Experimental Models to Investigate Viral and Cellular Dynamics in Respiratory Viral Co-Infections
Abstract
1. Introduction
2. Experimental Models to Study Respiratory Viral Co-Infections
2.1. 2D Cell Culture Models
| Cell Type | Description | References |
|---|---|---|
| A549 (Human alveolar basal epithelial cells) | Used for studying respiratory viruses such as SARS-CoV-2, and RSV. A549 cells are known for their susceptibility to viral infections and are used in viral co-infection studies. | [33] |
| Calu-3 (Human lung adenocarcinoma cells) | Used for studying respiratory viruses, especially SARS-CoV-1, 2 and RSV. Calu-3 cells replicate human lung tissue and are ideal for co-infection models with respiratory viruses. | [29,42] |
| BEAS-2B (Immortalized human bronchial epithelial cells) | Commonly used for respiratory viral studies including RSV, and SARS-CoV-2. BEAS-2B cells mimic bronchial epithelium and are often used to study viral replication and immune response. | [43,44] |
| H1299/ACE2 (Human non-small cell lung carcinoma cells) | Use of non-small cell lung carcinoma cell line in respiratory virus research, especially for SARS-CoV-2. | [45] |
| Vero (African green monkey kidney epithelial cells) | Widely used in respiratory virus research, including efficient replication of SARS-CoV-2 and adapted strains of influenza virus. | [46,47] |
| LLC-MK2 (Monkey kidney cells) | Used for studying respiratory viruses, including co-infection models for viruses like Human Metapneumovirus (HMPV) and RSV. | [48] |
| HEp-2 (Human epithelial cells) | Frequently used for respiratory viral replication studies, such as those involving influenza, RSV, and coronaviruses. | [39,49] |
| MDCK (Madin-Darby Canine Kidney cells) | Commonly used for influenza virus research and respiratory viral co-infection studies. MDCK cells are often employed to study viral replication and virus–host interactions. | [50] |
| Huh7 (Human hepatocellular carcinoma cells) | Although primarily used for HCV research, Huh7 cells can be utilized for respiratory viral infections and co-infection models involving influenza or coronaviruses. | [51] |
| 293T (Human embryonic kidney cells) | Can be used to study viral replication in respiratory infections, including co-infection studies, due to their ability to support viral gene expression. | [52] |
| NCI-H292 (Human pulmonary epithelial cells) | Can be used for respiratory viral co-infection studies, particularly those studying respiratory tract infections. | [53] |
| THP-1 (Human monocytic leukemia cell line) | Can be used for studying immune responses in viral co-infection models, especially involving respiratory viruses like influenza and rhinovirus. | [54] |
| U937 (Human histiocytic lymphoma cell line) | Can be used to study immune responses during respiratory viral co-infections, often in models involving macrophage activation. | [55] |
| HeLa (Human cervical epithelial cells) | Used for studying viral replication and host responses, including rhinovirus A, and RSV. | [56,57] |
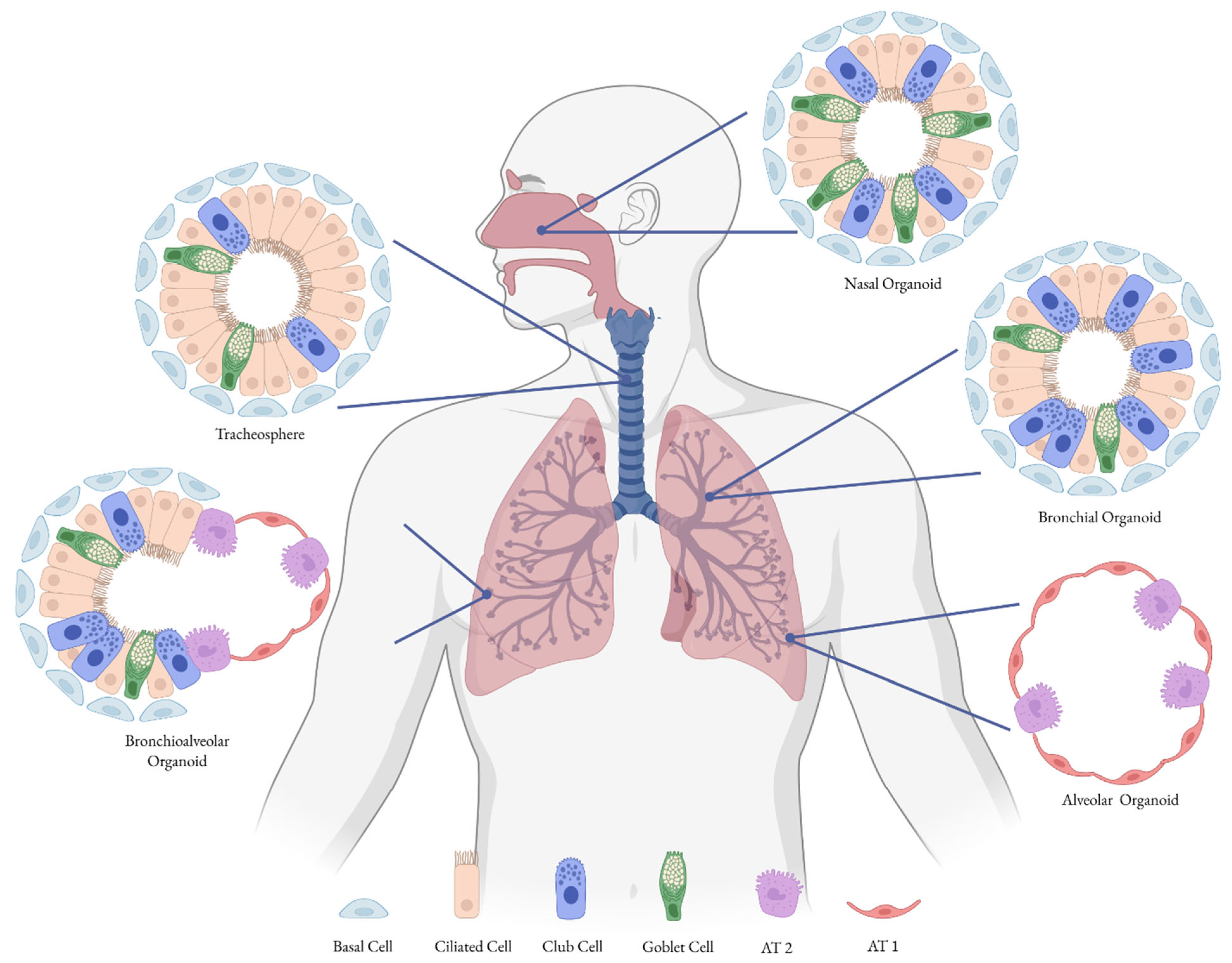
2.2. 3D Cell Culture Models
| Organoid Type | Description/Origin | Viruses Used for Infection |
|---|---|---|
| Tracheospheres | Spheroids grown from tracheal stem cells | Used to study differentiation and to assess infectivity of the influenza virus [71]. |
| Bronchiospheres/Bronchial Organoids | Derived from progenitor cells of bronchi, mainly basal cells; AT2 cells co-cultured with lung mesenchymal cells | Used to model influenza and SARS-CoV-2 infections [72]. |
| Alveolar Organoids | Derived from alveolar progenitor cells (AT2 cells) | Used as a model for respiratory viruses, SARS-CoV-2 [72,73]. |
| Bronchioalveolar Organoids | Lung organoids with CHIR99021-induced SCGB1A1+ bronchiolar cells | Employed to study infections caused by influenza virus and SARS-CoV-2 [72]. |
| Lung Bud Organoids | Derived from hPSCs (mesoderm and pulmonary endoderm); develop into airway organoids | Used in RSV infection studies [74]. |
| Nasal Organoids | Generated from human nasal epithelial stem/progenitor cells; recapitulate nasal mucosa structure and function | Used to study SARS-CoV-2 and RSV; valuable for modeling viral entry, replication, and host responses [67,75,76,77] |
2.3. Alternative Cell Culture Models to Study Respiratory Viral Co-Infections
2.4. Animal Models
3. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Subbarao, K.; Mahanty, S. Respiratory Virus Infections: Understanding COVID-19. Immunity 2020, 52, 905–909. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, L.; Deng, X.; Liang, R.; Su, M.; He, C.; Hu, L.; Su, Y.; Ren, J.; Yu, F.; et al. Recent Advances in the Detection of Respiratory Virus Infection in Humans. J. Med. Virol. 2020, 92, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.; Zambon, M. Influenza, Respiratory Syncytial Virus and SARS. Medicine 2009, 37, 679–685. [Google Scholar] [CrossRef]
- Bakaletz, L.O. Viral–Bacterial Co-Infections in the Respiratory Tract. Curr. Opin. Microbiol. 2017, 35, 30–35. [Google Scholar] [CrossRef]
