Hepatitis B Virus-Related Liver Disease and Gut Microbiota: An Updated Review
Abstract
1. Introduction
2. Gut Microbiota and Gut–Liver Axis in Humans
3. Gut Microbiota Modulate Immune Response During HBV Infection
3.1. Gut Microbiota and Host Immune Response
3.2. Gut Microbiota Modulates the Immune Response Against HBV Infection
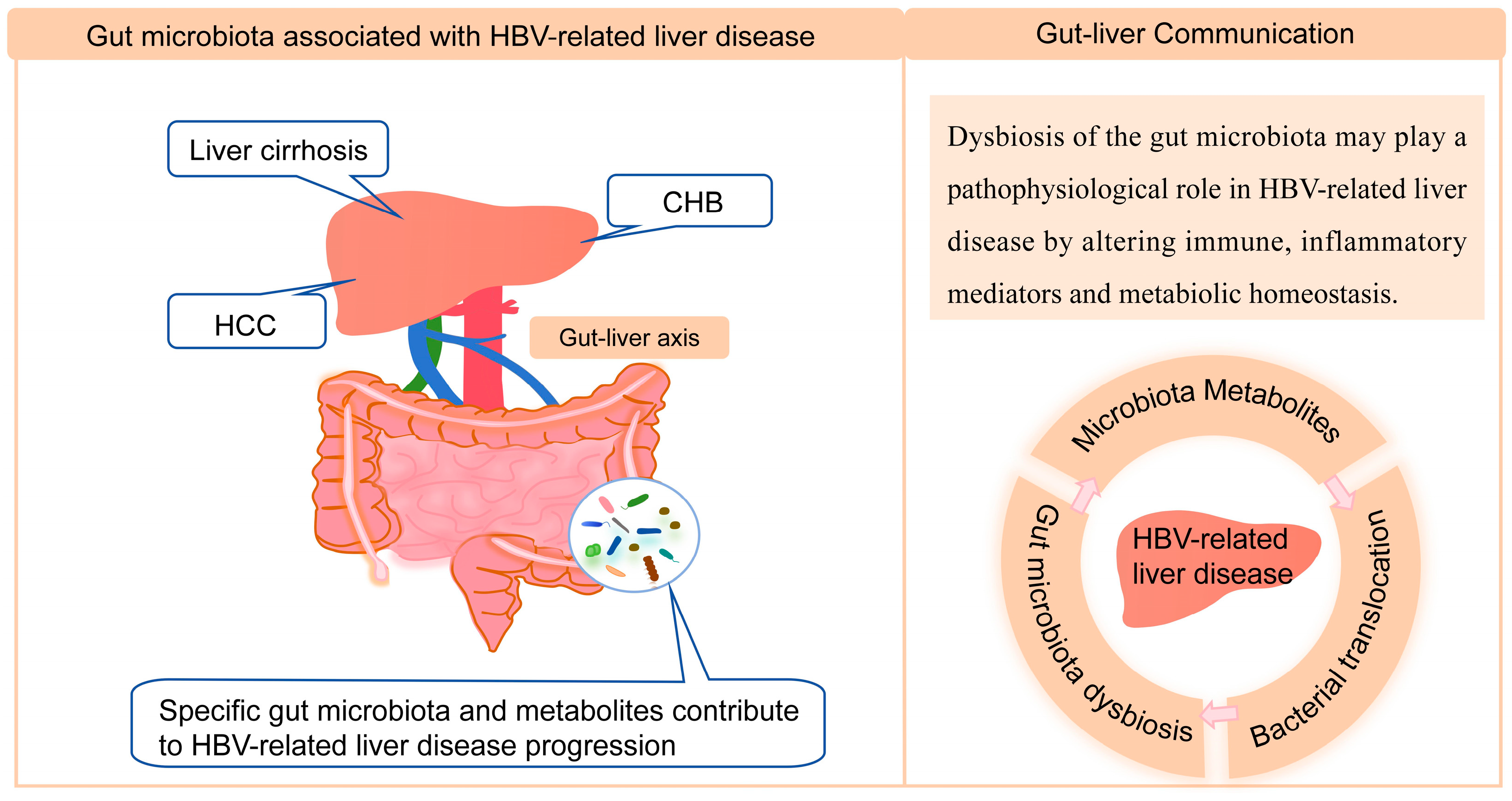
4. Gut Microbiota Associated with HBV-Related Liver Disease
4.1. Chronic HBV Infection
4.2. Liver Cirrhosis
4.3. Hepatocellular Carcinoma
5. Gut Microbiota and Pharmacotherapy
6. Gut Microbiota Modulation in CHB Therapy
6.1. Targeted Therapy with Probiotics
6.2. Fecal Microbiota Transplantation
7. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Progress Report on HIV, Viral Hepatitis and Sexually Transmitted Infections 2021. WHO. 2021. Available online: https://www.who.int/publications/i/item/9789240027077 (accessed on 9 September 2025).
