Probiotic Potential of Traditional and Emerging Microbial Strains in Functional Foods: From Characterization to Applications and Health Benefits
Abstract
1. Introduction
1.1. Background
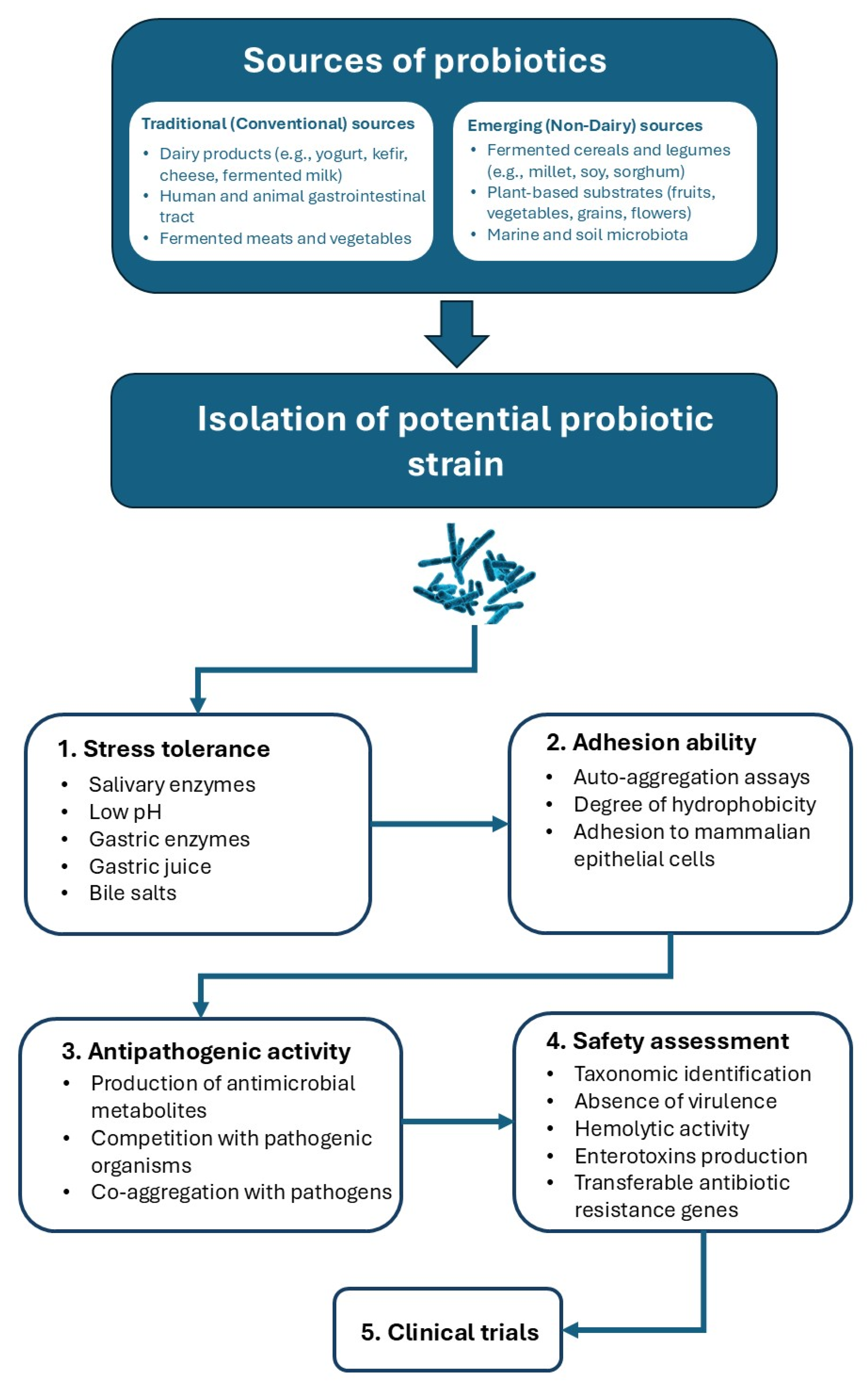
1.2. Sources of Novel and Conventional Probiotic Strains
1.3. Conventional vs. Emerging Probiotic Strains
2. Biological and Functional Characteristics of Probiotic Strains
2.1. Ability to Tolerate Stress
2.2. Adhesion Ability
2.3. Antipathogenic Activity
2.4. Safety and Validation Assessment
3. Emerging Probiotics
3.1. Emerging Probiotics from Non-Dairy Fermented Foods
3.2. Emerging Probiotics from Dairy Fermented Foods
3.3. Emerging Probiotics from Other Unconventional Sources
4. Application of Probiotic Strains in Functional Food Development
4.1. Food Matrix Compatibility
4.2. Encapsulation of Probiotics to Improve Shelf-Life and Viability
4.3. Role of Artificial Intelligence in the Discovery, Characterization, and Application of New Probiotics
5. Health Benefits and Mechanisms of Action of Probiotics
5.1. Immunomodulatory and Anti-Inflammatory Effects
5.2. Effect on Cardiovascular Health
5.3. Anti-Anxiety and Anti-Depression
5.4. Antimicrobial Activity and Modulation of Gut Microbiota
6. Translational Insights: Market Trends and Consumer Acceptance of Probiotics in Functional Foods
6.1. Market Growth of Functional Foods and Probiotics
6.2. Consumer Awareness and Preferences
6.3. Personalized Nutrition and Microbiome-Based Products
7. Challenges and Future Perspectives
7.1. Regulatory and Safety Considerations
7.2. Research Gaps and Future Directions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Birch, C.S.; Bonwick, G.A. Ensuring the future of functional foods. Int. J. Food Sci. Technol. 2019, 54, 1467–1485. [Google Scholar] [CrossRef]
- Binda, S.; Hill, C.; Johansen, E.; Obis, D.; Pot, B.; Sanders, M.E.; Tremblay, A.; Ouwehand, A.C. Criteria to qualify microorganisms as “probiotic” in foods and dietary supplements. Front. Microbiol. 2020, 11, 1662. [Google Scholar] [CrossRef]
- Shimizu, M. History and current status of functional food regulations in Japan. In Nutraceutical and Functional Food Regulations in the United States and Around the World; Elsevier: Amsterdam, The Netherlands, 2019; pp. 337–344. [Google Scholar]
- Manach, C.; Milenkovic, D.; Van de Wiele, T.; Rodriguez-Mateos, A.; de Roos, B.; Garcia-Conesa, M.T.; Landberg, R.; Gibney, E.R.; Heinonen, M.; Tomás-Barberán, F. Addressing the inter-individual variation in response to consumption of plant food bioactives: Towards a better understanding of their role in healthy aging and cardiometabolic risk reduction. Mol. Nutr. Food Res. 2017, 61, 1600557. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Li, M.; Yue, X. Current research on probiotics and fermented products. Foods 2024, 13, 1406. [Google Scholar] [CrossRef]
- Oyeniran, A.; Gyawali, R.; Aljaloud, S.O.; Krastanov, A.; Ibrahim, S.A. Probiotic characteristics and health benefits of the yogurt bacterium Lactobacillus delbrueckii sp. bulgaricus. In Current Issues and Challenges in the Dairy Industry; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar]
- Khan, R.S.; Grigor, J.; Winger, R.; Win, A. Functional food product development–Opportunities and challenges for food manufacturers. Trends Food Sci. Technol. 2013, 30, 27–37. [Google Scholar] [CrossRef]
- Vlaicu, P.A.; Untea, A.E.; Varzaru, I.; Saracila, M.; Oancea, A.G. Designing nutrition for health—Incorporating dietary by-products into poultry feeds to create functional foods with insights into health benefits, risks, bioactive compounds, food component functionality and safety regulations. Foods 2023, 12, 4001. [Google Scholar] [CrossRef]
- Latif, A.; Shehzad, A.; Niazi, S.; Zahid, A.; Ashraf, W.; Iqbal, M.W.; Rehman, A.; Riaz, T.; Aadil, R.M.; Khan, I.M. Probiotics: Mechanism of action, health benefits and their application in food industries. Front. Microbiol. 2023, 14, 1216674, Erratum in Front. Microbiol. 2024, 14, 1216674. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- ISAPP. Minimum Criteria for Probiotics [Online]; International Scientific Association for Probiotics and Prebiotics: Sacramento, CA, USA, 2018. [Google Scholar]
- Champagne, C.P.; da Cruz, A.G.; Daga, M. Strategies to improve the functionality of probiotics in supplements and foods. Curr. Opin. Food Sci. 2018, 22, 160–166. [Google Scholar] [CrossRef]
- Granato, D.; Branco, G.F.; Nazzaro, F.; Cruz, A.G.; Faria, J.A. Functional foods and nondairy probiotic food development: Trends, concepts, and products. Compr. Rev. Food Sci. Food Saf. 2010, 9, 292–302. [Google Scholar] [CrossRef]
- Corbo, M.R.; Campaniello, D.; Speranza, B.; Altieri, C.; Sinigaglia, M.; Bevilacqua, A. Neutralisation of toxins by probiotics during the transit into the gut: Challenges and perspectives. Int. J. Food Sci. Technol. 2018, 53, 1339–1351. [Google Scholar] [CrossRef]
- Mokhtari, S.; Khomeiri, M.; Jafari, S.M.; Maghsoudlou, Y.; Ghorbani, M. Descriptive analysis of bacterial profile, physicochemical and sensory characteristics of grape juice containing Saccharomyces cerevisiae cell wall-coated probiotic microcapsules during storage. Int. J. Food Sci. Technol. 2017, 52, 1042–1048. [Google Scholar] [CrossRef]
- Campaniello, D.; Speranza, B.; Petruzzi, L.; Bevilacqua, A.; Corbo, M.R. How to routinely assess transition, adhesion and survival of probiotics into the gut: A case study on propionibacteria. Int. J. Food Sci. Technol. 2018, 53, 484–490. [Google Scholar] [CrossRef]
- Moumita, S.; Das, B.; Sundaray, A.; Satpathi, S.; Thangaraj, P.; Marimuthu, S.; Jayabalan, R. Study of soy-fortified green tea curd formulated using potential hypocholesterolemic and hypotensive probiotics isolated from locally made curd. Food Chem. 2018, 268, 558–566. [Google Scholar] [CrossRef]