- Santus, P.; Danzo, F.; Signorello, J.C.; Rizzo, A.; Gori, A.; Antinori, S.; Gismondo, M.R.; Brambilla, A.M.; Contoli, M.; Rizzardini, G.; et al. Burden and Risk Factors for Coinfections in Patients with a Viral Respiratory Tract Infection. Pathogens 2024, 13, 993. [Google Scholar] [CrossRef]
- Dikranian, L.; Barry, S.; Ata, A.; Chiotos, K.; Gist, K.; Bhalala, U.; Danesh, V.; Heavner, S.; Gharpure, V.; Bjornstad, E.C.; et al. SARS-CoV-2 With Concurrent Respiratory Viral Infection as a Risk Factor for a Higher Level of Care in Hospitalized Pediatric Patients. Pediatr. Emerg. Care 2022, 38, 472–476. [Google Scholar] [CrossRef]
- Krumbein, H.; Kümmel, L.S.; Fragkou, P.C.; Thölken, C.; Hünerbein, B.L.; Reiter, R.; Papathanasiou, K.A.; Renz, H.; Skevaki, C. Respiratory Viral Co-infections in Patients with COVID-19 and Associated Outcomes: A Systematic Review and Meta-analysis. Rev. Med. Virol. 2023, 33, e2365. [Google Scholar] [CrossRef]
- Aberle, J.H.; Aberle, S.W.; Pracher, E.; Hutter, H.-P.; Kundi, M.; Popow-Kraupp, T. Single Versus Dual Respiratory Virus Infections in Hospitalized Infants: Impact on Clinical Course of Disease and Interferon-γ Response. Pediatr. Infect. Dis. J. 2005, 24, 605–610. [Google Scholar] [CrossRef]
- Echenique, I.A.; Chan, P.A.; Chapin, K.C.; Andrea, S.B.; Fava, J.L.; Mermel, L.A. Clinical Characteristics and Outcomes in Hospitalized Patients with Respiratory Viral Co-Infection during the 2009 H1N1 Influenza Pandemic. PLoS ONE 2013, 8, e60845. [Google Scholar] [CrossRef] [PubMed]
- Asner, S.A.; Rose, W.; Petrich, A.; Richardson, S.; Tran, D.J. Is Virus Coinfection a Predictor of Severity in Children with Viral Respiratory Infections? Clin. Microbiol. Infect. 2015, 21, 264.e1–264.e6. [Google Scholar] [CrossRef] [PubMed]
- Esper, F.P.; Spahlinger, T.; Zhou, L. Rate and Influence of Respiratory Virus Co-Infection on Pandemic (H1N1) Influenza Disease. J. Infect. 2011, 63, 260–266. [Google Scholar] [CrossRef]
- Cong, B.; Deng, S.; Wang, X.; Li, Y. The Role of Respiratory Co-Infection with Influenza or Respiratory Syncytial Virus in the Clinical Severity of COVID-19 Patients: A Systematic Review and Meta-Analysis. J. Glob. Health 2022, 12, 05040. [Google Scholar] [CrossRef] [PubMed]
- Swets, M.C.; Russell, C.D.; Harrison, E.M.; Docherty, A.B.; Lone, N.; Girvan, M.; Hardwick, H.E.; Visser, L.G.; Openshaw, P.J.M.; Groeneveld, G.H.; et al. SARS-CoV-2 Co-Infection with Influenza Viruses, Respiratory Syncytial Virus, or Adenoviruses. Lancet 2022, 399, 1463–1464. [Google Scholar] [CrossRef] [PubMed]
- Goka, E.A.; Vallely, P.J.; Mutton, K.J.; Klapper, P.E. Single, Dual and Multiple Respiratory Virus Infections and Risk of Hospitalization and Mortality. Epidemiol. Infect. 2015, 143, 37–47. [Google Scholar] [CrossRef]
- Maltezou, H.C.; Papanikolopoulou, A.; Vassiliu, S.; Theodoridou, K.; Nikolopoulou, G.; Sipsas, N.V. COVID-19 and Respiratory Virus Co-Infections: A Systematic Review of the Literature. Viruses 2023, 15, 865. [Google Scholar] [CrossRef]
- Chow, E.J.; Uyeki, T.M.; Chu, H.Y. The Effects of the COVID-19 Pandemic on Community Respiratory Virus Activity. Nat. Rev. Microbiol. 2022, 21, 195–210. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, X.; Cong, B.; Deng, S.; Feikin, D.R.; Nair, H. Understanding the Potential Drivers for Respiratory Syncytial Virus Rebound During the Coronavirus Disease 2019 Pandemic. J. Infect. Dis. 2022, 225, 957–964. [Google Scholar] [CrossRef]
- Nott, R.; Fuller, T.L.; Brasil, P.; Nielsen-Saines, K. Out-of-Season Influenza during a COVID-19 Void in the State of Rio de Janeiro, Brazil: Temperature Matters. Vaccines 2022, 10, 821. [Google Scholar] [CrossRef]
- Piret, J.; Boivin, G. Viral Interference between Respiratory Viruses. Emerg. Infect. Dis. 2022, 28, 273–281. [Google Scholar] [CrossRef]
- Trepat, K.; Gibeaud, A.; Trouillet-Assant, S.; Terrier, O. Exploring Viral Respiratory Coinfections: Shedding Light on Pathogen Interactions. PLOS Pathog. 2024, 20, e1012556. [Google Scholar] [CrossRef]
- Kim, E.-H.; Nguyen, T.-Q.; Casel, M.A.B.; Rollon, R.; Kim, S.-M.; Kim, Y.-I.; Yu, K.-M.; Jang, S.-G.; Yang, J.; Poo, H.; et al. Coinfection with SARS-CoV-2 and Influenza A Virus Increases Disease Severity and Impairs Neutralizing Antibody and CD4+ T Cell Responses. J. Virol. 2022, 96, e0187321. [Google Scholar] [CrossRef]
- Hurme, P.; Sahla, R.; Rückert, B.; Vahlberg, T.; Turunen, R.; Vuorinen, T.; Akdis, M.; Söderlund-Venermo, M.; Akdis, C.; Jartti, T. Human Bocavirus 1 Coinfection Is Associated with Decreased Cytokine Expression in the Rhinovirus-induced First Wheezing Episode in Children. Clin. Transl. Allergy 2023, 13, e12311. [Google Scholar] [CrossRef]
- Meskill, S.D.; O’Bryant, S.C. Respiratory Virus Co-Infection in Acute Respiratory Infections in Children. Curr. Infect. Dis. Rep. 2020, 22, 3. [Google Scholar] [CrossRef]
- Wu, H.; Zhou, H.-Y.; Zheng, H.; Wu, A. Towards Understanding and Identification of Human Viral Co-Infections. Viruses 2024, 16, 673. [Google Scholar] [CrossRef]