- Rizzo, G.E.M.; Cabibbo, G.; Craxi, A. Hepatitis B Virus-Associated Hepatocellular Carcinoma. Viruses 2022, 14, 986. [Google Scholar] [CrossRef]
- Nguyen, M.H.; Wong, G.; Gane, E.; Kao, J.H.; Dusheiko, G. Hepatitis B Virus: Advances in Prevention, Diagnosis, and Therapy. Clin. Microbiol. Rev. 2020, 33, e00046-19. [Google Scholar] [CrossRef]
- Locarnini, S.; Hatzakis, A.; Chen, D.S.; Lok, A. Strategies to control hepatitis B: Public policy, epidemiology, vaccine and drugs. J. Hepatol. 2015, 62, S76–S86. [Google Scholar] [CrossRef]
- Tojo, R.; Suarez, A.; Clemente, M.G.; de los Reyes-Gavilan, C.G.; Margolles, A.; Gueimonde, M.; Ruas-Madiedo, P. Intestinal microbiota in health and disease: Role of bifidobacteria in gut homeostasis. World J. Gastroenterol. 2014, 20, 15163–15176. [Google Scholar] [CrossRef] [PubMed]
- Arab, J.P.; Arrese, M.; Shah, V.H. Gut microbiota in non-alcoholic fatty liver disease and alcohol-related liver disease: Current concepts and perspectives. Hepatol. Res. Off. J. Jpn. Soc. Hepatol. 2020, 50, 407–418. [Google Scholar] [CrossRef]
- Zheng, Z.; Wang, B. The Gut-Liver Axis in Health and Disease: The Role of Gut Microbiota-Derived Signals in Liver Injury and Regeneration. Front. Immunol. 2021, 12, 775526. [Google Scholar] [CrossRef]
- Pabst, O.; Hornef, M.W.; Schaap, F.G.; Cerovic, V.; Clavel, T.; Bruns, T. Gut-liver axis: Barriers and functional circuits. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 447–461. [Google Scholar] [CrossRef]
- Heymann, F.; Tacke, F. Immunology in the liver--from homeostasis to disease. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 88–110. [Google Scholar] [CrossRef]
- Ma, C.; Han, M.; Heinrich, B.; Fu, Q.; Zhang, Q.; Sandhu, M.; Agdashian, D.; Terabe, M.; Berzofsky, J.A.; Fako, V.; et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science 2018, 360, eaan5931. [Google Scholar] [CrossRef]
- Yang, Y.; Nguyen, M.; Khetrapal, V.; Sonnert, N.D.; Martin, A.L.; Chen, H.; Kriegel, M.A.; Palm, N.W. Within-host evolution of a gut pathobiont facilitates liver translocation. Nature 2022, 607, 563–570. [Google Scholar] [CrossRef]
- Son, G.; Kremer, M.; Hines, I.N. Contribution of gut bacteria to liver pathobiology. Gastroenterol. Res. Pract. 2010, 2010, 453563. [Google Scholar] [CrossRef]
- Schneider, K.M.; Mohs, A.; Gui, W.; Galvez, E.J.C.; Candels, L.S.; Hoenicke, L.; Muthukumarasamy, U.; Holland, C.H.; Elfers, C.; Kilic, K.; et al. Imbalanced gut microbiota fuels hepatocellular carcinoma development by shaping the hepatic inflammatory microenvironment. Nat. Commun. 2022, 13, 3964. [Google Scholar] [CrossRef]
- Hapfelmeier, S.; Lawson, M.A.; Slack, E.; Kirundi, J.K.; Stoel, M.; Heikenwalder, M.; Cahenzli, J.; Velykoredko, Y.; Balmer, M.L.; Endt, K.; et al. Reversible microbial colonization of germ-free mice reveals the dynamics of IgA immune responses. Science 2010, 328, 1705–1709. [Google Scholar] [CrossRef]
- Choo, J.M.; Rogers, G.B. Gut microbiota transplantation for colonization of germ-free mice. STAR Protoc. 2021, 2, 100610. [Google Scholar] [CrossRef] [PubMed]