- Mohammadi-Sartang, M.; Bellissimo, N.; de Zepetnek, J.T.; Brett, N.; Mazloomi, S.; Fararouie, M.; Bedeltavana, A.; Famouri, M.; Mazloom, Z. The effect of daily fortified yogurt consumption on weight loss in adults with metabolic syndrome: A 10-week randomized controlled trial. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Ephrem, E.; Najjar, A.; Charcosset, C.; Greige-Gerges, H. Encapsulation of natural active compounds, enzymes, and probiotics for fruit juice fortification, preservation, and processing: An overview. J. Funct. Foods 2018, 48, 65–84. [Google Scholar] [CrossRef]
- Wardill, H.R.; Van Sebille, Y.Z.; Ciorba, M.A.; Bowen, J.M. Prophylactic probiotics for cancer therapy-induced diarrhoea: A meta-analysis. Curr. Opin. Support. Palliat. Care 2018, 12, 187–197. [Google Scholar] [CrossRef]
- Pimentel, T.C.; de Oliveira, L.I.G.; Macedo, E.d.L.C.; Costa, G.N.; Dias, D.R.; Schwan, R.F.; Magnani, M. Understanding the potential of fruits, flowers, and ethnic beverages as valuable sources of techno-functional and probiotics strains: Current scenario and main challenges. Trends Food Sci. Technol. 2021, 114, 25–59. [Google Scholar] [CrossRef]
- Lakra, A.K.; Domdi, L.; Hanjon, G.; Tilwani, Y.M.; Arul, V. Some probiotic potential of Weissella confusa MD1 and Weissella cibaria MD2 isolated from fermented batter. LWT 2020, 125, 109261. [Google Scholar] [CrossRef]
- Vasiee, A.; Alizadeh Behbahani, B.; Tabatabaei Yazdi, F.; Mortazavi, S.A.; Noorbakhsh, H. Diversity and probiotic potential of lactic acid bacteria isolated from horreh, a traditional Iranian fermented food. Probiotics Antimicrob. Proteins 2018, 10, 258–268. [Google Scholar] [CrossRef]
- Cao, J.; Yu, Z.; Liu, W.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Probiotic characteristics of Bacillus coagulans and associated implications for human health and diseases. J. Funct. Foods 2020, 64, 103643. [Google Scholar] [CrossRef]
- Lee, N.-K.; Kim, S.-Y.; Han, K.J.; Eom, S.J.; Paik, H.-D. Probiotic potential of Lactobacillus strains with anti-allergic effects from kimchi for yogurt starters. LWT-Food Sci. Technol. 2014, 58, 130–134. [Google Scholar] [CrossRef]
- Aarti, C.; Khusro, A. Functional and technological properties of exopolysaccharide producing autochthonous Lactobacillus plantarum strain AAS3 from dry fish based fermented food. LWT 2019, 114, 108387. [Google Scholar] [CrossRef]
- Son, S.-H.; Yang, S.-J.; Jeon, H.-L.; Yu, H.-S.; Lee, N.-K.; Park, Y.-S.; Paik, H.-D. Antioxidant and immunostimulatory effect of potential probiotic Lactobacillus paraplantarum SC61 isolated from Korean traditional fermented food, jangajji. Microb. Pathog. 2018, 125, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Woraprayote, W.; Pumpuang, L.; Tosukhowong, A.; Roytrakul, S.; Perez, R.H.; Zendo, T.; Sonomoto, K.; Benjakul, S.; Visessanguan, W. Two putatively novel bacteriocins active against Gram-negative food borne pathogens produced by Weissella hellenica BCC 7293. Food Control 2015, 55, 176–184. [Google Scholar] [CrossRef]
- Patil, M.; Jadhav, A.; Patil, U. Functional characterization and in vitro screening of Fructobacillus fructosus MCC 3996 isolated from Butea monosperma flower for probiotic potential. Lett. Appl. Microbiol. 2020, 70, 331–339. [Google Scholar] [CrossRef]
- Lee, N.-K.; Hong, J.-Y.; Yi, S.-H.; Hong, S.-P.; Lee, J.-E.; Paik, H.-D. Bioactive compounds of probiotic Saccharomyces cerevisiae strains isolated from cucumber jangajji. J. Funct. Foods 2019, 58, 324–329. [Google Scholar] [CrossRef]
- Bonatsou, S.; Karamouza, M.; Zoumpopoulou, G.; Mavrogonatou, E.; Kletsas, D.; Papadimitriou, K.; Tsakalidou, E.; Nychas, G.-J.E.; Panagou, E.Ζ. Evaluating the probiotic potential and technological characteristics of yeasts implicated in cv. Kalamata natural black olive fermentation. Int. J. Food Microbiol. 2018, 271, 48–59. [Google Scholar] [CrossRef]
- Das, P.; Khowala, S.; Biswas, S. In vitro probiotic characterization of Lactobacillus casei isolated from marine samples. LWT 2016, 73, 383–390. [Google Scholar] [CrossRef]
- Kim, Y.; Choi, S.-I.; Jeong, Y.; Kang, C.-H. Evaluation of safety and probiotic potential of Enterococcus faecalis MG5206 and Enterococcus faecium MG5232 isolated from kimchi, a Korean fermented cabbage. Microorganisms 2022, 10, 2070. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Li, Q.; Cheng, L.; Buch, H.; Zhang, F. Akkermansia muciniphila is a promising probiotic. Microb. Biotechnol. 2019, 12, 1109–1125. [Google Scholar] [CrossRef] [PubMed]
- Martín, R.; Miquel, S.; Benevides, L.; Bridonneau, C.; Robert, V.; Hudault, S.; Chain, F.; Berteau, O.; Azevedo, V.; Chatel, J.M. Functional characterization of novel Faecalibacterium prausnitzii strains isolated from healthy volunteers: A step forward in the use of F. prausnitzii as a next-generation probiotic. Front. Microbiol. 2017, 8, 1226. [Google Scholar] [CrossRef]
- Brodmann, T.; Endo, A.; Gueimonde, M.; Vinderola, G.; Kneifel, W.; de Vos, W.M.; Salminen, S.; Gómez-Gallego, C. Safety of novel microbes for human consumption: Practical examples of assessment in the European Union. Front. Microbiol. 2017, 8, 1725. [Google Scholar] [CrossRef]
- Bubnov, R.V.; Babenko, L.P.; Lazarenko, L.M.; Mokrozub, V.V.; Spivak, M.Y. Specific properties of probiotic strains: Relevance and benefits for the host. EPMA J. 2018, 9, 205–223. [Google Scholar] [CrossRef] [PubMed]
- García-Ruiz, A.; de Llano, D.G.; Esteban-Fernández, A.; Requena, T.; Bartolomé, B.; Moreno-Arribas, M.V. Assessment of probiotic properties in lactic acid bacteria isolated from wine. Food Microbiol. 2014, 44, 220–225. [Google Scholar] [CrossRef]
- Salmerón, I. Fermented cereal beverages: From probiotic, prebiotic and synbiotic towards Nanoscience designed healthy drinks. Lett. Appl. Microbiol. 2017, 65, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Abu-Ghannam, N. Probiotic fermentation of plant based products: Possibilities and opportunities. Crit. Rev. Food Sci. Nutr. 2012, 52, 183–199. [Google Scholar] [CrossRef]
- Betoret, N.; Puente, L.; Dıaz, M.; Pagán, M.; Garcıa, M.; Gras, M.; Martínez-Monzó, J.; Fito, P. Development of probiotic-enriched dried fruits by vacuum impregnation. J. Food Eng. 2003, 56, 273–277. [Google Scholar] [CrossRef]
