Abstract
Ziziphus lotus (L.) Lam., (Rhamnaceae) a resilient shrub native to Moroccan’s arid regions, functions as a keystone species by creating microhabitats that buffer temperature extremes, retain soil moisture, and accumulate organic matter. However, its role in structuring soil fungal diversity and community composition in these environments remains largely unexplored. This study investigated the spatial distribution of fungal communities associated with Z. lotus in barley-planted and non-planted fields. Soil samples were collected at 0, 3, and 6 m from shrub clusters during the barley harvest. The fungal community was dominated by Ascomycota (93.5%). Alpha diversity indices (Shannon–Wiener and Simpson) were significantly higher near shrub bases (0 and 3 m) compared to more distant soils (6 m), indicating a clear decline in diversity with distance (0 m vs. 6 m: p = 0.0012; 3 m vs. 6 m: p = 0.0007). Soil physicochemical parameters, including calcium carbonate content, nitrate, and salinity, significantly influenced fungal diversity (p ≤ 0.05). Beta diversity analysis revealed significant spatial differentiation in fungal community composition (PERMANOVA: p = 0.001). Overall, fungal richness and diversity were highest near shrub. Genera such as Cladosporium, Fusarium, and Alternaria were more abundant near shrub bases, while taxa like Didymellaceae and Alfaria were specially restricted. Functional predictions indicated dominance of fungi with mixed trophic modes (pathotroph–saprotroph–symbiotroph), suggesting ecological plasticity. Despite barley cultivation, the fungal community structure remained largely similar between the planted and non-planted fields. Overall, our findings underscore the ecological importance of Z. lotus as a reservoir of stress-tolerant fungi and as a potential keystone species for restoring degraded arid ecosystems.
Keywords:
arid ecosystems; community structure; diversity; fungi; spatial distribution; soil; Ziziphus lotus 1. Introduction
Ziziphus lotus (L.) Lam. (Rhamnaceae) commonly known as wild jujube, is a perennial shrub widely distributed across arid and semi-arid regions of North Africa, where it plays a key ecological role [1]. Renowned for resilience, Z. lotus withstand harsh abiotic conditions, including prolonged drought, high temperatures, and poor soil fertility [2], as well as biotic stressors such as parasitism by Cuscuta epithymum (L.) L. [3]. As nurse plant, it contributes to ecological stability by forming dense vegetation clusters that provide shade, lower surface temperatures, and retain soil moisture [4,5]. These microhabitats support the establishment of other plant species and offer refuge to insects, birds, reptiles, and small mammals [6]. Furthermore, the shrub’s deep root system stabilizes soil, mitigates erosion, and promotes nutrient cycling [7], thereby contributing to the formation of fertile “resource islands” [8]. Due to these functions, Z. lotus is considered a potential keystone species for the conservation and restoration of degraded drylands [9].
Like many desert plants, Z. lotus hosts a diverse fungal community that includes foliar endophytes, root-associated fungi, and rhizosphere inhabitants. Nearly all plants in natural ecosystems form associations with mycorrhizal or endophytic fungi, which enhance their adaptability under environmental stress [10]. Several studies have demonstrated the rich fungal diversity associated with Ziziphus species. For example, a survey in Oman reported over 80 endophytic fungal species from healthy Ziziphus leaves, including Alternaria spp., Aspergillus spp., Cladosporium spp., and Fusarium spp. [11]. Similar findings from North Africa confirmed the predominance of Ascomycota (~78%), followed by Basidiomycota, suggesting a conserved foliar mycobiota across regions [12].
These fungi often engage in mutualistic interactions enhancing host fitness through stress mitigation, growth promotion, and production of bioactive metabolites [12]. In particular, Z. lotus is known to form arbuscular mycorrhizal (AM) associations with glomeromycetes, which aid in water and nutrient uptake, especially phosphorus, crucial for survival in nutrient-depleted soils [13]. Inoculation studies with Rhizophagus irregularis have demonstrated a ~70% increase in biomass and phosphorus uptake in Ziziphus under AM colonization [14], underscoring the functional relevance of its fungal symbionts.
Despite these benefits, Z. lotus poses challenges in agroecosystems. Its extensive root system competes with crops for water and nutrients, potentially reducing yields and complicating land management [15]. Its thorny branches can damage agricultural machinery and hinder field operations. Therefore, while conservation is essential in natural habitats, integrated management strategies are required in agricultural contexts to balance ecological functions with productivity.
The role of Z. lotus in structuring soil fungal diversity in agricultural landscapes remains poorly understood. This study investigates whether wild jujube shrubs harbor fungal communities of ecological relevance in arid agroecosystems and whether these communities exhibit spatial structuring across shrub gradients. Given the well-known fertile island effect of desert shrubs, characterized by the accumulation of organic matter and improved microclimate beneath the shrubs, we expect fungal diversity to be highest under or near the Z. lotus canopy and to decline with increasing distance into the open field. Our objectives were (i) to assess spatial patterns of fungal diversity across Z. lotus shrub gradients; (ii) to identify the dominant fungal taxa and their potential ecological functions under both cultivated and fallow conditions; and (iii) to evaluate differences in fungal community composition between cultivated (planted) and fallow (non-planted) fields.
2. Materials and Methods
2.1. Study Site and Sample Collection
Sampling was conducted on 16 May 2023, in Rhamna Province, Morocco (GPS coordinates: 31°59′44″ N, 8°01′02″ W), approximately 100 m from Highway A3 [16]. The study site comprised two adjacent 1-hectare fields: one actively planted with barley and occasionally irrigated during the growing season, and the other left non-planted (Figure S1).
The Rhamna region lies at the interface between Mediterranean and Saharan climatic zones, exhibiting a semi-arid to arid climate. Summers are long, hot and dry, with daytime temperatures exceeding 40 °C in July and August. Winters are short and mild, with average temperatures ranging from 10 to 15 °C, and occasional nighttime lows below 5 °C. Annual precipitation ranges from 200 to 400 mm, concentrated primarily between November and March, with negligible rainfall during summer months [17].
To investigate the influence of Z. lotus on soil fungal communities, a spatially explicit sampling design was implemented. Five shrub patches were sampled within the planted field and two in the adjacent non-planted field. Each patch was sampled at 0 m (beneath the shrub canopy), 3 m, and 6 m representing a distances gradient from immediate shrub influence to surrounding field soil. These distances were chosen because the average spacing between distinct shrub patches was ~12 m; thus 6 m represents roughly half the inter-patch distance (minimizing overlap between neighboring shrubs’ influence), and 3 m serves as an intermediate point just outside the canopy. Each sample was a composite of four subsamples (~250 g each), collected at a depth of 20 cm (Figure S2). These distances were chosen based on the minimum distance (12 m) between shrub patches. In total, 84 soil samples were collected: 60 from the planted field and 24 from the non-planted field. Samples were sealed in Ziplock plastic bags (30 × 20 cm), stored on ice, and transported to the laboratory for analysis.
