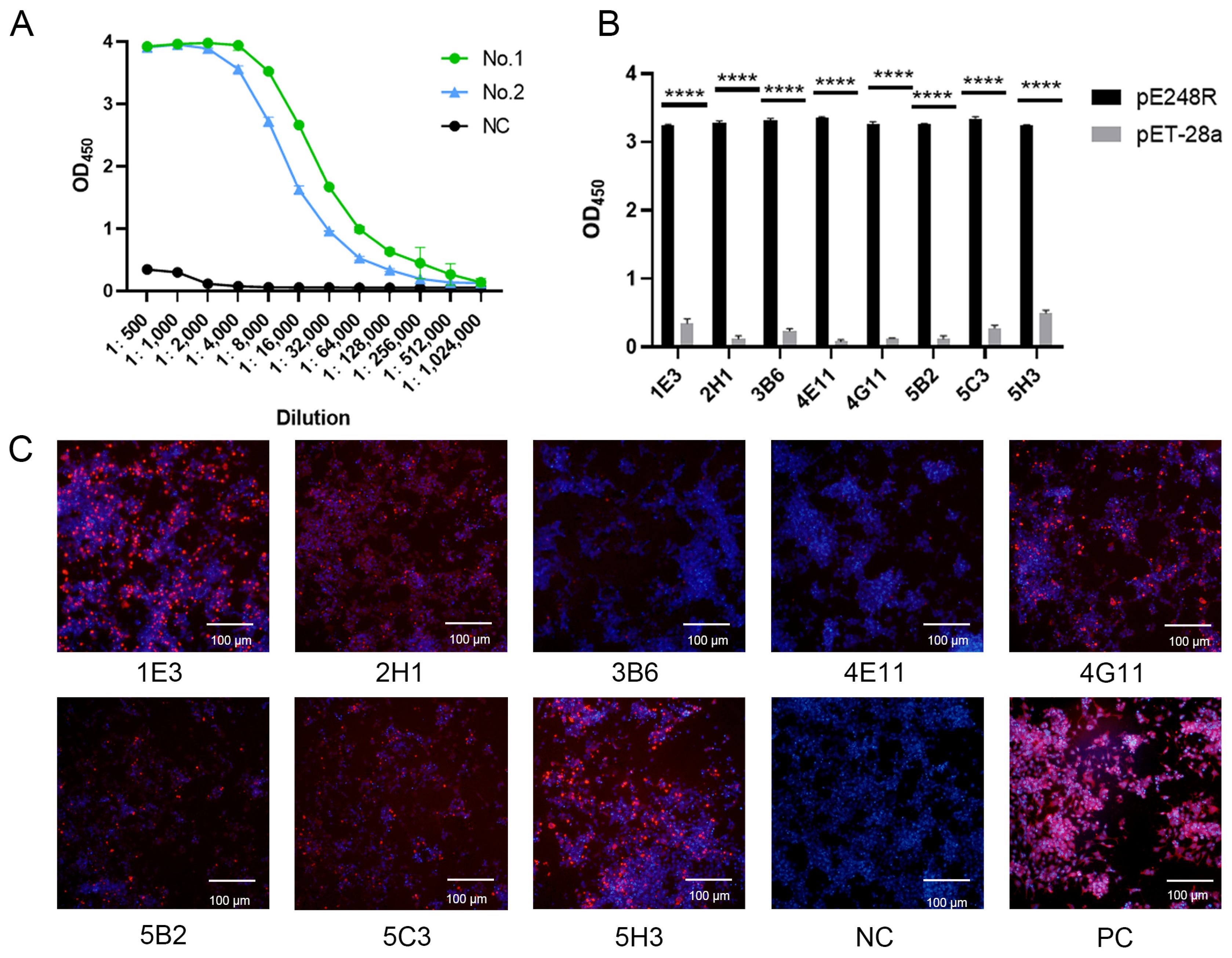
2.5. Indirect Enzyme-Linked Immunosorbent Assay (iELISA)
2.6. Purification and Characterization of Monoclonal Antibodies
2.7. Immunofluorescence Assay (IFA)
2.8. Peptide Synthesis and Epitope Mapping
2.9. Peptide-ELISA and Dot-Blot
2.10. Conservation Analysis and Spatial Localization of the Epitope
3. Results
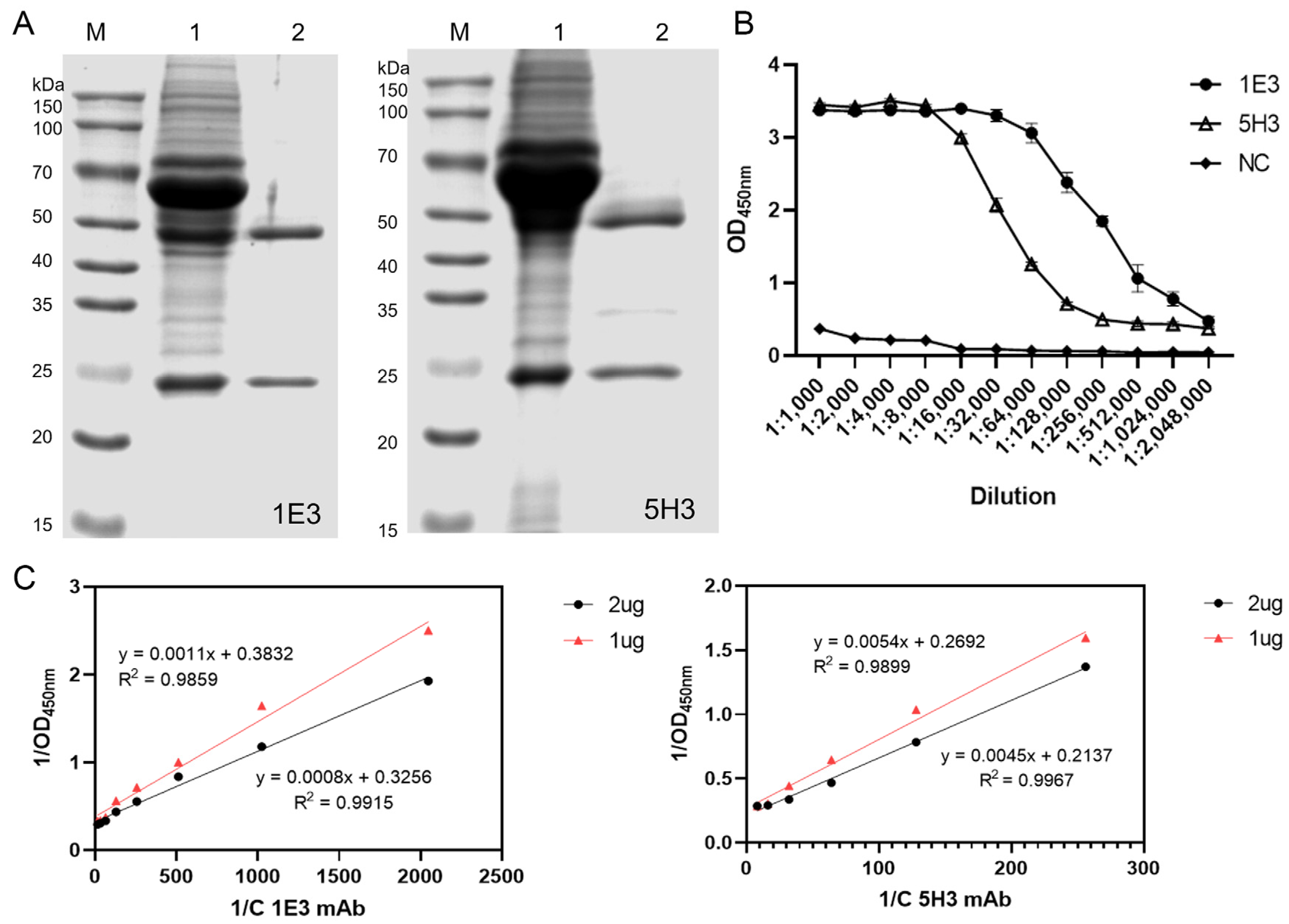
3.1. Expression of Recombinant pE248R Protein
3.2. Screening of mAbs Against the ASFV pE248R Protein
3.3. Purification and Characterization of mAbs
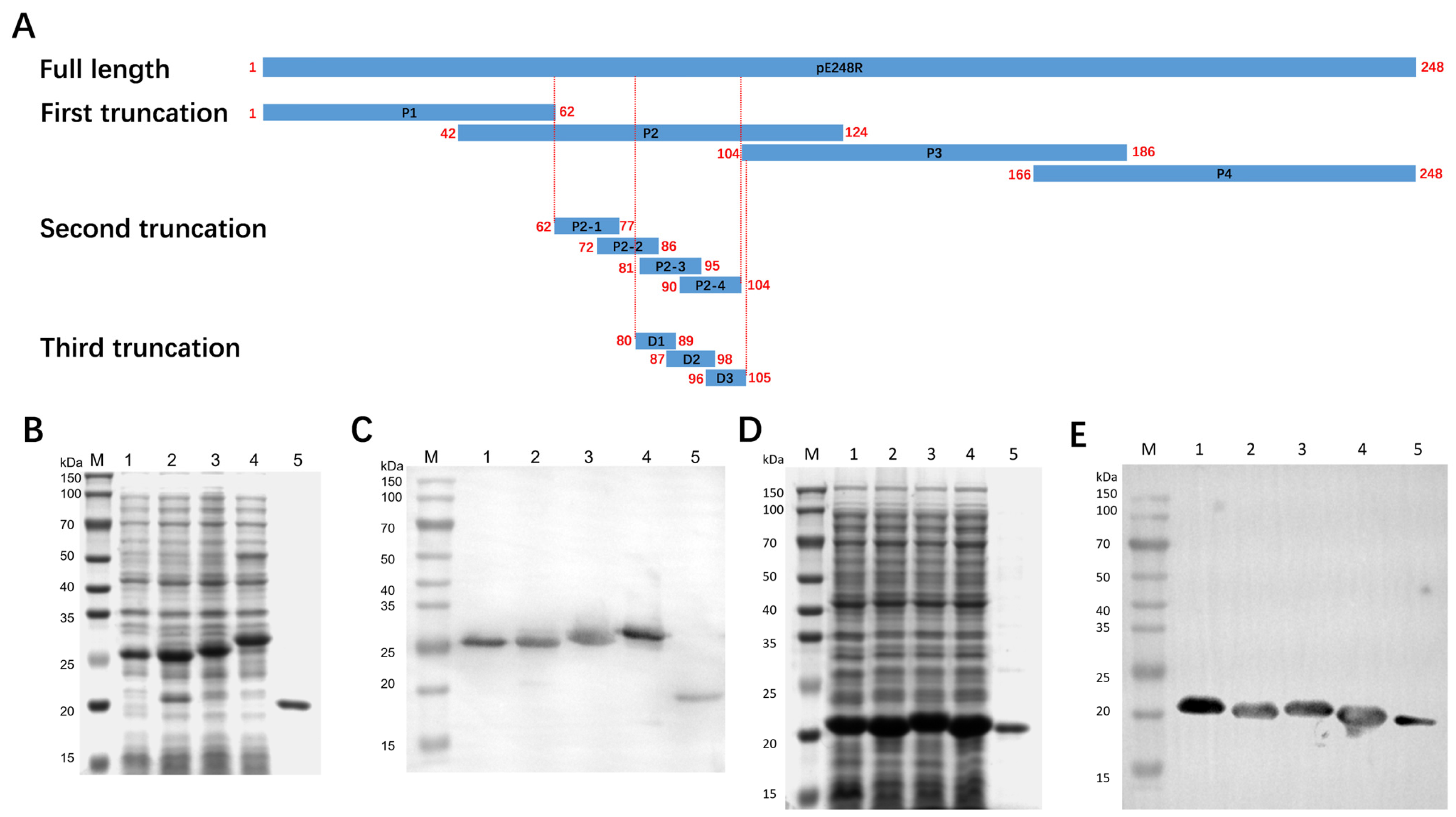
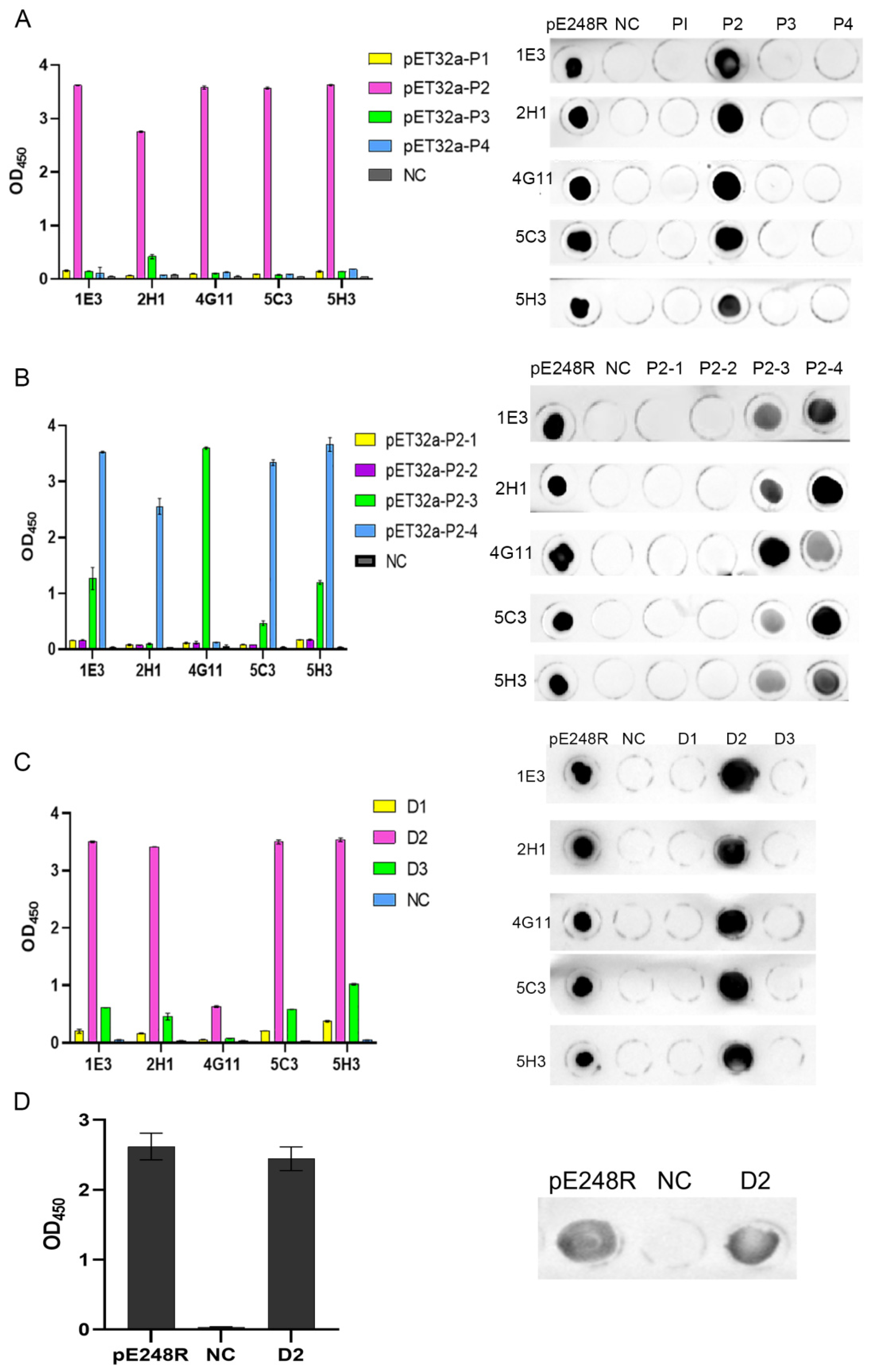
3.4. Expression and Identification of Truncated Proteins
3.5. Antigenic Epitope Mapping and Identification of pE248R
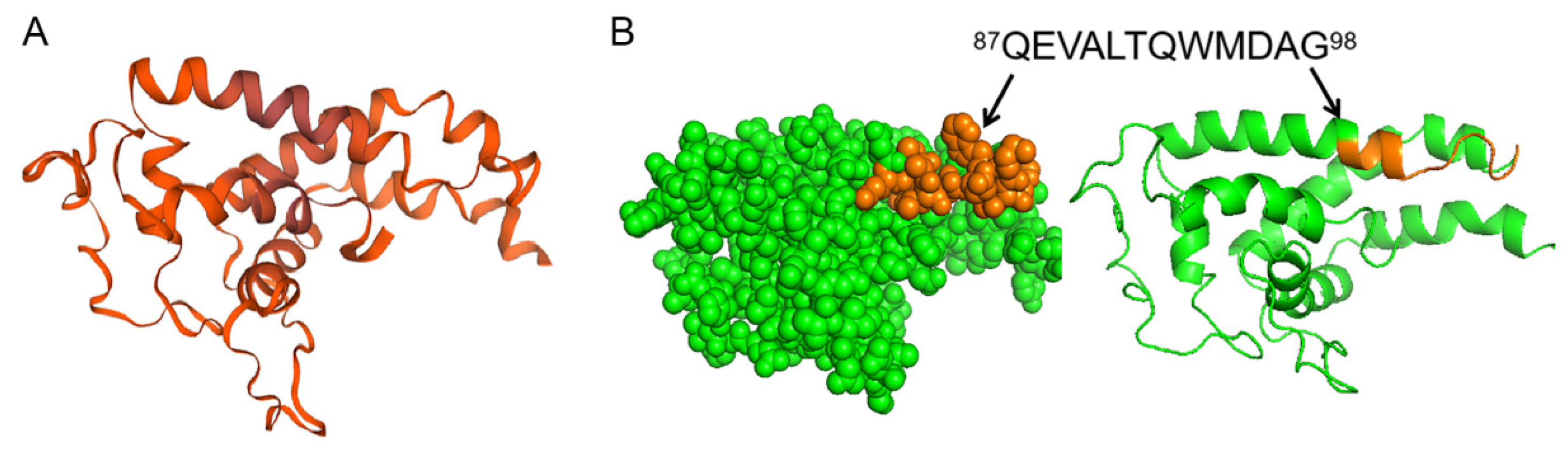
3.6. Conservation Analysis and Spatial Localization of the Linear B-Cell Epitope of pE248R Protein
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sánchez-Cordón, P.J.; Montoya, M.; Reis, A.L.; Dixon, L.K. African swine fever: A re-emerging viral disease threatening the global pig industry. Vet. J. 2018, 233, 41–48. [Google Scholar] [CrossRef]
- Liu, J.; Liu, B.; Shan, B.; Wei, S.; An, T.; Shen, G.; Chen, Z. Prevalence of African Swine Fever in China, 2018–2019. J. Med. Virol. 2020, 92, 1023–1034. [Google Scholar] [CrossRef]
- Alonso, C.; Borca, M.; Dixon, L.; Revilla, Y.; Rodriguez, F.; Escribano, J.M.; Ictv Report, C. ICTV Virus Taxonomy Profile: Asfarviridae. J. Gen. Virol. 2018, 99, 613–614. [Google Scholar] [CrossRef] [PubMed]
