Effect of High-Temperature Stress on Fatty Acid Composition and Undecylprodiginine Biosynthesis in Streptomyces coelicolor M511
Abstract
1. Introduction
2. Materials and Methods
2.1. Strain Information and Culture Conditions
2.2. Leucine, Isoleucine, Valine, and Chemical Feeding Studies in High Temperature Stress Conditions
2.3. HPLC Analysis of Prodiginine Extract from M511 Strain
2.4. Verification of Undecylprodiginine in the Spectrum Using LC-QTOF-MS
2.5. Confirmation of Fatty Acid Profiling in Each Culture Group Using GC-MS
3. Results
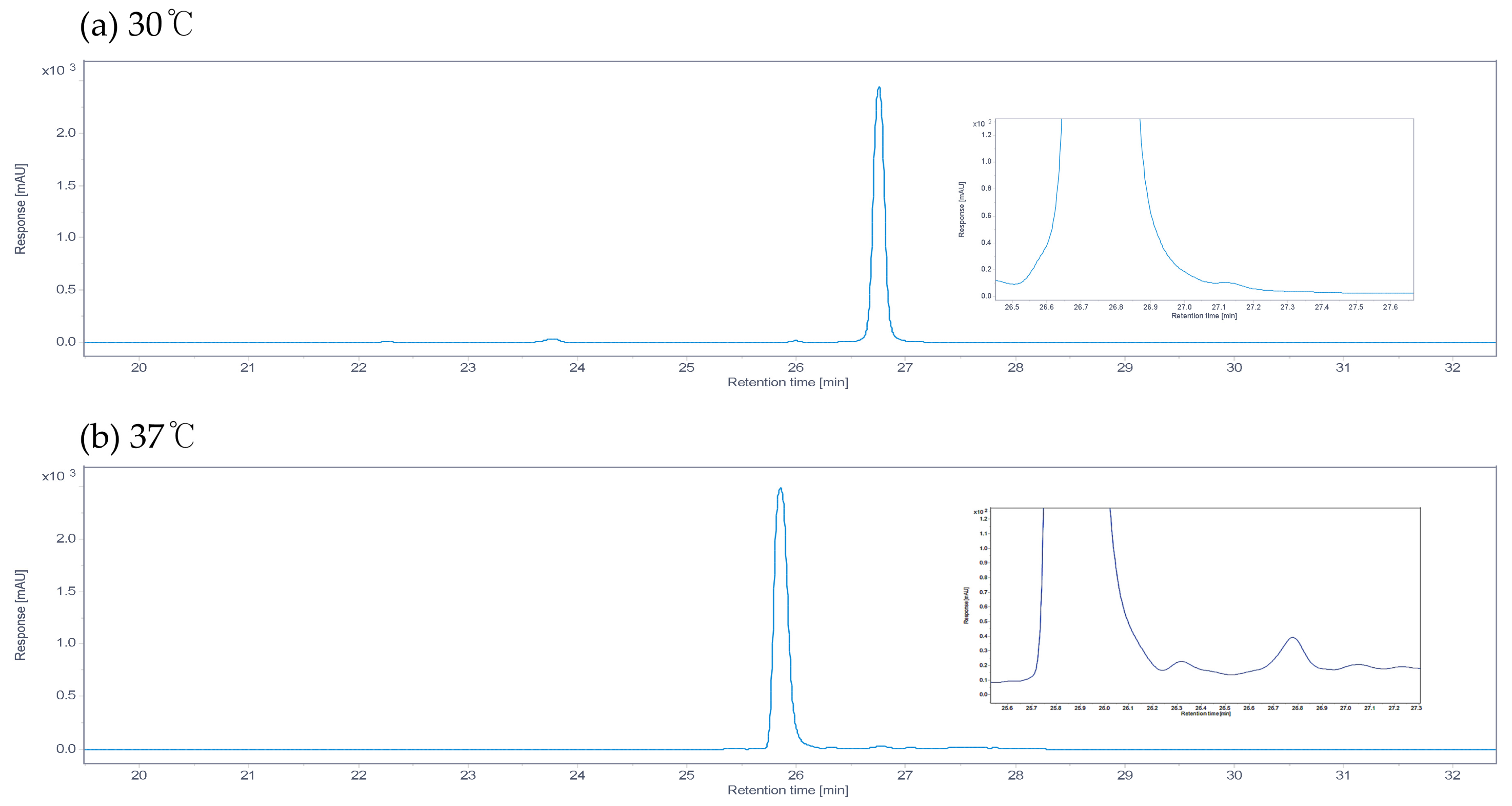
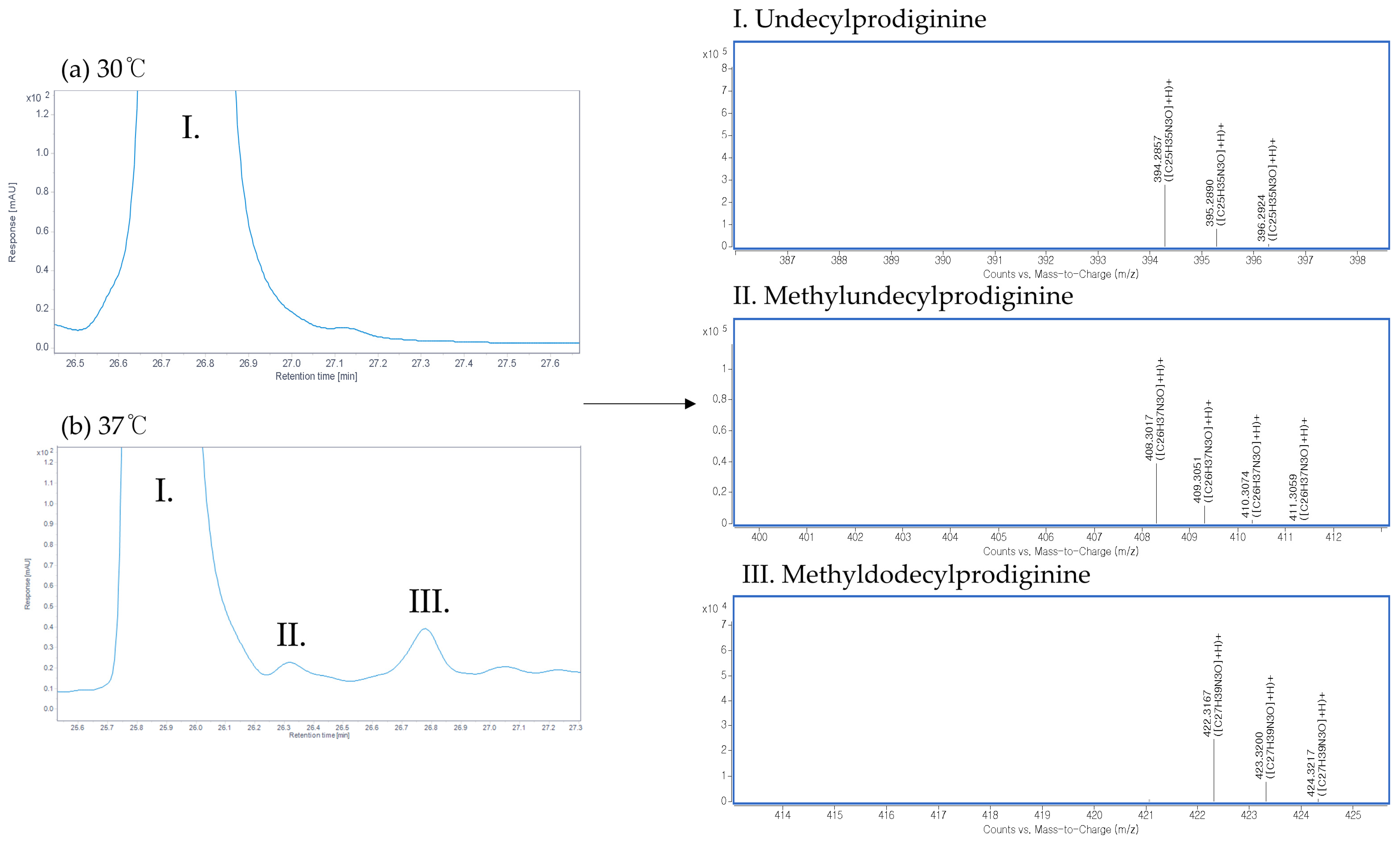
3.1. Heat Stress During the Growth of the M511 Strain Reduces the Production of Undecylprodiginine and Generates Branched-Chain Alkylprodiginines
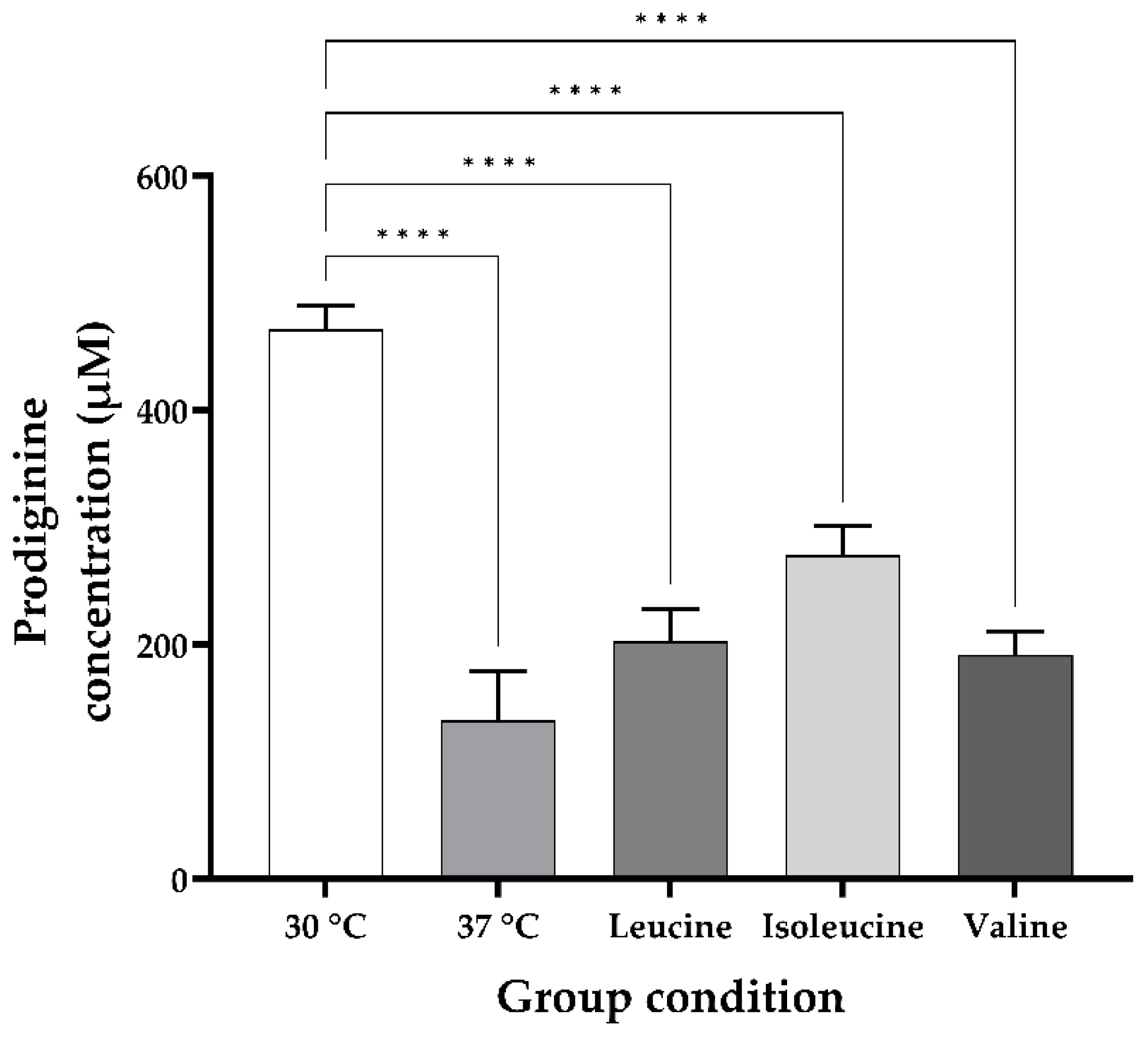
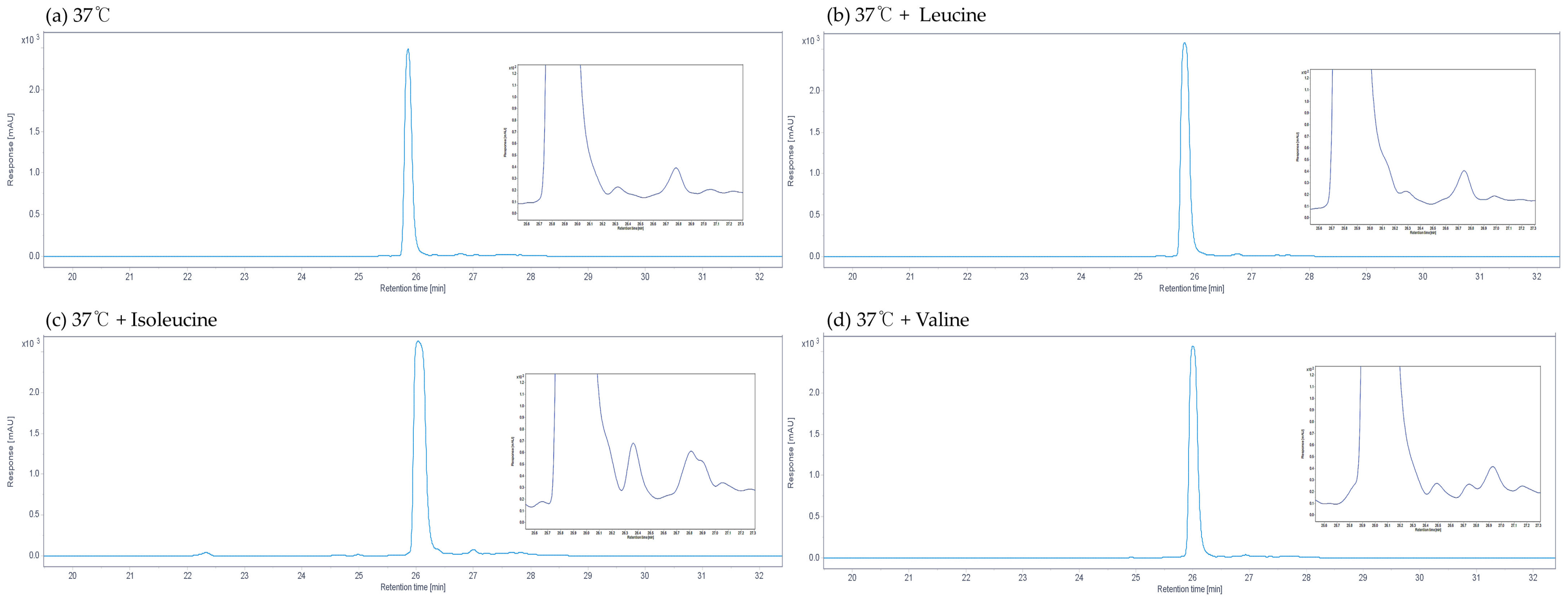
3.2. Changes in the Undecylprodiginine Production Pattern When BCAA Is Added in a Heat Stress Environment
3.3. Observation of Changes in Fatty Acid Composition Within Strains Under Heat Stress Conditions and with BCAAs Supplementation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hoskisson, P.A.; van Wezel, G.P. Streptomyces coelicolor. Trends Microbiol. 2019, 27, 468–469. [Google Scholar] [CrossRef] [PubMed]
- Ultee, E.; van der Aart, L.T.; Zhang, L.; van Dissel, D.; Diebolder, C.A.; van Wezel, G.P.; Claessen, D.; Briegel, A. Teichoic Acids Anchor Distinct Cell Wall Lamellae in an Apically Growing Bacterium. Commun. Biol. 2020, 3, 314. [Google Scholar] [CrossRef] [PubMed]
- Kämpfer, P.; Glaeser, S.P.; Parkes, L.; van Keulen, G.; Dyson, P. The Family Streptomycetaceae. In The Prokaryotes; Springer: Berlin/Heidelberg, Germany, 2014; pp. 889–1010. ISBN 9783642301377. [Google Scholar]