2.2. Soil Physicochemical Analysis
Composite samples representing each distance class (0 m, 3 m, and 6 m) within each Z. lotus patch were homogenized and analyzed at the AITTC laboratory, University Mohammed VI Polytechnic (Benguerir, Morocco). The analysis included measurements of physical properties of soil (Clay, Silt and Sand) using the NF X 31-107 protocol [18]. Soil pH was measured according to NF ISO 10390 [18], while electrical conductivity (EC) was determined following NF ISO 1 [18]. Available nutrients (nitrate-N, P2O5, K2O) were analyzed using NF ISO 11263 [18]. Micronutrients (Fe, Zn), cation exchange capacity (CEC), organic matter (OM), calcium carbonate (CaCO3), sodium oxide (Na2O), magnesium oxide (MgO), and calcium oxide (CaO) were determined according to NF X 31-108, NF X 31-121, and NF X 31-130, respectively, following standard soil analysis protocols (Table S1) [16].
2.3. DNA Extraction, PCR Amplification, and Sequencing
Genomic DNA was extracted from 250 mg of soil from each of the 84 samples using the Soil Pro Kit (Qiagen; distributed by Global Diagnostic Distribution, Témara, Morocco), following the manufacturer’s instructions. Samples were homogenized using a TissueLyser II (Qiagen) with 2 mm tungsten beads at 24 Hz for 15 min. DNA integrity was assessed via agarose gel electrophoresis, and concentration was measured with a BioSpectrophotometer (Eppendorf, Hamburg, Germany). Fungal ITS2 region amplification was performed using the primers CS1_ITS3_KYO2 (5′-ACACTGACGACATGGTTCTACAGATGAAGAACGYAGYRAA-3′) and CS2_ITS4 (5′-TACGGTAGCAGAGACTTGGTCTTCCTCCGCTTATTGATATGC-3′) (Alpha DNA, Montreal, Canada) as described in Legeay et al. [19]. Each 25 μL PCR reaction contained 1× Platinum Direct PCR Universal Master Mix (ThermoFisher, Rabat, Morocco), 0.25 μM of each primer, and ~10 ng of DNA template. Amplifications were performed in duplicate on a Mastercycler X50s (Eppendorf) using the following thermal profile: initial denaturation at 94 °C for 3 min; 30 cycles of 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 1 min; final elongation at 72 °C for 7 min. Negative (sterile water) and positive controls were included.
The fungal ITS amplicon library preparation was performed as described in Legeay et al. [19]. Briefly, the PCR products were purified using Agencourt AMPure XP beads (Beckman Coulter, Brea, CA, USA). After two ethanol washes and air drying, amplicons were resuspended in 10 mM Tris (pH 8.5). A second PCR was conducted to add Illumina sequencing adapters and index tags, using 5 µL purified PCR product, 2.5 µL Fluidigm Access Array Barcode 384, and 1× KAPA HiFi HotStart ReadyMix (Roche Sequencing Solutions, Santa Clara, CA, USA). Thermocycling was as follows: 95 °C for 3 min; 8 cycles of 95 °C for 30 s, 55 °C for 30 s, 72 °C for 30 s; and a final extension at 72 °C for 5 min.
Final libraries were purified with AMPure XP beads, quantified using the Qubit dsDNA HS Assay Kit (ThermoFisher, Témara, Morocco), and normalized and pooled according to Illumina protocols. Paired-end sequencing (2 × 300 bp) was performed using a MiSeq V3 reagent kit on an Illumina MiSeq platform (Illumina, Paris, France).
2.4. Bioinformatic Analysis
Raw sequence data was processed using the DADA2 pipeline in R v4.3.3 [20]. Low reads (Q < 30) were discarded. Primers and adapters were trimmed and reads denoised based on DADA2’s error model amplicon sequence variants (ASVs). Taxonomic assignment of ASVs was performed using BLAST against the UNITE database [21], via assignTaxonomy function.
2.5. Statistical Analysis
ASVs abundances were transformed into compositional data using the transform function of the phyloseq package (v1.46.0) with the “compositional” option to normalize sequencing depth.
Alpha diversity was calculated using Shannon–Wiener, inverse-Simpson, Pielou’s evenness, Simpson evenness, observed richness, Chao1, and ACE indices with the phyloseq and vegan packages (v2.6-8) [22]. Beta diversity was assessed using Bray–Curtis dissimilarity matrices and tested for significance using PERMANOVA (adonis function, vegan package) [23]. Soil variables were analyzed using univariate and multivariate ANOVAs to assess spatial trends across distance gradients. We also performed one-way ANOVA to compare diversity across the three distances, followed by post hoc tests to identify pairwise differences. Network analyses were conducted using SpiecEasi (v1.1.2) [24] and igraph (v2.0.2) axon-level betweenness centrality was used to identify potential hub taxa mediating community interactions.
Random forest models were built using the randomForest package (v4.7-1.2) [25] with 100 trees per model to classify fungal community patterns across conditions. The purpose of this model was to classify fungal community composition across different conditions (in this case, distances from the shrub), and to identify the taxa most important for distinguishing among those conditions. Taxon importance was evaluated using the model’s importance scores, specifically the Mean Decrease in Accuracy metric. The most informative taxa were identified based on model importance scores. Indicator species analysis was performed using the indicspecies package (v1.9.0) [26] to identify taxa significantly associated with specific environmental conditions or sampling distances.
3. Results
3.1. Soil Physicochemical Composition
Multivariate analysis revealed several soil variables as significant predictors of alpha diversity (Table S2). For the Shannon–Wiener index, calcium carbonate (CaCO3), sodium oxide (Na2O), and nitrate (NO3−) were significant (p ≤ 0.05), with Na2O showing the strongest influence (p = 0.001 ***), suggesting that sodium oxide has a substantial impact on fungal diversity. The same three variables were also significant for the Simpson index, underscoring their consistent influence on community structure.
In univariate analyses (Table S3), organic matter, Na2O, and total nitrogen (Total N) were significantly correlated with both Shannon–Wiener and Simpson indices (p ≤ 0.05), highlighting the individual contributions of nutrient content and sodium concentration. Magnesium oxide (MgO) exhibited particularly strong effects (p ≤ 0.001 for Shannon–Wiener; p < 0.001 for Simpson), indicating magnesium’s central role in shaping diversity. Additionally, calcium oxide (CaO) was significant for the Simpson index (p = 0.045), suggesting a potential influence on community evenness and dominance.