- Dixon, L.K.; Chapman, D.A.; Netherton, C.L.; Upton, C. African swine fever virus replication and genomics. Virus Res. 2013, 173, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Xie, M.; Wu, W.; Chen, Z. Structures and Functional Diversities of ASFV Proteins. Viruses 2021, 13, 2124. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhao, D.; Wang, J.; Zhang, Y.; Wang, M.; Gao, Y.; Li, F.; Wang, J.; Bu, Z.; Rao, Z.; et al. Architecture of African swine fever virus and implications for viral assembly. Science 2019, 366, 640–644. [Google Scholar] [CrossRef]
- Liu, S.; Luo, Y.; Wang, Y.; Li, S.; Zhao, Z.; Bi, Y.; Sun, J.; Peng, R.; Song, H.; Zhu, D.; et al. Cryo-EM Structure of the African Swine Fever Virus. Cell Host Microbe 2019, 26, 836–843.e3. [Google Scholar] [CrossRef]
- Andrés, G.; García-Escudero, R.; Salas, M.L.; Rodríguez, J.M. Repression of African swine fever virus polyprotein pp220-encoding gene leads to the assembly of icosahedral core-less particles. J. Virol. 2002, 76, 2654–2666. [Google Scholar] [CrossRef]
- Cackett, G.; Matelska, D.; Sýkora, M.; Portugal, R.; Malecki, M.; Bähler, J.; Dixon, L.; Werner, F. The African Swine Fever Virus Transcriptome. J. Virol. 2020, 94, e00119-20. [Google Scholar] [CrossRef]
- Kumar, N.; Bajiya, N.; Patiyal, S.; Raghava, G.P.S. Multi-perspectives and challenges in identifying B-cell epitopes. Protein Sci. 2023, 32, e4785. [Google Scholar] [CrossRef]