- Geva-Zatorsky, N.; Sefik, E.; Kua, L.; Pasman, L.; Tan, T.G.; Ortiz-Lopez, A.; Yanortsang, T.B.; Yang, L.; Jupp, R.; Mathis, D.; et al. Mining the Human Gut Microbiota for Immunomodulatory Organisms. Cell 2017, 168, 928–943. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Zarzycka, A.; Preussner, M.; Fischer, F.; Hain, T.; Herrmann, J.P.; Roth, K.; Keber, C.U.; Suryamohan, K.; Raifer, H.; et al. Selected commensals educate the intestinal vascular and immune system for immunocompetence. Microbiome 2022, 10, 158. [Google Scholar] [CrossRef] [PubMed]
- Mazmanian, S.K.; Liu, C.H.; Tzianabos, A.O.; Kasper, D.L. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 2005, 122, 107–118. [Google Scholar] [CrossRef]
- Sun, M.; Wu, W.; Chen, L.; Yang, W.; Huang, X.; Ma, C.; Chen, F.; Xiao, Y.; Zhao, Y.; Ma, C.; et al. Microbiota-derived short-chain fatty acids promote Th1 cell IL-10 production to maintain intestinal homeostasis. Nat. Commun. 2018, 9, 3555. [Google Scholar] [CrossRef]
- Smits, H.H.; Engering, A.; van der Kleij, D.; de Jong, E.C.; Schipper, K.; van Capel, T.M.; Zaat, B.A.; Yazdanbakhsh, M.; Wierenga, E.A.; van Kooyk, Y.; et al. Selective probiotic bacteria induce IL-10-producing regulatory T cells in vitro by modulating dendritic cell function through dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin. J. Allergy Clin. Immunol. 2005, 115, 1260–1267. [Google Scholar] [CrossRef]
- Gaboriau-Routhiau, V.; Rakotobe, S.; Lecuyer, E.; Mulder, I.; Lan, A.; Bridonneau, C.; Rochet, V.; Pisi, A.; De Paepe, M.; Brandi, G.; et al. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity 2009, 31, 677–689. [Google Scholar] [CrossRef]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Schulthess, J.; Pandey, S.; Capitani, M.; Rue-Albrecht, K.C.; Arnold, I.; Franchini, F.; Chomka, A.; Ilott, N.E.; Johnston, D.G.W.; Pires, E.; et al. The Short Chain Fatty Acid Butyrate Imprints an Antimicrobial Program in Macrophages. Immunity 2019, 50, 432–445 e437. [Google Scholar] [CrossRef] [PubMed]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottiere, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and functional importance in the gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Kang, S.G.; Park, J.H.; Yanagisawa, M.; Kim, C.H. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology 2013, 145, 396–406.e10. [Google Scholar] [CrossRef]
- Park, J.; Kim, M.; Kang, S.G.; Jannasch, A.H.; Cooper, B.; Patterson, J.; Kim, C.H. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol. 2015, 8, 80–93. [Google Scholar] [CrossRef]
- Thimme, R.; Wieland, S.; Steiger, C.; Ghrayeb, J.; Reimann, K.A.; Purcell, R.H.; Chisari, F.V. CD8(+) T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J. Virol. 2003, 77, 68–76. [Google Scholar] [CrossRef]
- Cheng, X.; Xia, Y.; Serti, E.; Block, P.D.; Chung, M.; Chayama, K.; Rehermann, B.; Liang, T.J. Hepatitis B virus evades innate immunity of hepatocytes but activates cytokine production by macrophages. Hepatology 2017, 66, 1779–1793. [Google Scholar] [CrossRef]
- Iannacone, M.; Guidotti, L.G. Immunobiology and pathogenesis of hepatitis B virus infection. Nat. Rev. Immunol. 2022, 22, 19–32. [Google Scholar] [CrossRef]
- Hong, M.; Bertoletti, A. Tolerance and immunity to pathogens in early life: Insights from HBV infection. Semin. Immunopathol. 2017, 39, 643–652. [Google Scholar] [CrossRef]
- Thio, C.L.; Thomas, D.L.; Carrington, M. Chronic viral hepatitis and the human genome. Hepatology 2000, 31, 819–827. [Google Scholar] [CrossRef]