- Omura, S.; Ikeda, H.; Ishikawa, J.; Hanamoto, A.; Takahashi, C.; Shinose, M.; Takahashi, Y.; Horikawa, H.; Nakazawa, H.; Osonoe, T.; et al. Genome Sequence of an Industrial Microorganism Streptomyces avermitilis: Deducing the Ability of Producing Secondary Metabolites. Proc. Natl. Acad. Sci. USA 2001, 98, 12215–12220. [Google Scholar] [CrossRef]
- Demain, A.L. Pharmaceutically Active Secondary Metabolites of Microorganisms. Appl. Microbiol. Biotechnol. 1999, 52, 455–463. [Google Scholar] [CrossRef]
- Ryding, N.J.; Anderson, T.B.; Champness, W.C. Regulation of the Streptomyces coelicolor Calcium-Dependent Antibiotic by absA, Encoding a Cluster-Linked Two-Component System. J. Bacteriol. 2002, 184, 794–805. [Google Scholar] [CrossRef]
- Bibb, M. 1995 Colworth Prize Lecture. The Regulation of Antibiotic Production in Streptomyces coelicolor A3(2). Microbiology 1996, 142, 1335–1344. [Google Scholar] [CrossRef]
- Luti, K.J.K.; Mavituna, F. Streptomyces coelicolor Increases the Production of Undecylprodigiosin When Interacted with Bacillus Subtilis. Biotechnol. Lett. 2011, 33, 113–118. [Google Scholar] [CrossRef]
- Stankovic, N.; Senerovic, L.; Ilic-Tomic, T.; Vasiljevic, B.; Nikodinovic-Runic, J. Properties and Applications of Undecylprodigiosin and Other Bacterial Prodigiosins. Appl. Microbiol. Biotechnol. 2014, 98, 3841–3858. [Google Scholar] [CrossRef]
- Shin, J.-H.; Singh, A.K.; Cheon, D.-J.; Roe, J.-H. Activation of the SoxR Regulon in Streptomyces coelicolor by the Extracellular Form of the Pigmented Antibiotic Actinorhodin. J. Bacteriol. 2011, 193, 75–81. [Google Scholar] [CrossRef]
