Mechanisms of Microorganisms Alleviating Drought and Salt Stresses in Plants
Abstract
1. Introduction
2. Plant Regulatory Pathways in Response to Drought and Salt Stresses
2.1. Stress Signal Perception and Transduction
2.2. Stress-Responsive Gene Expression Regulation
2.3. Antioxidant System Activation
3. Advances in the Study of Microbial Strategies Against Drought and Salt Stresses in Plants
3.1. Advances in Microorganisms Involved in Plant Drought Stress Response
3.2. Advancements in Microorganisms Involved in Plant Salt Stress Response
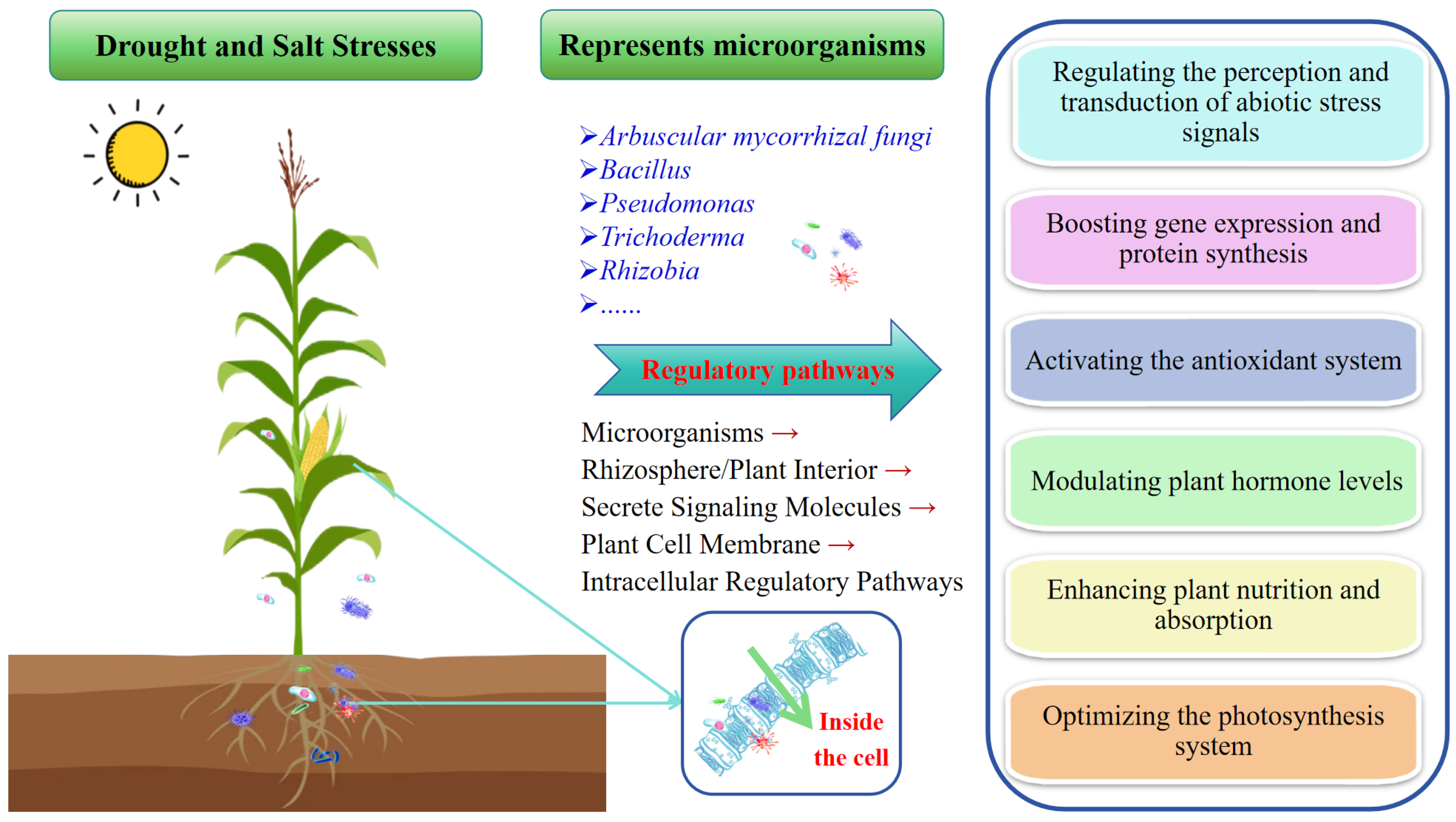
4. Mechanisms Behind Microbial Regulation of Plant Stress
4.1. Regulating the Perception and Transduction of Abiotic Stress Signals to Enhance Plant Adaptive Responses
4.2. Boosting Gene Expression and Protein Synthesis for Overall Plant Metabolic Regulation
4.3. Activating the Antioxidant System to Strengthen Plant Tolerance
4.4. Modulating Plant Hormone Levels to Stimulate Growth in Response to Adversity
4.5. Enhancing Plant Nutrition and Absorption to Improve Tolerance
4.6. Optimizing the Photosynthesis System to Promote the Synthesis of Essential Substances, Safeguarding Plant Growth and Development Amidst Adversity
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, W.; Vinocur, B.; Altman, A. Plant responses to drought, salinity and extreme temperatures: Towards genetic engineering for stress tolerance. Planta 2003, 218, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Song, T. Green finance and food production: Evidence from cities in China. J. Clean. Prod. 2024, 458, 142423. [Google Scholar] [CrossRef]
- Ahmad, A.; Aslam, Z.; BelliTürk, K.; Hussain, S.; Bibi, I. Soil application of cellulolytic Microbe–Enriched vermicompost modulated the morpho-physiological and biochemical responses of wheat cultivars under different moisture regimes. J. Soil Sci. Plant Nutr. 2022, 22, 4153–4167. [Google Scholar] [CrossRef]
- Chang, Y.; Chen, Z.; Jiang, J.; Zhang, H.; Zhu, J.; Duan, C. Epigenetic regulation in plant abiotic stress responses. J. Integr. Plant Biol. 2020, 62, 563–580. [Google Scholar] [CrossRef]
- Huot, B.; Yao, J.; Montgomery, B.L.; He, S.Y. Growth–Defense Tradeoffs in Plants: A balancing act to optimize fitness. Mol. Plant 2014, 7, 1267–1287. [Google Scholar] [CrossRef]
- Cruz, C.C.; Cardoso, P.; Santos, J.; Matos, D.; Figueira, E. Bioprospecting soil bacteria from srid zones to increase plant tolerance to drought: Growth and biochemical status of maize inoculated with plant growth-promoting bacteria isolated from sal island, cape verde. Plants 2022, 11, 2912. [Google Scholar] [CrossRef]
- Liu, H.; Carvalhais, L.C.; Crawford, M.; Singh, E.; Dennis, P.G.; Pieterse, C.M.J. Inner plant values: Diversity, colonization and benefits from endophytic bacteria. Front. Microbiol. 2017, 8, 2552. [Google Scholar] [CrossRef]
- Eichmann, R.; Richards, L.; Schäfer, P. Hormones as go-betweens in plant microbiome assembly. Plant J. 2021, 105, 518–541. [Google Scholar] [CrossRef]
- Christensen, M.J.; Bennett, R.J.; Ansari, H.A.; Koga, H.; Johnson, R.D.; Bryan, G.T.; Simpson, W.R.; Koolaard, J.; Nickless, E.; Voisey, C.R. Epichloë endophytes grow by intercalary hyphal extension in elongating grass leaves. Fungal Genet. Biol. 2008, 45, 84–93. [Google Scholar] [CrossRef]
- Khan, A.L.; Hamayun, M.; Radhakrishnan, R.; Waqas, M.; Kang, S.M.; Kim, Y.H.; Shin, J.H.; Choo, Y.S.; Kim, J.G.; Lee, I.J. Mutualistic association of Paecilomyces formosus LHL10 offers thermotolerance to Cucumis sativus. Antonie Van Leeuwenhoek 2012, 101, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Verma, J.P. Does plant—Microbe interaction confer stress tolerance in plants: A review? Microbiol. Res. 2018, 207, 41–52. [Google Scholar] [CrossRef]
- Lata, R.; Chowdhury, S.; Gond, S.K.; White, J.F. Induction of abiotic stress tolerance in plants by endophytic microbes. Lett. Appl. Microbiol. 2018, 66, 268–276. [Google Scholar] [CrossRef]
- Lynch, J.M.; Moffat, A. Bioremediation—Prospects for the future application of innovative applied biological research. Ann. Appl. Biol. 2005, 146, 217–221. [Google Scholar] [CrossRef]
- Feng, D.; Gao, Q.; Liu, J.; Tang, J.; Hua, Z.; Sun, X. Categories of exogenous substances and their effect on alleviation of plant salt stress. Eur. J. Agron. 2023, 142, 126656. [Google Scholar] [CrossRef]