PERMANOVA (adonis2) analysis for Total P, Total N, and Total K (Table 1) showed that at 0 m, these variables significantly shaped community structure (p = 0.029, 0.004, and 0.024, respectively). However, no significant effects were observed at 3 m (p = 0.547–0.784), and at 6 m, while p-values were lower (0.195–0.506), none reached statistical significance. These results suggest that nutrient-driven structuring of microbial communities is strongest at the plant origin point, diminishing with distance.

Table 1.
Influence of selected chemical parameters on community structure at different distance levels (0 m, 3 m, and 6 m). Significance levels (p-values) are provided, with asterisks indicating statistically significant differences (* p ≤ 0.05, ** p ≤ 0.01, ns: Non significance).
3.2. Fungal Diversity and Richness Vary with Special Distribution
Sequencing yielded 282,652 reads, which were clustered into fungal ASVs (Table S4), spanning 204 genera, 107 families, 52 orders, 22 classes, and 7 phyla. ASV counts varied by distance: 774 ASV at 0 m, 978 at 3 m, and 579 at 6 m. Uniquely, 28.6% of ASVs were exclusive to 0 m, and 39.9% were unique to 3 m. Ascomycota dominated across all samples (93.5% of ASVs), followed by Basidiomycota (5.2%) and Mortierellomycota (0.6%). The most abundant genera overall were, Alternaria (23.7%), Ascobolus (11.4%), and Fusarium (10.5%) (Figure 1).
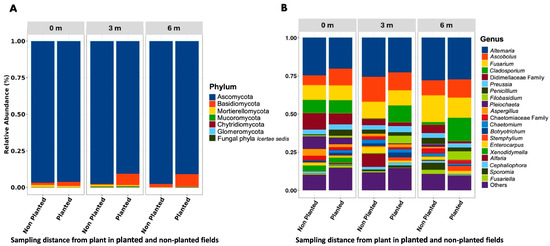
Figure 1.
Distribution of the 20 most prevalent taxa, categorized by (A) phylum and (B) genus. Bars represent mean relative abundance by field type (non-planted vs. planted) within each distance class (0 m, 3 m, 6 m). Taxa not ranked among the top 20 are grouped under ‘Others’.
At the individual sampling distances, the composition of fungal phyla and dominant genera showed notable variation. At 0 m, the community was overwhelmingly dominated by Ascomycota (96.3%), followed by Basidiomycota (2.4%) and Mortierellomycota (1.0%). The most abundant genera at this distance were Alternaria (21.9%), Ascobolus (10.1%), and Fusarium (9.2%). At 3 m, Ascomycota remained dominant but decreased slightly to 92.0%, while Basidiomycota increased to 6.3%, and Mucoromycota appeared at 0.9%. The leading genera were Alternaria (23.8%), Ascobolus (12.5%), and Fusarium (9.9%). At 6 m, the relative abundance of Ascomycota was similar (92.1%), but Basidiomycota increased further to 7.3%, and Mucoromycota declined to 0.5%. The top genera at this distance shifted to Alternaria (26.3%), Fusarium (13.4%), and Cladosporium (13.1%), suggesting a compositional shift in fungal communities with increasing distance from the plant. Beyond the effect of distance, overall fungal diversity was higher in the barley-planted field compared to the non-planted field. Shannon–Wiener diversity in planted soil was significantly greater (p = 0.0051) than in non-planted soil, and other indices, such as richness and evenness, also showed higher values in the planted field (p ≈ 0.01–0.04).
PERMANOVA on Bray–Curtis distances confirmed significant differences in community composition across distances and field conditions (p = 0.001; Table 2), through the interaction between distance and field distance was marginal (p = 0.056). Alpha diversity indices further highlighted spatial trends. The Shannon–Wiener index did not differ significantly between the 0 m and 3 m (p = 0.747) but was significantly higher in both compared to 6 m (p = 0.001 and p = 0.0007, respectively (Table 3)). Richness indices, including Richness (ACE, and Chao1, and observed richness) followed the same trend, with 6 m showing markedly lower diversity (p ≤ 0.001). In contrast, evenness indices such (Pielou’s and Simpson) did not vary significantly by distance. Planted plots exhibited significantly higher diversity than non-planted ones in terms of Shannon–Wiener (p = 0.0051), richness (p = 0.034), Pielou evenness (p = 0.012), and ACE (p = 0.039), while no differences were found for Simpson evenness or Chao1. The contrast between 3 m and 6 m was particularly pronounced (p = 0.001624), while 0 m vs. 3 m remained non-significant. These trends are visualized in Figure 2, where only comparisons involving 6 m revealed significant differences.

Table 2.
PERMANOVA test of distance using the Bray–Curtis method. NA: not applicable.

Table 3.
Factors significantly influencing alpha diversity, assessed by the ACE, Chao1, Pielou evenness, richness, Shannon–Wiener, and Simpson evenness indices, across different sampling distances and origins. Significance levels (p-values) are shown, with asterisks (* p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001, ns: not significant) indicating significant differences.
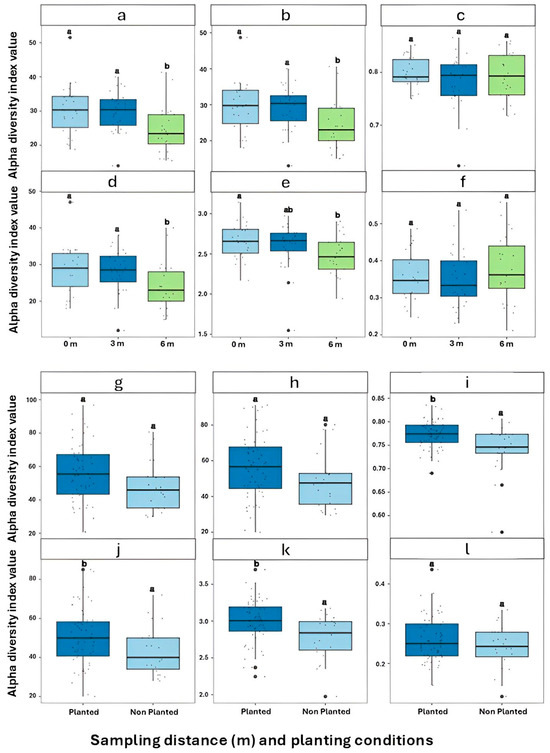
Figure 2.