- Parsons, J.B.; Rock, C.O. Bacterial Lipids: Metabolism and Membrane Homeostasis. Prog. Lipid Res. 2013, 52, 249–276. [Google Scholar] [CrossRef]
- Schweizer, E.; Hofmann, J. Microbial Type I Fatty Acid Synthases (FAS): Major Players in a Network of Cellular FAS Systems. Microbiol. Mol. Biol. Rev. 2004, 68, 501–517. [Google Scholar] [CrossRef]
- Gago, G.; Diacovich, L.; Arabolaza, A.; Tsai, S.-C.; Gramajo, H. Fatty Acid Biosynthesis in Actinomycetes. FEMS Microbiol. Rev. 2011, 35, 475–497. [Google Scholar] [CrossRef]
- Kaneda, T. Iso- and Anteiso-Fatty Acids in Bacteria: Biosynthesis, Function, and Taxonomic Significance. Microbiol. Rev. 1991, 55, 288–302. [Google Scholar] [CrossRef]
- Wallace, K.K.; Zhao, B.; McArthur, H.A.I.; Reynolds, K.A. In Vivo Analysis of Straight-Chain and Branched-Chain Fatty Acid Biosynthesis in Three Actinomycetes. FEMS Microbiol. Lett. 1995, 131, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Yu, Y.; Luo, L.; Huang, M.; Zhang, Y.; Su, J.; Zhang, W.; Ma, J.; Hu, Z.; Wang, H. 3-Ketoacyl-ACP Synthase III FabH1 Is Essential for Branched-Chain DSF Family Signals in Xanthomonas oryzae Pv. Oryzae. Phytopathol. Res. 2023, 5, 26. [Google Scholar] [CrossRef]
- Chen, A.; Re, R.N.; Burkart, M.D. Type II Fatty Acid and Polyketide Synthases: Deciphering Protein-Protein and Protein-Substrate Interactions. Nat. Prod. Rep. 2018, 35, 1029–1045. [Google Scholar] [CrossRef] [PubMed]
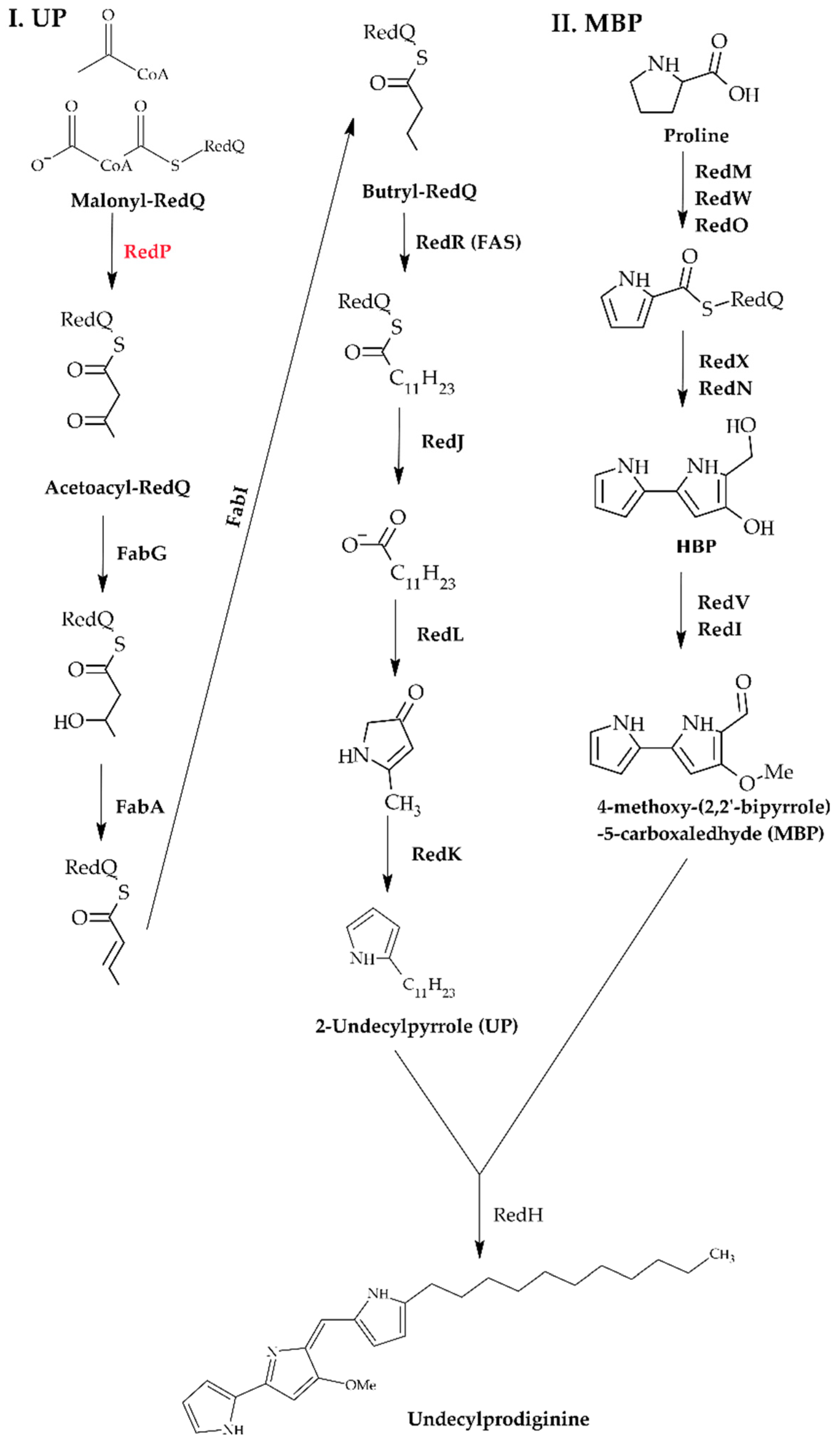
- Cerdeño, A.M.; Bibb, M.J.; Challis, G.L. Analysis of the Prodiginine Biosynthesis Gene Cluster of Streptomyces coelicolor A3(2): New Mechanisms for Chain Initiation and Termination in Modular Multienzymes. Chem. Biol. 2001, 8, 817–829. [Google Scholar] [CrossRef]