- Feng, D.; Liu, W.; Chen, K.; Ning, S.; Gao, Q.; Chen, J.; Liu, J.; Sun, X.; Xu, W. Exogenous substances used to relieve plants from drought stress and their associated underlying mechanisms. Int. J. Mol. Sci. 2024, 25, 9249. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh, S.; Gaurav, A.K.; Srivastava, S.; Verma, J.P. Plant growth-promoting bacteria: Biological tools for the mitigation of salinity stress in plants. Front. Microbiol. 2020, 11, 1216. [Google Scholar] [CrossRef] [PubMed]
- Braud, A.; Jézéquel, K.; Bazot, S.; Lebeau, T. Enhanced phytoextraction of an agricultural Cr- and Pb-contaminated soil by bioaugmentation with siderophore-producing bacteria. Chemosphere 2009, 74, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Hayat, R.; Ali, S.; Amara, U.; Khalid, R.; Ahmed, I. Soil beneficial bacteria and their role in plant growth promotion: A review. Ann. Microbiol. 2010, 60, 579–598. [Google Scholar] [CrossRef]
- Gupta, A.; Mishra, R.; Rai, S.; Bano, A.; Pathak, N.; Fujita, M.; Sharma, M.; Hasanuzzaman, M. Mechanistic insights of plant growth promoting bacteria mediated drought and salt stress tolerance in plants for sustainable agriculture. Int. J. Mol. Sci. 2022, 23, 3741. [Google Scholar] [CrossRef]
- Sabagh, A.E.; Islam, M.S.; Hossain, A.; Iqbal, M.A.; Mubeen, M.; Waleed, M.; Reginato, M.; Battaglia, M.L.; Ahmed, S.; Rehman, A.; et al. Phytohormones as growth regulators during abiotic stress tolerance in plants. Front. Agron. 2022, 4, 765068. [Google Scholar] [CrossRef]
- Chen, X.; Ding, Y.; Yang, Y.; Song, C.; Wang, B.; Yang, S.; Guo, Y.; Gong, Z. Protein kinases in plant responses to drought, salt, and cold stress. J. Integr. Plant Biol. 2021, 63, 53–78. [Google Scholar] [CrossRef] [PubMed]
- Castro, B.; Citterico, M.; Kimura, S.; Stevens, D.M.; Wrzaczek, M.; Coaker, G. Stress-induced reactive oxygen species compartmentalization, perception and signalling. Nat. Plants 2021, 7, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Niazi, A.; Akhtar, J.; Saifullah; Farooq, M.; Souri, Z.; Karimi, N.; Rengel, Z. Acquiring control: The evolution of ROS-Induced oxidative stress and redox signaling pathways in plant stress responses. Plant Physiol. Biochem. 2019, 141, 353–369. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Gul, N.; Wani, I.A.; Mir, R.A.; Nowshehri, J.A.; Aslam, S.; Gupta, R.; Verma, S.; Aslam, S. Plant growth promoting microorganisms mediated abiotic stress tolerance in crop plants: A critical appraisal. Plant Growth Regul. 2023, 100, 7–24. [Google Scholar] [CrossRef]
- Issa, A.A.; Abd-Alla, M.H.; Mahmoud, A.M. Effect of biological treatments on growth and some metabolic activities of barley plants grown in saline soil. Microbiol. Res. 1994, 149, 317–320. [Google Scholar] [CrossRef]
- Aggarwal, A.; Kadian, N.; Karishma, K.; Neetu, N.; Tanwar, A.; Gupta, K. Arbuscular mycorrhizal symbiosis and alleviation of salinity stress. J. Appl. Nat. Sci. 2012, 4, 144–155. [Google Scholar] [CrossRef]
- Ahmad, P.; Hashem, A.; Abd_Allah, E.F.; John, R.; Egamberdieva, D.; Gücel, S. Role of Trichoderma harzianum in mitigating NaCl stress in Indian mustard (Brassica juncea L.) through antioxidative defense system. Front. Plant Sci. 2015, 6, 868. [Google Scholar] [CrossRef]
- Tiwari, S.; Lata, C. Heavy metal stress, signaling, and tolerance due to plant-associated microbes: An overview. Front. Plant Sci. 2018, 9, 452. [Google Scholar] [CrossRef]
- Lesk, C.; Rowhani, P.; Ramankutty, N. Influence of extreme weather disasters on global crop production. Nature 2016, 529, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.S.; Singh, A.K.; Ansari, M.I. Effect of drought stress on crop production. In New Frontiers in Stress Management for Durable Agriculture; Rakshit, A., Singh, H., Singh, A., Singh, U., Fraceto, L., Eds.; Springer: Singapore, 2020; pp. 35–47. [Google Scholar] [CrossRef]
- Iqbal, S.; Iqbal, M.; Li, C.; Iqbal, A.; Abbas, R.Z. Overviewing drought and heat stress amelioration—From plant responses to microbe-mediated mitigation. Sustainability 2023, 15, 1671. [Google Scholar] [CrossRef]
- Liu, F.; Ma, H.; Ma, B.; Du, Z.; Jing, D.; Xing, S. Effect of plant growth-promoting rhizobacteria on photosynthetic characteristics in walnut seedlings under drought stress. Sci. Silvae Sin. 2015, 51, 84–90. [Google Scholar]