- Rijsbergen, L.C.; Van Dijk, L.L.A.; Engel, M.F.M.; De Vries, R.D.; De Swart, R.L. In Vitro Modelling of Respiratory Virus Infections in Human Airway Epithelial Cells—A Systematic Review. Front. Immunol. 2021, 12, 683002. [Google Scholar] [CrossRef]
- Mohtasham, L.; Auais, A.; Piedimonte, G. Advances in Viral Respiratory Infections: New Experimental Models. Drug Discov. Today Dis. Models 2004, 1, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Sura, T.; Gering, V.; Cammann, C.; Hammerschmidt, S.; Maaß, S.; Seifert, U.; Becher, D. Streptococcus Pneumoniae and Influenza A Virus Co-Infection Induces Altered Polyubiquitination in A549 Cells. Front. Cell. Infect. Microbiol. 2022, 12, 817532. [Google Scholar] [CrossRef]
- Rajan, A.; Piedra, F.-A.; Aideyan, L.; McBride, T.; Robertson, M.; Johnson, H.L.; Aloisio, G.M.; Henke, D.; Coarfa, C.; Stossi, F.; et al. Multiple Respiratory Syncytial Virus (RSV) Strains Infecting HEp-2 and A549 Cells Reveal Cell Line-Dependent Differences in Resistance to RSV Infection. J. Virol. 2022, 96, e01904-21. [Google Scholar] [CrossRef] [PubMed]
- Harcourt, J.L.; Caidi, H.; Anderson, L.J.; Haynes, L.M. Evaluation of the Calu-3 Cell Line as a Model of in Vitro Respiratory Syncytial Virus Infection. J. Virol. Methods 2011, 174, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Hillyer, P.; Shepard, R.; Uehling, M.; Krenz, M.; Sheikh, F.; Thayer, K.R.; Huang, L.; Yan, L.; Panda, D.; Luongo, C.; et al. Differential Responses by Human Respiratory Epithelial Cell Lines to Respiratory Syncytial Virus Reflect Distinct Patterns of Infection Control. J. Virol. 2018, 92, e02202-17. [Google Scholar] [CrossRef]
- Seng, L.-G.; Daly, J.; Chang, K.-C.; Kuchipudi, S.V. High Basal Expression of Interferon-Stimulated Genes in Human Bronchial Epithelial (BEAS-2B) Cells Contributes to Influenza A Virus Resistance. PLoS ONE 2014, 9, e109023. [Google Scholar] [CrossRef]
- Lee, D.C.; Mok, C.K.; Law, A.H.; Peiris, M.; Lau, A.S. Differential Replication of Avian Influenza H9N2 Viruses in Human Alveolar Epithelial A549 Cells. Virol. J. 2010, 7, 71. [Google Scholar] [CrossRef]
- Vanetti, C.; Saulle, I.; Artusa, V.; Moscheni, C.; Cappelletti, G.; Zecchini, S.; Strizzi, S.; Garziano, M.; Fenizia, C.; Tosoni, A.; et al. A Complex Remodeling of Cellular Homeostasis Distinguishes RSV/SARS-CoV-2 Co-Infected A549-hACE2 Expressing Cell Lines. Microb. Cell 2024, 11, 353–367. [Google Scholar] [CrossRef]
- Goto, H.; Ihira, H.; Morishita, K.; Tsuchiya, M.; Ohta, K.; Yumine, N.; Tsurudome, M.; Nishio, M. Enhanced Growth of Influenza A Virus by Coinfection with Human Parainfluenza Virus Type 2. Med. Microbiol. Immunol. 2016, 205, 209–218. [Google Scholar] [CrossRef]
- Malausse, N.; Van Der Werf, S.; Naffakh, N.; Munier, S. Influenza B Virus Infection Is Enhanced Upon Heterotypic Co-Infection with Influenza A Virus. Front. Microbiol. 2021, 12, 631346. [Google Scholar] [CrossRef]
- Bai, L.; Zhao, Y.; Dong, J.; Liang, S.; Guo, M.; Liu, X.; Wang, X.; Huang, Z.; Sun, X.; Zhang, Z.; et al. Coinfection with Influenza A Virus Enhances SARS-CoV-2 Infectivity. Cell Res. 2021, 31, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Wanitchang, A.; Narkpuk, J.; Jaru-ampornpan, P.; Jengarn, J.; Jongkaewwattana, A. Inhibition of Influenza A Virus Replication by Influenza B Virus Nucleoprotein: An Insight into Interference between Influenza A and B Viruses. Virology 2012, 432, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Narkpuk, J.; Teeravechyan, S.; Puthavathana, P.; Jongkaewwattana, A.; Jaru-Ampornpan, P. Single Nucleoprotein Residue Determines Influenza A Virus Sensitivity to an Intertypic Suppression Mechanism. Virology 2017, 506, 99–109. [Google Scholar] [CrossRef]
- Drori, Y.; Jacob-Hirsch, J.; Pando, R.; Glatman-Freedman, A.; Friedman, N.; Mendelson, E.; Mandelboim, M. Influenza A Virus Inhibits RSV Infection via a Two-Wave Expression of IFIT Proteins. Viruses 2020, 12, 1171. [Google Scholar] [CrossRef] [PubMed]
- Haney, J.; Vijayakrishnan, S.; Streetley, J.; Dee, K.; Goldfarb, D.M.; Clarke, M.; Mullin, M.; Carter, S.D.; Bhella, D.; Murcia, P.R. Coinfection by Influenza A Virus and Respiratory Syncytial Virus Produces Hybrid Virus Particles. Nat. Microbiol. 2022, 7, 1879–1890. [Google Scholar] [CrossRef]
- Thiam, F.; Yazeedi, S.A.; Feng, K.; Phogat, S.; Demirsoy, E.; Brussow, J.; Abokor, F.A.; Osei, E.T. Understanding Fibroblast-Immune Cell Interactions via Co-Culture Models and Their Role in Asthma Pathogenesis. Front. Immunol. 2023, 14, 1128023. [Google Scholar] [CrossRef]
- Tat, V.Y.; Drelich, A.K.; Huang, P.; Khanipov, K.; Hsu, J.C.; Widen, S.G.; Tseng, C.-T.K.; Golovko, G. Characterizing Temporal and Global Host Innate Immune Responses against SARS-CoV-1 and -2 Infection in Pathologically Relevant Human Lung Epithelial Cells. PLoS ONE 2025, 20, e0317921. [Google Scholar] [CrossRef]
- Zhuang, X.; Gallo, G.; Sharma, P.; Ha, J.; Magri, A.; Borrmann, H.; Harris, J.M.; Tsukuda, S.; Bentley, E.; Kirby, A.; et al. Hypoxia Inducible Factors Inhibit Respiratory Syncytial Virus Infection by Modulation of Nucleolin Expression. iScience 2024, 27, 108763. [Google Scholar] [CrossRef] [PubMed]