- Song, J.; Wang, M.; Du, Y.; Wan, B.; Zhang, A.; Zhang, Y.; Zhuang, G.; Ji, P.; Wu, Y.; Zhang, G. Identification of a linear B-cell epitope on the African swine fever virus CD2v protein. Int. J. Biol. Macromol. 2023, 232, 123264. [Google Scholar] [CrossRef]
- Yin, D.; Geng, R.; Shao, H.; Ye, J.; Qian, K.; Chen, H.; Qin, A. Identification of novel linear epitopes in P72 protein of African swine fever virus recognized by monoclonal antibodies. Front. Microbiol. 2022, 13, 1055820. [Google Scholar] [CrossRef]
- Zhao, H.J.; Wang, G.J.; Dong, H.X.; Wu, S.Y.; Du, Y.K.; Wan, B.; Ji, P.C.; Wu, Y.A.; Jiang, D.W.; Zhuang, G.Q.; et al. Identification of a Linear B Cell Epitope on p54 of African Swine Fever Virus Using Nanobodies as a Novel Tool. Microbiol. Spectr. 2023, 11, e0336222. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.; Sun, Z.; Wang, M.; Song, J.; Sun, J.; Zhou, L.; Jiang, D.; Zhang, A.; Wu, Y.; Zhang, G. Identification of a novel linear B-cell epitope on the p30 protein of African swine fever virus using monoclonal antibodies. Virus Res. 2024, 341, 199328. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Shao, J.; Liu, W.; Gao, S.; Zhou, G.; Ning, X.; Huang, H.; Liu, Y.; Chang, H. Screening and identification of linear B-cell epitopes on structural proteins of African Swine Fever Virus. Virus Res. 2024, 350, 199465. [Google Scholar] [CrossRef] [PubMed]
