Diversity of the Alongshan Virus in Ixodes Ticks Collected in the Russian Federation in 2023
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Processing of Ticks
2.2. Reverse Transcriptase PCR (RT-PCR) and Sequencing of Amplified Products
2.3. NGS Sequencing and NGS Data Analysis
2.4. Phylogenetic Analysis
2.5. Nucleotide Sequence Accession Numbers
2.6. Biosafety
3. Results
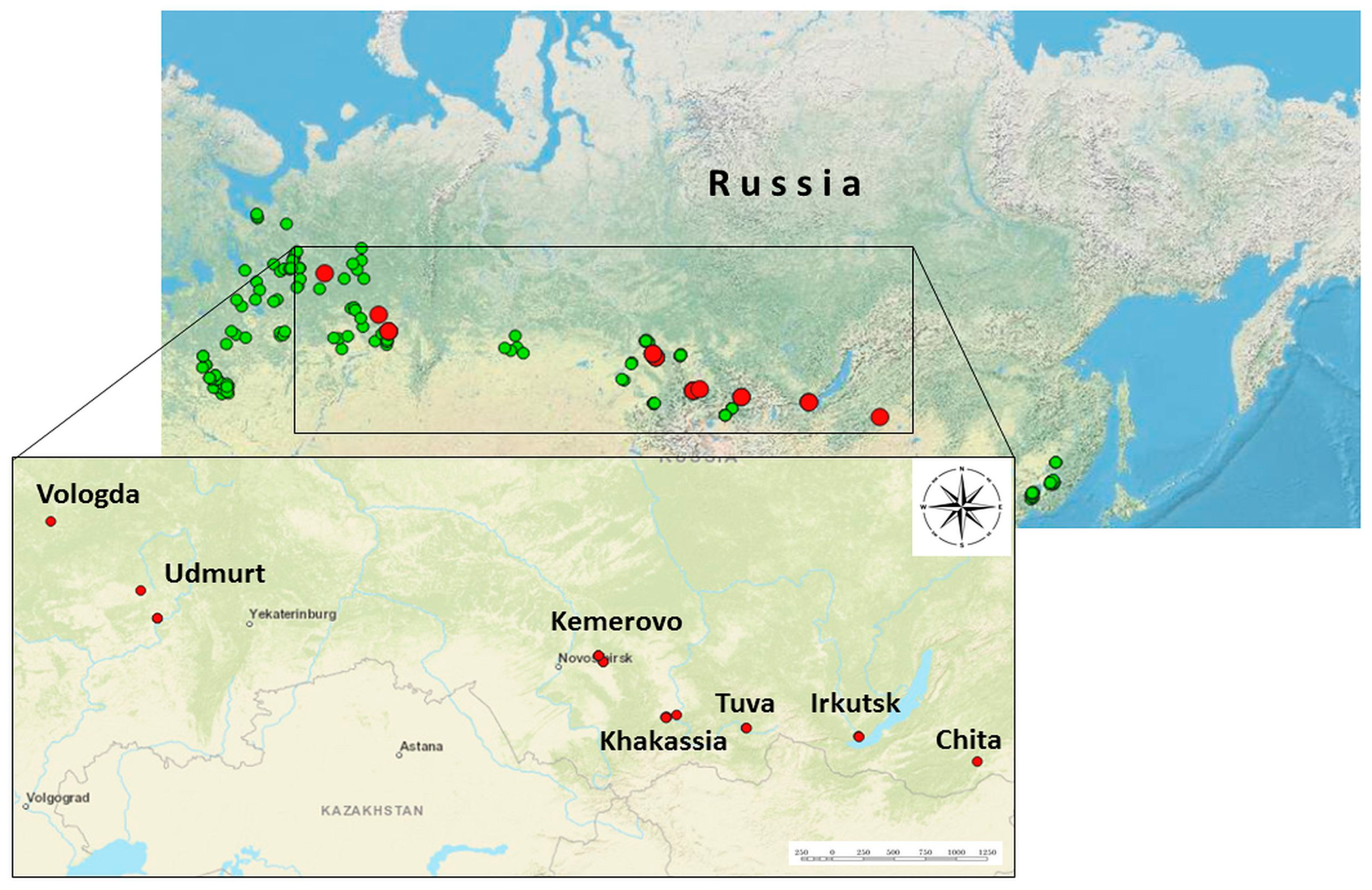
3.1. Tick Collection and ALSV Detection
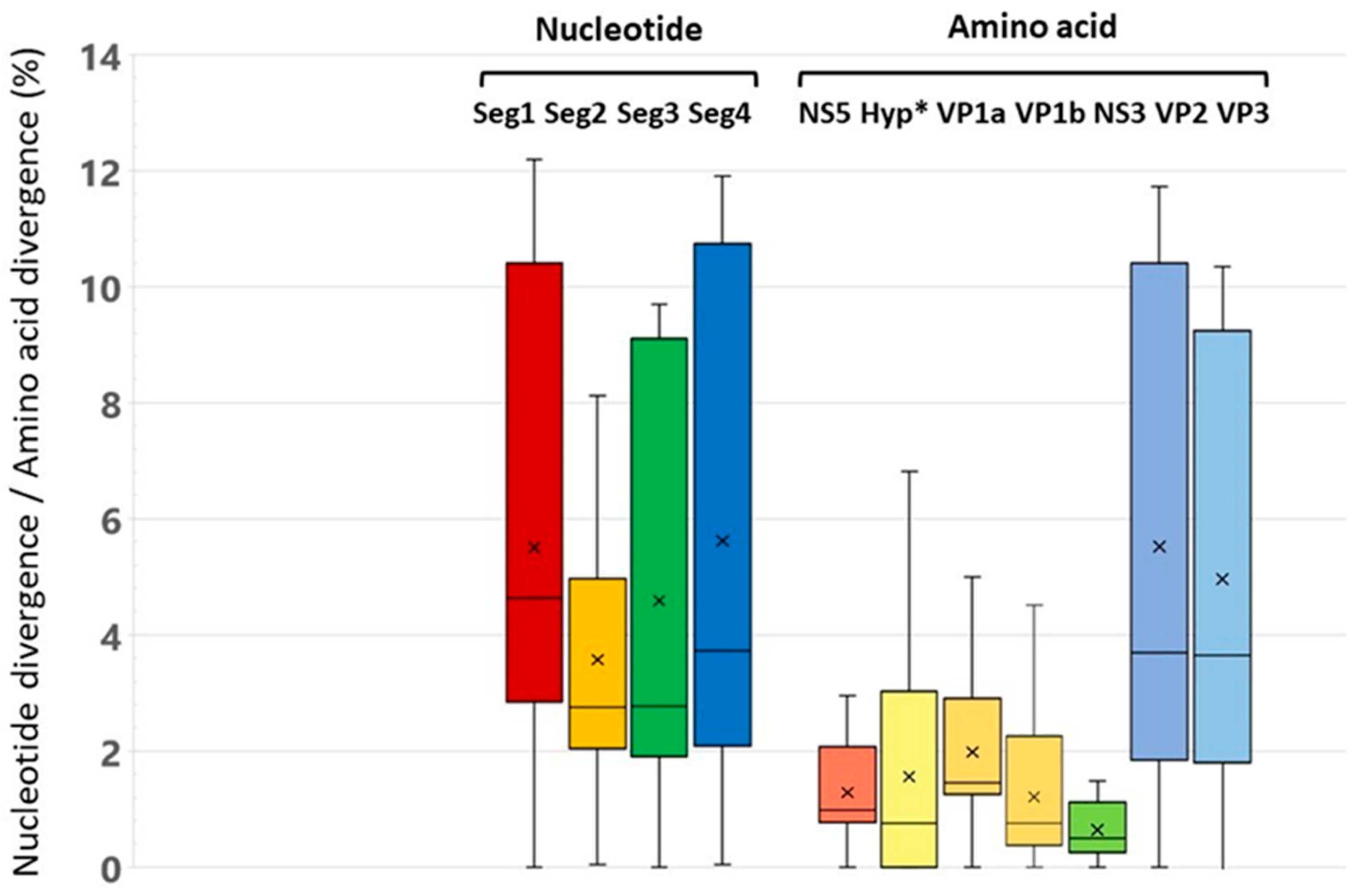
3.2. Analysis of Genome Identity
3.3. Phylogenetic Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALSV | Alongshan virus |
| ssRNA | single-stranded RNA |
| dsDNA | double-stranded DNA |
References
- Pierson, T.C.; Diamond, M.S. The continued threat of emerging flaviviruses. Nat. Microbiol. 2020, 5, 796–812. [Google Scholar] [CrossRef] [PubMed]
- Leonova, G.N.; Kondratov, I.G.; Ternovoi, V.A.; Romanova, E.V.; Protopopova, E.V.; Chausov, E.V.; Pavlenko, E.V.; Ryabchikova, E.I.; Belikov, S.I.; Loktev, V.B. Characterization of Powassan viruses from Far Eastern Russia. Arch. Virol. 2009, 154, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Ruzek, D.; Avšič Županc, T.; Borde, J.; Chrdle, A.; Eyer, L.; Karganova, G.; Kholodilov, I.; Knap, N.; Kozlovskaya, L.; Matveev, A.; et al. Tick-borne encephalitis in Europe and Russia: Review of pathogenesis, clinical features, therapy, and vaccines. Antivir. Res. 2019, 164, 23–51. [Google Scholar] [CrossRef] [PubMed]
- Tyulko, Z.S.; Fadeev, A.V.; Vasilenko, A.G.; Gradoboeva, E.A.; Yakimenko, V.V.; Komissarov, A.B. Analysis of changes in the genome of the Omsk hemorrhagic fever virus (Flaviviridae: Orthoflavivirus) during laboratory practices for virus preservation. Probl. Virol. (Vopr. Virusol.) 2024, 69, 509–523. (In Russian) [Google Scholar] [CrossRef]
- Ternovoi, V.A.; Gladysheva, A.V.; Ponomareva, E.P.; Mikryukova, T.P.; Protopopova, E.V.; Shvalov, A.N.; Konovalova, S.N.; Chausov, E.V.; Loktev, V.B. Variability in the 3′ untranslated regions of the genomes of the different tick-borne encephalitis virus subtypes. Virus Genes 2019, 55, 448–457. [Google Scholar] [CrossRef]