- Verhoog, S.; Taneri, P.E.; Roa Díaz, Z.M.; Marques-Vidal, P.; Troup, J.P.; Bally, L.; Franco, O.H.; Glisic, M.; Muka, T. Dietary factors and modulation of bacteria strains of Akkermansia muciniphila and Faecalibacterium prausnitzii: A systematic review. Nutrients 2019, 11, 1565. [Google Scholar] [CrossRef]
- Pabari, K.; Pithva, S.; Kothari, C.; Purama, R.K.; Kondepudi, K.K.; Vyas, B.R.M.; Kothari, R.; Ambalam, P. Evaluation of probiotic properties and prebiotic utilization potential of Weissella paramesenteroides isolated from fruits. Probiotics Antimicrob. Proteins 2020, 12, 1126–1138. [Google Scholar] [CrossRef]
- Mei, L.; Wang, J.; Hao, Y.; Zeng, X.; Yang, Y.; Wu, Z.; Ji, Y. A comprehensive update on the immunoregulatory mechanisms of Akkermansia muciniphila: Insights into active ingredients, metabolites, and nutrient-driven modulation. Crit. Rev. Food Sci. Nutr. 2024, 65, 5487–5504. [Google Scholar] [CrossRef]
- Day, R.L.; Harper, A.J.; Woods, R.M.; Davies, O.G.; Heaney, L.M. Probiotics: Current landscape and future horizons. Future Sci. OA 2019, 5, FSO391. [Google Scholar] [CrossRef]
- FAO/WHO. Guidelines for the evaluation of probiotics in food. In Food and Agriculture Organization of the United Nations and World Health Organization Working Group Report; WHO: Geneva, Switzerland, 2002. [Google Scholar]
- de Albuquerque, T.M.R.; Garcia, E.F.; de Oliveira Araújo, A.; Magnani, M.; Saarela, M.; de Souza, E.L. In vitro characterization of Lactobacillus strains isolated from fruit processing by-products as potential probiotics. Probiotics Antimicrob. Proteins 2018, 10, 704–716. [Google Scholar] [CrossRef]
- Wattam, A.R.; Davis, J.J.; Assaf, R.; Boisvert, S.; Brettin, T.; Bun, C.; Conrad, N.; Dietrich, E.M.; Disz, T.; Gabbard, J.L. Improvements to PATRIC, the all-bacterial bioinformatics database and analysis resource center. Nucleic Acids Res. 2017, 45, D535–D542. [Google Scholar] [CrossRef] [PubMed]
- Kõll-Klais, P.; Mändar, R.; Leibur, E.; Marcotte, H.; Hammarström, L.; Mikelsaar, M. Oral lactobacilli in chronic periodontitis and periodontal health: Species composition and antimicrobial activity. Oral Microbiol. Immunol. 2005, 20, 354–361. [Google Scholar] [CrossRef]
- de Melo Pereira, G.V.; de Oliveira Coelho, B.; Júnior, A.I.M.; Thomaz-Soccol, V.; Soccol, C.R. How to select a probiotic? A review and update of methods and criteria. Biotechnol. Adv. 2018, 36, 2060–2076. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhou, T.; Tang, H.; Li, X.; Chen, Y.; Zhang, L.; Zhang, J. Probiotic potential and amylolytic properties of lactic acid bacteria isolated from Chinese fermented cereal foods. Food Control 2020, 111, 107057. [Google Scholar] [CrossRef]
- Garcia, E.F.; Luciano, W.A.; Xavier, D.E.; da Costa, W.C.; de Sousa Oliveira, K.; Franco, O.L.; de Morais Junior, M.A.; Lucena, B.T.; Picao, R.C.; Magnani, M. Identification of lactic acid bacteria in fruit pulp processing byproducts and potential probiotic properties of selected Lactobacillus strains. Front. Microbiol. 2016, 7, 1371. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Pacheco, P.; García-Béjar, B.; Jiménez-del Castillo, M.; Carreño-Domínguez, J.; Briones Pérez, A.; Arévalo-Villena, M. Potential probiotic and food protection role of wild yeasts isolated from pistachio fruits (Pistacia vera). J. Sci. Food Agric. 2021, 101, 2201–2209. [Google Scholar] [CrossRef]
- Maragkoudakis, P.A.; Zoumpopoulou, G.; Miaris, C.; Kalantzopoulos, G.; Pot, B.; Tsakalidou, E. Probiotic potential of Lactobacillus strains isolated from dairy products. Int. Dairy J. 2006, 16, 189–199. [Google Scholar] [CrossRef]
- Ogunremi, O.; Sanni, A.; Agrawal, R. Probiotic potentials of yeasts isolated from some cereal-based Nigerian traditional fermented food products. J. Appl. Microbiol. 2015, 119, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Boonaert, C.J.; Rouxhet, P.G. Surface of lactic acid bacteria: Relationships between chemical composition and physicochemical properties. Appl. Environ. Microbiol. 2000, 66, 2548–2554. [Google Scholar] [CrossRef]
- Duary, R.K.; Rajput, Y.S.; Batish, V.K.; Grover, S. Assessing the adhesion of putative indigenous probiotic lactobacilli to human colonic epithelial cells. Indian J. Med. Res. 2011, 134, 664–671. [Google Scholar] [CrossRef]
- Tuo, Y.; Yu, H.; Ai, L.; Wu, Z.; Guo, B.; Chen, W. Aggregation and adhesion properties of 22 Lactobacillus strains. J. Dairy Sci. 2013, 96, 4252–4257. [Google Scholar] [CrossRef] [PubMed]
- Chelliah, R.; Ramakrishnan, S.R.; Prabhu, P.R.; Antony, U. Evaluation of antimicrobial activity and probiotic properties of wild-strain Pichia kudriavzevii isolated from frozen idli batter. Yeast 2016, 33, 385–401. [Google Scholar] [CrossRef]
- Leite, A.M.; Miguel, M.; Peixoto, R.; Ruas-Madiedo, P.; Paschoalin, V.; Mayo, B.; Delgado, S. Probiotic potential of selected lactic acid bacteria strains isolated from Brazilian kefir grains. J. Dairy Sci. 2015, 98, 3622–3632. [Google Scholar] [CrossRef]
- Ramos, C.L.; Thorsen, L.; Schwan, R.F.; Jespersen, L. Strain-specific probiotics properties of Lactobacillus fermentum, Lactobacillus plantarum and Lactobacillus brevis isolates from Brazilian food products. Food Microbiol. 2013, 36, 22–29. [Google Scholar] [CrossRef]
- Mihaylova-Garnizova, R.; Davidova, S.; Hodzhev, Y.; Satchanska, G. Antimicrobial peptides derived from bacteria: Classification, sources, and mechanism of action against multidrug-resistant bacteria. Int. J. Mol. Sci. 2024, 25, 10788. [Google Scholar] [CrossRef]
- Vera-Pingitore, E.; Jimenez, M.E.; Dallagnol, A.; Belfiore, C.; Fontana, C.; Fontana, P.; von Wright, A.; Vignolo, G.; Plumed-Ferrer, C. Screening and characterization of potential probiotic and starter bacteria for plant fermentations. LWT-Food Sci. Technol. 2016, 71, 288–294. [Google Scholar] [CrossRef]
- Divya, J.B.; Varsha, K.K.; Nampoothiri, K.M. Newly isolated lactic acid bacteria with probiotic features for potential application in food industry. Appl. Biochem. Biotechnol. 2012, 167, 1314–1324. [Google Scholar] [CrossRef] [PubMed]