- Zhou, C.; Ma, Z.; Zhu, L.; Xiao, X.; Xie, Y.; Zhu, J.; Wang, J. Rhizobacterial strain bacillus megaterium BOFC15 induces cellular polyamine changes that improve plant growth and drought resistance. Int. J. Mol. Sci. 2016, 17, 976. [Google Scholar] [CrossRef]
- Ravanbakhsh, M.; Sasidharan, R.; Voesenek, L.A.C.J.; Kowalchuk, G.A.; Jousset, A. Microbial modulation of plant ethylene signaling: Ecological and evolutionary consequences. Microbiome 2018, 6, 52. [Google Scholar] [CrossRef] [PubMed]
- Timmusk, S.; El-Daim, I.A.A.; Copolovici, L.; Tanilas, T.; Kännaste, A.; Behers, L.; Nevo, E.; Seisenbaeva, G.; Stenström, E.; Niinemets, Ü. Drought-tolerance of wheat improved by rhizosphere bacteria from harsh environments: Enhanced biomass production and reduced emissions of stress volatiles. PLoS ONE 2014, 9, e96086. [Google Scholar] [CrossRef] [PubMed]
- De Vries, F.T.; Griffiths, R.I.; Knight, C.; Nicolitch, O.; Williams, A.H. Harnessing rhizosphere microbiomes for drought-resilient crop production. Science 2020, 368, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Khan, W.; Zhu, Y.; Khan, A.; Zhao, L.; Yang, Y.; Wang, N.; Hao, M.; Ma, Y.; Nepal, J.; Ullah, F.; et al. Above-and below-ground feedback loop of maize is jointly enhanced by plant growth-promoting rhizobacteria and arbuscular mycorrhizal fungi in drier soil. Sci. Total Environ. 2024, 917, 170417. [Google Scholar] [CrossRef]
- Cong, G.; Yin, C.; He, B.; Li, L.; Gao, K. Effect of the endophytic fungus Chaetomium globosum ND35 on the growth and resistance to drought of winter wheat at the seedling stage under water stress. Acta Ecol. Sin. 2015, 35, 6120–6128. [Google Scholar] [CrossRef]
- Arzanesh, M.H.; Alikhani, H.A.; Khavazi, K.; Rahimian, H.; Miransari, M. Wheat (Triticum aestivum L.) growth enhancement by Azospirillum sp. under drought stress. World J. Microbiol. Biotechnol. 2011, 27, 197–205. [Google Scholar] [CrossRef]
- Barnawal, D.; Bharti, N.; Pandey, S.S.; Pandey, A.; Chanotiya, C.S.; Kalra, A. Plant growth-promoting rhizobacteria enhance wheat salt and drought stress tolerance by altering endogenous phytohormone levels and TaCTR1/TaDREB2 expression. Physiol. Plant. 2017, 161, 502–514. [Google Scholar] [CrossRef]
- Zhang, B.; Chang, S.X.; Anyia, A.O. Mycorrhizal inoculation and nitrogen fertilization affect the physiology and growth of spring wheat under two contrasting water regimes. Plant Soil 2015, 398, 47–57. [Google Scholar] [CrossRef]
- Getahun, A.; Muleta, D.; Assefa, F.; Kiros, S. Plant growth-promoting rhizobacteria isolated from degraded habitat enhance drought tolerance of acacia (Acacia abyssinica hochst. ex benth.) seedlings. Int. J. Microbiol. 2020, 29, 8897998. [Google Scholar] [CrossRef] [PubMed]
- Tiepo, A.N.; Constantino, L.V.; Madeira, T.B.; Gonçalves, L.S.A.; Pimenta, J.A.; Bianchini, E.; De Oliveira, A.L.M.; Oliveira, H.C.; Stolf-Moreira, R. Plant growth-promoting bacteria improve leaf antioxidant metabolism of drought-stressed Neotropical trees. Planta 2020, 251, 83. [Google Scholar] [CrossRef]
- Van Zelm, E.; Zhang, Y.; Testerink, C. Salt tolerance mechanisms of plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef]
- Navarro-Torre, S.; Barcia-Piedras, J.M.; Mateos-Naranjo, E.; Redondo-Gómez, S.; Camacho, M.; Caviedes, M.A.; Pajuelo, E.; Rodríguez-Llorente, I.D. Assessing the role of endophytic bacteria in the halophyte arthrocnemum macrostachyumsalt tolerance. Plant Biol. 2016, 19, 249–256. [Google Scholar] [CrossRef]
- Singh, S.; Singh, U.P.; Trivedi, M.; Sahu, P.K.; Paul, S.; Sivanesan, I.; Saxena, A.K. Seed biopriming with salt-tolerant endophytic pseudomonas geniculata-modulated biochemical responses provide ecological fitness in maize (Zea mays L.) grown in saline sodic soil. Int. J. Environ. Res. Public Health 2019, 17, 253. [Google Scholar] [CrossRef]
- Hameed, A.; Egamberdieva, D.; Abd-Allah, E.F.; Hashem, A.; Kumar, A.; Ahmad, P. Salinity stress and arbuscular mycorrhizal symbiosis in plants. In Use of Microbes for the Alleviation of Soil Stresses; Miransari, M., Ed.; Springer: New York, NY, USA, 2014; pp. 139–159. [Google Scholar] [CrossRef]
- Afridi, M.S.; Van Hamme, J.D.; Bundschuh, J.; Khan, M.N.; Salam, A.; Waqar, M.; Chaudhary, H.J. Biotechnological approaches in agriculture and environmental management-bacterium Kocuria rhizophila 14ASP as heavy metal and salt tolerant plant growth-promoting strain. Biologia 2021, 76, 3091–3105. [Google Scholar] [CrossRef]