- Neilan, J.G.; Zsak, L.; Lu, Z.; Burrage, T.G.; Kutish, G.F.; Rock, D.L. Neutralizing antibodies to African swine fever virus proteins p30, p54, and p72 are not sufficient for antibody-mediated protection. Virology 2004, 319, 337–342. [Google Scholar] [CrossRef]
- Rodríguez, I.; Redrejo-Rodríguez, M.; Rodríguez, J.M.; Alejo, A.; Salas, J.; Salas, M.L. African swine fever virus pB119L protein is a flavin adenine dinucleotide-linked sulfhydryl oxidase. J. Virol. 2006, 80, 3157–3166. [Google Scholar] [CrossRef]
- Maurer-Stroh, S.; Eisenhaber, F. Myristoylation of viral and bacterial proteins. Trends Microbiol. 2004, 12, 178–185. [Google Scholar] [CrossRef]
- Rodríguez, I.; Nogal, M.L.; Redrejo-Rodríguez, M.; Bustos, M.J.; Salas, M.L. The African Swine Fever Virus Virion Membrane Protein pE248R Is Required for Virus Infectivity and an Early Postentry Event. J. Virol. 2009, 83, 12290–12300. [Google Scholar] [CrossRef]
- Hernáez, B.; Guerra, M.; Salas, M.L.; Andrés, G. African Swine Fever Virus Undergoes Outer Envelope Disruption, Capsid Disassembly and Inner Envelope Fusion before Core Release from Multivesicular Endosomes. PLoS Pathog. 2016, 12, e1005595. [Google Scholar] [CrossRef]
- Mazloum, A.; Zhukov, I.U.; Aronova, E.B.; Igolkin, A.S.; Vlasova, N.N. ASF virus replication features in the presence of recombinant proteins CD2v, pX69R and pE248R. Vopr. Virusol. 2019, 64, 193–200. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, W.; Wen, Y.; Niu, Q.; Yang, J.; Guan, G.; Yin, H.; Zheng, H.; Li, D.; Liu, Z. The E248R protein of African swine fever virus inhibits the cGAS-STING-mediated innate immunity. Chin. J. Biotechnol. 2022, 38, 1837–1846. [Google Scholar] [CrossRef]
- Zhao, J.; Mou, C.; Zhu, L.; Sun, X.; Chen, Z. African swine fever virus-encoded pE248R protein inhibits interferon production via blocking RIG-I-mediated antiviral signaling. Int. J. Biol. Macromol. 2025, 327 Pt 1, 147383. [Google Scholar] [CrossRef] [PubMed]
- Moss, B. Poxvirus cell entry: How many proteins does it take? Viruses 2012, 4, 688–707. [Google Scholar] [CrossRef] [PubMed]
- Senkevich, T.G.; Ojeda, S.; Townsley, A.; Nelson, G.E.; Moss, B. Poxvirus multiprotein entry–fusion complex. Proc. Natl. Acad. Sci. USA 2005, 102, 18572–18577. [Google Scholar] [CrossRef]
- Senkevich, T.G.; White, C.L.; Koonin, E.V.; Moss, B. Complete pathway for protein disulfide bond formation encoded by poxviruses. Proc. Natl. Acad. Sci. USA 2002, 99, 6667–6672. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, W.; Yang, S.; Song, S.; Ma, Y.; Zhou, G.; Liang, X.; Miao, C.; Li, J.; Liu, Y.; et al. Evaluation of humoral and cellular immune responses induced by a cocktail of recombinant African swine fever virus antigens fused with OprI in domestic pigs. Virol. J. 2023, 20, 104. [Google Scholar] [CrossRef]