- Wang, B.; Li, L. Who determines the outcomes of HBV exposure? Trends Microbiol. 2015, 23, 328–329. [Google Scholar] [CrossRef]
- Chou, H.H.; Chien, W.H.; Wu, L.L.; Cheng, C.H.; Chung, C.H.; Horng, J.H.; Ni, Y.H.; Tseng, H.T.; Wu, D.; Lu, X.; et al. Age-related immune clearance of hepatitis B virus infection requires the establishment of gut microbiota. Proc. Natl. Acad. Sci. USA 2015, 112, 2175–2180. [Google Scholar] [CrossRef] [PubMed]
- Sjogren, Y.M.; Jenmalm, M.C.; Bottcher, M.F.; Bjorksten, B.; Sverremark-Ekstrom, E. Altered early infant gut microbiota in children developing allergy up to 5 years of age. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2009, 39, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Prendergast, A.J.; Klenerman, P.; Goulder, P.J. The impact of differential antiviral immunity in children and adults. Nat. Rev. Immunol. 2012, 12, 636–648. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.L.; Huang, T.S.; Shyu, Y.C.; Wang, C.L.; Wang, H.Y.; Chen, P.J. Gut microbiota in the innate immunity against hepatitis B virus—Implication in age-dependent HBV clearance. Curr. Opin. Virol. 2021, 49, 194–202. [Google Scholar] [CrossRef]
- Wu, T.; Li, F.; Chen, Y.; Wei, H.; Tian, Z.; Sun, C.; Sun, R. CD4(+) T Cells Play a Critical Role in Microbiota-Maintained Anti-HBV Immunity in a Mouse Model. Front. Immunol. 2019, 10, 927. [Google Scholar] [CrossRef]
- Wang, T.; Fan, Y.; Tan, S.; Wang, Z.; Li, M.; Guo, X.; Yu, X.; Lin, Q.; Song, X.; Xu, L.; et al. Probiotics and their metabolite spermidine enhance IFN-gamma(+)CD4(+) T cell immunity to inhibit hepatitis B virus. Cell Rep. Med. 2024, 5, 101822. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Zhang, X.; Liu, J.; Zhang, Q.; Zhao, Y.; Peng, J.; Feng, Q.; Dai, J.; Sun, S.; et al. Gut Microbial Dysbiosis Is Associated with Altered Hepatic Functions and Serum Metabolites in Chronic Hepatitis B Patients. Front. Microbiol. 2017, 8, 2222. [Google Scholar] [CrossRef]
- Schnabl, B.; Brenner, D.A. Interactions between the intestinal microbiome and liver diseases. Gastroenterology 2014, 146, 1513–1524. [Google Scholar] [CrossRef]
- Wei, X.; Yan, X.; Zou, D.; Yang, Z.; Wang, X.; Liu, W.; Wang, S.; Li, X.; Han, J.; Huang, L.; et al. Abnormal fecal microbiota community and functions in patients with hepatitis B liver cirrhosis as revealed by a metagenomic approach. BMC Gastroenterol. 2013, 13, 175. [Google Scholar] [CrossRef]
- Liu, Q.; Li, F.; Zhuang, Y.; Xu, J.; Wang, J.; Mao, X.; Zhang, Y.; Liu, X. Alteration in gut microbiota associated with hepatitis B and non-hepatitis virus related hepatocellular carcinoma. Gut Pathog. 2019, 11, 1. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Z.; Guo, R.; Chen, N.; Lu, H.; Huang, S.; Wang, J.; Li, L. Correlation between gastrointestinal fungi and varying degrees of chronic hepatitis B virus infection. Diagn. Microbiol. Infect. Dis. 2011, 70, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wang, B.; Fu, Y.; Chen, Y.; Yang, F.; Lu, H.; Chen, Y.; Xu, J.; Li, L. Changes of fecal Bifidobacterium species in adult patients with hepatitis B virus-induced chronic liver disease. Microb. Ecol. 2012, 63, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.W.; Lu, H.F.; Wu, J.; Zuo, J.; Chen, P.; Sheng, J.F.; Zheng, S.S.; Li, L.J. Assessment