- Inturri, R.; Stivala, A.; Furneri, P.; Blandino, G. Growth and adhesion to HT-29 cells inhibition of Gram-negatives by Bifidobacterium longum BB536 e Lactobacillus rhamnosus HN001 alone and in combination. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4943–4949. [Google Scholar] [PubMed]
- Benítez-Cabello, A.; Calero-Delgado, B.; Rodríguez-Gómez, F.; Garrido-Fernández, A.; Jiménez-Díaz, R.; Arroyo-López, F.N. Biodiversity and multifunctional features of lactic acid bacteria isolated from table olive biofilms. Front. Microbiol. 2019, 10, 836. [Google Scholar] [CrossRef]
- Sanders, M.E.; Shane, A.L.; Merenstein, D.J. Advancing probiotic research in humans in the United States: Challenges and strategies. Gut Microbes 2016, 7, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.; Shukla, P. An overview of advanced technologies for selection of probiotics and their expediency: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 3233–3242. [Google Scholar] [CrossRef]
- Bagheripoor-Fallah, N.; Mortazavian, A.; Hosseini, H.; Khoshgozaran-Abras, S.; Rad, A.H. Comparison of molecular techniques with other methods for identification and enumeration of probiotics in fermented milk products. Crit. Rev. Food Sci. Nutr. 2015, 55, 396–413. [Google Scholar] [CrossRef]
- Temmerman, R.; Huys, G.; Swings, J. Identification of lactic acid bacteria: Culture-dependent and culture-independent methods. Trends Food Sci. Technol. 2004, 15, 348–359. [Google Scholar] [CrossRef]
- Chen, Y.; Hsiao, P.; Hong, W.; Dai, T.; Chen, M. Lactobacillus kefiranofaciens M1 isolated from milk kefir grains ameliorates experimental colitis in vitro and in vivo. J. Dairy Sci. 2012, 95, 63–74. [Google Scholar] [CrossRef]
- Sanders, M.E.; Akkermans, L.M.; Haller, D.; Hammerman, C.; Heimbach, J.T.; Hörmannsperger, G.; Huys, G. Safety assessment of probiotics for human use. Gut Microbes 2010, 1, 164–185. [Google Scholar] [CrossRef]
- Ashraf, R.; Shah, N.P. Antibiotic resistance of probiotic organisms and safety of probiotic dairy products. Int. Food Res. J. 2011, 18, 837–853. [Google Scholar]
- Klare, I.; Konstabel, C.; Werner, G.; Huys, G.; Vankerckhoven, V.; Kahlmeter, G.; Hildebrandt, B.; Müller-Bertling, S.; Witte, W.; Goossens, H. Antimicrobial susceptibilities of Lactobacillus, Pediococcus and Lactococcus human isolates and cultures intended for probiotic or nutritional use. J. Antimicrob. Chemother. 2007, 59, 900–912. [Google Scholar] [CrossRef]
- Nawaz, M.; Wang, J.; Zhou, A.; Ma, C.; Wu, X.; Moore, J.E.; Cherie Millar, B.; Xu, J. Characterization and transfer of antibiotic resistance in lactic acid bacteria from fermented food products. Curr. Microbiol. 2011, 62, 1081–1089. [Google Scholar] [CrossRef]
- Senan, S.; Prajapati, J.; Joshi, C. Feasibility of genome-wide screening for biosafety assessment of probiotics: A case study of Lactobacillus helveticus MTCC 5463. Probiotics Antimicrob. Proteins 2015, 7, 249–258. [Google Scholar] [CrossRef]
- Schulz, K.F.; Altman, D.G.; Moher, D.; The CONSORT Group. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomized trials. Ann. Intern. Med. 2010, 152, 726–732. [Google Scholar] [CrossRef]
- Hutchinson, A.N.; Bergh, C.; Kruger, K.; Sűsserová, M.; Allen, J.; Améen, S.; Tingö, L. The effect of probiotics on health outcomes in the elderly: A systematic review of randomized, placebo-controlled studies. Microorganisms 2021, 9, 1344. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, D.; Ołdak, A.; Rzepkowska, A.; Zieliński, K. Enumeration and identification of probiotic bacteria in food matrices. In Advances in Biotechnology for Food Industry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 167–196. [Google Scholar]
- Sornplang, P.; Piyadeatsoontorn, S. Probiotic isolates from unconventional sources: A review. J. Anim. Sci. Technol. 2016, 58, 26. [Google Scholar] [CrossRef] [PubMed]
- Nanasombat, S.; Phunpruch, S.; Jaichalad, T. Screening and identification of lactic acid bacteria from raw seafoods and Thai fermented seafood products for their potential use as starter cultures. Songklanakarin J. Sci. Technol. 2012, 34, 255–262. [Google Scholar]
- Senthong, R.; Chanthachum, S.; Sumpavapol, P. Screening and identification of probiotic lactic acid bacteria isolated from Poo-Khem, A traditional salted crab. In Proceedings of the International Conference on Nutrition and Food Sciences, Singapore, 23–24 July 2012; pp. 111–115. [Google Scholar]
- Miyashita, M.; Yukphan, P.; Chaipitakchonlatarn, W.; Malimas, T.; Sugimoto, M.; Yoshino, M.; Potacharoen, W.; Tanasupawat, S.; Nakagawa, Y.; Kirtikara, K. 16S rRNA gene sequence analysis of lactic acid bacteria isolated from fermented foods in Thailand. Microbiol. Cult. Coll. 2012, 28, 1–9. [Google Scholar]
- Siripornadulsil, W.; Tasaku, S.; Buahorm, J.; Siripornadulsil, S. Probiotic properties of lactic acid bacteria isolated from fermented food. Intl. J. Biol. Food Vet. Agri. Eng. 2014, 8, 364–366. [Google Scholar]
- Bacha, K.; Mehari, T.; Ashenafi, M. In-vitro probiotic potential of lactic acid bacteria isolated from ‘Wakalim’, a traditional Ethiopian fermented beef sausage. Ethiop. J. Health Sci. 2009, 19, 21–27. [Google Scholar]
- Agaliya, P.J.; Jeevaratnam, K. Screening of Lactobacillus plantarum isolated from fermented idli batter for probiotic properties. Afr. J. Biotechnol. 2012, 11, 12856–12864. [Google Scholar] [CrossRef]
- Oluwajoba, S.O.; Akinyosoye, F.A.; Oyetayo, V.O. In vitro screening and selection of probiotic lactic acid bacteria isolated from spontaneously fermenting Kunu-Zaki. Adv. Microbiol. 2013, 3, 309. [Google Scholar] [CrossRef]
- Sarıtaş, S.; Mondragon Portocarrero, A.d.C.; Miranda, J.M.; Witkowska, A.M.; Karav, S. Functional Yogurt: Types and Health Benefits. Appl. Sci. 2024, 14, 11798. [Google Scholar] [CrossRef]
- Sharifi Yazdi, M.K.; Davoodabadi, A.; Khesht Zarin, H.R.; Tajabadi Ebrahimi, M.; Soltan Dallal, M.M. Characterisation and probiotic potential of lactic acid bacteria isolated from Iranian traditional yogurts. Ital. J. Anim. Sci. 2017, 16, 185–188. [Google Scholar] [CrossRef]
- Anumudu, C.K.; Ikimi, C.G.; Zige, D.V.; Omeje, F.I.; Gbodo, E.E. Production of Bacteriocins by Lactobacillus plantarum and Pediococcus acidilactici Isolated from Cow Milk. Niger. J. Microbiol. 2019, 33, 4373–4379. [Google Scholar]