- Postler, T.S.; Beer, M.; Blitvich, B.J.; Bukh, J.; de Lamballerie, X.; Drexler, J.F.; Imrie, A.; Kapoor, A.; Karganova, G.G.; Lemey, P.; et al. Renaming of the genus Flavivirus to Orthoflavivirus and extension of binomial species names within the family Flaviviridae. Arch. Virol. 2023, 168, 224. [Google Scholar] [CrossRef]
- Qin, X.C.; Shi, M.; Tian, J.H.; Lin, X.D.; Gao, D.Y.; He, J.R.; Wang, J.B.; Li, C.X.; Kang, Y.J.; Yu, B.; et al. A tick-borne segmented RNA virus contains genome segments derived from unsegmented viral ancestors. Proc. Natl. Acad. Sci. USA 2014, 111, 6744–6749. [Google Scholar] [CrossRef]
- Villa, E.C.; Maruyama, S.R.; de Miranda-Santos, I.K.F.; Palacios, G.; Ladner, J.T. Complete Coding Genome Sequence for Mogiana Tick Virus, a Jingmenvirus Isolated from Ticks in Brazil. Genome Announc. 2017, 5, e00232-17. [Google Scholar] [CrossRef]
- Colmant, A.M.G.; Charrel, R.N.; Coutard, B. Jingmenviruses: Ubiquitous, understudied, segmented flavi-like viruses. Front. Microbiol. 2022, 13, 997058. [Google Scholar] [CrossRef]
- Ogola, E.O.; Roy, A.; Wollenberg, K.; Ochwoto, M.; Bloom, M.E. Strange relatives: The enigmatic arbo-jingmenviruses and orthoflaviviruses. npj Viruses 2025, 3, 24. [Google Scholar] [CrossRef]
- Temmam, S.; Bigot, T.; Chrétien, D.; Gondard, M.; Pérot, P.; Pommelet, V.; Dufour, E.; Petres, S.; Devillers, E.; Hoem, T.; et al. Insights into the host range, genetic diversity, and geographical distribution of Jingmenviruses. mSphere 2019, 4, e00645-19. [Google Scholar] [CrossRef]
- Valle, C.; Parry, R.H.; Coutard, B.; Colmant, A.M.G. Discovery of additional genomic segments reveals the fluidity of jingmenvirus genomic organization. Virus Evol. 2025, 11, veaf023. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.D.; Wang, B.; Wei, F.; Han, S.Z.; Zhang, L.; Yang, Z.T.; Yan, Y.; Lv, X.L.; Li, L.; Wang, S.C.; et al. A New Segmented Virus Associated with Human Febrile Illness in China. N. Engl. J. Med. 2019, 380, 2116–2125. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.D.; Wang, W.; Wang, N.N.; Qiu, K.; Zhang, X.; Tana, G.; Liu, Q.; Zhu, X.Q. Prevalence of the emerging novel Alongshan virus infection in sheep and cattle in Inner Mongolia, northeastern China. Parasites Vectors 2019, 12, 450. [Google Scholar] [CrossRef] [PubMed]
- Kuivanen, S.; Levanov, L.; Kareinen, L.; Sironen, T.; Jääskeläinen, A.J.; Plyusnin, I.; Zakham, F.; Emmerich, P.; Schmidt-Chanasit, J.; Hepojoki, J.; et al. Detection of novel tick-borne pathogen, Alongshan virus, in Ixodes ricinus ticks, south-eastern Finland, 2019. Eurosurveillance 2019, 24, 1900394. [Google Scholar] [CrossRef]
- Stanojević, M.; Li, K.; Stamenković, G.; Ilić, B.; Paunović, M.; Pešić, B.; Maslovara, I.Đ.; Šiljić, M.; Ćirković, V.; Zhang, Y. Depicting the RNA virome of hematophagous arthropods from Belgrade, Serbia. Viruses 2020, 12, 975. [Google Scholar] [CrossRef]
- Ebert, C.L.; Söder, L.; Kubinski, M.; Glanz, J.; Gregersen, E.; Dümmer, K.; Grund, D.; Wöhler, A.S.; Könenkamp, L.; Liebig, K.; et al. Detection and characterization of Alongshan virus in ticks and tick saliva from Lower Saxony, Germany with serological evidence for viral transmission to game and domestic animals. Microorganisms 2023, 11, 543. [Google Scholar] [CrossRef]
- Stegmüller, S.; Fraefel, C.; Kubacki, J. Genome Sequence of Alongshan virus from Ixodes ricinus ticks collected in Switzerland. Microbiol. Resour. Announc. 2023, 12, e0128722. [Google Scholar] [CrossRef]
- Kholodilov, I.S.; Litov, A.G.; Klimentov, A.S.; Belova, O.A.; Polienko, A.E.; Nikitin, N.A.; Shchetinin, A.M.; Ivannikova, A.Y.; Bell-Sakyi, L.; Yakovlev, A.S.; et al. Isolation and characterisation of Alongshan virus in Russia. Viruses 2020, 12, 362. [Google Scholar] [CrossRef]
- Kholodilov, I.S.; Belova, O.A.; Morozkin, E.S.; Litov, A.G.; Ivannikova, A.Y.; Makenov, M.T.; Shchetinin, A.M.; Aibulatov, S.V.; Bazarova, G.K.; Bell-Sakyi, L.; et al. Geographical and tick-dependent distribution of flavi-like Alongshan and Yanggou tick viruses in Russia. Viruses 2021, 13, 458. [Google Scholar] [CrossRef]