- Moon, Y.; Ali, S. Possible mechanisms for the equilibrium of ACC and role of ACC deaminase-producing bacteria. Appl. Microbiol. Biotechnol. 2022, 106, 877–887. [Google Scholar] [CrossRef]
- Kumari, S.; Vaishnav, A.; Jain, S.; Varma, A.; Choudhary, D.K. Bacterial-mediated induction of systemic tolerance to salinity with expression of stress alleviating enzymes in soybean (Glycine max L. Merrill). J. Plant Growth Regul. 2015, 34, 558–573. [Google Scholar] [CrossRef]
- Mukherjee, P.; Mitra, A.; Roy, M. Halomonas rhizobacteria of avicennia marina of indian sundarbans promote rice growth under saline and heavy metal stresses through exopolysaccharide production. Front. Microbiol. 2019, 10, 1207. [Google Scholar] [CrossRef]
- Ren, C.; Dai, C. Jasmonic acid is involved in the signaling pathway for fungal endophyte-induced volatile oil accumulation of Atractylodes lancea plantlets. BMC Plant Biol. 2012, 12, 128. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.M.; Yang, B.; Ren, C.G.; Wang, H.W.; Wang, J.Y.; Dai, C.C. Involvement of abscisic acid and salicylic acid in signal cascade regulating bacterial endophyte-induced volatile oil biosynthesis in plantlets of Atractylodes lancea. Physiol. Plant. 2015, 153, 30–42. [Google Scholar] [CrossRef]
- Shao, M.; Zhao, W.; Su, Z.; Dong, L.; Guo, Q.; Ma, P. Effect of Bacillus subtilis NCD-2 on the growth of tomato and the microbial community structure of rhizosphere soil under salt stress. Sci. Agric. Sin. 2021, 54, 4573–4584. [Google Scholar]
- Mahmud-Ur-Rahman, N.; Naser, I.B.; Abd-Elsalam, K.A.; Sarker, A.; Hoque, M.; Islam, T. A highly salt-tolerant bacterium brevibacterium sediminis promotes the growth of rice (Oryza sativa L.) seedlings. Stresses 2022, 2, 275–289. [Google Scholar] [CrossRef]
- Akhtar, S.S.; Andersen, M.N.; Naveed, M.; Zahir, Z.A.; Liu, F. Interactive effect of biochar and plant growth-promoting bacterial endophytes on ameliorating salinity stress in maize. Funct. Plant Biol. 2015, 42, 770–781. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Charles, T.C.; Glick, B.R. Amelioration of high salinity stress damage by plant growth-promoting bacterial endophytes that contain ACC deaminase. Plant Physiol. Biochem. 2014, 80, 160–167. [Google Scholar] [CrossRef]
- Sharma, S.; Kulkarni, J.; Jha, B. Halotolerant rhizobacteria promote growth and enhance salinity tolerance in peanut. Front. Microbiol. 2016, 7, 1600. [Google Scholar] [CrossRef] [PubMed]
- Lastochkina, O.; Pusenkova, L.; Yuldashev, R.; Babaev, M.; Garipova, S.; Blagova, D.; Khairullin, R.; Aliniaeifard, S. Effects of Bacillus subtilis on some physiological and biochemical parameters of Triticum aestivum L. (wheat) under salinity. Plant Physiol. Biochem. 2017, 121, 80–88. [Google Scholar] [CrossRef]
- Shahzad, R.; Khan, A.L.; Bilal, S.; Waqas, M.; Kang, S.M.; Lee, I.J. Inoculation of abscisic acid-producing endophytic bacteria enhances salinity stress tolerance in Oryza sativa. Environ. Exp. Bot. 2017, 136, 68–77. [Google Scholar] [CrossRef]
- Islam, F.; Yasmeen, T.; Arif, M.; Ali, S.; Ali, B.; Hameed, S.; Zhou, W. Plant growth promoting bacteria confer salt tolerance in Vigna radiata by up-regulating antioxidant defense and biological soil fertility. Plant Growth Regul. 2016, 80, 23–36. [Google Scholar] [CrossRef]
- Vega, C.; Rodríguez, M.; Llamas, I.; Béjar, V.; Sampedro, I. Silencing of phytopathogen communication by the halotolerant PGPR Staphylococcus equorum strain EN21. Microorganisms 2019, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Baltruschat, H.; Fodor, J.; Harrach, B.D.; Niemczyk, E.; Barna, B.; Gullner, G.; Janeczko, A.; Kogel, K.; Schäfer, P.; Schwarczinger, I.; et al. Salt tolerance of barley induced by the root endophyte Piriformospora indica is associated with a strong increase in antioxidants. New Phytol. 2008, 180, 501–510. [Google Scholar] [CrossRef]
- Sen, S.; Chandrasekhar, C.N. Effect of PGPR on growth promotion of rice (Oryza sativa L.) under salt stress. Asian J. Plant Sci. Res. 2014, 4, 62–67. [Google Scholar]
- Conway, J.; Walton, W.G.; Salas-González, I.; Law, T.F.; Lindberg, C.A.; Crook, L.; Kosina, S.M.; Fitzpatrick, C.R.; Lietzan, A.; Northen, T.; et al. Diverse MarR bacterial regulators of auxin catabolism in the plant microbiome. Nat. Microbiol. 2022, 7, 1817–1833. [Google Scholar] [CrossRef]