- Wang, A.; Chen, Z.; Zhou, J.; Chen, Y.; Liu, Y.; Liu, H.; Liang, C.; Zhu, X.; Zhang, Y.; Xin, C.; et al. Development and characterization of monoclonal antibodies against p37 protein of African swine fever virus. Int. J. Biol. Macromol. 2024, 264 Pt 2, 130689. [Google Scholar] [CrossRef]
- Wang, A.; Yin, J.; Liu, Y.; Zhu, R.; Zhao, J.; Zhou, J.; Liu, H.; Ding, P.; Zhang, G. Identification of linear B-cell epitope on the structure protein p49 of African Swine Fever Virus (ASFV). Int. J. Biol. Macromol. 2024, 280 Pt 3, 135983. [Google Scholar] [CrossRef]
- Goldberg, M.E.; Djavadi-Ohaniance, L. Methods for measurement of antibody/antigen affinity based on ELISA and RIA. Curr. Opin. Immunol. 1993, 5, 278–281. [Google Scholar] [CrossRef]
- Farfan-Castro, S.; Garcia-Soto, M.J.; Comas-Garcia, M.; Arevalo-Villalobos, J.I.; Palestino, G.; Gonzalez-Ortega, O.; Rosales-Mendoza, S. Synthesis and immunogenicity assessment of a gold nanoparticle conjugate for the delivery of a peptide from SARS-CoV-2. Nanomedicine 2021, 34, 102372. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, A.; Yan, W.; Li, J.; Meng, X.; Chen, L.; Li, S.; Tong, W.; Kong, N.; Yu, L.; et al. Identification of Linear Epitopes in the C-Terminal Region of ASFV p72 Protein. Microorganisms 2023, 11, 2846. [Google Scholar] [CrossRef] [PubMed]
- Zheng, N.; Li, C.; Hou, H.; Chen, Y.; Zhang, A.; Han, S.; Wan, B.; Wu, Y.; He, H.; Wang, N.; et al. A Novel Linear B-Cell Epitope on the P54 Protein of African Swine Fever Virus Identified Using Monoclonal Antibodies. Viruses 2023, 15, 867. [Google Scholar] [CrossRef]