- Kholodilov, I.S.; Belova, O.A.; Ivannikova, A.Y.; Gadzhikurbanov, M.N.; Makenov, M.T.; Yakovlev, A.S.; Polienko, A.E.; Dereventsova, A.V.; Litov, A.G.; Gmyl, L.V.; et al. Distribution and characterisation of tick-borne flavi-, flavi-like, and phenuiviruses in the Chelyabinsk Region of Russia. Viruses 2022, 14, 2699. [Google Scholar] [CrossRef]
- Kartashov, M.Y.; Krivosheina, E.I.; Kurushina, V.Y.; Moshkin, A.B.; Khankhareev, S.S.; Biche-ool, C.R.; Pelevina, O.N.; Popov, N.V.; Bogomazova, O.L.; Ternovoi, V.A. Prevalence and genetic diversity of the Alongshan virus (Flaviviridae) circulating in ticks in the south of Eastern Siberia. Probl. Virol. (Vopr. Virusol.) 2024, 69, 151–161. (In Russian) [Google Scholar] [CrossRef]
- Tamura, K.; Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Ladner, J.T.; Wiley, M.R.; Beitzel, B.; Auguste, A.J.; Dupuis, A.P.; Lindquist, M.E.; Sibley, S.D.; Kota, K.P.; Fetterer, D.; Eastwood, G.; et al. A Multicomponent Animal Virus Isolated from Mosquitoes. Cell Host Microbe 2016, 20, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Souza, W.M.; Fumagalli, M.J.; Torres Carrasco, A.O.; Romeiro, M.F.; Modha, S.; Seki, M.C.; Gheller, J.M.; Daffre, S.; Nunes, M.R.T.; Murcia, P.R.; et al. Viral diversity of Rhipicephalus microplus parasitizing cattle in southern Brazil. Sci. Rep. 2018, 8, 16315. [Google Scholar] [CrossRef]
- Emmerich, P.; Jakupi, X.; von Possel, R.; Berisha, L.; Halili, B.; Günther, S.; Cadar, D.; Ahmeti, S.; Schmidt-Chanasit, J. Viral metagenomics, genetic and evolutionary characteristics of Crimean-Congo hemorrhagic fever orthonairovirus in humans, Kosovo. Infect. Genet. Evol. 2018, 65, 6–11. [Google Scholar] [CrossRef]
- Kartashov, M.Y.; Krivosheina, E.I.; Naidenova, E.V.; Zakharov, K.S.; Shvalov, A.N.; Boumbaly, S.; Ternovoi, V.A.; Loktev, V.B. Simultaneous Detection and Genome Analysis of the Kindia Tick Virus in Cattle and Rhipicephalus Ticks in the Republic of Guinea. Vector Borne Zoonotic Dis. 2025, 25, 470–475. [Google Scholar] [CrossRef]