- Whicher, J.R.; Florova, G.; Sydor, P.K.; Singh, R.; Alhamadsheh, M.; Challis, G.L.; Reynolds, K.A.; Smith, J.L. Structure and Function of the RedJ Protein, a Thioesterase from the Prodiginine Biosynthetic Pathway in Streptomyces coelicolor. J. Biol. Chem. 2011, 286, 22558–22569. [Google Scholar] [CrossRef]
- Mo, S.; Sydor, P.K.; Corre, C.; Alhamadsheh, M.M.; Stanley, A.E.; Haynes, S.W.; Song, L.; Reynolds, K.A.; Challis, G.L. Elucidation of the Streptomyces coelicolor Pathway to 2-Undecylpyrrole, a Key Intermediate in Undecylprodiginine and Streptorubin B Biosynthesis. Chem. Biol. 2008, 15, 137–148. [Google Scholar] [CrossRef]
- Singh, R. Enzymatic Control of the Related Pathways of Fatty Acid and Undecylprodiginine Biosynthesis in Streptomyces coelicolor. Ph.D. Thesis, Portland State University, Portland, OR, USA, 2014. [Google Scholar]
- Singh, R.; Mo, S.; Florova, G.; Reynolds, K.A. Streptomyces coelicolor RedP and FabH Enzymes, Initiating Undecylprodiginine and Fatty Acid Biosynthesis, Exhibit Distinct Acyl-CoA and Malonyl-Acyl Carrier Protein Substrate Specificities. FEMS Microbiol. Lett. 2012, 328, 32–38. [Google Scholar] [CrossRef]
- Mo, S.; Kim, B.S.; Reynolds, K.A. Production of Branched-Chain Alkylprodiginines in S. coelicolor by Replacement of the 3-Ketoacyl ACP Synthase III Initiation Enzyme, RedP. Chem. Biol. 2005, 12, 191–200. [Google Scholar] [CrossRef]
- Bucca, G.; Pothi, R.; Hesketh, A.; Möller-Levet, C.; Hodgson, D.A.; Laing, E.E.; Stewart, G.R.; Smith, C.P. Translational Control Plays an Important Role in the Adaptive Heat-Shock Response of Streptomyces coelicolor. Nucleic Acids Res. 2018, 46, 5692–5703. [Google Scholar] [CrossRef]