Alpha diversity indices of fungal communities across (a–f) sampling distance gradients (0 m, 3 m, 6 m) and (g–l) planting conditions (Planted vs. Non-planted) in Ziziphus lotus rhizosphere soils. (a,g) Observed richness; (b,h) Chao1; (c,i) Pielou’s evenness; (d,j) Shannon–Wiener index; (e,k) Inverse Simpson index; (f,l) Simpson’s evenness. Different letters (a, b) above boxplots indicate significant differences among groups.
3.3. Fungal Communities Are Structurally Shaped by Distance
Beta diversity analysis confirmed a clear special distribution of fungal communities. PCoA based on Bray–Curtis dissimilarities (Figure 3) revealed loose clustering at 0 m, while 3 m and 6 m samples were more widely scattered. An ADONIS test confirmed significant effects of distance on community composition (p = 0.002). Although the effect size was modest, these results indicate that agricultural cultivation exerts a detectable influence on community composition. Planted fields tended to cluster separately from non-planted field, reflecting differences in the relative abundances of several taxa. Nonetheless, the substantial overlap between groups suggests the presence of a shared core fungal community across both land-use types.
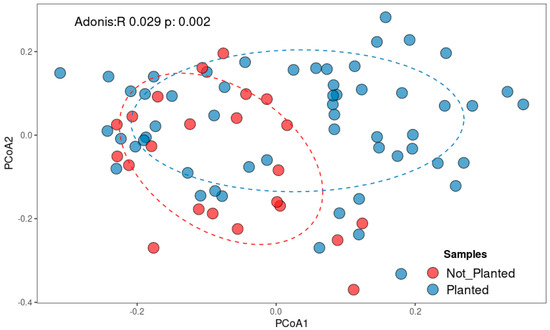
Figure 3.
Spatial distribution patterns across the sample origins, visualized using principal coordinate analysis (PCoA) using the Bray–Curtis dissimilarities.
3.4. A Shared Core Coexists with Distance-Specific Fungal Assemblages
At the genus level (Figure 4A), 3 m samples harbored the most unique genera (37, 18.1%), followed by 0 m (34, 16.7%) and 6 m (8, 3.9%). 72 genera (35.3%) were shared across all distances. At the ASV level (Figure 4B), 3 m again showed the highest number of unique ASVs (637, 35.5%), compared to 467 (26.0%) at 0 m and 304 (16.9%) at 6 m. Only 149 ASVs (8.3%) were common for all distances.
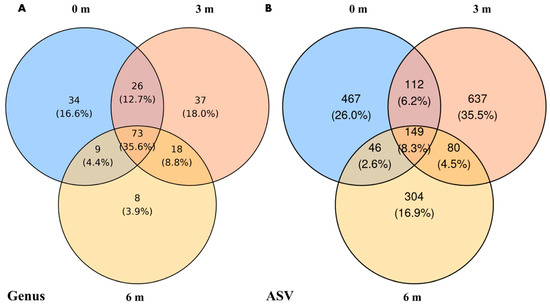
Figure 4.
Venn diagrams showing the distribution of taxa across different sampling distances, categorized by (A) genus level and (B) ASVs.
Random forest classification identified the top 20 ASVs most responsible for differentiating distances (Table S4, Figure 5). ASV_96 (Dothidotthiaceae, unassigned genus) ranked highest in importance (MeanDecreaseAccuracy = 10.55). Other key taxa included ASV_8 (Pleiochaeta), ASV_93 (Aureobasidium), and ASV_62 (Chaetomiaceae), pointing to the roles of Dothideomycetes and Sordariomycetes in spatial differentiation.
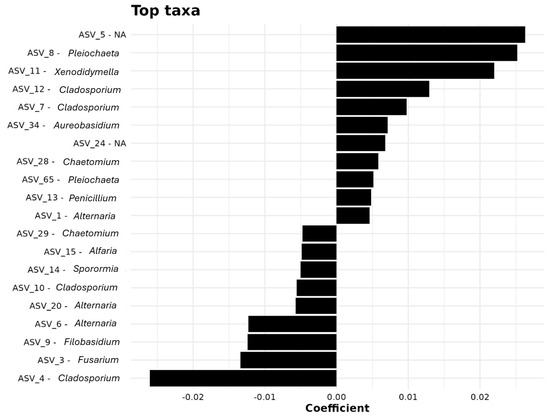
Figure 5.
Key taxa influencing microbial community structure, identified through coefficient analysis.
Multiple ASVs from Pleosporales (ASV_106, ASV_122, ASV_24) and Fusarium-related taxa (ASV_60) were important markers, highlighting the sensitivity of Hypocreales to spatial gradients. ASVs from Saccotheciaceae (ASV_30, ASV_34) also differentiated near-origin from distal sites.
Figure 5 visualizes these associations, showing taxa such as ASV_8 (Pleiochaeta) and ASV_5 (unassigned) as strongly responsive to distance. Genera including Xenodidymella, Alternaria, and Cladosporium contributed moderately, suggesting widespread but spatially variable presence.
3.5. Functional Potential Varies with Spatial and Planting Conditions
FUNGuild functional prediction (Top 20 taxa shown) revealed that most fungi exhibited mixed trophic modes, primarily pathotroph-saprotroph or pathotroph-saprotroph-symbiotroph, indicating potential roles as decomposers, pathogens, and symbionts. At the near-shrub (0 m), multitrophic taxa were most prevalent, including Pleiochaeta, Xenodidymella, Aureobasidium, and Cladosporium (pathotroph–saprotroph [±symbiotroph] assignments), consistent with a resource-rich microhabitat favoring ecological generalists. Typical fast-growing saprotrophs such as Chaetomium and Penicillium were also enriched under the canopy, reflecting litter/OM inputs and rapid turnover. By contrast, at 3–6 m, we observed higher relative representation of oligotrophy-tolerant taxa such as Alfaria and Myriococcum, consistent with lower nutrient availability away from shrubs. Across land-use types, the cultivated field was enriched in Cystofilobasidiaceae (yeast-like taxa often classified as saprotrophs or mixed modes), whereas Stephanosporaceae, Myriococcum, and Alfaria were relatively more abundant in the fallow field (Figure 6).
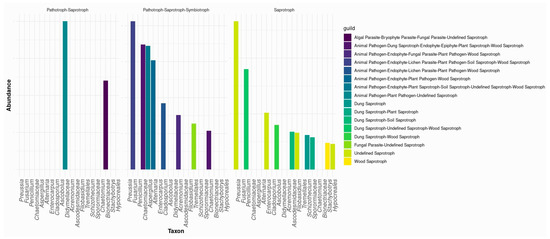
Figure 6.