- Ternovoi, V.A.; Gladysheva, A.V.; Sementsova, A.O.; Zaykovskaya, A.V.; Volynkina, A.S.; Kotenev, E.S.; Agafonov, A.P.; Loktev, V.B. Detection of the RNA for new multicomponent virus in patients with Crimean-Congo hemorrhagic fever in southern Russia. Ann. Russ. Acad. Med. Sci. 2020, 75, 129–134. (In Russian) [Google Scholar] [CrossRef]
- Su, S.; Cui, M.Y.; Xing, L.L.; Gao, R.J.; Mu, L.; Hong, M.; Guo, Q.Q.; Ren, H.; Yu, J.F.; Si, X.Y.; et al. Metatranscriptomic analysis reveals the diversity of RNA viruses in ticks in Inner Mongolia, China. PLoS Negl. Trop. Dis. 2024, 18, e0012706. [Google Scholar] [CrossRef]
- Xu, W.; Wang, W.; Li, L.; Li, N.; Liu, Z.; Che, L.; Wang, G.; Zhang, K.; Feng, X.; Wang, W.J.; et al. Alongshan Virus Infection in Rangifer tarandus Reindeer, Northeastern China. Emerg. Infect. Dis. 2024, 30, 1434–1437. [Google Scholar] [CrossRef]
- Korobitsyn, I.G.; Moskvitina, N.S.; Tyutenkov, O.Y.; Gashkov, S.I.; Kononova, Y.V.; Moskvitin, S.S.; Romanenko, V.N.; Mikryukova, T.P.; Protopopova, E.V.; Kartashov, M.Y.; et al. Detection of tick-borne pathogens in wild birds and their ticks in Western Siberia and high level of their mismatch. Folia Parasitol. (Praha) 2021, 68, 024. [Google Scholar] [CrossRef]
- Kartashov, M.Y.; Gladysheva, A.V.; Shvalov, A.N.; Tupota, N.L.; Chernikova, A.A.; Ternovoi, V.A.; Loktev, V.B. Novel Flavi-like virus in ixodid ticks and patients in Russia. Ticks Tick Borne Dis. 2023, 14, 102101. [Google Scholar] [CrossRef]

| Description | Primers | Sequences (5′–3′) | Size (bp) |
|---|---|---|---|
| Segment 1 | AL1_1F | GCCATGATTGTCCTGATAGTG | |
| AL1_1R | GCCCTGTCCATCTTCATTTCC | 982 | |
| AL1_2F | AGGAAAGACAGATCACTCAC | ||
| AL1_2R | GGACATCATGGACTTCTCCT | 1038 | |
| AL1_3F | AGAAGTCCATGATGTCCTCC | ||
| AL1_3R | GTTCATCCAGTCCTTGTAGTTTCC | 840 | |
| Segment 2 | AL2_1F | GTAACCTCCGTAGACTGTCCA | |