- Noll, J.C.G.; Rani, R.; Butt, S.L.; Fernandes, M.H.V.; do Nascimento, G.M.; Martins, M.; Caserta, L.C.; Covaleda, L.; Diel, D.G. Identification of an Immunodominant B-Cell Epitope in African Swine Fever Virus p30 Protein and Evidence of p30 Antibody-Mediated Antibody Dependent Cellular Cytotoxicity. Viruses 2024, 16, 758. [Google Scholar] [CrossRef] [PubMed]
- Tran, X.H.; Le, T.T.P.; Nguyen, Q.H.; Do, T.T.; Nguyen, V.D.; Gay, C.G.; Borca, M.V.; Gladue, D.P. African swine fever virus vaccine candidate ASFV-G-ΔI177L efficiently protects European and native pig breeds against circulating Vietnamese field strain. Transbound. Emerg. Dis. 2022, 69, E497–E504. [Google Scholar] [CrossRef] [PubMed]
| Primers | Sequence (5′–3′) | Position (Amino Acid) |
|---|---|---|
| P1-F | CGCGGATCCATGGGCGGCTCTACCTC | 1–62 aa |
| P1-R | CCGCTCGAGTTAACAGGAGGTGTTCAGAGA | |
| P2-F | CGCGGATCCATGGGCGACGGCAACATCC | 42–124 aa |
| P2-R | CCGCTCGAGTTAAGACACGCAGTTCTGGAT | |
| P3-F | CGCGGATCCATGACAGACATTGAGGAGAAC | 104–186 aa |
| P3-R | CCGCTCGAGTTAAAAGAAATTCTTGCTGGT | |
| P4-F | CGCGGATCCATGACCACCACCGGCATCA | 166–248 aa |
| P4-R | CCGCTCGAGTTAGCTCACGGCGGCGTT | |
| P2-1-F | CGCGGATCCATGGTGCAGAAGCATGTGA | 62–77 aa |
| P2-1-R | CCGCTCGAGTTAGCTCAGATTGGTGATAAA | |
| P2-2-F | CGCGGATCCATGTTTATCACCAATCTGAGC | 72–86 aa |
| P2-2-R | CCGCTCGAGTTAGTCCTTCAGGTTCTGT | |