- Apriliana, P.; Kahar, P.; Rachmadona, N.; Restu, W.K.; Kondo, A.; Ogino, C. Lipid Production in Streptomyces Jeddahensis Is Enhanced by Glucose and Fatty Acid Derivatives, with Temperature Variations Influencing Gene Expression and Biosynthesis. Fermentation 2025, 11, 45. [Google Scholar] [CrossRef]
- Yuk, H.-G.; Marshall, D.L. Heat Adaptation Alters Escherichia coli O157:H7 Membrane Lipid Composition and Verotoxin Production. Appl. Environ. Microbiol. 2003, 69, 5115–5119. [Google Scholar] [CrossRef] [PubMed]
- Min, B.; Kim, K.; Li, V.; Cho, S.; Kim, H. Changes in Cell Membrane Fatty Acid Composition of Streptococcus thermophilus in Response to Gradually Increasing Heat Temperature. J. Microbiol. Biotechnol. 2020, 30, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.J.; Bruheim, P.; Jovetic, S.; Marinelli, F.; Postma, P.W.; Bibb, M.J. Engineering of Primary Carbon Metabolism for Improved Antibiotic Production in Streptomyces Lividans. Appl. Environ. Microbiol. 2002, 68, 4731–4739. [Google Scholar] [CrossRef] [PubMed]
- Stankovic, N.; Radulovic, V.; Petkovic, M.; Vuckovic, I.; Jadranin, M.; Vasiljevic, B.; Nikodinovic-Runic, J. Streptomyces sp. JS520 Produces Exceptionally High Quantities of Undecylprodigiosin with Antibacterial, Antioxidative, and UV-Protective Properties. Appl. Microbiol. Biotechnol. 2012, 96, 1217–1231. [Google Scholar] [CrossRef]
- Jung, M.Y.; Kim, G.B.; Jang, E.S.; Jung, Y.K.; Park, S.Y.; Lee, B.H. Improved Extraction Method with Hexane for Gas Chromatographic Analysis of Conjugated Linoleic Acids. J. Dairy Sci. 2006, 89, 90–94. [Google Scholar] [CrossRef]
