Comparison of Adjuvant Potency of Alum, AddaVax, and ISA 71 VG on the Seasonal Split Influenza Vaccine in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Cells and Viruses
2.3. Preparation of Influenza Vaccines and Adjuvants
2.4. Immunization and Challenge with MA-CA04
2.5. ELISA Detection for the Antibody Level in Sera
2.6. Statistical Analysis
3. Results
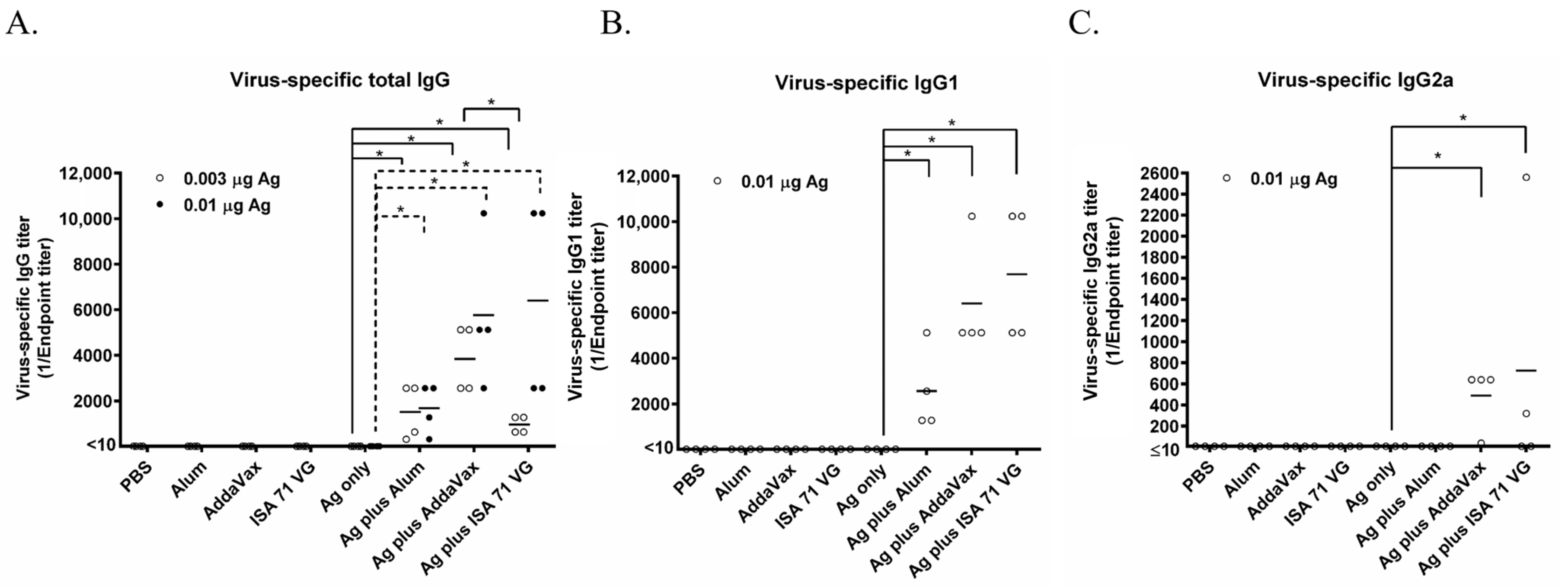
3.1. Alum, AddaVax, and ISA71 VG Enhance the Immunogenicity of the Split Seasonal Influenza Vaccine
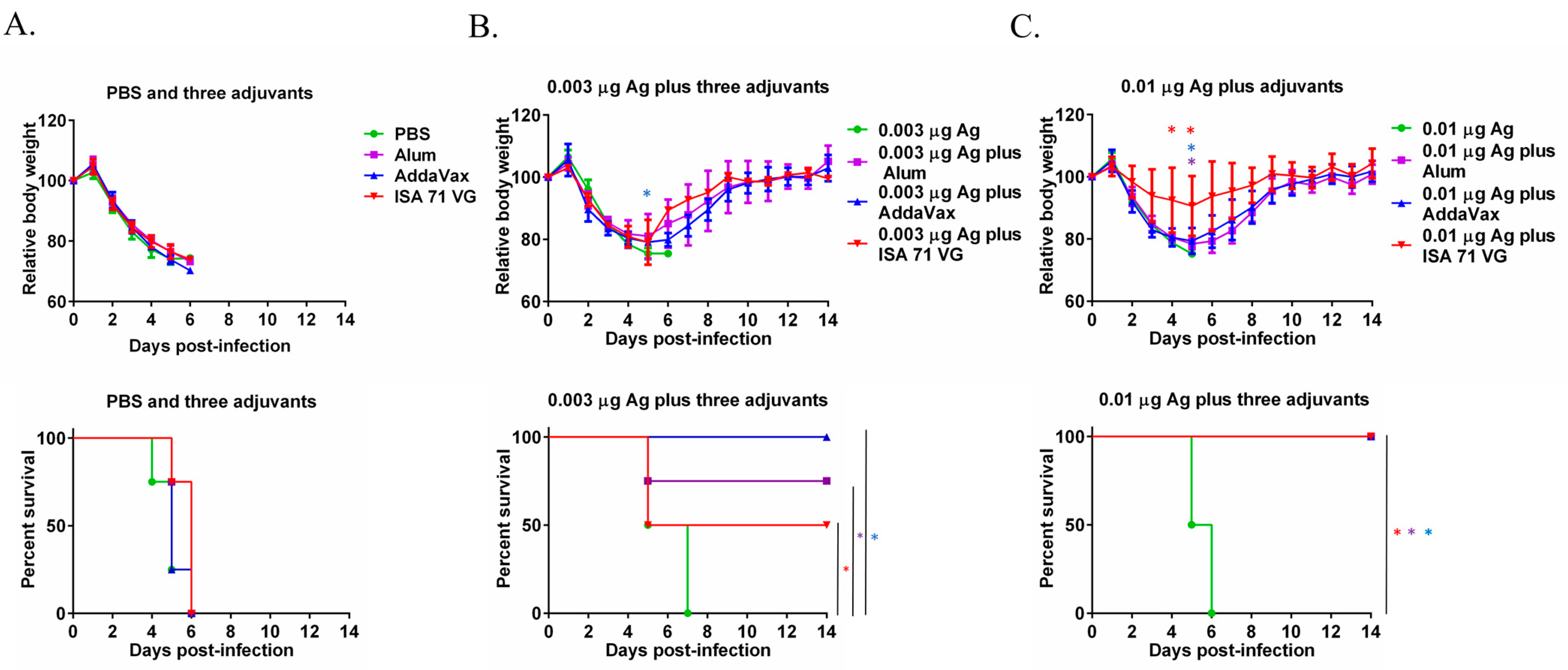
3.2. Alum, AddaVax, and ISA 71 VG Improve the Efficacy of the Split Influenza Vaccine Against Lethal Challenge
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alvarez, F.; Froes, F.; Rojas, A.G.; Moreno-Perez, D.; Martinon-Torres, F. The challenges of influenza for public health. Future Microbiol. 2019, 14, 1429–1436. [Google Scholar] [CrossRef] [PubMed]
- Fineberg, H.V. Pandemic preparedness and response—lessons from the H1N1 influenza of 2009. N. Engl. J. Med. 2014, 370, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.I.; Park, S.; Bae, J.Y.; Lee, S.; Kim, J.; Kim, G.; Yoo, K.; Heo, J.; Kim, Y.S.; Shin, J.S.; et al. Glycosylation generates an efficacious and immunogenic vaccine against H7N9 influenza virus. PLoS Biol. 2020, 18, e3001024. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Deng, G.; Zeng, X.; Cui, P.; Hou, Y.; Liu, Y.; Fang, J.; Pan, S.; Wang, D.; Chen, X.; et al. Genetic and biological properties of H7N9 avian influenza viruses detected after application of the H7N9 poultry vaccine in China. PLoS Pathog. 2021, 17, e1009561. [Google Scholar] [CrossRef]
- CDC. First H5 Bird Flu Death Reported in United States. Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/media/releases/2025/m0106-h5-birdflu-death.html (accessed on 28 March 2025).