of the fecal lactobacilli population in patients with hepatitis B virus-related decompensated cirrhosis and hepatitis B cirrhosis treated with liver transplant. Microb. Ecol. 2012, 63, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Chen, S.; Fu, Y.; Wu, W.; Chen, T.; Chen, J.; Yang, B.; Ou, Q. Gut microbiota dysbiosis in patients with hepatitis B virus-induced chronic liver disease covering chronic hepatitis, liver cirrhosis and hepatocellular carcinoma. J. Viral Hepat. 2020, 27, 143–155. [Google Scholar] [CrossRef]
- Honda, T.; Ishigami, M.; Ishizu, Y.; Imai, N.; Ito, T.; Yamamoto, K.; Yokoyama, S.; Muto, H.; Inukai, Y.; Kato, A.; et al. Gut microbes associated with functional cure of chronic hepatitis B. Hepatol. Int. 2025, 19, 519–528. [Google Scholar] [CrossRef]
- Ye, Y.; Xie, X.; Yu, J.; Zhou, L.; Xie, H.; Jiang, G.; Yu, X.; Zhang, W.; Wu, J.; Zheng, S. Involvement of Th17 and Th1 effector responses in patients with Hepatitis B. J. Clin. Immunol. 2010, 30, 546–555. [Google Scholar] [CrossRef]
- Yang, R.; Xu, Y.; Dai, Z.; Lin, X.; Wang, H. The Immunologic Role of Gut Microbiota in Patients with Chronic HBV Infection. J. Immunol. Res. 2018, 2018, 2361963. [Google Scholar] [CrossRef]
- Sozinov, A.S. Systemic endotoxemia during chronic viral hepatitis. Bull. Exp. Biol. Med. 2002, 133, 153–155. [Google Scholar] [CrossRef]
- Pan, C.; Gu, Y.; Zhang, W.; Zheng, Y.; Peng, L.; Deng, H.; Chen, Y.; Chen, L.; Chen, S.; Zhang, M.; et al. Dynamic changes of lipopolysaccharide levels in different phases of acute on chronic hepatitis B liver failure. PLoS ONE 2012, 7, e49460. [Google Scholar] [CrossRef]
- Xing, H.C.; Li, L.J.; Xu, K.J.; Shen, T.; Chen, Y.B.; Sheng, J.F.; Chen, Y.; Fu, S.Z.; Chen, C.L.; Wang, J.G.; et al. Protective role of supplement with foreign Bifidobacterium and Lactobacillus in experimental hepatic ischemia-reperfusion injury. J. Gastroenterol. Hepatol. 2006, 21, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Wu, Z.; Xu, W.; Yang, J.; Chen, Y.; Li, L. Intestinal microbiota was assessed in cirrhotic patients with hepatitis B virus infection. Intestinal microbiota of HBV cirrhotic patients. Microb. Ecol. 2011, 61, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.L.; McKay, D.M. The immunophysiological impact of bacterial CpG DNA on the gut. Clin. Chim. Acta; Int. J. Clin. Chem. 2006, 364, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.S.; Shah, V.H. The role of gut-liver axis in the pathogenesis of liver cirrhosis and portal hypertension. Clin. Mol. Hepatol. 2012, 18, 337–346. [Google Scholar] [CrossRef]
- Chen, F.; Yao, Y.; Li, Z.; Deng, L.; He, R. Assessment of compensated advanced chronic liver disease based on serum bile acids in chronic hepatitis B patients. Sci. Rep. 2023, 13, 12834. [Google Scholar] [CrossRef]
- Cai, J.; Sun, L.; Gonzalez, F.J. Gut microbiota-derived bile acids in intestinal immunity, inflammation, and tumorigenesis. Cell Host Microbe 2022, 30, 289–300. [Google Scholar] [CrossRef]
- Boullier, S.; Tanguy, M.; Kadaoui, K.A.; Caubet, C.; Sansonetti, P.; Corthesy, B.; Phalipon, A. Secretory IgA-mediated neutralization of Shigella flexneri prevents intestinal tissue destruction by down-regulating inflammatory circuits. J. Immunol. 2009, 183, 5879–5885. [Google Scholar] [CrossRef]