- Birkenbihl, R.P.; Kracher, B.; Ross, A.; Kramer, K.; Finkemeier, I.; Somssich, I.E. Principles and characteristics of the Arabidopsis WRKY regulatory network during early MAMP-triggered immunity. Plant J. 2018, 96, 487–502. [Google Scholar] [CrossRef]
- Meena, K.K.; Sorty, A.M.; Bitla, U.M.; Choudhary, K.; Gupta, P.; Pareek, A.; Singh, D.P.; Prabha, R.; Sahu, P.K.; Gupta, V.K.; et al. Abiotic stress responses and microbe-mediated mitigation in plants: The omics strategies. Front. Plant Sci. 2017, 8, 172. [Google Scholar] [CrossRef]
- Zhalnina, K.; Louie, K.; Hao, Z.; Mansoori, N.; Da Rocha, U.N.; Shi, S.; Cho, H.; Karaöz, U.; Loqué, D.; Bowen, B.P.; et al. Dynamic root exudate chemistry and microbial substrate preferences drive patterns in rhizosphere microbial community assembly. Nat. Microbiol. 2018, 3, 470–480. [Google Scholar] [CrossRef]
- Preece, C.; Peñuelas, J. Rhizodeposition under drought and consequences for soil communities and ecosystem resilience. Plant Soil 2016, 409, 1–17. [Google Scholar] [CrossRef]
- Williams, A.; De Vries, F.T. Plant root exudation under drought: Implications for ecosystem functioning. New Phytol. 2019, 225, 1899–1905. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Cao, X.; Zhou, Y.; Ma, S.; Wang, Y.; Li, Z.; Zhao, D.; Yang, Y.; Zhang, H.; Meng, C.; et al. Purines enrich root-associated Pseudomonas and improve wild soybean growth under salt stress. Nat. Commun. 2024, 15, 3520. [Google Scholar] [CrossRef] [PubMed]
- Khokhar-Voytas, A.; Shahbaz, M.; Maqsood, M.F.; Zulfiqar, U.; Naz, N.; Iqbal, U.; Sarà, M.; Aqeel, M.; Khalid, N.; Noman, A.; et al. Genetic modification strategies for enhancing plant resilience to abiotic stresses in the context of climate change. Funct. Integr. Genom. 2023, 23, 283. [Google Scholar] [CrossRef]
- Olejnik, P.; Madrzak, C.J.; Nuc, K. Cyclophilins and their functions in abiotic stress and Plant–Microbe interactions. Biomolecules 2021, 11, 1390. [Google Scholar] [CrossRef]
- Nikolić, B.; Schwab, H.; Sessitsch, A. Metagenomic analysis of the 1-aminocyclopropane-1-carboxylate deaminase gene (acdS) operon of an uncultured bacterial endophyte colonizing Solanum tuberosum L. Arch. Microbiol. 2011, 193, 665–676. [Google Scholar] [CrossRef]
- Ghorbanpour, A.; Salimi, A.; Ghanbary, M.A.T.; Pirdashti, H.; Dehestani, A. The effect of Trichoderma harzianum in mitigating low temperature stress in tomato (Solanum lycopersicum L.) plants. Sci. Hortic. 2018, 230, 134–141. [Google Scholar] [CrossRef]
- Abuqamar, S.F.; Luo, H.; Laluk, K.; Mickelbart, M.V.; Mengiste, T. Crosstalk between biotic and abiotic stress responses in tomato is mediated by the AIM1 transcription factor. Plant J. 2009, 58, 347–360. [Google Scholar] [CrossRef]
- Sziderics, A.H.; Rasche, F.; Trognitz, F.; Sessitsch, A.; Wilhelm, E. Bacterial endophytes contribute to abiotic stress adaptation in pepper plants (Capsicum annuum L.). Can. J. Microbiol. 2007, 53, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Rosenblueth, M.; Martínez-Romero, E. Rhizobium etli maize populations and their competitiveness for root colonization. Arch. Microbiol. 2004, 181, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Arora, N.K.; Fatima, T.; Mishra, I.; Verma, S. Microbe-based inoculants: Role in next Green Revolution. In Environmental Concerns and Sustainable Development; Shukla, V., Kumar, N., Eds.; Springer: Singapore, 2020; pp. 191–246. [Google Scholar] [CrossRef]
- Waadt, R.; Seller, C.A.; Hsu, P.K.; Takahashi, Y.; Munemasa, S.; Schroeder, J.I. Plant hormone regulation of abiotic stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 680–694. [Google Scholar] [CrossRef]
- Bastías, D.A.; Balestrini, R.; Pollmann, S.; Gundel, P.E. Environmental interference of plant−microbe interactions. Plant Cell Environ. 2022, 45, 3387–3398. [Google Scholar] [CrossRef] [PubMed]
- Hammerbacher, A.; Coutinho, T.A.; Gershenzon, J. Roles of plant volatiles in defence against microbial pathogens and microbial exploitation of volatiles. Plant Cell Environ. 2019, 42, 2827–2843. [Google Scholar] [CrossRef]