- Hoque, M.; Akter, F.; Hossain, K.; Rahman, M.; Billah, M.; Islam, K. Isolation, identification and analysis of probiotic properties of Lactobacillus spp. from selective regional yoghurts. World J. Dairy Food Sci. 2010, 5, 39–46. [Google Scholar]
- Plessas, S.; Nouska, C.; Mantzourani, I.; Kourkoutas, Y.; Alexopoulos, A.; Bezirtzoglou, E. Microbiological exploration of different types of kefir grains. Fermentation 2017, 3, 1. [Google Scholar] [CrossRef]
- Diosma, G.; Romanin, D.E.; Rey-Burusco, M.F.; Londero, A.; Garrote, G.L. Yeasts from kefir grains: Isolation, identification, and probiotic characterization. World J. Microbiol. Biotechnol. 2014, 30, 43–53. [Google Scholar] [CrossRef]
- Maeno, S.; Kajikawa, A.; Dicks, L.; Endo, A. Introduction of bifunctional alcohol/acetaldehyde dehydrogenase gene (adhE) in Fructobacillus fructosus settled its fructophilic characteristics. Res. Microbiol. 2019, 170, 35–42. [Google Scholar] [CrossRef]
- Behare, P.V.; Mazhar, S.; Pennone, V.; McAuliffe, O. Evaluation of lactic acid bacteria strains isolated from fructose-rich environments for their mannitol-production and milk-gelation abilities. J. Dairy Sci. 2020, 103, 11138–11151. [Google Scholar] [CrossRef]
- Sakandar, H.A.; Kubow, S.; Sadiq, F.A. Isolation and in-vitro probiotic characterization of fructophilic lactic acid bacteria from Chinese fruits and flowers. Lwt 2019, 104, 70–75. [Google Scholar] [CrossRef]
- Adetunji, C.O.; Akram, M.; Michael, O.S.; Shahzad, K.; Ayeni, A.E.; Hasan, S.; Adetunji, J.B.; Hasan, S.M.; Inamuddin; Olaniyan, M. Polysaccharides derived from natural sources: A panacea to health and nutritional challenges. In Polysaccharides: Properties and Applications; Wiley: Hoboken, NJ, USA, 2021; pp. 701–738. [Google Scholar] [CrossRef]
- Rodrigues, N.P.A.; Garcia, E.F.; de Souza, E.L. Selection of lactic acid bacteria with promising probiotic aptitudes from fruit and ability to survive in different food matrices. Braz. J. Microbiol. 2021, 52, 2257–2269. [Google Scholar] [CrossRef] [PubMed]
- Tenea, G.N.; Perugachi, E. Innovative functional juice enriched with native probiotics for enhanced nutrition and antimicrobial properties. Front. Nutr. 2025, 12, 1552745. [Google Scholar] [CrossRef] [PubMed]
- Sarao, L.K.; Arora, M. Probiotics, prebiotics, and microencapsulation: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 344–371. [Google Scholar] [CrossRef]
- WGO. Global Guidelines: Probiotics and Prebiotics; WGO: Hong Kong, China, 2017. [Google Scholar]
- Flach, J.; van der Waal, M.B.; van den Nieuwboer, M.; Claassen, E.; Larsen, O.F. The underexposed role of food matrices in probiotic products: Reviewing the relationship between carrier matrices and product parameters. Crit. Rev. Food Sci. Nutr. 2018, 58, 2570–2584. [Google Scholar] [CrossRef]
- Yao, M.; Xie, J.; Du, H.; McClements, D.J.; Xiao, H.; Li, L. Progress in microencapsulation of probiotics: A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 857–874. [Google Scholar] [CrossRef] [PubMed]
- Reque, P.M.; Brandelli, A. Encapsulation of probiotics and nutraceuticals: Applications in functional food industry. Trends Food Sci. Technol. 2021, 114, 1–10. [Google Scholar] [CrossRef]
- Fenster, K.; Freeburg, B.; Hollard, C.; Wong, C.; Rønhave Laursen, R.; Ouwehand, A.C. The production and delivery of probiotics: A review of a practical approach. Microorganisms 2019, 7, 83. [Google Scholar] [CrossRef]
- Terpou, A.; Papadaki, A.; Lappa, I.K.; Kachrimanidou, V.; Bosnea, L.A.; Kopsahelis, N. Probiotics in food systems: Significance and emerging strategies towards improved viability and delivery of enhanced beneficial value. Nutrients 2019, 11, 1591. [Google Scholar] [CrossRef]
- Singh, S.; Gupta, R.; Chawla, S.; Gauba, P.; Singh, M.; Tiwari, R.K.; Upadhyay, S.; Sharma, S.; Chanda, S.; Gaur, S. Natural sources and encapsulating materials for probiotics delivery systems: Recent applications and challenges in functional food development. Front. Nutr. 2022, 9, 971784. [Google Scholar] [CrossRef]
- Ta, L.P.; Bujna, E.; Antal, O.; Ladányi, M.; Juhász, R.; Szécsi, A.; Kun, S.; Sudheer, S.; Gupta, V.K.; Nguyen, Q.D. Effects of various polysaccharides (alginate, carrageenan, gums, chitosan) and their combination with prebiotic saccharides (resistant starch, lactosucrose, lactulose) on the encapsulation of probiotic bacteria Lactobacillus casei 01 strain. Int. J. Biol. Macromol. 2021, 183, 1136–1144. [Google Scholar] [CrossRef]
- Anumudu, C.; Miri, T.; Onyeaka, H. Sporicidal Activity of Micro-Encapsulated Nisin-like Bacteriocins Obtained from Lactococcus lactis against Bacillus cereus spores. In Proceedings of the 2024 European Symposium on Food Safety, Geneva, Switzerland, 30 April–2 May 2024; IAFP: Urbandale, IA, USA, 2024. [Google Scholar]
- Heunis, T.; Botes, M.; Dicks, L. Encapsulation of Lactobacillus plantarum 423 and its bacteriocin in nanofibers. Probiotics Antimicrob. Proteins 2010, 2, 46–51. [Google Scholar] [CrossRef]
- Afzaal, M.; Khan, A.U.; Saeed, F.; Ahmed, A.; Ahmad, M.H.; Maan, A.A.; Tufail, T.; Anjum, F.M.; Hussain, S. Functional exploration of free and encapsulated probiotic bacteria in yogurt and simulated gastrointestinal conditions. Food Sci. Nutr. 2019, 7, 3931–3940. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, T.; Song, Y.; Shu, G.; Chen, H. Effect of xanthan-chitosan-xanthan double layer encapsulation on survival of Bifidobacterium BB01 in simulated gastrointestinal conditions, bile salt solution and yogurt. LWT-Food Sci. Technol. 2017, 81, 274–280. [Google Scholar] [CrossRef]
- Dias, C.O.; de Almeida, J.d.S.O.; Pinto, S.S.; de Oliveira Santana, F.C.; Verruck, S.; Müller, C.M.O.; Prudêncio, E.S.; Amboni, R.D.d.M.C. Development and physico-chemical characterization of microencapsulated bifidobacteria in passion fruit juice: A functional non-dairy product for probiotic delivery. Food Biosci. 2018, 24, 26–36. [Google Scholar] [CrossRef]