- Macpherson, A.J.; Uhr, T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science 2004, 303, 1662–1665. [Google Scholar] [CrossRef]
- Mou, H.; Yang, F.; Zhou, J.; Bao, C. Correlation of liver function with intestinal flora, vitamin deficiency and IL-17A in patients with liver cirrhosis. Exp. Ther. Med. 2018, 16, 4082–4088. [Google Scholar] [CrossRef]
- Liu, B.; Zhou, Z.; Jin, Y.; Lu, J.; Feng, D.; Peng, R.; Sun, H.; Mu, X.; Li, C.; Chen, Y. Hepatic stellate cell activation and senescence induced by intrahepatic microbiota disturbances drive progression of liver cirrhosis toward hepatocellular carcinoma. J. Immunother. Cancer 2022, 10, e003069. [Google Scholar] [CrossRef]
- Ray, K. Gut microbiota: Obesity-induced microbial metabolite promotes HCC. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 442. [Google Scholar] [CrossRef] [PubMed]
- Dapito, D.H.; Mencin, A.; Gwak, G.Y.; Pradere, J.P.; Jang, M.K.; Mederacke, I.; Caviglia, J.M.; Khiabanian, H.; Adeyemi, A.; Bataller, R.; et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell 2012, 21, 504–516. [Google Scholar] [CrossRef] [PubMed]
- Jinato, T.; Anuntakarun, S.; Satthawiwat, N.; Chuaypen, N.; Tangkijvanich, P. Distinct alterations of gut microbiota between viral- and non-viral-related hepatocellular carcinoma. Appl. Microbiol. Biotechnol. 2024, 108, 34. [Google Scholar] [CrossRef]
- Tarao, K.; Ohkawa, S.; Miyagi, Y.; Morinaga, S.; Ohshige, K.; Yamamoto, N.; Ueno, M.; Kobayashi, S.; Kameda, R.; Tamai, S.; et al. Inflammation in background cirrhosis evokes malignant progression in HCC development from HCV-associated liver cirrhosis. Scand. J. Gastroenterol. 2013, 48, 729–735. [Google Scholar] [CrossRef]
- He, Y.; Fu, L.; Li, Y.; Wang, W.; Gong, M.; Zhang, J.; Dong, X.; Huang, J.; Wang, Q.; Mackay, C.R.; et al. Gut microbial metabolites facilitate anticancer therapy efficacy by modulating cytotoxic CD8(+) T cell immunity. Cell Metab. 2021, 33, 988–1000.e7. [Google Scholar] [CrossRef]
- Shen, Y.; Wu, S.D.; Chen, Y.; Li, X.Y.; Zhu, Q.; Nakayama, K.; Zhang, W.Q.; Weng, C.Z.; Zhang, J.; Wang, H.K.; et al. Alterations in gut microbiome and metabolomics in chronic hepatitis B infection-associated liver disease and their impact on peripheral immune response. Gut Microbes 2023, 15, 2155018. [Google Scholar] [CrossRef]
- Zeng, Y.L.; Qin, L.; Wei, W.J.; Cai, H.; Yu, X.F.; Zhang, W.; Wu, X.L.; Liu, X.B.; Chen, W.M.; You, P.; et al. Meta-omics characteristics of intestinal microbiota associated to HBeAg seroconversion induced by oral antiviral therapy. Sci. Rep. 2021, 11, 3253. [Google Scholar] [CrossRef]
- Long, J.; Gong, J.; Zhu, H.; Liu, X.; Li, L.; Chen, B.; Ren, H.; Liu, C.; Lu, H.; Zhang, J.; et al. Difference of gut microbiota between patients with negative and positive HBeAg in chronic hepatitis B and the effect of tenofovir alafenamide on intestinal flora. Front. Microbiol. 2023, 14, 1232180. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Kassam, Z.; Fagan, A.; Gavis, E.A.; Liu, E.; Cox, I.J.; Kheradman, R.; Heuman, D.; Wang, J.; Gurry, T.; et al. Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: A randomized clinical trial. Hepatology 2017, 66, 1727–1738. [Google Scholar] [CrossRef]