| AL2_1R | GTCCCTTCCGTTTGGTTGTG | 477 | |
| AL2_2F | CTTGCTACATCGGAATCATGCC | ||
| AL2_2R | GATAAGCCCTCTCGATACCTC | 1091 | |
| AL2_3F | TGGTACGACTGGCTTTCGAG | ||
| AL2_3R | ACTTGTTGTAGTCTGCAACCC | 1098 | |
| Segment 3 | AL3_1F | TCGTCCAAGACTACTTAACAG | |
| AL3_1R | GTATCGCCTGTCCTCTATCC | 721 | |
| AL3_2F | TGCTGTCCATAGCAATCATACC | ||
| AL3_2R | GTAGGACACGTCCTTTGCGA | 865 | |
| AL3_3F | GCAAAGGACGTGTCCTACGT | ||
| AL3_3R | TTACCACTTGCTGGTCACAG | 1314 | |
| Segment 4 | AL4_1F | ACTTTGATCTACATCCTCGCC | |
| AL4_1R | GTATCCAGCTCTTCCCTTCTC | 824 | |
| AL4_2F | GGAAGAGCTGGATACCGAACTG | ||
| AL4_2R | TGCCAGATGTGTAGCTTCCC | 1274 | |
| AL4_3F | CAGCACTGGCGAAGATAACC | ||
| AL4_3R | TGCCCTGATACCTCCTAGCA | 503 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kartashov, M.Y.; Svirin, K.A.; Antonets, M.E.; Zheleznova, A.S.; Kurushina, V.Y.; Agafonov, A.P.; Ternovoi, V.A.; Loktev, V.B. Diversity of the Alongshan Virus in Ixodes Ticks Collected in the Russian Federation in 2023. Microorganisms 2025, 13, 2564. https://doi.org/10.3390/microorganisms13112564
Kartashov MY, Svirin KA, Antonets ME, Zheleznova AS, Kurushina VY, Agafonov AP, Ternovoi VA, Loktev VB. Diversity of the Alongshan Virus in Ixodes Ticks Collected in the Russian Federation in 2023. Microorganisms. 2025; 13(11):2564. https://doi.org/10.3390/microorganisms13112564
Chicago/Turabian StyleKartashov, Mikhail Y., Kirill A. Svirin, Maria E. Antonets, Alina S. Zheleznova, Valentina Y. Kurushina, Alexander P. Agafonov, Vladimir A. Ternovoi, and Valery B. Loktev. 2025. "Diversity of the Alongshan Virus in Ixodes Ticks Collected in the Russian Federation in 2023" Microorganisms 13, no. 11: 2564. https://doi.org/10.3390/microorganisms13112564
APA StyleKartashov, M. Y., Svirin, K. A., Antonets, M. E., Zheleznova, A. S., Kurushina, V. Y., Agafonov, A. P., Ternovoi, V. A., & Loktev, V. B. (2025). Diversity of the Alongshan Virus in Ixodes Ticks Collected in the Russian Federation in 2023. Microorganisms, 13(11), 2564. https://doi.org/10.3390/microorganisms13112564