- Mokhtari, S.; Jafari, S.M.; Khomeiri, M. Survival of encapsulated probiotics in pasteurized grape juice and evaluation of their properties during storage. Food Sci. Technol. Int. 2019, 25, 120–129. [Google Scholar] [CrossRef]
- Asar, R.; Erenler, S.; Devecioglu, D.; Ispirli, H.; Karbancioglu-Guler, F.; Ozturk, H.I.; Dertli, E. Understanding the Functionality of Probiotics on the Edge of Artificial Intelligence (AI) Era. Fermentation 2025, 11, 259. [Google Scholar] [CrossRef]
- Westfall, S.; Carracci, F.; Estill, M.; Zhao, D.; Wu, Q.-l.; Shen, L.; Simon, J.; Pasinetti, G.M. Optimization of probiotic therapeutics using machine learning in an artificial human gastrointestinal tract. Sci. Rep. 2021, 11, 1067. [Google Scholar] [CrossRef]
- Sadeghi, M.; Panahi, B.; Mazlumi, A.; Hejazi, M.A.; Komi, D.E.A.; Nami, Y. Screening of potential probiotic lactic acid bacteria with antimicrobial properties and selection of superior bacteria for application as biocontrol using machine learning models. LWT 2022, 162, 113471. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, J.; Kalimuthu, S.; Liu, J.; Song, Z.-M.; He, B.-b.; Cai, P.; Zhong, Z.; Feng, C.; Neelakantan, P. A systematically biosynthetic investigation of lactic acid bacteria reveals diverse antagonistic bacteriocins that potentially shape the human microbiome. Microbiome 2023, 11, 91. [Google Scholar] [CrossRef] [PubMed]
- van Heel, A.J.; de Jong, A.; Song, C.; Viel, J.H.; Kok, J.; Kuipers, O.P. BAGEL4: A user-friendly web server to thoroughly mine RiPPs and bacteriocins. Nucleic Acids Res. 2018, 46, W278–W281. [Google Scholar] [CrossRef]
- Arango-Argoty, G.; Garner, E.; Pruden, A.; Heath, L.S.; Vikesland, P.; Zhang, L. DeepARG: A deep learning approach for predicting antibiotic resistance genes from metagenomic data. Microbiome 2018, 6, 23. [Google Scholar] [CrossRef]
- Krawczyk, P.S.; Lipinski, L.; Dziembowski, A. PlasFlow: Predicting plasmid sequences in metagenomic data using genome signatures. Nucleic Acids Res. 2018, 46, e35. [Google Scholar] [CrossRef]
- Wu, S.; Feng, T.; Tang, W.; Qi, C.; Gao, J.; He, X.; Wang, J.; Zhou, H.; Fang, Z. metaProbiotics: A tool for mining probiotic from metagenomic binning data based on a language model. Brief. Bioinform. 2024, 25, bbae085. [Google Scholar] [CrossRef]
- González-Sendino, R.; Serrano, E.; Bajo, J. Mitigating bias in artificial intelligence: Fair data generation via causal models for transparent and explainable decision-making. Future Gener. Comput. Syst. 2024, 155, 384–401. [Google Scholar] [CrossRef]
- D’Urso, F.; Broccolo, F. Applications of artificial intelligence in microbiome analysis and probiotic interventions—An overview and perspective based on the current state of the art. Appl. Sci. 2024, 14, 8627. [Google Scholar] [CrossRef]
- Song, M.W.; Chung, Y.; Kim, K.-T.; Hong, W.S.; Chang, H.J.; Paik, H.-D. Probiotic characteristics of Lactobacillus brevis B13-2 isolated from kimchi and investigation of antioxidant and immune-modulating abilities of its heat-killed cells. LWT 2020, 128, 109452. [Google Scholar] [CrossRef]
- Fakruddin, M.; Hossain, M.N.; Ahmed, M.M. Antimicrobial and antioxidant activities of Saccharomyces cerevisiae IFST062013, a potential probiotic. BMC Complement. Altern. Med. 2017, 17, 64. [Google Scholar] [CrossRef]
- Ornellas, R.M.S.; Santos, T.T.; Arcucio, L.B.; Sandes, S.H.C.; Oliveira, M.M.; Dias, C.V.; de Carvalho Silva, S.; Uetanabaro, A.P.T.; Vinderola, G.; Nicoli, J.R. Selection of lactic acid bacteria with probiotic potential isolated from the fermentation process of “Cupuaçu”(Theobroma grandiflorum). Adv. Microbiol. Infect. Dis. Public Health 2017, 7, 1–16. [Google Scholar]
- Ooi, L.-G.; Liong, M.-T. Cholesterol-lowering effects of probiotics and prebiotics: A review of in vivo and in vitro findings. Int. J. Mol. Sci. 2010, 11, 2499–2522. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Nagpal, R.; Kumar, R.; Hemalatha, R.; Verma, V.; Kumar, A.; Chakraborty, C.; Singh, B.; Marotta, F.; Jain, S. Cholesterol-lowering probiotics as potential biotherapeutics for metabolic diseases. J. Diabetes Res. 2012, 2012, 902917, Erratum in J. Diabetes Res. 2022, 2022, 3952529. [Google Scholar] [CrossRef]
- Cavalcante, R.G.; de Albuquerque, T.M.; de Luna Freire, M.O.; Ferreira, G.A.; Dos Santos, L.A.C.; Magnani, M.; Cruz, J.C.; Braga, V.A.; de Souza, E.L.; de Brito Alves, J.L. The probiotic Lactobacillus fermentum 296 attenuates cardiometabolic disorders in high fat diet-treated rats. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 1408–1417. [Google Scholar] [CrossRef]
- Costabile, A.; Buttarazzi, I.; Kolida, S.; Quercia, S.; Baldini, J.; Swann, J.R.; Brigidi, P.; Gibson, G.R. An in vivo assessment of the cholesterol-lowering efficacy of Lactobacillus plantarum ECGC 13110402 in normal to mildly hypercholesterolaemic adults. PLoS ONE 2017, 12, e0187964. [Google Scholar]
- Tannock, G.W.; Savage, D.C. Influences of dietary and environmental stress on microbial populations in the murine gastrointestinal tract. Infect. Immun. 1974, 9, 591–598. [Google Scholar] [CrossRef]
- Luna, R.A.; Foster, J.A. Gut brain axis: Diet microbiota interactions and implications for modulation of anxiety and depression. Curr. Opin. Biotechnol. 2015, 32, 35–41. [Google Scholar] [CrossRef]
- Collins, S.M.; Surette, M.; Bercik, P. The interplay between the intestinal microbiota and the brain. Nat. Rev. Microbiol. 2012, 10, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Dinan, T.G.; Cryan, J.F. Melancholic microbes: A link between gut microbiota and depression? Neurogastroenterol. Motil. 2013, 25, 713–719. [Google Scholar] [CrossRef]
- Akkasheh, G.; Kashani-Poor, Z.; Tajabadi-Ebrahimi, M.; Jafari, P.; Akbari, H.; Taghizadeh, M.; Memarzadeh, M.R.; Asemi, Z.; Esmaillzadeh, A. Clinical and metabolic response to probiotic administration in patients with major depressive disorder: A randomized, double-blind, placebo-controlled trial. Nutrition 2016, 32, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Kouitcheu Mabeku, L.B.; Ngue, S.; Bonsou Nguemo, I.; Leundji, H. Potential of selected lactic acid bacteria from Theobroma cacao fermented fruit juice and cell-free supernatants from cultures as inhibitors of Helicobacter pylori and as good probiotic. BMC Res. Notes 2020, 13, 64. [Google Scholar] [CrossRef]