- Sorty, A.M.; Meena, K.K.; Choudhary, K.; Bitla, U.M.; Minhas, P.S.; Krishnani, K.K. Effect of plant growth promoting bacteria associated with halophytic weed (Psoralea corylifolia L.) on germination and seedling growth of wheat under saline conditions. Appl. Biochem. Biotechnol. 2016, 180, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Marra, R.; Barbetti, M.J.; Li, H.; Woo, S.L.; Lorito, M. A novel role for Trichoderma secondary metabolites in the interactions with plants. Physiol. Mol. Plant Pathol. 2008, 72, 80–86. [Google Scholar] [CrossRef]
- Glick, B.R.; Penrose, D.M.; Li, J. A model for the lowering of plant ethylene concentrations by plant growth-promoting bacteria. J. Theor. Biol. 1998, 190, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, K.; Yamaguchi-Shinozaki, K. Gene networks involved in drought stress response and tolerance. J. Exp. Bot. 2006, 58, 221–227. [Google Scholar] [CrossRef]
- Zhou, C.; Li, F.; Xie, Y.; Zhu, L.; Xiao, X.; Ma, Z.; Wang, J. Involvement of abscisic acid in microbe-induced saline-alkaline resistance in plants. Plant Signal. Behav. 2017, 12, e1367465. [Google Scholar] [CrossRef]
- Shah, S.; Chen, C.; Sun, Y.; Wang, D.; Nawaz, T.; El-Kahtany, K.; Fahad, S. Mechanisms of nitric oxide involvement in plant-microbe interaction and its enhancement of stress resistance. Plant Stress 2023, 10, 100191. [Google Scholar] [CrossRef]
- Ghosh, D.; Gupta, A.; Mohapatra, S. Dynamics of endogenous hormone regulation in plants by phytohormone secreting rhizobacteria under water-stress. Symbiosis 2019, 77, 265–278. [Google Scholar] [CrossRef]
- Mendes, R.E.; Garbeva, P.; Raaijmakers, J.M. The rhizosphere microbiome: Significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 2013, 37, 634–663. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Cortés-Penagos, C.; López-Bucio, J. Trichoderma virens, a plant beneficial fungus, enhances biomass production and promotes lateral root growth through an auxin-dependent mechanism in arabidopsis. Plant Physiol. 2009, 149, 1579–1592. [Google Scholar] [CrossRef]
- He, X.; Shao, C.; Wu, A.; Xia, L.; Li, T.; Pei, J.; Zhang, N.; Wang, Y. Arbuscular mycorrhizal fungi enhance nutrient acquisition and reduce aluminum toxicity in Lespedeza formosa under acid rain. Environ. Sci. Pollut. Res. 2022, 29, 29904–29916. [Google Scholar] [CrossRef]
- Riquelme, M.; Aguirre, J.; Bartnicki-García, S.; Braus, G.H.; Feldbrügge, M.; Fleig, U.; Hansberg, W.; Herrera-Estrella, A.; Kämper, J.; Kück, U.; et al. Fungal morphogenesis, from the polarized growth of hyphae to complex reproduction and infection structures. Microbiol. Mol. Biol. Rev. 2018, 82, e00068-17. [Google Scholar] [CrossRef]
- Zhu, X.; Song, F.; Liu, S.; Liu, T. Effects of arbuscular mycorrhizal fungus on photosynthesis and water status of maize under high temperature stress. Plant Soil 2011, 346, 189–199. [Google Scholar] [CrossRef]
- Karthik, C.; Elangovan, N.; Kumar, T.S.; Govindharaju, S.; Barathi, S.; Oves, M.; Arulselvi, P.I. Characterization of multifarious plant growth promoting traits of rhizobacterial strain AR6 under Chromium (VI) stress. Microbiol. Res. 2017, 204, 65–71. [Google Scholar] [CrossRef]
- Mathesius, U.; Mulders, S.; Gao, M.; Teplitski, M.; Caetano-Anollés, G.; Rolfe, B.G.; Bauer, W. Extensive and specific responses of a eukaryote to bacterial quorum-sensing signals. Proc. Natl. Acad. Sci. USA 2003, 100, 1444–1449. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, H.; Fraga, R. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol. Adv. 1999, 17, 319–339. [Google Scholar] [CrossRef] [PubMed]
- Collavino, M.M.; Sansberro, P.A.; Mroginski, L.A.; Aguilar, O.M. Comparison of in vitro solubilization activity of diverse phosphate-solubilizing bacteria native to acid soil and their ability to promote Phaseolus vulgaris growth. Biol. Fertil. Soils 2010, 46, 727–738. [Google Scholar] [CrossRef]
- Liu, F.; Ma, H.; Peng, L.; Du, Z.; Ma, B.; Liu, X. Effect of the inoculation of plant growth-promoting rhizobacteria on the photosynthetic characteristics of Sambucus williamsii Hance container seedlings under drought stress. AMB Express 2019, 9, 169. [Google Scholar] [CrossRef]
- Ratajczak, K.; Sulewska, H.; Błaszczyk, L.; Basińska-Barczak, A.; Mikołajczak, K.; Salamon, S.; Szymańska, G.; Dryjański, L. Growth and photosynthetic activity of selected spelt varieties (Triticum aestivum ssp. spelta L.) cultivated under drought conditions with different endophytic core microbiomes. Int. J. Mol. Sci. 2020, 21, 7987. [Google Scholar] [CrossRef]