The top 20 fungal taxa and their predicted trophic modes and functions.
Differential abundance analysis (Figure S3) revealed significant compositional shifts between planted and non-planted fields. Several taxa, including Cystofilobasidiaceae, Filobasidium, Stempellium, and Cladosporium, were significantly enriched under planted conditions, suggesting that crop presence and associated root inputs promote these fungal groups. In contrast, taxa such as Stephanosporaceae, Myriococcum, and Alfaria were more abundant in non-planted field, reflecting adaptation to resource-poor environments or ecological specialization in the absence of cultivation. These contrasting patterns indicate that plant presence shapes fungal community assembly by favoring distinct ecological strategies.
Co-occurrence patterns (Figure S4) reinforced these trends. In planted fields, ASV_8 (Pleiochaeta) was a dominant member, potentially reflecting adaptation to the rhizosphere. In non-planted fields, ASV_57 (Aspergillus) prevailed, consistent with its saprophytic lifestyle in plant-free soils. These patterns suggest that fungal taxa differentially respond to the presence or absence of vegetation, likely due to changes in root exudates, soil nutrients, or organic inputs.
4. Discussion
This study investigated the spatial distribution of fungal communities surrounding wild jujube shrubs in arid environment, highlighting their potential ecological roles under contrasting land uses. The results revealed a heterogeneous fungal community structure, with clear spatial patterns in both cultivated and uncultivated fields. Together with the findings of Radouane et al. [16], who reported spatial structuring of bacterial communities around Z. lotus patches and demonstrated the shrub’s substantial influence on bacterial dynamics in arid ecosystems, our results support the hypothesis that fungal richness and composition vary with distance from Z. lotus and that the shrub exerts ecological effects extending beyond its immediate root zone.
As a keystone species in arid ecosystems, Z. lotus creates localized microhabitats that enhance fungal diversity compared to surrounding barren soils. By accumulating litter and nutrients under its canopy, the shrub promotes microbial activity and alters soil conditions in ways that support diverse fungal communities [27]. Similar patterns of higher fungal richness under shrub canopies compared to open soils have been reported for other desert shrubs [28]. This effect is likely mediated through organic matter inputs, shade, and moisture retention, which allow a broader range of fungal taxa to persist.
The fungal communities associated with Z. lotus displayed both spatial and functional differentiation, shaped by resource gradients and land use. Our findings confirmed elevated levels of organic matter, nitrogen (N), and phosphorus (P) at 0 m compared to farther distances, validating this shrub-mediated nutrient enrichment. Notably, the abundance of Alternaria, Fusarium, and Cladosporium near roots (0 m), and the exclusive presence of Didymellaceae at 0 m and Alfaria at 6 m, point to niche specialization influenced by root exudates and local organic matter availability. Functional predictions from FUNGuild further demonstrated that many dominant taxa exhibit mixed trophic strategies, suggesting high ecological plasticity, a necessary trait in environments where nutrients and water are limiting [29].
The dominance of Ascomycota across all distances is consistent with previous studies in arid regions [28,30]. This phylum is well known for its tolerance to extreme stressors, including drought, salinity, and high temperatures, owing to adaptations such as melanized cell walls and stress-responsive metabolisms [31,32]. Our data also revealed the presence of unclassified fungal taxa (e.g., ASV_115: Ascobolaceae incertae sedis), reinforcing the idea that Z. lotus hosts underexplored fungal lineages potentially adapted to harsh conditions.
Specific classes within Ascomycota, Dothideomycetes, Sordariomycetes, and Pezizomycetes, emerged as spatial indictors. Genera like Pleiochaeta, Neomicrosphaeropsis, and Alternaria showed clear spatial trends, with intra-generic variability (e.g., Fusarium) contributing to spatial differentiation. The detection of Rhizophlyctis (Chytridiomycota) also suggests that, despite Ascomycota dominance, other fungal lineages contribute to community structuring. These patterns reflect fungal sensitivity to fine-scale environmental gradients and suggest that certain taxa can serve as bioindicators of spatial habitat variation.
In this study, our results demonstrate that Z. lotus strongly influences the composition and diversity of soil fungal communities through localized environmental modifications. Nutrient enrichment beneath shrub canopies provides resources that support a richer fungal assemblage. In addition, the shrub canopy likely buffers soil microclimate by moderating temperature and conserving moisture, conditions known to favor microbial persistence in arid soils. Root exudates and potential mycorrhizal associations may further select for specific taxa, such as Pleiochaeta and Aureobasidium, that thrive in rhizosphere-enriched niches. While fungi are well documented to enhance plant stress tolerance in other systems, our study did not measure plant performance, so we cannot conclude a direct benefit to Z. lotus. Instead, our findings highlight the intrinsic mechanisms by which shrubs shape soil fungal communities, creating fertile islands that sustain microbial diversity under harsh environmental conditions. Fungal partners can facilitate nutrient acquisition and improve drought tolerance [33].
Alpha diversity was highest in soils immediately beneath Z. lotus, decreasing with distance, a pattern consistent with the “fertile-island” effect, where shrubs enrich microbial life beneath canopies [28]. Conversely, soils farther from the shrub were more exposed and nutrient-poor, supporting less diverse communities. Despite these general trends, beta diversity analyses revealed significant differences between communities even just a few meters apart, highlighting rapid spatial turnover, likely driven by patchy resource distribution and niche filtering [27,34].
A distance-decay relationship, wherein community similarity decreases with spatial separation, was evident, aligning with established patterns in soil fungal biogeography [35].
Dispersal limitation and localized environmental filtering appear to jointly shape fungal communities [36], even at fine spatial scales. Similar observations in ectomycorrhizal fungi and other arid soil microbiomes support this finding [37].
Several mechanisms may explain spatial changes in fungal composition around Z. lotus. The shrub’s roots improve water retention, release exudates that serve as microbial substrates, and trap litter, all contributing to a richer soil environment [38]. Our findings confirmed elevated levels of organic matter, nitrogen (N) and phosphorus (P) at 0 m [27], validating this shrub-mediated nutrient enrichment.
We also found that specific edaphic factors, CaCO3, NO3−, and Na2O, strongly influenced fungal composition. High CaCO3, indicative of alkaline conditions, is known to shape fungal communities [39]. Nitrate availability, as a key microbial nutrient, may favor copiotrophic fungi, whereas oligotrophs dominate in N-depleted zones. Interestingly, our study suggests moderate nitrogen levels near Z. lotus support a balanced and diverse community. High Na2O levels reflect salinity stress, which selectively favors salt-tolerant taxa such as Ascomycota [30]. However, it is important to note that our study did not directly measure litter accumulation, root biomass, or soil moisture; factors known to influence soil fungi. We infer their effects indirectly, but future studies should quantify these variables. Which will help disentangle the mechanisms behind the patterns we observed.