- Taaffe, J.; Ostrowsky, J.T.; Mott, J.; Goldin, S.; Friede, M.; Gsell, P.; Chadwick, C. Advancing influenza vaccines: A review of next-generation candidates and their potential for global health impact. Vaccine 2024, 42, 126408. [Google Scholar] [CrossRef]
- FDA. Food and Drug Administration. Available online: https://www.fda.gov/vaccines-blood-biologics/lot-release/use-trivalent-influenza-vaccines-2024-2025-us-influenza-season (accessed on 23 April 2025).
- Barr, I.G.; Subbarao, K. Implications of the apparent extinction of B/Yamagata-lineage human influenza viruses. npj Vaccines 2024, 9, 219. [Google Scholar] [CrossRef]
- CDC. Nonhuman Primates Policy Statements. Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/importation/laws-regulations/nonhuman-primates-policy-statements.html (accessed on 25 August 2025).
- Frutos, A.M.; Cleary, S.; Reeves, E.L.; Ahmad, H.M.; Price, A.M.; Self, W.H.; Zhu, Y.; Safdar, B.; Peltan, I.D.; Gibbs, K.W.; et al. Interim Estimates of 2024-2025 Seasonal Influenza Vaccine Effectiveness—Four Vaccine Effectiveness Networks, United States, October 2024-February 2025. MMWR. Morb. Mortal. Wkly. Rep. 2025, 74, 83–90. [Google Scholar] [CrossRef]
- Tregoning, J.S.; Russell, R.F.; Kinnear, E. Adjuvanted influenza vaccines. Hum. Vaccines Immunother. 2018, 14, 550–564. [Google Scholar] [CrossRef]
- Hotez, P.J.; Corry, D.B.; Strych, U.; Bottazzi, M.E. COVID-19 vaccines: Neutralizing antibodies and the alum advantage. Nat. Rev. Immunol. 2020, 20, 399–400. [Google Scholar] [CrossRef]
- Pulendran, B.; Arunachalam, P.S.; O’Hagan, D.T. Emerging concepts in the science of vaccine adjuvants. Nat. Rev. Drug Discov. 2021, 20, 454–475. [Google Scholar] [CrossRef]
- Beck, L.; Spiegelberg, H.L. The polyclonal and antigen-specific IgE and IgG subclass response of mice injected with ovalbumin in alum or complete Freund’s adjuvant. Cell. Immunol. 1989, 123, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Oleszycka, E.; Lavelle, E.C. Immunomodulatory properties of the vaccine adjuvant alum. Curr. Opin. Immunol. 2014, 28, 1–5. [Google Scholar] [CrossRef]
- Tomar, J.; Patil, H.P.; Bracho, G.; Tonnis, W.F.; Frijlink, H.W.; Petrovsky, N.; Vanbever, R.; Huckriede, A.; Hinrichs, W.L.J. Advax augments B and T cell responses upon influenza vaccination via the respiratory tract and enables complete protection of mice against lethal influenza virus challenge. J. Control. Release Off. J. Control. Release Soc. 2018, 288, 199–211. [Google Scholar] [CrossRef]
- Tsai, T.F. Fluad(R)-MF59(R)-Adjuvanted Influenza Vaccine in Older Adults. Infect. Chemother. 2013, 45, 159–174. [Google Scholar] [CrossRef]
- Zhang, W.; Cui, H.; Xu, J.; Shi, M.; Bian, L.; Cui, L.; Jiang, C.; Zhang, Y. Biodistribution and mechanisms of action of MF59 and MF59-like adjuvants. J. Control. Release Off. J. Control. Release Soc. 2025, 378, 573–587. [Google Scholar] [CrossRef]
- Essink, B.; Fierro, C.; Rosen, J.; Figueroa, A.L.; Zhang, B.; Verhoeven, C.; Edelman, J.; Smolenov, I. Immunogenicity and safety of MF59-adjuvanted quadrivalent influenza vaccine versus standard and alternate B strain MF59-adjuvanted trivalent influenza vaccines in older adults. Vaccine 2020, 38, 242–250. [Google Scholar] [CrossRef]