- Bartolomeo, C.S.; Lemes, R.M.R.; Morais, R.L.; Pereria, G.C.; Nunes, T.A.; Costa, A.J.; De Barros Maciel, R.M.; Braconi, C.T.; Maricato, J.T.; Janini, L.M.R.; et al. SARS-CoV-2 Infection and Replication Kinetics in Different Human Cell Types: The Role of Autophagy, Cellular Metabolism and ACE2 Expression. Life Sci. 2022, 308, 120930. [Google Scholar] [CrossRef] [PubMed]
- Salgado-Benvindo, C.; Tas, A.; Zevenhoven-Dobbe, J.C.; Van Der Meer, Y.; Sidorov, I.A.; Leijs, A.A.; Wanningen, P.; Gelderloos, A.T.; Van Kasteren, P.B.; Snijder, E.J.; et al. Characterization of SARS-CoV-2 Replication in Human H1299/ACE2 Cells: A Versatile and Practical Infection Model for Antiviral Research and Beyond. Antivir. Res. 2024, 227, 105903. [Google Scholar] [CrossRef] [PubMed]
- Barreto-Vieira, D.F.; Da Silva, M.A.N.; Garcia, C.C.; Miranda, M.D.; Matos, A.D.R.; Caetano, B.C.; Resende, P.C.; Motta, F.C.; Siqueira, M.M.; Girard-Dias, W.; et al. Morphology and Morphogenesis of SARS-CoV-2 in Vero-E6 Cells. Mem. Inst. Oswaldo Cruz 2021, 116, e200443. [Google Scholar] [CrossRef]
- Ozaki, H.; Govorkova, E.A.; Li, C.; Xiong, X.; Webster, R.G.; Webby, R.J. Generation of High-Yielding Influenza A Viruses in African Green Monkey Kidney (Vero) Cells by Reverse Genetics. J. Virol. 2004, 78, 1851–1857. [Google Scholar] [CrossRef]
- Kinder, J. Pneumovirus Infections: Understanding RSV and HMPV Entry, Replication, and Spread; University of Kentucky Libraries: Lexington, KY, USA, 2020. [Google Scholar]
- Wang, L.; Fan, X.; Bonenfant, G.; Cui, D.; Hossain, J.; Jiang, N.; Larson, G.; Currier, M.; Liddell, J.; Wilson, M.; et al. Susceptibility to SARS-CoV-2 of Cell Lines and Substrates Commonly Used to Diagnose and Isolate Influenza and Other Viruses. Emerg. Infect. Dis. 2021, 27, 1380–1392. [Google Scholar] [CrossRef]
- Martin, B.E.; Harris, J.D.; Sun, J.; Koelle, K.; Brooke, C.B. Cellular Co-Infection Can Modulate the Efficiency of Influenza A Virus Production and Shape the Interferon Response. PLoS Pathog. 2020, 16, e1008974. [Google Scholar] [CrossRef]
- Freymuth, F.; Vabret, A.; Rozenberg, F.; Dina, J.; Petitjean, J.; Gouarin, S.; Legrand, L.; Corbet, S.; Brouard, J.; Lebon, P. Replication of Respiratory Viruses, Particularly Influenza Virus, Rhinovirus, and Coronavirus in HuH7 Hepatocarcinoma Cell Line. J. Med. Virol. 2005, 77, 295–301. [Google Scholar] [CrossRef]
- Oliveira, A.P.; Simabuco, F.M.; Tamura, R.E.; Guerrero, M.C.; Ribeiro, P.G.G.; Libermann, T.A.; Zerbini, L.F.; Ventura, A.M. Human Respiratory Syncytial Virus N, P and M Protein Interactions in HEK-293T Cells. Virus Res. 2013, 177, 108–112. [Google Scholar] [CrossRef]
- Castells, E.; George, V.G.; Hierholzer, J.C. NCI-H292 as an Alternative Cell Line for the Isolation and Propagation of the Human Paramyxoviruses. Arch. Virol. 1990, 115, 277–288. [Google Scholar] [CrossRef]
- Rosas-Taraco, A.G.; Martinez-Castilla, A.M.; Salado-Gamez, J.H.; Flores-Torres, A.S.; Flores-Aldaba, K.A.; Torres-Lopez, E.; Salinas-Carmona, M.C. Human Rhinovirus and Influenza A H1N1 Monoinfection and Co-Infection Induce Both Proinflammatory and Anti-Inflammatory Responses in Macrophages THP-1. J. Immunol. 2020, 204, 93.22. [Google Scholar] [CrossRef]
- Zhao, D.-C.; Yan, T.; Li, L.; You, S.; Zhang, C. Respiratory Syncytial Virus Inhibits Interferon-α-Inducible Signaling in Macrophage-like U937 Cells. J. Infect. 2007, 54, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Amineva, S.P.; Aminev, A.G.; Gern, J.E.; Palmenberg, A.C. Comparison of Rhinovirus A Infection in Human Primary Epithelial and HeLa Cells. J. Gen. Virol. 2011, 92, 2549–2557. [Google Scholar] [CrossRef]
- Krzyzaniak, M.A.; Zumstein, M.T.; Gerez, J.A.; Picotti, P.; Helenius, A. Host Cell Entry of Respiratory Syncytial Virus Involves Macropinocytosis Followed by Proteolytic Activation of the F Protein. PLoS Pathog. 2013, 9, e1003309. [Google Scholar] [CrossRef] [PubMed]
- Tindle, C.; Fuller, M.; Fonseca, A.; Taheri, S.; Ibeawuchi, S.-R.; Beutler, N.; Katkar, G.D.; Claire, A.; Castillo, V.; Hernandez, M.; et al. Adult Stem Cell-Derived Complete Lung Organoid Models Emulate Lung Disease in COVID-19. eLife 2021, 10, e66417. [Google Scholar] [CrossRef] [PubMed]
- Van Riet, S.; Van Schadewijk, A.; Khedoe, P.P.S.J.; Limpens, R.W.A.L.; Bárcena, M.; Stolk, J.; Hiemstra, P.S.; Van Der Does, A.M. Organoid-Based Expansion of Patient-Derived Primary Alveolar Type 2 Cells for Establishment of Alveolus Epithelial Lung-Chip Cultures. Am. J. Physiol. Lung Cell. Mol. Physiol. 2022, 322, L526–L538. [Google Scholar] [CrossRef]
- Edwards, C.E.; Tata, A.; Baric, R.S. Human Lung Organoids as a Model for Respiratory Virus Replication and Countermeasure Performance in Human Hosts. Transl. Res. 2022, 250, 36–45. [Google Scholar] [CrossRef]
- Miller, A.J.; Hill, D.R.; Nagy, M.S.; Aoki, Y.; Dye, B.R.; Chin, A.M.; Huang, S.; Zhu, F.; White, E.S.; Lama, V.; et al. In Vitro Induction and In Vivo Engraftment of Lung Bud Tip Progenitor Cells Derived from Human Pluripotent Stem Cells. Stem Cell Rep. 2018, 10, 101–119. [Google Scholar] [CrossRef]