- Martínez, P.M.; Ortiz-Martínez, V.M.; Sánchez Segado, S.; Salar-García, M.J.; de los Ríos, A.P.; Hernández Fernández, F.J.; Lozano-Blanco, L.J.; Godínez, C. Deep Eutectic Solvents for the Extraction of Fatty Acids from Microalgae Biomass: Recovery of Omega-3 Eicosapentaenoic Acid. Sep. Purif. Technol. 2022, 300, 121842. [Google Scholar] [CrossRef]
- Chico Retrato, M.D.; Qiu, S.; Lundquist, A.; Muratovic, A.Z.; Rad, F.M.; Ubhayasekera, S.J.K.A.; Bergquist, J. Simultaneous Determination of 22 Fatty Acids in Total Parenteral Nutrition (TPN) Components by Gas Chromatography-Mass Spectrometry (GC-MS). Anal. Methods 2023, 15, 2480–2489. [Google Scholar] [CrossRef]
- Choi, K.-H.; Heath, R.J.; Rock, C.O. β-Ketoacyl-Acyl Carrier Protein Synthase III (FabH) Is a Determining Factor in Branched-Chain Fatty Acid Biosynthesis. J. Bacteriol. 2000, 182, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Tsay, J.T.; Oh, W.; Larson, T.J.; Jackowski, S.; Rock, C.O. Isolation and Characterization of the Beta-Ketoacyl-Acyl Carrier Protein Synthase III Gene (fabH) from Escherichia coli K-12. J. Biol. Chem. 1992, 267, 6807–6814. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, W.M. The Fatty Acids of Bacteria. Bacteriol. Rev. 1962, 26, 421–447. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Chai, Q.; Wang, Y.; Chen, Y.; Dong, H.; Shen, J.; Qi, Y.; Yu, H.; Wang, F.; Wen, Q. Effects of Heat Stress and Exogenous Salicylic Acid on Secondary Metabolites Biosynthesis in Pleurotus Ostreatus (Jacq.) P. Kumm. Life 2022, 12, 915. [Google Scholar] [CrossRef]