- Klimka, A.; Michels, L.; Glowalla, E.; Tosetti, B.; Kronke, M.; Krut, O. Montanide ISA 71 VG is Advantageous to Freund’s Adjuvant in Immunization Against S. aureus Infection of Mice. Scand. J. Immunol. 2015, 81, 291–297. [Google Scholar] [CrossRef]
- Ali, Z.M.; Hassan, M.; Hussein, H.A.; Ahmed, B.M.; Sanousi, A. Protective efficacy of combined trivalent inactivated ISA 71 oil adjuvant vaccine against avian influenza virus subtypes (H9N2 and H5N1) and Newcastle disease virus. Vet. World 2017, 10, 1212–1220. [Google Scholar] [CrossRef]
- Neumann, G.; Watanabe, T.; Ito, H.; Watanabe, S.; Goto, H.; Gao, P.; Hughes, M.; Perez, D.R.; Donis, R.; Hoffmann, E.; et al. Generation of influenza A viruses entirely from cloned cDNAs. Proc. Natl. Acad. Sci. USA 1999, 96, 9345–9350. [Google Scholar] [CrossRef] [PubMed]
- Sakabe, S.; Ozawa, M.; Takano, R.; Iwastuki-Horimoto, K.; Kawaoka, Y. Mutations in PA, NP, and HA of a pandemic (H1N1) 2009 influenza virus contribute to its adaptation to mice. Virus Res. 2011, 158, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Even-Or, O.; Samira, S.; Rochlin, E.; Balasingam, S.; Mann, A.J.; Lambkin-Williams, R.; Spira, J.; Goldwaser, I.; Ellis, R.; Barenholz, Y. Immunogenicity, protective efficacy and mechanism of novel CCS adjuvanted influenza vaccine. Vaccine 2010, 28, 6527–6541. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Kang, Y.; Chen, W.; Xie, H.; Zhang, L.; Lv, M.; Wang, J.; Wu, J.; Zhao, W. Comparison of Immunogenicity of Alum and MF59-Like Adjuvant Inactivated SARS-CoV-2 Vaccines Against SARS-CoV-2 Variants in Elderly Mice. Viral Immunol. 2023, 36, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Ko, E.J.; Kang, S.M. Immunology and efficacy of MF59-adjuvanted vaccines. Hum. Vaccines Immunother. 2018, 14, 3041–3045. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Liu, X.; Dang, Y.; Duan, P.; Xu, W.; Zhang, X.; Wang, S.; Luo, J.; Li, X. AddaVax-Adjuvanted H5N8 Inactivated Vaccine Induces Robust Humoral Immune Response against Different Clades of H5 Viruses. Vaccines 2022, 10, 1683. [Google Scholar] [CrossRef]
- Jang, S.I.; Lillehoj, H.S.; Lee, S.H.; Lee, K.W.; Lillehoj, E.P.; Bertrand, F.; Dupuis, L.; Deville, S. Montanide ISA 71 VG adjuvant enhances antibody and cell-mediated immune responses to profilin subunit antigen vaccination and promotes protection against Eimeria acervulina and Eimeria tenella. Exp. Parasitol. 2011, 127, 178–183. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, L.; Silacci, C.; Thom, M.; Boyington, J.C.; Druz, A.; Joyce, M.G.; Guzman, E.; Kong, W.P.; Lai, Y.T.; et al. Protection of calves by a prefusion-stabilized bovine RSV F vaccine. Npj Vaccines 2017, 2, 7. [Google Scholar] [CrossRef]
- Cajaraville, A.; Gomes, M.P.B.; Azamor, T.; Pereira, R.C.; Neves, P.; De Luca, P.M.; Lima, S.M.B.; Gaspar, L.P.; Caride, E.; Freire, M.D.S.; et al. Evaluation of Two Adjuvant Formulations for an Inactivated Yellow Fever 17DD Vaccine Candidate in Mice. Vaccines 2022, 11, 73. [Google Scholar] [CrossRef]