- Kühl, L.; Graichen, P.; Von Daacke, N.; Mende, A.; Wygrecka, M.; Potaczek, D.P.; Miethe, S.; Garn, H. Human Lung Organoids—A Novel Experimental and Precision Medicine Approach. Cells 2023, 12, 2067. [Google Scholar] [CrossRef] [PubMed]
- Vieira Braga, F.A.; Kar, G.; Berg, M.; Carpaij, O.A.; Polanski, K.; Simon, L.M.; Brouwer, S.; Gomes, T.; Hesse, L.; Jiang, J.; et al. A Cellular Census of Human Lungs Identifies Novel Cell States in Health and in Asthma. Nat. Med. 2019, 25, 1153–1163. [Google Scholar] [CrossRef]
- Rajan, A.; Weaver, A.M.; Aloisio, G.M.; Jelinski, J.; Johnson, H.L.; Venable, S.F.; McBride, T.; Aideyan, L.; Piedra, F.-A.; Ye, X.; et al. The Human Nose Organoid Respiratory Virus Model: An Ex Vivo Human Challenge Model to Study Respiratory Syncytial Virus (RSV) and Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Pathogenesis and Evaluate Therapeutics. mBio 2022, 13, e03511-21. [Google Scholar] [CrossRef]
- Aloisio, G.M.; Nagaraj, D.; Murray, A.M.; Schultz, E.M.; McBride, T.; Aideyan, L.; Nicholson, E.G.; Henke, D.; Ferlic-Stark, L.; Rajan, A.; et al. Infant-Derived Human Nasal Organoids Exhibit Relatively Increased Susceptibility, Epithelial Responses, and Cytotoxicity during RSV Infection. J. Infect. 2024, 89, 106305. [Google Scholar] [CrossRef]
- Rajan, A.; Nagaraj, D.; Bomidi, C.; Aloisio, G.M.; Murray, A.M.; Schultz, E.M.; Kambal, A.; Estes, M.K.; Nicholson, E.; Avadhanula, V.; et al. Single Cell Sequencing Analysis of Respiratory Syncytial Virus–Infected Pediatric and Adult Human Nose Organoids Reveals Age Differences, Proliferative Diversity and Identifies Novel Cellular Tropism. J. Infect. 2025, 91, 106617. [Google Scholar] [CrossRef]
- Aloisio, G.M.; McBride, T.J.; Aideyan, L.; Schultz, E.M.; Murray, A.M.; Rajan, A.; Nicholson, E.G.; Henke, D.; Ferlic-Stark, L.; Kambal, A.; et al. Strain-Specific Variability in Viral Kinetics, Cytokine Response, and Cellular Damage in Air–Liquid Cultures of Human Nasal Organoids After Infection with SARS-CoV-2. Viruses 2025, 17, 1343. [Google Scholar] [CrossRef]
- Sachs, N.; Papaspyropoulos, A.; Zomer-van Ommen, D.D.; Heo, I.; Böttinger, L.; Klay, D.; Weeber, F.; Huelsz-Prince, G.; Iakobachvili, N.; Amatngalim, G.D.; et al. Long-term Expanding Human Airway Organoids for Disease Modeling. EMBO J. 2019, 38, e100300. [Google Scholar] [CrossRef]
- Turner, D.L.; Amoozadeh, S.; Baric, H.; Stanley, E.; Werder, R.B. Building a Human Lung from Pluripotent Stem Cells to Model Respiratory Viral Infections. Respir. Res. 2024, 25, 277. [Google Scholar] [CrossRef]
- Barkauskas, C.E.; Chung, M.-I.; Fioret, B.; Gao, X.; Katsura, H.; Hogan, B.L.M. Lung Organoids: Current Uses and Future Promise. Development 2017, 144, 986–997. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, C.; Sachs, N.; Chiu, M.C.; Wong, B.H.-Y.; Chu, H.; Poon, V.K.-M.; Wang, D.; Zhao, X.; Wen, L.; et al. Differentiated Human Airway Organoids to Assess Infectivity of Emerging Influenza Virus. Proc. Natl. Acad. Sci. USA 2018, 115, 6822–6827. [Google Scholar] [CrossRef] [PubMed]
- Ekanger, C.T.; Zhou, F.; Bohan, D.; Lotsberg, M.L.; Ramnefjell, M.; Hoareau, L.; Røsland, G.V.; Lu, N.; Aanerud, M.; Gärtner, F.; et al. Human Organotypic Airway and Lung Organoid Cells of Bronchiolar and Alveolar Differentiation Are Permissive to Infection by Influenza and SARS-CoV-2 Respiratory Virus. Front. Cell. Infect. Microbiol. 2022, 12, 841447. [Google Scholar] [CrossRef]
- Youk, J.; Kim, T.; Evans, K.V.; Jeong, Y.-I.; Hur, Y.; Hong, S.P.; Kim, J.H.; Yi, K.; Kim, S.Y.; Na, K.J.; et al. Three-Dimensional Human Alveolar Stem Cell Culture Models Reveal Infection Response to SARS-CoV-2. Cell Stem Cell 2020, 27, 905–919.e10. [Google Scholar] [CrossRef]
- Chen, Y.-W.; Huang, S.X.; De Carvalho, A.L.R.T.; Ho, S.-H.; Islam, M.N.; Volpi, S.; Notarangelo, L.D.; Ciancanelli, M.; Casanova, J.-L.; Bhattacharya, J.; et al. A Three-Dimensional Model of Human Lung Development and Disease from Pluripotent Stem Cells. Nat. Cell Biol. 2017, 19, 542–549. [Google Scholar] [CrossRef]
- Chiu, M.C.; Li, C.; Liu, X.; Song, W.; Wan, Z.; Yu, Y.; Huang, J.; Xiao, D.; Chu, H.; Cai, J.-P.; et al. Human Nasal Organoids Model SARS-CoV-2 Upper Respiratory Infection and Recapitulate the Differential Infectivity of Emerging Variants. mBio 2022, 13, e01944-22. [Google Scholar] [CrossRef] [PubMed]
- Rajan, A.; Marie Aloisio, G.; Weaver, A.M.; Jelinski, J.; Johnson, H.; McBride, T.; Robertson, M.; Melicoff-Portillo, E.; Kanthi Yerramilli, M.R.; Zeng, X.-L.; et al. #17: Disease and Immunoprophylaxis Model of Human Nose Organoids to Study SARS-CoV-2 and RSV Infection. J. Pediatr. Infect. Dis. Soc. 2021, 10, S8. [Google Scholar] [CrossRef]
- Li, C.; Huang, J.; Yu, Y.; Wan, Z.; Chiu, M.C.; Liu, X.; Zhang, S.; Cai, J.-P.; Chu, H.; Li, G.; et al. Human Airway and Nasal Organoids Reveal Escalating Replicative Fitness of SARS-CoV-2 Emerging Variants. Proc. Natl. Acad. Sci. USA 2023, 120, e2300376120. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, R.; Watanabe, Y.; Keshta, A.; Sugiyama, M.; Kitai, Y.; Hirabayashi, A.; Yasuhara, N.; Morimoto, S.; Sakamoto, A.; Matsumura, Y.; et al. Human iPS Cell–Derived Respiratory Organoids as a Model for Respiratory Syncytial Virus Infection. Life Sci. Alliance 2025, 8, e202402837. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, N.; Fan, S.; Zhao, L.; Song, W.; Gong, Y.; Shen, Q.; Zhang, C.; Ren, P.; Lin, C.; et al. Establishment of Human Distal Lung Organoids for SARS-CoV-2 Infection. Cell Discov. 2021, 7, 108. [Google Scholar] [CrossRef]