- Di Cagno, R.; Filannino, P.; Cantatore, V.; Polo, A.; Celano, G.; Martinovic, A.; Cavoski, I.; Gobbetti, M. Design of potential probiotic yeast starters tailored for making a cornelian cherry (Cornus mas L.) functional beverage. Int. J. Food Microbiol. 2020, 323, 108591. [Google Scholar] [CrossRef] [PubMed]
- Sardar, D.; Morol, I.; Bari, J.; Sarkar, A.; Habib, A. Optimization of cryoprotectants and storage temperatures for preserving viability and probiotic properties of lyophilized bacterial strains from chicken gut. PLoS ONE 2025, 20, e0328216. [Google Scholar] [CrossRef]
- Abdul Manan, M. Progress in Probiotic Science: Prospects of Functional Probiotic-Based Foods and Beverages. Int. J. Food Sci. 2025, 2025, 5567567. [Google Scholar] [CrossRef] [PubMed]
- Gore, A. Probiotics Market Report 2025 (Global Edition). Available online: https://www.cognitivemarketresearch.com/probiotics-market-report (accessed on 3 September 2025).
- Hill, P.; Muir, J.G.; Gibson, P.R. Controversies and Recent Developments of the Low-FODMAP Diet. Gastroenterol. Hepatol. 2017, 13, 36–45. [Google Scholar]
- Murillo, A.Z.; Arévalo, F.E.; Jáuregui, E.P. Diet low in fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAPs) in the treatment of irritable bowel syndrome: Indications and design. Endocrinol. Nutr. (Engl. Ed.) 2016, 63, 132–138. [Google Scholar] [CrossRef]
- Fredua-Agyeman, M.; Larbi, E.A. Inaccurate labelling practices in probiotic products: A regulatory shortfall in Accra, Ghana. PLoS ONE 2025, 20, e0322194. [Google Scholar] [CrossRef]
- Siegrist, M.; Shi, J.; Giusto, A.; Hartmann, C. Worlds apart. Consumer acceptance of functional foods and beverages in Germany and China. Appetite 2015, 92, 87–93. [Google Scholar] [CrossRef]
- Chang, M.Y.; Chen, H.S. Understanding Consumers’ Intentions to Purchase Clean Label Products: Evidence from Taiwan. Nutrients 2022, 14, 3684. [Google Scholar] [CrossRef]
- Bimbo, F.; Bonanno, A.; Nocella, G.; Viscecchia, R.; Nardone, G.; De Devitiis, B.; Carlucci, D. Consumers’ acceptance and preferences for nutrition-modified and functional dairy products: A systematic review. Appetite 2017, 113, 141–154. [Google Scholar] [CrossRef]
- Kraus, A.; Annunziata, A.; Vecchio, R. Sociodemographic factors differentiating the consumer and the motivations for functional food consumption. J. Am. Coll. Nutr. 2017, 36, 116–126. [Google Scholar] [CrossRef]
- Roman, S.; Sánchez-Siles, L.M.; Siegrist, M. The importance of food naturalness for consumers: Results of a systematic review. Trends Food Sci. Technol. 2017, 67, 44–57. [Google Scholar] [CrossRef]
- Rendahl, J.; Korp, P.; Ekström, M.P.; Berg, C. Adolescents’ trust in food messages and their sources. Br. Food J. 2017, 119, 2712–2723. [Google Scholar] [CrossRef]
- De Simone, C. The unregulated probiotic market. Clin. Gastroenterol. Hepatol. 2019, 17, 809–817. [Google Scholar] [CrossRef]
- Liang, L.; Yi, Y.; Lv, Y.; Qian, J.; Lei, X.; Zhang, G. A comprehensive genome survey provides novel insights into bile salt hydrolase (BSH) in Lactobacillaceae. Molecules 2018, 23, 1157. [Google Scholar] [CrossRef]
- Abdul Manan, M. The role of probiotics in personalized therapeutics: Advances in gut microbe-driven interventions. Microbe 2025, 8, 100497. [Google Scholar] [CrossRef]
- Abouelela, M.E.; Helmy, Y.A. Next-Generation Probiotics as Novel Therapeutics for Improving Human Health: Current Trends and Future Perspectives. Microorganisms 2024, 12, 430. [Google Scholar] [CrossRef] [PubMed]
- McCoubrey, L.E.; Elbadawi, M.; Orlu, M.; Gaisford, S.; Basit, A.W. Harnessing machine learning for development of microbiome therapeutics. Gut Microbes 2021, 13, 1872323. [Google Scholar] [CrossRef]
- Li, P.; Luo, H.; Ji, B.; Nielsen, J. Machine learning for data integration in human gut microbiome. Microb. Cell Fact. 2022, 21, 241. [Google Scholar] [CrossRef]
- Pedroza Matute, S.; Iyavoo, S. Exploring the gut microbiota: Lifestyle choices, disease associations, and personal genomics. Front Nutr 2023, 10, 1225120. [Google Scholar] [CrossRef]
- Leuschner, R.G.; Robinson, T.P.; Hugas, M.; Cocconcelli, P.S.; Richard-Forget, F.; Klein, G.; Licht, T.R.; Nguyen-The, C.; Querol, A.; Richardson, M. Qualified presumption of safety (QPS): A generic risk assessment approach for biological agents notified to the European Food Safety Authority (EFSA). Trends Food Sci. Technol. 2010, 21, 425–435, Erratum in Trends Food Sci. Technol. 2011, 22, 51–52. [Google Scholar] [CrossRef]
- EFSA-BIOHAZ; Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Hilbert, F. Update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA 10: Suitability of taxonomic units notified to EFSA until March 2019. EFSA J. 2019, 17, e05753. [Google Scholar] [PubMed]
- Huys, G.; Vancanneyt, M.; d’Haene, K.; Vankerckhoven, V.; Goossens, H.; Swings, J. Accuracy of species identity of commercial bacterial cultures intended for probiotic or nutritional use. Res. Microbiol. 2006, 157, 803–810. [Google Scholar] [CrossRef]
- Vankerckhoven, V.; Moreillon, P.; Piu, S.; Giddey, M.; Huys, G.; Vancanneyt, M.; Goossens, H.; Entenza, J.M. Infectivity of Lactobacillus rhamnosus and Lactobacillus paracasei isolates in a rat model of experimental endocarditis. J. Med. Microbiol. 2007, 56, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Koirala, S.; Anal, A.K. Probiotics-based foods and beverages as future foods and their overall safety and regulatory claims. Future Foods 2021, 3, 100013. [Google Scholar] [CrossRef]
- Sanders, M.E. How do we know when something called “probiotic” is really a probiotic? A guideline for consumers and health care professionals. Funct. Food Rev. 2009, 1, 3–12. [Google Scholar]
- Douillard, F.P.; Ribbera, A.; Kant, R.; Pietilä, T.E.; Järvinen, H.M.; Messing, M.; Randazzo, C.L.; Paulin, L.; Laine, P.; Ritari, J. Comparative genomic and functional analysis of 100 Lactobacillus rhamnosus strains and their comparison with strain GG. PLoS Genet. 2013, 9, e1003683. [Google Scholar] [CrossRef] [PubMed]