- Gou, W.; Li, T.; Ruan, Z.; Zheng, P.; Chen, F.; Zhang, L.; Zhiyan, C.; Zheng, P.; Zheng, L.; Gao, M.; et al. Accumulation of choline and glycinebetaine and drought stress tolerance induced in maize (Zea mays) by three plant growth promoting rhizobacteria (PGPR) strains. Pak. J. Bot. 2015, 47, 581–586. [Google Scholar]
- Zareyan, M.; Mockevičiūtė, R.; Jurkonienė, S.; Gavelienė, V.; Paškevičius, A.; Šveikauskas, V. Physiological, biochemical, and genetic reactions of winter wheat to drought under the influence of plant growth promoting microorganisms and calcium. Microorganisms 2025, 13, 1042. [Google Scholar] [CrossRef]
- Heidarianpour, M.B.; Aliasgharzad, N.; Olsson, P.A. Positive effects of co-inoculation with Rhizophagus irregularis and Serendipita indica on tomato growth under saline conditions, and their individual colonization estimated by signature lipids. Mycorrhiza 2020, 30, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Li, Y.W.; Ling, K.J.; Wang, J.Q.; Yan, H.; Mou, L.J.; Jiang, J.L.; Fang, F.; Liu, R.Q. Effects of arbuscular mycorrhizal fungi inoculation on salt-tolerance of tomato plants. Fujian J. Agric. Sci. 2022, 37, 188–196. [Google Scholar]
| Name of Microorganism | Test Crop | Level of Stress | Microorganism Dosage Usage | Response of Microbial Inoculated Plants to Drought Stress as Compared to Controls | Mechanism of Action | Reference |
|---|---|---|---|---|---|---|
| Arbuscular mycorrhizal fungi (AMF) | Maize (Zea mays L.) | 35%, 55%, 80% field water holding capacity | 10 mL 108 CFU/mL | Enhances root colonization, water utilization and root hydraulic conductivity, thereby improving nutrient uptake in the corn root system and aboveground | (3) (4) (5) (6) | [38] |
| Chaetomium globosum ND35 | Wheat (Triticum aestivum L.) | 30% of maximum water holding capacity | 106 CFU/mL | Promote root and plant development during the seedling stage of winter wheat, allowing wheat to enter the three-leaf stage earlier, enhance drought avoidance, and at the same time improve root activity and increase drought resistance | (2) (3) (5) | [39] |
| Glomous mosseae | Chinese wildrye (Leymus chinensis) | 10% PEG | 5 g AMF strain | Inhibition of Na+, Cl− uptake, enhancement of Ka+ uptake, elevated proline content, elevated antioxidant defense enzyme content | (1) (3) (5) | [35] |
| Azospirillum lipoferum AZ1, Azospirillum lipoferum AZ45, Azospirillum lipoferum AZ9 | Wheat (Triticum aestivum L.) | 80, 50, 25% field water holding capacity | 3.2 × 109 CFU/mL | Indole-3-acetic acid (IAA), and proteins, polyamines, nitrogen fixation, root growth promotion | (1) (4) (5) | [40] |
| Bacillus cereus L90 | Walnut (Juglans regia L.) | Water content 34.64% | 2 × 108 CFU/mL | Promotes secretion of cytokinin (CTK), which increases net photosynthetic rate, stomatal conductance, intercellular CO2 concentration and chlorophyll content | (1) (3) (4) (6) | [33] |
| Bacillus megatherium BOFC15 | Arabidopsis thaliana (Arabidopsis thaliana L. Heynh.) | 200 min of dehydration | 1 mL bacterial diluent | Increase plant biomass, improved root structure, and enhanced photosynthetic capacity. | (1) (2) (3) (4) (6) | [34] |
| Sarthrobacter protophormiae SA3 Dietzia natronolimnaea STR1 Bacillus subtilis LDR2 | Wheat (Triticum aestivum L.) | 10% PEG | 25 mL 105 CFU/mL | Increase IAA content, photosynthetic efficiency, reduced abscisic acid and Enzyme 1-amino-cyclopropane-1-carboxylate (ACC) content | (1) (2) (4) (6) | [41] |
| AMF | Spring wheat (Triticum aestivum L.) | 40% soil moisture content | Inoculums (1600 propagules/g) were mixed with wheat seeds at 10 mL/kg pre-wetting rate. | Increase N and P concentrations in stems and grains resulted in a significant increase in the plant’s water use efficiency | (1) (5) (6) | [42] |
| Pseudomonas fluorescens strains FB-49 | Acacia martius (Acacia farnesiana L. Willd.) | Keep 20% water content | 15 mL 108 CFU/mL | Increase root length, aboveground node length and dry biomass of plants | (1) (5) | [43] |
| Azotobacter brazilensis Bacillus sp. | Tropical trees (Pinus tropicalis Morelet) | 14, 30% humidity | 50 mL 106 CFU/mL | Induce greater accumulation of secondary compounds and increased leaf area. | (1) (3) | [44] |
| Name of Microorganism | Test Crop | Level of Stress | Microorganism Dosage Usage | Response of Microbial Inoculated Plants to High Salt Stress as Compared to Controls | Mechanism of Action | Reference |