- Li, C.; Yu, Y.; Wan, Z.; Chiu, M.C.; Huang, J.; Zhang, S.; Zhu, X.; Lan, Q.; Deng, Y.; Zhou, Y.; et al. Human Respiratory Organoids Sustained Reproducible Propagation of Human Rhinovirus C and Elucidation of Virus-Host Interaction. Nat. Commun. 2024, 15, 10772. [Google Scholar] [CrossRef]
- Chen, Q.; Qi, H.; Guo, J. Applications of Human Lung Organoids to Human Respiratory Virus Research: Advances, Limitations and Future Directions. Infection 2025, 53, 1663–1675. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, S.; Kim, H.; Gil, D.; Han, H.-J.; Thimmulappa, R.K.; Choi, J.-H.; Kim, J.-H. Reciprocal Enhancement of SARS-CoV-2 and Influenza Virus Replication in Human Pluripotent Stem Cell-Derived Lung Organoids. Emerg. Microbes Infect. 2023, 12, 2211685. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Hill, T.; Li, K.; Peters, C.J.; Tseng, C.-T.K. Severe Acute Respiratory Syndrome (SARS) Coronavirus-Induced Lung Epithelial Cytokines Exacerbate SARS Pathogenesis by Modulating Intrinsic Functions of Monocyte-Derived Macrophages and Dendritic Cells. J. Virol. 2009, 83, 3039–3048. [Google Scholar] [CrossRef]
- Baldassi, D.; Gabold, B.; Merkel, O.M. Air−Liquid Interface Cultures of the Healthy and Diseased Human Respiratory Tract: Promises, Challenges, and Future Directions. Adv. NanoBiomed Res. 2021, 1, 2000111. [Google Scholar] [CrossRef]
- Carlier, F.M.; De Fays, C.; Pilette, C. Epithelial Barrier Dysfunction in Chronic Respiratory Diseases. Front. Physiol. 2021, 12, 691227. [Google Scholar] [CrossRef]
- Dvorak, A.; Tilley, A.E.; Shaykhiev, R.; Wang, R.; Crystal, R.G. Do Airway Epithelium Air–Liquid Cultures Represent the In Vivo Airway Epithelium Transcriptome? Am. J. Respir. Cell Mol. Biol. 2011, 44, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Ekanger, C.T.; Dinesh Kumar, N.; Koutstaal, R.W.; Zhou, F.; Beukema, M.; Waldock, J.; Jochems, S.P.; Mulder, N.; Van Els, C.A.C.M.; Engelhardt, O.G.; et al. Comparison of Air-Liquid Interface Transwell and Airway Organoid Models for Human Respiratory Virus Infection Studies. Front. Immunol. 2025, 16, 1532144. [Google Scholar] [CrossRef] [PubMed]
- Vanderwall, E.R.; Barrow, K.A.; Rich, L.M.; Read, D.F.; Trapnell, C.; Okoloko, O.; Ziegler, S.F.; Hallstrand, T.S.; White, M.P.; Debley, J.S. Airway Epithelial Interferon Response to SARS-CoV-2 Is Inferior to Rhinovirus and Heterologous Rhinovirus Infection Suppresses SARS-CoV-2 Replication. Sci. Rep. 2022, 12, 6972. [Google Scholar] [CrossRef]
- Essaidi-Laziosi, M.; Alvarez, C.; Puhach, O.; Sattonnet-Roche, P.; Torriani, G.; Tapparel, C.; Kaiser, L.; Eckerle, I. Sequential Infections with Rhinovirus and Influenza Modulate the Replicative Capacity of SARS-CoV-2 in the Upper Respiratory Tract. Emerg. Microbes Infect. 2022, 11, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Cheemarla, N.R.; Watkins, T.A.; Mihaylova, V.T.; Wang, B.; Zhao, D.; Wang, G.; Landry, M.L.; Foxman, E.F. Dynamic Innate Immune Response Determines Susceptibility to SARS-CoV-2 Infection and Early Replication Kinetics. J. Exp. Med. 2021, 218, e20210583. [Google Scholar] [CrossRef]
- Dee, K.; Goldfarb, D.M.; Haney, J.; Amat, J.A.R.; Herder, V.; Stewart, M.; Szemiel, A.M.; Baguelin, M.; Murcia, P.R. Human Rhinovirus Infection Blocks Severe Acute Respiratory Syndrome Coronavirus 2 Replication Within the Respiratory Epithelium: Implications for COVID-19 Epidemiology. J. Infect. Dis. 2021, 224, 31–38. [Google Scholar] [CrossRef]
- Dee, K.; Schultz, V.; Haney, J.; Bissett, L.A.; Magill, C.; Murcia, P.R. Influenza A and Respiratory Syncytial Virus Trigger a Cellular Response That Blocks Severe Acute Respiratory Syndrome Virus 2 Infection in the Respiratory Tract. J. Infect. Dis. 2023, 227, 1396–1406. [Google Scholar] [CrossRef] [PubMed]
- Cheemarla, N.R.; Watkins, T.A.; Mihaylova, V.T.; Foxman, E.F. Viral Interference During Influenza A–SARS-CoV-2 Coinfection of the Human Airway Epithelium and Reversal by Oseltamivir. J. Infect. Dis. 2024, 229, 1430–1434. [Google Scholar] [CrossRef]
- Pizzorno, A.; Padey, B.; Dulière, V.; Mouton, W.; Oliva, J.; Laurent, E.; Milesi, C.; Lina, B.; Traversier, A.; Julien, T.; et al. Interactions Between Severe Acute Respiratory Syndrome Coronavirus 2 Replication and Major Respiratory Viruses in Human Nasal Epithelium. J. Infect. Dis. 2022, 226, 2095–2104. [Google Scholar] [CrossRef]
- Wu, A.; Mihaylova, V.T.; Landry, M.L.; Foxman, E.F. Interference between Rhinovirus and Influenza A Virus: A Clinical Data Analysis and Experimental Infection Study. Lancet Microbe 2020, 1, e254–e262. [Google Scholar] [CrossRef]