- Straus, D.B.; Walter, W.A.; Gross, C.A. The Activity of Sigma 32 Is Reduced under Conditions of Excess Heat Shock Protein Production in Escherichia coli. Genes Dev. 1989, 3, 2003–2010. [Google Scholar] [CrossRef]
- Revill, W.P.; Bibb, M.J.; Hopwood, D.A. Purification of a Malonyltransferase from Streptomyces coelicolor A3(2) and Analysis of Its Genetic Determinant. J. Bacteriol. 1995, 177, 3946–3952. [Google Scholar] [CrossRef]
- Rey, F.J.; Chamorro, O.; Martín Gil, F.J.; Martín Gil, J. Characterization of Fatty Acid Methyl Esters by Thermal Analysis. J. Therm. Anal. Calorim. 1993, 40, 463–473. [Google Scholar] [CrossRef]
- Li, K.-H.; Yu, Y.-H.; Dong, H.-J.; Zhang, W.-B.; Ma, J.-C.; Wang, H.-H. Biological Functions of ilvC in Branched-Chain Fatty Acid Synthesis and Diffusible Signal Factor Family Production in Xanthomonas campestris. Front. Microbiol. 2017, 8, 2486. [Google Scholar] [CrossRef]
- Han, L.; Lobo, S.; Reynolds, K.A. Characterization of β-Ketoacyl-Acyl Carrier Protein Synthase III from Streptomyces glaucescens and Its Role in Initiation of Fatty Acid Biosynthesis. J. Bacteriol. 1998, 180, 4481–4486. [Google Scholar] [CrossRef]


| Fatty Acid Type | % of Total Fatty Acid Pool in: | ||||
|---|---|---|---|---|---|
| 30 °C | 37 °C | Leucine | Isoleucine | Valine | |
| Total BCFAs | 93.63 | 85.63 | 89.946 | 93.69 | 87.66 |
| 10-methyl-dodecanoic acid (aiC13) | 0.89 ± 0.05 | 2.45 ± 0.34 | 1.97 ± 2.45 | 1.96 ± 0.94 | 0.37 ± 0.33 |
| 12-methyl-tridecanoic acid (iC14) | 10.8 ± 0.3 | 11.33 ± 0.97 | 6.54 ± 2.47 | 1.45 ± 0.48 | 12.75 ± 5.29 |
| 13-methyl-tetradecanoic acid (iC15) | 5.19 ± 0.73 | 15.34 ± 1.59 | 20.87 ± 2.18 | 4.15 ± 1.3 | 9.2 ± 2.77 |