| Species/Strains | Isolation Source | Key Functional Traits | References |
|---|---|---|---|
| Weissella confusa MD1 and Weissella cibaria MD2 | Fermented batter, traditional fermented foods such as horreh | Co-aggregation with pathogens; lysozyme and acid tolerance; cholesterol reduction; antimicrobial potential; exopolysaccharide production; antioxidant activity. | [22,23] |
| Bacillus coagulans | Milk | Spore-forming abilities, gastrointestinal survivability; enzyme production; heat tolerance. | [24] |
| Lactobacillus plantarum (e.g., SY11, SY12, AAS3) | Kimchi, dry fish based fermented food | Acid and bile tolerance; adhesion to intestinal cells; cholesterol reducing property; antimicrobial and antioxidant activity. | [25,26] |
| L. paraplantarum (e.g., SC61) | Jangajii (fermented vegetable) | Antioxidant and immunostimulatory activity; stability in artificial gastric and bile conditions, non-production of β-glucuronidase, suitable antibiotic susceptibility, and attachment to intestinal cells. | [27] |
| Weissella hellenica BCC 7239 | Nham (fermented pork sausage) | Production of bacteriocins, bactericidal effects against both Gram-positive and Gram-negative organisms | [28] |
| Fructobacillus fructosus MCC 3996 | Flower nectar | Resistance to gastric conditions; co-aggregation with pathogens, hydrophobicity, and the absence of hemolytic activity. | [29] |
| Saccharomyces cerevisiae (e.g., KU200270, KU200280, and KU200284) | Cucumber jangajji and other fermented foods | Antioxidative properties; gastric and bile resistance; adhesion to epithelial cells. | [30] |
| Aureobasidium pullulans (e.g., Y39, Y40, Y41, Y43) | Kalamata table olive | Auto-aggregation ability; hydrophobicity; adhesion to Caco-2 cells; absence of hemolytic activity. | [31] |
| Lacticaseibacillus casei (e.g., SB71, SB73 and SB93) | Marine ecosystem | Inability to form biogenic amines; adherence to Caco-2 cells; cholesterol assimilation; and tolerance to NaCl, bile and low pH. | [32] |
| Leuconostoc (citreum and mesenteroides subsp. mesenteroides) | Traditional fermented foods such as Horreh | Exopolysaccharide production; antioxidant activity; acid tolerance | [23] |
| Pediococcus pentosaceus | Traditional fermented foods | Exopolysaccharide production; antioxidant activity; acid tolerance | [23] |
| Enterococcus (faecium and faecalis) | Traditional fermented foods (e.g., Kimchi, Horreh) | Absence of antibiotic resistance or virulence factors; auto aggregation ability; hydrophobicity; resistance to gastrointestinal conditions. | [23,33] |
| Akkermansia muciniphila | Human intestinal microbiota | Mucin degradation; modulation of host metabolism; gut barrier reinforcement | [34] |
| Faecalibacterium prausnitzii | Human gut | Butyrate production; anti-inflammatory and gut-protective effects | [35] |
| Criteria | Conventional Probiotics (e.g., Lactobacillus, Bifidobacterium) | Emerging Probiotics (e.g., Akkermansia muciniphila, Faecalibacterium prausnitzii) |
|---|---|---|
| Safety status | Well-established (GRAS/QPS) [2,10] | Ongoing safety evaluation and limited regulatory approval [21] |
| Isolation sources | Traditional fermented foods (yogurt, kefir, sauerkraut), dairy products, and human/animal gut [5,6] | Novel environments (soil, plants, insects, marine microbiota, human gut) [21,32,35] |
| Health benefits (evidence base) | Well-documented gut health and anti-diarrheal effects, lactose intolerance relief; multiple clinical trials [5,10] | Limited but growing number of clinical studies; promising roles in obesity, diabetes, inflammatory bowel disease, and metabolic syndrome [42] |
| Functional traits | Antimicrobial activity; acid and bile salt tolerance, epithelial adhesion [25,26] | Immune modulation, gut barrier enhancement, production of short-chain fatty acid (SCFA) [43,44] |
| Mechanistic understanding | Mechanisms relatively well-characterized (competition with pathogens, production of antimicrobials, adhesion, immune modulation) [2]. | Mechanisms still being evaluated (mucin degradation, signaling via metabolites like SCFAs, anti-inflammatory pathways) [42,44] |
| Industrial application | Widely commercialized in yogurts, cheeses, beverages, infant formula, dietary supplements [38]. | Limited commercial applications; potential in next-generation probiotics (capsules, synbiotics, functional beverages) [42] |
| Challenges | Strain-specific variability; genetic instability in industrial settings [45] | Difficulties in cultivation, safety uncertainties, lack of regulatory approval, stability issues in food matrices [21] |
| Future perspectives | Continued use in conventional foods and nutraceuticals; exploration of strain engineering for enhanced traits | Potential game-changers in personalized nutrition, microbiome-targeted therapies, and precision probiotics once validated |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uhegwu, C.C.; Anumudu, C.K. Probiotic Potential of Traditional and Emerging Microbial Strains in Functional Foods: From Characterization to Applications and Health Benefits. Microorganisms 2025, 13, 2521. https://doi.org/10.3390/microorganisms13112521
Uhegwu CC, Anumudu CK. Probiotic Potential of Traditional and Emerging Microbial Strains in Functional Foods: From Characterization to Applications and Health Benefits. Microorganisms. 2025; 13(11):2521. https://doi.org/10.3390/microorganisms13112521
Chicago/Turabian StyleUhegwu, Chijioke Christopher, and Christian Kosisochukwu Anumudu. 2025. "Probiotic Potential of Traditional and Emerging Microbial Strains in Functional Foods: From Characterization to Applications and Health Benefits" Microorganisms 13, no. 11: 2521. https://doi.org/10.3390/microorganisms13112521
APA StyleUhegwu, C. C., & Anumudu, C. K. (2025). Probiotic Potential of Traditional and Emerging Microbial Strains in Functional Foods: From Characterization to Applications and Health Benefits. Microorganisms, 13(11), 2521. https://doi.org/10.3390/microorganisms13112521