Shannon–Wiener and Simpson indices indicated that fungal diversity decreased with distance from Z. lotus (0 m > 3 m > 6 m). Root-proximal soils hosted enriched communities of multitrophic fungi such as Pleiochaeta, Xenodidymella, and Aureobasidium, many of which produce extracellular polymers for stress resistance [40]. Fast-growing saprotrophs (Chaetomium, Penicillium) thrived near organic matter-rich zones, while oligotrophs like Alfaria emerged at 6 m, consistent with resource gradient theory [41,42].
Interestingly, despite barley cultivation, fungal composition remained relatively stable around Z. lotus, possibly due to microbial buffering by root exudates or allelopathic effects. In contrast to studies where tillage disrupts fungal networks [43], we observed higher richness in cultivated plots, perhaps due to barley roots and inputs enhancing microbial niches. However, copiotrophic taxa such as Fusarium and Alternaria did not increase at 6 m under cultivation, suggesting inhibitory effects, potentially from allelochemicals derived from Z. lotus [44].
FUNGuild analyses confirmed functional redundancy in dominant genera. Many taxa shift among saprotrophic, symbiotic, and pathogenic modes, a strategy also seen in Mediterranean shrubs [45] For example, Fusarium can become pathogenic under host stress, and Cladosporium spores resist desiccation. Such conditional mutualisms, where opportunists are tolerated for their ecological services, may be essential for plant survival in semi-arid ecosystems [46].
Co-occurrence network analysis revealed key taxa under different land-use conditions. Pleiochaeta dominated planted fields, likely benefiting from root-associated substrates, while Aspergillus was central in non-planted soils, consistent with its saprotrophic lifestyle and dominance in undisturbed soils [47,48]. These patterns illustrate how cultivation shifts community composition toward plant-associated fungi, while non-planted soils support generalist decomposers.
5. Conclusions
This study demonstrates that Ziziphus lotus significantly influences fungal community structure in arid soils. Fungal richness and diversity were highest beneath the shrub canopy and declined with distance, with clear shifts in community composition associated with edaphic variables such as nitrate, salinity, and calcium carbonate. The shrub’s microenvironment supported fungi with diverse ecological roles, ranging from saprotrophs to multitrophic taxa, reflecting adaptations to arid and nutrient-limited conditions. Notably, when comparing land-use types, fungal diversity was generally higher in the planted fields than in the non-planted field, although overall community structure remained broadly similar. This indicates that Z. lotus maintains a relatively stable and resilient fungal community even under cultivation, underscoring its role as a keystone shrub that facilitates soil microbial diversity and may contribute to sustainable land management in drylands.
However, we caution that these findings are based on a single region and two adjacent fields. While the results offer important insights, they should not be overgeneralized to other ecosystems without further validation. Future studies incorporating broader geographic sampling, temporal replication, and functional assays will be essential to confirm and expand upon the patterns observed here.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms13112489/s1, Figure S1: (A) Location of the sampling site in Rhamna Province. between Ben Guerir and Marrakech. Morocco retrieved from Google Maps. along the A3 highway. (B) Image of a barley-planted field with patches of wild jujube shrubs. (C) Close-up view of a wild jujube shrub patch; Figure S2: Experimental sampling in barley-planted and non-planted fields. The diagram illustrates the sampling design. with soil samples collected at three distances (0 m. 3 m. and 6 m) from Ziziphus lotus patches. Each sampling cluster consists of 12 samples; Figure S3: Differential abundance analysis of fungal taxa between planted and non-planted fields; Figure S4: Cooccurrence analysis of planted and non-planted sampling sites. the triangles are the hub taxa over top ASV in 10% of the samples. the key taxa are encircled in blue. node size indicate the abundance of taxa; Table S1: Physicochemical characteristics of different Ziziphus lotus clusters in barley-planted and non-barley-planted fields across varying sampling distances. EC: electrical conductivity; OM: organic matter; CEC: cation exchange capacity. Cluster codes begin with ‘C’ for barley-planted fields. followed by the cluster number (1–5) and the sampling distance (0. 3. or 6 m). Similarly, ‘T’ denotes clusters from unplanted fields. followed by the cluster number (1–2) and the sampling distance; Table S2: Results of multivariate ANOVA showing the influence of soil physicochemical properties on microbial community structure. Significance levels (p-values) are indicated. with asterisks denoting statistical significance (* p ≤ 0.05. ** p ≤ 0.01. *** p ≤ 0.001); Table S3: Results of univariate ANOVA demonstrating the influence of soil physicochemical properties on microbial community structure. Significance levels (p-values) are indicated. with asterisks denoting statistically significant differences (* p ≤ 0.05. ** p ≤ 0.01. *** p ≤ 0.001); Table S4: Rarefaction analysis of the samples showing the original sequence reads and corresponding diversity indices; Table S5: Top 20 fungal ASVs shared across distance (0 m, 3 m, 6 m) identified by random forest.
Author Contributions
N.R. performed the experiments, analyzed the data and wrote the manuscript draft. Z.M. performed the experiments. K.E. performed the MiSeq sequencing. S.M. and K.A.S.M. contributed to sample preparation and performing experiments. M.H. contributed to the conceptualization, design, supervision, and manuscript writing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the OCP Nutricrops (Project AS-85) and UM6P.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw sequencing reads have been deposited in NCBI (https://www.ncbi.nlm.nih.gov/) with accession numbers provided under the BioProject ID: PRJNA1253578.