- Woodhouse, C.A.; Patel, V.C.; Singanayagam, A.; Shawcross, D.L. Review article: The gut microbiome as a therapeutic target in the pathogenesis and treatment of chronic liver disease. Aliment. Pharmacol. Ther. 2018, 47, 192–202. [Google Scholar] [CrossRef]
- Loguercio, C.; Federico, A.; Tuccillo, C.; Terracciano, F.; D’Auria, M.V.; De Simone, C.; Del Vecchio Blanco, C. Beneficial effects of a probiotic VSL#3 on parameters of liver dysfunction in chronic liver diseases. J. Clin. Gastroenterol. 2005, 39, 540–543. [Google Scholar] [CrossRef]
- Suez, J.; Zmora, N.; Segal, E.; Elinav, E. The pros, cons, and many unknowns of probiotics. Nat. Med. 2019, 25, 716–729. [Google Scholar] [CrossRef]
- Xia, X.; Chen, J.; Xia, J.; Wang, B.; Liu, H.; Yang, L.; Wang, Y.; Ling, Z. Role of probiotics in the treatment of minimal hepatic encephalopathy in patients with HBV-induced liver cirrhosis. J. Int. Med. Res. 2018, 46, 3596–3604. [Google Scholar] [CrossRef] [PubMed]
- Piano, S.; Bunchorntavakul, C.; Marciano, S.; Rajender Reddy, K. Infections in cirrhosis. Lancet Gastroenterol. Hepatol. 2024, 9, 745–757. [Google Scholar] [CrossRef] [PubMed]
- Kunz, A.N.; Fairchok, M.P.; Noel, J.M. Lactobacillus sepsis associated with probiotic therapy. Pediatrics 2005, 116, 517. [Google Scholar] [CrossRef] [PubMed]
- Thomas, H. IBD: FMT induces clinical remission in ulcerative colitis. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 196. [Google Scholar] [CrossRef]
- Borody, T.J.; Eslick, G.D.; Clancy, R.L. Fecal microbiota transplantation as a new therapy: From Clostridioides difficile infection to inflammatory bowel disease, irritable bowel syndrome, and colon cancer. Curr. Opin. Pharmacol. 2019, 49, 43–51. [Google Scholar] [CrossRef]
- Kim, Y.K.; Shin, C. The Microbiota-Gut-Brain Axis in Neuropsychiatric Disorders: Pathophysiological Mechanisms and Novel Treatments. Curr. Neuropharmacol. 2018, 16, 559–573. [Google Scholar] [CrossRef]
- Hu, X.F.; Zhang, W.Y.; Wen, Q.; Chen, W.J.; Wang, Z.M.; Chen, J.; Zhu, F.; Liu, K.; Cheng, L.X.; Yang, J.; et al. Fecal microbiota transplantation alleviates myocardial damage in myocarditis by restoring the microbiota composition. Pharmacol. Res. 2019, 139, 412–421. [Google Scholar] [CrossRef]
- Ren, Y.D.; Ye, Z.S.; Yang, L.Z.; Jin, L.X.; Wei, W.J.; Deng, Y.Y.; Chen, X.X.; Xiao, C.X.; Yu, X.F.; Xu, H.Z.; et al. Fecal microbiota transplantation induces hepatitis B virus e-antigen (HBeAg) clearance in patients with positive HBeAg after long-term antiviral therapy. Hepatology 2017, 65, 1765–1768. [Google Scholar] [CrossRef]
- Hadi, D.K.; Baines, K.J.; Jabbarizadeh, B.; Miller, W.H.; Jamal, R.; Ernst, S.; Logan, D.; Belanger, K.; Esfahani, K.; Elkrief, A.; et al. Improved survival in advanced melanoma patients treated with fecal microbiota transplantation using healthy donor stool in combination with anti-PD1: Final results of the MIMic phase 1 trial. J. Immunother. Cancer 2025, 13, e012659. [Google Scholar] [CrossRef]
- Wang, X.; Fang, Y.; Liang, W.; Wong, C.C.; Qin, H.; Gao, Y.; Liang, M.; Song, L.; Zhang, Y.; Fan, M.; et al. Fusobacterium nucleatum facilitates anti-PD-1 therapy in microsatellite stable colorectal cancer. Cancer Cell 2024, 42, 1729–1746.e8. [Google Scholar] [CrossRef]