- Tao, K.P.; Chong, M.K.C.; Chan, K.Y.Y.; Pun, J.C.S.; Tsun, J.G.S.; Chow, S.M.W.; Ng, C.S.H.; Wang, M.H.T.; Chan, P.K.S.; Li, A.M.; et al. Suppression of Influenza Virus Infection by Rhinovirus Interference—At the Population, Individual and Cellular Levels. Curr. Res. Microb. Sci. 2022, 3, 100147. [Google Scholar] [CrossRef]
- Essaidi-Laziosi, M.; Geiser, J.; Huang, S.; Constant, S.; Kaiser, L.; Tapparel, C. Interferon-Dependent and Respiratory Virus-Specific Interference in Dual Infections of Airway Epithelia. Sci. Rep. 2020, 10, 10246, Erratum in Sci. Rep. 2020, 10, 12523. [Google Scholar] [CrossRef]
- Gohy, S.; Carlier, F.M.; Fregimilicka, C.; Detry, B.; Lecocq, M.; Ladjemi, M.Z.; Verleden, S.; Hoton, D.; Weynand, B.; Bouzin, C.; et al. Altered Generation of Ciliated Cells in Chronic Obstructive Pulmonary Disease. Sci. Rep. 2019, 9, 17963. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.E.; Reidel, B.; Nelson, M.R.; Macdonald, J.K.; Kesimer, M.; Randell, S.H. Air-Liquid Interface Cultures to Model Drug Delivery through the Mucociliary Epithelial Barrier. Adv. Drug Deliv. Rev. 2023, 198, 114866. [Google Scholar] [CrossRef] [PubMed]
- Blom, R.A.M.; Erni, S.T.; Krempaská, K.; Schaerer, O.; Van Dijk, R.M.; Amacker, M.; Moser, C.; Hall, S.R.R.; Von Garnier, C.; Blank, F. A Triple Co-Culture Model of the Human Respiratory Tract to Study Immune-Modulatory Effects of Liposomes and Virosomes. PLoS ONE 2016, 11, e0163539. [Google Scholar] [CrossRef]
- Bluhmki, T.; Traub, S.; Müller, A.-K.; Bitzer, S.; Schruf, E.; Bammert, M.-T.; Leist, M.; Gantner, F.; Garnett, J.P.; Heilker, R. Functional Human iPSC-Derived Alveolar-like Cells Cultured in a Miniaturized 96-Transwell Air–Liquid Interface Model. Sci. Rep. 2021, 11, 17028. [Google Scholar] [CrossRef]
- Huang, J.; Hume, A.J.; Abo, K.M.; Werder, R.B.; Villacorta-Martin, C.; Alysandratos, K.-D.; Beermann, M.L.; Simone-Roach, C.; Lindstrom-Vautrin, J.; Olejnik, J.; et al. SARS-CoV-2 Infection of Pluripotent Stem Cell-Derived Human Lung Alveolar Type 2 Cells Elicits a Rapid Epithelial-Intrinsic Inflammatory Response. Cell Stem Cell 2020, 27, 962–973.e7. [Google Scholar] [CrossRef]
- Akram, K.M.; Yates, L.L.; Mongey, R.; Rothery, S.; Gaboriau, D.C.A.; Sanderson, J.; Hind, M.; Griffiths, M.; Dean, C.H. Live Imaging of Alveologenesis in Precision-Cut Lung Slices Reveals Dynamic Epithelial Cell Behaviour. Nat. Commun. 2019, 10, 1178. [Google Scholar] [CrossRef]
- Koziol-White, C.; Gebski, E.; Cao, G.; Panettieri, R.A. Precision Cut Lung Slices: An Integrated Ex Vivo Model for Studying Lung Physiology, Pharmacology, Disease Pathogenesis and Drug Discovery. Respir. Res. 2024, 25, 231, Erratum in Respir. Res. 2025, 26, 42. [Google Scholar] [CrossRef] [PubMed]
- Michalaki, C.; Dean, C.; Johansson, C. The Use of Precision-Cut Lung Slices for Studying Innate Immunity to Viral Infections. Curr. Protoc. 2022, 2, e505. [Google Scholar] [CrossRef] [PubMed]
- Viana, F.; O’Kane, C.M.; Schroeder, G.N. Precision-cut Lung Slices: A Powerful Ex Vivo Model to Investigate Respiratory Infectious Diseases. Mol. Microbiol. 2022, 117, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Krimmling, T.; Schwegmann-Weßels, C. Comparison of Mono- and Co-Infection by Swine Influenza A Viruses and Porcine Respiratory Coronavirus in Porcine Precision-Cut Lung Slices. Res. Vet. Sci. 2017, 115, 470–477. [Google Scholar] [CrossRef]
- Huh, D. (Dan) A Human Breathing Lung-on-a-Chip. Ann. Am. Thorac. Soc. 2015, 12, S42–S44. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Yang, Y.; Yan, J.; Xiong, Y.; Wang, W.; Lei, J.; Jiang, T. Recapitulating Essential Pathophysiological Characteristics in Lung-on-a-Chip for Disease Studies. Front. Immunol. 2023, 14, 1093460. [Google Scholar] [CrossRef]
- Si, L.; Bai, H.; Rodas, M.; Cao, W.; Oh, C.Y.; Jiang, A.; Moller, R.; Hoagland, D.; Oishi, K.; Horiuchi, S.; et al. A Human-Airway-on-a-Chip for the Rapid Identification of Candidate Antiviral Therapeutics and Prophylactics. Nat. Biomed. Eng. 2021, 5, 815–829. [Google Scholar] [CrossRef]
- Man, Y.; Zhai, Y.; Jiang, A.; Bai, H.; Gulati, A.; Plebani, R.; Mannix, R.J.; Merry, G.E.; Goyal, G.; Belgur, C.; et al. Exacerbation of Influenza Virus Induced Lung Injury by Alveolar Macrophages and Its Suppression by Pyroptosis Blockade in a Human Lung Alveolus Chip. bioRxiv 2024. [Google Scholar] [CrossRef]
- George, J.A.; AlShamsi, S.H.; Alhammadi, M.H.; Alsuwaidi, A.R. Exacerbation of Influenza A Virus Disease Severity by Respiratory Syncytial Virus Co-Infection in a Mouse Model. Viruses 2021, 13, 1630. [Google Scholar] [CrossRef]
- Hartwig, S.M.; Miller, A.M.; Varga, S.M. Respiratory Syncytial Virus Provides Protection against a Subsequent Influenza A Virus Infection. J. Immunol. 2022, 208, 720–731. [Google Scholar] [CrossRef]
- Cox, G.; Gonzalez, A.J.; Ijezie, E.C.; Rodriguez, A.; Miller, C.R.; Van Leuven, J.T.; Miura, T.A. Priming with Rhinovirus Protects Mice Against a Lethal Pulmonary Coronavirus Infection. Front. Immunol. 2022, 13, 886611. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.J.; Ijezie, E.C.; Balemba, O.B.; Miura, T.A. Attenuation of Influenza A Virus Disease Severity by Viral Coinfection in a Mouse Model. J. Virol. 2018, 92, e00881-18. [Google Scholar] [CrossRef]