| P2-3-F | CGCGGATCCATGACACAGAACCTGAAGG | 81–95 aa |
| P2-3-R | CCGCTCGAGTTACATCCACTGGGTCAG | |
| P2-4-F | CGCGGATCCATGGCCCTGACCCAGTG | 90–104 aa |
| P2-4-R | CCGCTCGAGTTATTTCTGATCGTGGGTGC |
| Name | Sequence |
|---|---|
| D1 | 80ITQNLKDQEV89 |
| D2 | 87QEVALTQWMDAG98 |
| D3 | 96DAGTHDQKTD105 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, E.; Liu, X.; Chen, Y.; Liu, H.; Zhou, J.; Wang, A. Identification of a Linear B-Cell Epitope in the African Swine Fever Virus pE248R Protein Targeted by Monoclonal Antibodies. Microorganisms 2025, 13, 2616. https://doi.org/10.3390/microorganisms13112616
Liu E, Liu X, Chen Y, Liu H, Zhou J, Wang A. Identification of a Linear B-Cell Epitope in the African Swine Fever Virus pE248R Protein Targeted by Monoclonal Antibodies. Microorganisms. 2025; 13(11):2616. https://doi.org/10.3390/microorganisms13112616
Chicago/Turabian StyleLiu, Enping, Xinyue Liu, Yumei Chen, Hongliang Liu, Jingming Zhou, and Aiping Wang. 2025. "Identification of a Linear B-Cell Epitope in the African Swine Fever Virus pE248R Protein Targeted by Monoclonal Antibodies" Microorganisms 13, no. 11: 2616. https://doi.org/10.3390/microorganisms13112616
APA StyleLiu, E., Liu, X., Chen, Y., Liu, H., Zhou, J., & Wang, A. (2025). Identification of a Linear B-Cell Epitope in the African Swine Fever Virus pE248R Protein Targeted by Monoclonal Antibodies. Microorganisms, 13(11), 2616. https://doi.org/10.3390/microorganisms13112616