Acknowledgments
We express our gratitude to Hicham Benslim and Imand Khatour, Rachid Elfermi for their help and support with sample collection, sample preparation, and bioinformatic analysis. We also thank SIMLAB of UMs6P for providing the computational infrastructure used for bioinformatics data processing.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rsaissi, N.; Bouhache, M.; Bencharki, B. Importance and agro-economical impact of wild jujube (Ziziphus lotus) in Chaouia region. Rev. Marocaine Prot. Plantes 2012, 3, 13–27. [Google Scholar]
- Wang, B.; Huang, Q.; Venkitasamy, C.; Chai, H.; Gao, H.; Cheng, N.; Cao, W.; Lv, X.; Pan, Z. Changes in phenolic compounds and their antioxidant capacities in jujube (Ziziphus jujuba Miller) during three edible maturity stages. LWT-Food Sci. Technol. 2016, 66, 56–62. [Google Scholar] [CrossRef]
- Radouane, N.; Errafii, K.; Mouhib, S.; Mhand, K.A.; Legeay, J.; Hijri, M. Potential Plant-To-Plant Transmission: Shared Endophytic Bacterial Community Between Ziziphus lotus and Its Parasite Cuscuta epithymum. Microb. Ecol. 2024, 87, 119. [Google Scholar] [CrossRef]
- Abdoul-Azize, S. Potential Benefits of Jujube (Zizyphus lotus L.) Bioactive Compounds for Nutrition and Health. J. Nutr. Metab. 2016, 2016, 2867470. [Google Scholar] [CrossRef]
- El Maaiden, E.; El Kharrassi, Y.; Moustaid, K.; Essamadi, A.K.; Nasser, B. Comparative study of phytochemical profile between Ziziphus spina christi and Ziziphus lotus from Morocco. J. Food Meas. Charact. 2018, 13, 121–130. [Google Scholar] [CrossRef]
- Danthu, P.; Soloviev, P.; Totté, A.; Tine, E.; Ayessou, N.; Gaye, A.; Niang, T.D.; Seck, M.; Fall, M. Caractères physico-chimiques et organoleptiques comparés de jujubes sauvages et des fruits de la variété Gola introduite au Sénégal. Fruits 2002, 57, 173–182. [Google Scholar] [CrossRef]
- Benmehdi, H.; Hasnaoui, O.; Benali, O.; Salhi, F. Phytochemical investigation of leaves and fruits extracts of Chamaerops humilis L. J. Mater. Environ. Sci. 2012, 3, 320–327. [Google Scholar]
- Aboudrar, W.; Schwartz, C.; Benizri, E.; Morel, J.L.; Boularbah, A. Soil microbial diversity as affected by the rhizosphere of the hyperaccumulator Thlaspi caerulescens under natural conditions. Int. J. Phytoremediat. 2007, 9, 41–52. [Google Scholar] [CrossRef]
- Koubaa, M.; Barba, F.J.; Grimi, N.; Mhemdi, H.; Koubaa, W.; Boussetta, N.; Vorobiev, E. Recovery of colorants from red prickly pear peels and pulps enhanced by pulsed electric field and ultrasound. Innov. Food Sci. Emerg. Technol. 2016, 37, 336–344. [Google Scholar] [CrossRef]
- Al-Neami, F.; Radwan, O.; Al-Neami, F.; Radwan, O. A Prospective Implementation of Plant-Associated Microbes for a Sustainable Agriculture in Qatar. In Proceedings of the Qatar Foundation Annual Research Conference Proceedings, Doha, Qatar, 22–23 March 2016; Volume 2016. [Google Scholar] [CrossRef]
- El-Nagerabi, S.A.F.; Elshafie, A.E.; Alkhanjari, S.S. Endophytic fungi associated with Ziziphus species and new records from mountainous area of Oman. Biodiversitas J. Biol. Divers. 1970, 14, 10–16. [Google Scholar] [CrossRef]
- Ghazi-Yaker, A.; Kraak, B.; Houbraken, J.; Houali, K.; Saadoun, N. Diversity of epiphytic and endophytic fungal communities associated with leaves of Ziziphus lotus (L.) Lam. from Algeria. Pol. J. Ecol. 2022, 70, 159–174. [Google Scholar] [CrossRef]
- Ducousso-Detrez, A.; Fontaine, J.; Lounes-Hadj Sahraoui, A.; Hijri, M. Diversity of Phosphate Chemical Forms in Soils and Their Contributions on Soil Microbial Community Structure Changes. Microorganisms 2022, 10, 609. [Google Scholar] [CrossRef]
- Thioye, B.; Mania, S.; Kane, A.; Ndiaye, C.; Soule, A.O.; Falls, D.; Duponnois, R.; Sylla, S.N.; Bâ, A.M. Growth response of different species of Ziziphus to inoculation with arbuscular mycorrhizal fungi. Fruits 2017, 72, 174–181. [Google Scholar] [CrossRef]
- Regehr, D.L.; El Brahli, A. Wild Jujube (Ziziphus Lotus) Control in Morocco. Weed Technol. 1995, 9, 326–330. [Google Scholar] [CrossRef]
- Radouane, N.; Meliane, Z.; Errafii, K.; Ait Si Mhand, K.; Mouhib, S.; Hijri, M. Influence of Ziziphus lotus (Rhamnaceae) Plants on the Spatial Distribution of Soil Bacterial Communities in Semi-Arid Ecosystems. Microorganisms 2025, 13, 1740. [Google Scholar] [CrossRef] [PubMed]
- Outbakat, M.B.; Bouray, M.; Choukr-Allah, R.; El Gharous, M.; El Omari, K.; El Mejahed, K. Phosphogypsum as Fertilizer: Impacts on Soil Fertility, Barley Yield Components, and Heavy Metals Contents. Plants 2024, 14, 16. [Google Scholar] [CrossRef]
- Morvan, T.; Lambert, Y.; Germain, P.; Lemercier, B.; Moreira, M.; Beff, L. A dataset of physico-chemical properties, extractable organic N, N mineralization and physical organic matter fractionation of soils developed on loess silts, crystalline rocks and sedimentary rocks. Data Brief 2023, 51, 109776. [Google Scholar] [CrossRef]
- Legeay, J.; Basiru, S.; Ziami, A.; Errafii, K.; Hijri, M. Response of Alternaria and Fusarium Species to Low Precipitation in a Drought-Tolerant Plant in Morocco. Microb. Ecol. 2024, 87, 127. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Abarenkov, K.; Nilsson, R.H.; Larsson, K.H.; Taylor, A.F.S.; May, T.W.; Froslev, T.G.; Pawlowska, J.; Lindahl, B.; Poldmaa, K.; Truong, C.; et al. The UNITE database for molecular identification and taxonomic communication of fungi and other eukaryotes: Sequences, taxa and classifications reconsidered. Nucleic Acids Res. 2024, 52, D791–D797. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. Vegan: Community Ecology Package; R Package Version 2.2-0; The R Foundation: Vienna, Austria, 2014. [Google Scholar]