- Zhao, T.; Cai, Y.; Jiang, Y.; He, X.; Wei, Y.; Yu, Y.; Tian, X. Vaccine adjuvants: Mechanisms and platforms. Signal Transduct. Target. Ther. 2023, 8, 283. [Google Scholar] [CrossRef]
- Vono, M.; Taccone, M.; Caccin, P.; Gallotta, M.; Donvito, G.; Falzoni, S.; Palmieri, E.; Pallaoro, M.; Rappuoli, R.; Di Virgilio, F.; et al. The adjuvant MF59 induces ATP release from muscle that potentiates response to vaccination. Proc. Natl. Acad. Sci. USA 2013, 110, 21095–21100. [Google Scholar] [CrossRef]
- Kim, E.H.; Woodruff, M.C.; Grigoryan, L.; Maier, B.; Lee, S.H.; Mandal, P.; Cortese, M.; Natrajan, M.S.; Ravindran, R.; Ma, H.; et al. Squalene emulsion-based vaccine adjuvants stimulate CD8 T cell, but not antibody responses, through a RIPK3-dependent pathway. eLife 2020, 9, e52687. [Google Scholar] [CrossRef]
- Mastelic Gavillet, B.; Eberhardt, C.S.; Auderset, F.; Castellino, F.; Seubert, A.; Tregoning, J.S.; Lambert, P.H.; de Gregorio, E.; Del Giudice, G.; Siegrist, C.A. MF59 Mediates Its B Cell Adjuvanticity by Promoting T Follicular Helper Cells and Thus Germinal Center Responses in Adult and Early Life. J. Immunol. 2015, 194, 4836–4845. [Google Scholar] [CrossRef]
- Bretscher, P.A. On the mechanism determining the TH1/TH2 phenotype of an immune response, and its pertinence to strategies for the prevention, and treatment, of certain infectious diseases. Scand. J. Immunol. 2014, 79, 361–376. [Google Scholar] [CrossRef]
- Zahmati, S.; Taghizadeh, M.; Haghighat, S.; Jalalirad, R.; Mahdavi, M. Recombinant hemagglutinin of swine H1N1 influenza virus expression in the insect cells: Formulation in Montanide ISA71 adjuvant and the potency studies. Iran. J. Basic Med. Sci. 2021, 24, 1546–1553. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, L.; Yang, R.; Wu, H.; Yang, B.; Zhang, X.; Tao, Y.; Wu, X.; Li, S.; Shu, J.; He, Y.; et al. Comparison of Adjuvant Potency of Alum, AddaVax, and ISA 71 VG on the Seasonal Split Influenza Vaccine in Mice. Microorganisms 2025, 13, 2542. https://doi.org/10.3390/microorganisms13112542
Wu L, Yang R, Wu H, Yang B, Zhang X, Tao Y, Wu X, Li S, Shu J, He Y, et al. Comparison of Adjuvant Potency of Alum, AddaVax, and ISA 71 VG on the Seasonal Split Influenza Vaccine in Mice. Microorganisms. 2025; 13(11):2542. https://doi.org/10.3390/microorganisms13112542
Chicago/Turabian StyleWu, Li, Rui Yang, Huimin Wu, Beibei Yang, Xin Zhang, Yingying Tao, Xing Wu, Shaozhen Li, Jianhong Shu, Yulong He, and et al. 2025. "Comparison of Adjuvant Potency of Alum, AddaVax, and ISA 71 VG on the Seasonal Split Influenza Vaccine in Mice" Microorganisms 13, no. 11: 2542. https://doi.org/10.3390/microorganisms13112542
APA StyleWu, L., Yang, R., Wu, H., Yang, B., Zhang, X., Tao, Y., Wu, X., Li, S., Shu, J., He, Y., & Feng, H. (2025). Comparison of Adjuvant Potency of Alum, AddaVax, and ISA 71 VG on the Seasonal Split Influenza Vaccine in Mice. Microorganisms, 13(11), 2542. https://doi.org/10.3390/microorganisms13112542