|---|---|---|---|---|---|---|
| Bacillus subtilis NCD-2 | Tomato (Solanum lycopersicum L.) | 100 mmol/L NaCl | 1.0 × 109 CFU/mL | Enhanced resistance enzyme activity, increased ABA content, and enriched rhizosphere beneficial microbes | (1) (3) (4) | [55] |
| Brevibacterium sediminis Strain IBGE3C | Rice (Oryza sativa L.) | 0.2–1.2% NaCl | Seed soaking | Improve rice varieties with different levels of salt tolerance | (1) (3) (4) | [56] |
| Burkholderia phytofirmans PsJN Enterobacter sp. FD17 | Maize (Zea mays L.) | 25 mmol/L NaCl | Mix 20 mL of bacterial suspension with 100 g of sterilized peat | Reduce xylem Na+ concentration uptake, thereby maintaining nutrient balance and promoting plant growth | (1) (4) (5) | [57] |
| Pseudomonas fluorescens YsS6 Pseudomonas migulae 8R6 | Tomato (Solanum lycopersicum L.) | 165, 185 mmol/L NaCl | 1.75 × 108–1.97 × 108 CFU/ml | Higher fresh and dried biomass, higher chlorophyll content and more flowers and buds reduce salt stress | (1) (4) (6) | [58] |
| Klebsiella pseudomonas Agrobacterium ochrobactrum | Peanut (Arachis hypogaea L.) | 4, 8% NaCl | 108 cells/mL | Increase solubilization of phosphorus; promotes stem length, root length, shoot and root growth in peanut plants | (1) (2) (3) (4) (5) | [59] |
| Bacillus subtilis 10-4 | Babury Wolfberry Fruit (Lycium chinense Miller) | 2% NaCl | 105 CFU/mL | Inhibition of Salicylic acid (SA) accumulation, increase in water storage capacity in leaf tissues | (1) (3) (4) (5) | [60] |
| Bacillus amyloliquefaciens RWL-1 | Rice (Oryza sativa L.) | 40 g/L NaCl | 108 CFU/mL | Increase essential amino acids and SA, decreased ABA levels | (1) (4) (5) | [61] |
| Bacillus cereous Pb25 | Mung bean (Vigna radiata (L.) R. Wilczek) | Electric conductivity 9 dS/m | 107–108 CFU/mL | Increase plant antioxidant enzyme activity, proline, potassium, nitrogen, and phosphorus accumulation; decreased sodium accumulation | (1) (3) (4) (5) | [62] |
| Staphylococcus equorum strain EN21 | Tomato (Solanum lycopersicum L.) | 30% NaCl | 109 CFU/mL | Increase seed vigor index, branch length and root dry weight of plants | (1) (3) (4) (5) (6) | [63] |
| Pseudomonas strains AK-1 Bacillus strains SJ-5 | Soybean (Glycine max (L.) Merr.) | 100 mmol/L NaCl | 108 CFU/mL | Increase plant biomass, leaf water content, photosynthetic activity; increased proline accumulation and peroxidase (POX) activity | (1) (2) (3) (4) (5) (6) | [51] |
| Avicennia marina | Rice (Oryza sativa L.) | 0.5–22.5% NaCl | 108 CFU/mL | Promotes solubilization of inorganic phosphate and enhances nutrient uptake | (1) | [52] |
| Piriformospora indica | Barley (Hordeum vulgare L.) | 100, 300 mmol/L NaCl | The mycelial colonization rate is 50–60% | Enhancement of Ascorbate peroxidase (APX) activity in barley roots | (1) (3) (4) (6) | [64] |
| Pseudomonas strains PF1/TDK1 | Rice (Oryza sativa L.) | 100 mmol/L NaCl | 0.2 g/L | Plant height, root length, aboveground and root dry weight were significantly increased | (1) (3) (4) (6) | [65] |
| Trichoderma harzianum | Indian mustard (Brassica juncea L.) | 100, 200 mmol/L NaCl | 2 × 109 CFU/mL | Increase oil content improves absorption of essential nutrients, enhances antioxidant and osmotic agent accumulation, and reduces salt absorption | (1) (3) (4) (5) (6) | [28] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, D.; Li, W.; Huang, P.; Gu, M.; Tang, G.; Ding, Y.; Cao, G.; Xu, W. Mechanisms of Microorganisms Alleviating Drought and Salt Stresses in Plants. Microorganisms 2025, 13, 2565. https://doi.org/10.3390/microorganisms13112565
Feng D, Li W, Huang P, Gu M, Tang G, Ding Y, Cao G, Xu W. Mechanisms of Microorganisms Alleviating Drought and Salt Stresses in Plants. Microorganisms. 2025; 13(11):2565. https://doi.org/10.3390/microorganisms13112565
Chicago/Turabian StyleFeng, Di, Wenxiang Li, Pengfei Huang, Meiying Gu, Guangmu Tang, Yanhong Ding, Gang Cao, and Wanli Xu. 2025. "Mechanisms of Microorganisms Alleviating Drought and Salt Stresses in Plants" Microorganisms 13, no. 11: 2565. https://doi.org/10.3390/microorganisms13112565
APA StyleFeng, D., Li, W., Huang, P., Gu, M., Tang, G., Ding, Y., Cao, G., & Xu, W. (2025). Mechanisms of Microorganisms Alleviating Drought and Salt Stresses in Plants. Microorganisms, 13(11), 2565. https://doi.org/10.3390/microorganisms13112565