- Kurtz, Z.D.; Muller, C.L.; Miraldi, E.R.; Littman, D.R.; Blaser, M.J.; Bonneau, R.A. Sparse and compositionally robust inference of microbial ecological networks. PLoS Comput. Biol. 2015, 11, e1004226. [Google Scholar] [CrossRef]
- Liaw, A.; Wiener, M. Classification and regression by randomForest. R News 2002, 2, 18–22. [Google Scholar]
- De Caceres, M.; Legendre, P. Associations between species and groups of sites: Indices and statistical inference. Ecology 2009, 90, 3566–3574. [Google Scholar] [CrossRef]
- Liu, Z.; Shao, Y.; Cui, Q.; Ye, X.; Huang, Z. ‘Fertile island’ effects on the soil microbial community beneath the canopy of Tetraena mongolica, an endangered and dominant shrub in the West Ordos Desert, North China. BMC Plant Biol. 2024, 24, 178. [Google Scholar] [CrossRef]
- Suleiman, M.K.; Dixon, K.; Commander, L.; Nevill, P.; Quoreshi, A.M.; Bhat, N.R.; Manuvel, A.J.; Sivadasan, M.T. Assessment of the Diversity of Fungal Community Composition Associated With Vachellia pachyceras and Its Rhizosphere Soil From Kuwait Desert. Front. Microbiol. 2019, 10, 63. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Song, Z.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Yang, C.; Sun, J. Soil Salinity Drives the Distribution Patterns and Ecological Functions of Fungi in Saline-Alkali Land in the Yellow River Delta, China. Front. Microbiol. 2020, 11, 594284. [Google Scholar] [CrossRef]
- Tedersoo, L.; Mikryukov, V.; Anslan, S.; Bahram, M.; Khalid, A.N.; Corrales, A.; Agan, A.; Vasco-Palacios, A.-M.; Saitta, A.; Antonelli, A.; et al. The Global Soil Mycobiome consortium dataset for boosting fungal diversity research. Fungal Divers. 2021, 111, 573–588. [Google Scholar] [CrossRef]
- Liu, S.; Wang, F.; Xue, K.; Sun, B.; Zhang, Y.; He, Z.; Van Nostrand, J.D.; Zhou, J.; Yang, Y. The interactive effects of soil transplant into colder regions and cropping on soil microbiology and biogeochemistry. Environ. Microbiol. 2015, 17, 566–576. [Google Scholar] [CrossRef]
- Chavez-Gonzalez, J.D.; Flores-Nunez, V.M.; Merino-Espinoza, I.U.; Partida-Martinez, L.P. Desert plants, Arbuscular mycorrhizal fungi and associated bacteria: Exploring the diversity and role of symbiosis under drought. Environ. Microbiol. Rep. 2024, 16, e13300. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Huang, K.; Mumin, R.; Li, J.; Sun, Y.; Cui, B. Spatial variations impact the soil fungal communities of Larix gmelinii forests in Northeast China. Front. Plant Sci. 2024, 15, 1408272. [Google Scholar] [CrossRef]
- Wang, B.; Chen, C.; Xiao, Y.M.; Chen, K.Y.; Wang, J.; Zhao, S.; Liu, N.; Li, J.N.; Zhou, G.Y. Trophic relationships between protists and bacteria and fungi drive the biogeography of rhizosphere soil microbial community and impact plant physiological and ecological functions. Microbiol. Res. 2024, 280, 127603. [Google Scholar] [CrossRef]
- Matsuoka, S.; Kawaguchi, E.; Osono, T. Temporal distance decay of similarity of ectomycorrhizal fungal community composition in a subtropical evergreen forest in Japan. FEMS Microbiol. Ecol. 2016, 92, fiw061. [Google Scholar] [CrossRef]
- Bowman, E.A.; Arnold, A.E. Drivers and implications of distance decay differ for ectomycorrhizal and foliar endophytic fungi across an anciently fragmented landscape. ISME J. 2021, 15, 3437–3454. [Google Scholar] [CrossRef] [PubMed]
- Walker, L.R.; Thompson, D.B.; Landau, F.H. Experimental manipulations of fertile islands and nurse plant effects in the Mojave Desert, USA. West. N. Am. Nat. 2001, 61, 25–35. [Google Scholar]
- Zhang, T.; Wang, N.F.; Liu, H.Y.; Zhang, Y.Q.; Yu, L.Y. Soil pH is a Key Determinant of Soil Fungal Community Composition in the Ny-Alesund Region, Svalbard (High Arctic). Front. Microbiol. 2016, 7, 227. [Google Scholar] [CrossRef]
- Gostinčar, C.; Ohm, R.A.; Kogej, T.; Sonjak, S.; Turk, M.; Zajc, J.; Zalar, P.; Grube, M.; Sun, H.; Han, J. Genome sequencing of four Aureobasidium pullulans varieties: Biotechnological potential, stress tolerance, and description of new species. BMC Genom. 2014, 15, 549. [Google Scholar] [CrossRef]
- Fierer, N.; Schimel, J.; Holden, P. Influence of drying-rewetting frequency on soil bacterial community structure. Microb. Ecol. 2003, 45, 63–71. [Google Scholar] [CrossRef]
- Krijgsheld, P.; Bleichrodt, R.V.; Van Veluw, G.; Wang, F.; Müller, W.; Dijksterhuis, J.; Wösten, H. Development in Aspergillus. Stud. Mycol. 2013, 74, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, M.; Frey, B.; Mayer, J.; Mäder, P.; Widmer, F. Distinct soil microbial diversity under long-term organic and conventional farming. ISME J. 2015, 9, 1177–1194. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Asthir, B. Molecular responses to drought stress in plants. Biol. Plant. 2017, 61, 201–209. [Google Scholar] [CrossRef]
- Maciá-Vicente, J.G.; Nam, B.; Thines, M. Root filtering, rather than host identity or age, determines the composition of root-associated fungi and oomycetes in three naturally co-occurring Brassicaceae. Soil Biol. Biochem. 2020, 146, 107806. [Google Scholar] [CrossRef]
- Khidir, H.H.; Eudy, D.M.; Porras-Alfaro, A.; Herrera, J.; Natvig, D.O.; Sinsabaugh, R.L. A general suite of fungal endophytes dominate the roots of two dominant grasses in a semiarid grassland. J. Arid. Environ. 2010, 74, 35–42. [Google Scholar] [CrossRef]
- Samson, R.A.; Hong, S.; Peterson, S.W.; Frisvad, J.C.; Varga, J. Polyphasic taxonomy of Aspergillus section Fumigati and its teleomorph Neosartorya. Stud. Mycol. 2007, 59, 147–203. [Google Scholar] [CrossRef] [PubMed]
- Nji, Q.N.; Babalola, O.O.; Mwanza, M. Soil Aspergillus Species, Pathogenicity and Control Perspectives. J. Fungi 2023, 9, 766. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).