| 12-methyl-tetradecanoic acid (aiC15) | 20.94 ± 0.24 | 18.6 ± 1.54 | 11.41 ± 1.06 | 39.23 ± 8.03 | 9.9 ± 1.34 |
| 14-methyl-pentadecanoic acid (iC16) | 45.97 ± 0.35 | 17.24 ± 7.74 | 26.88 ± 6.02 | 18.15 ± 4.22 | 43.45 ± 13.42 |
| 15-methyl-hexadecanoic acid (iC17) | 8.24 ± 0.16 | 12.88 ± 1.36 | 14.26 ± 1.59 | 1.95 ± 0.71 | 6.67 ± 2.74 |
| 14-methyl-hexadecanoic acid (aiC17) | 1.6 ± 0.04 | 7.79 ± 0.89 | 8.02 ± 1.05 | 26.8 ± 0.6 | 5.32 ± 1.78 |
| Total SCFAs | 6.37 | 14.37 | 10.054 | 6.31 | 12.34 |
| Hexadecanoic acid (palmitic acid) (C16) | 6.37 ± 0.93 | 14.37 ± 1.16 | 10.06 ± 1.27 | 6.3 ± 1.7 | 12.34 ± 0.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Park, Y.; Han, K.; Mo, S. Effect of High-Temperature Stress on Fatty Acid Composition and Undecylprodiginine Biosynthesis in Streptomyces coelicolor M511. Microorganisms 2025, 13, 2520. https://doi.org/10.3390/microorganisms13112520
Han Y, Park Y, Han K, Mo S. Effect of High-Temperature Stress on Fatty Acid Composition and Undecylprodiginine Biosynthesis in Streptomyces coelicolor M511. Microorganisms. 2025; 13(11):2520. https://doi.org/10.3390/microorganisms13112520
Chicago/Turabian StyleHan, Youngjong, Yujun Park, Kyudong Han, and SangJoon Mo. 2025. "Effect of High-Temperature Stress on Fatty Acid Composition and Undecylprodiginine Biosynthesis in Streptomyces coelicolor M511" Microorganisms 13, no. 11: 2520. https://doi.org/10.3390/microorganisms13112520
APA StyleHan, Y., Park, Y., Han, K., & Mo, S. (2025). Effect of High-Temperature Stress on Fatty Acid Composition and Undecylprodiginine Biosynthesis in Streptomyces coelicolor M511. Microorganisms, 13(11), 2520. https://doi.org/10.3390/microorganisms13112520