- Riquelme, E.; Zhang, Y.; Zhang, L.; Montiel, M.; Zoltan, M.; Dong, W.; Quesada, P.; Sahin, I.; Chandra, V.; San Lucas, A.; et al. Tumor Microbiome Diversity and Composition Influence Pancreatic Cancer Outcomes. Cell 2019, 178, 795–806.e12. [Google Scholar] [CrossRef]
- Liang, Y.; Du, M.; Li, X.; Gao, J.; Li, Q.; Li, H.; Li, J.; Gao, X.; Cong, H.; Huang, Y.; et al. Upregulation of Lactobacillus spp. in gut microbiota as a novel mechanism for environmental eustress-induced anti-pancreatic cancer effects. Gut Microbes 2025, 17, 2470372. [Google Scholar] [CrossRef]
- Deng, Z.; Mei, S.; Ouyang, Z.; Wang, R.; Wang, L.; Zou, B.; Dai, J.; Mao, K.; Li, Q.; Guo, Q.; et al. Dysregulation of gut microbiota stimulates NETs-driven HCC intrahepatic metastasis: Therapeutic implications of healthy faecal microbiota transplantation. Gut Microbes 2025, 17, 2476561. [Google Scholar] [CrossRef]
- Chauhan, A.; Kumar, R.; Sharma, S.; Mahanta, M.; Vayuuru, S.K.; Nayak, B.; Kumar, S.; Shalimar. Fecal Microbiota Transplantation in Hepatitis B e Antigen-Positive Chronic Hepatitis B Patients: A Pilot Study. Dig. Dis. Sci. 2021, 66, 873–880. [Google Scholar] [CrossRef]

| Microbiota Dysbiosis | Outcomes | References |
|---|---|---|
| Veillonellaceae↑ Lachnospiraceae↑ Rikenellaceae↑ Porphyromonadaceae↓ Ruminococcaceae↓ | Detractive intestinal barrier against the colonization of pathogenic microbes by reducing the production of SCFAs and antimicrobial peptides | [39,40] |
| Enterobacteriaceae↑ Veillonella↑ Bacteroidetes↓ | Correlated with the Child-Turcotte-Pugh scores | [41] |
| Prevotella↑ Alloprevotella↑ Faecalibacterium↑ Ruminiclostridium↑ | Increased anti-inflammatory bacteria may be in response to HBV infection. | [42] |
| The detection rate of intestinal fungi and the diversity of enteric fungi increased | Positively correlated with the disease progression of patients with different degrees of chronic HBV infection | [43] |
| Bifidobacterium dysbiosis (B.dentium↑ B.catenulatum group↓ B. longum↓) | Intestinal Bifidobacterium species might shift from beneficial ones to opportunistic pathogens that associated with CHB and HBV-related cirrhosis | [44] |
| The diversity of lactobacilli population↓ | Disturbance of lactobacilli flora is associated with the liver injury | [45] |
| Increased the ratio of Bacteroidetes to Firmicutes (Firmicutes↓ and Bacteroidetes↑) | Associated with inflammatory disorders and may accelerate HBV-induced chronic liver disease progression | [46] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, D.; Han, N.; Liu, H.; Tang, H. Hepatitis B Virus-Related Liver Disease and Gut Microbiota: An Updated Review. Microorganisms 2025, 13, 2445. https://doi.org/10.3390/microorganisms13112445
Lv D, Han N, Liu H, Tang H. Hepatitis B Virus-Related Liver Disease and Gut Microbiota: An Updated Review. Microorganisms. 2025; 13(11):2445. https://doi.org/10.3390/microorganisms13112445
Chicago/Turabian StyleLv, Duoduo, Ning Han, Huan Liu, and Hong Tang. 2025. "Hepatitis B Virus-Related Liver Disease and Gut Microbiota: An Updated Review" Microorganisms 13, no. 11: 2445. https://doi.org/10.3390/microorganisms13112445
APA StyleLv, D., Han, N., Liu, H., & Tang, H. (2025). Hepatitis B Virus-Related Liver Disease and Gut Microbiota: An Updated Review. Microorganisms, 13(11), 2445. https://doi.org/10.3390/microorganisms13112445