- Van Leuven, J.T.; Gonzalez, A.J.; Ijezie, E.C.; Wixom, A.Q.; Clary, J.L.; Naranjo, M.N.; Ridenhour, B.J.; Miller, C.R.; Miura, T.A. Rhinovirus Reduces the Severity of Subsequent Respiratory Viral Infections by Interferon-Dependent and -Independent Mechanisms. mSphere 2021, 6, e00479-21. [Google Scholar] [CrossRef]
- Morris, D.R.; Qu, Y.; Thomason, K.S.; Haas De Mello, A.; Preble, R.; Menachery, V.D.; Casola, A.; Garofalo, R.P. The Impact of RSV/SARS-CoV-2 Co-Infection on Clinical Disease and Viral Replication: Insights from a BALB/c Mouse Model. bioRxiv 2023. [Google Scholar] [CrossRef]
- Sacco, R.E.; Durbin, R.K.; Durbin, J.E. Animal Models of Respiratory Syncytial Virus Infection and Disease. Curr. Opin. Virol. 2015, 13, 117–122. [Google Scholar] [CrossRef]
- Easton, A.J.; Scott, P.D.; Edworthy, N.L.; Meng, B.; Marriott, A.C.; Dimmock, N.J. A Novel Broad-Spectrum Treatment for Respiratory Virus Infections: Influenza-Based Defective Interfering Virus Provides Protection against Pneumovirus Infection in Vivo. Vaccine 2011, 29, 2777–2784. [Google Scholar] [CrossRef]
- Masciarella, A.D.; Di Gregorio, D.M.; Ramamoorthy, R.; Hussain, H.; Jayakumar, A.R.; Paidas, M.J. A Mouse Model of MHV-1 Virus Infection for Study of Acute and Long COVID Infection. Curr. Protoc. 2023, 3, e896. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.; Vijay, R.; Channappanvar, R.; Athmer, J.; Meyerholz, D.K.; Pagedar, N.; Tilley, S.; Perlman, S. Nasal Priming by a Murine Coronavirus Provides Protective Immunity against Lethal Heterologous Virus Pneumonia. JCI Insight 2018, 3, e99025. [Google Scholar] [CrossRef]
- Achdout, H.; Vitner, E.B.; Politi, B.; Melamed, S.; Yahalom-Ronen, Y.; Tamir, H.; Erez, N.; Avraham, R.; Weiss, S.; Cherry, L.; et al. Increased Lethality in Influenza and SARS-CoV-2 Coinfection Is Prevented by Influenza Immunity but Not SARS-CoV-2 Immunity. Nat. Commun. 2021, 12, 5819. [Google Scholar] [CrossRef]
- Zhang, A.J.; Lee, A.C.-Y.; Chan, J.F.-W.; Liu, F.; Li, C.; Chen, Y.; Chu, H.; Lau, S.-Y.; Wang, P.; Chan, C.C.-S.; et al. Coinfection by Severe Acute Respiratory Syndrome Coronavirus 2 and Influenza A(H1N1)Pdm09 Virus Enhances the Severity of Pneumonia in Golden Syrian Hamsters. Clin. Infect. Dis. 2021, 72, e978–e992. [Google Scholar] [CrossRef] [PubMed]
- Oishi, K.; Horiuchi, S.; Minkoff, J.M.; tenOever, B.R. The Host Response to Influenza A Virus Interferes with SARS-CoV-2 Replication during Coinfection. J. Virol. 2022, 96, e00765-22. [Google Scholar] [CrossRef]
- Di Pietro, C.; Haberman, A.M.; Lindenbach, B.D.; Smith, P.C.; Bruscia, E.M.; Allore, H.G.; Vander Wyk, B.; Tyagi, A.; Zeiss, C.J. Prior Influenza Infection Mitigates SARS-CoV-2 Disease in Syrian Hamsters. Viruses 2024, 16, 246. [Google Scholar] [CrossRef]
- Halfmann, P.J.; Nakajima, N.; Sato, Y.; Takahashi, K.; Accola, M.; Chiba, S.; Fan, S.; Neumann, G.; Rehrauer, W.; Suzuki, T.; et al. SARS-CoV-2 Interference of Influenza Virus Replication in Syrian Hamsters. J. Infect. Dis. 2022, 225, 282–286. [Google Scholar] [CrossRef]
- Carter, D.M.; Bloom, C.E.; Nascimento, E.J.M.; Marques, E.T.A.; Craigo, J.K.; Cherry, J.L.; Lipman, D.J.; Ross, T.M. Sequential Seasonal H1N1 Influenza Virus Infections Protect Ferrets against Novel 2009 H1N1 Influenza Virus. J. Virol. 2013, 87, 1400–1410. [Google Scholar] [CrossRef]
- Laurie, K.L.; Guarnaccia, T.A.; Carolan, L.A.; Yan, A.W.C.; Aban, M.; Petrie, S.; Cao, P.; Heffernan, J.M.; McVernon, J.; Mosse, J.; et al. Interval Between Infections and Viral Hierarchy Are Determinants of Viral Interference Following Influenza Virus Infection in a Ferret Model. J. Infect. Dis. 2015, 212, 1701–1710. [Google Scholar] [CrossRef] [PubMed]
- Camp, J.V.; Bagci, U.; Chu, Y.-K.; Squier, B.; Fraig, M.; Uriarte, S.M.; Guo, H.; Mollura, D.J.; Jonsson, C.B. Lower Respiratory Tract Infection of the Ferret by 2009 H1N1 Pandemic Influenza A Virus Triggers Biphasic, Systemic, and Local Recruitment of Neutrophils. J. Virol. 2015, 89, 8733–8748. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Skarlupka, A.L.; Jang, H.; Blas-Machado, U.; Holladay, N.; Hogan, R.J.; Ross, T.M. SARS-CoV-2 and Influenza A Virus Coinfections in Ferrets. J. Virol. 2022, 96, e01791-21. [Google Scholar] [CrossRef]
- Welch, M.; Krueger, K.; Zhang, J.; Piñeyro, P.; Patterson, A.; Gauger, P. Pathogenesis of an Experimental Coinfection of Porcine Parainfluenza Virus 1 and Influenza A Virus in Commercial Nursery Swine. Vet. Microbiol. 2023, 285, 109850. [Google Scholar] [CrossRef]
- Lemaitre, J.; Naninck, T.; Delache, B.; Creppy, J.; Huber, P.; Holzapfel, M.; Bouillier, C.; Contreras, V.; Martinon, F.; Kahlaoui, N.; et al. Non-Human Primate Models of Human Respiratory Infections. Mol. Immunol. 2021, 135, 147–164. [Google Scholar] [CrossRef] [PubMed]
- Munster, V.J.; Feldmann, F.; Williamson, B.; Doremalen, N.V.; Perez-Perez, L.; Schulz, J.; Meade-White, K.; Okumura, A.; Callison, J.; Brumbaugh, B.; et al. Respiratory Disease and Virus Shedding in Rhesus Macaques Inoculated with SARS-CoV-2 2020, 53027 Bytes. Nature 2020, 585, 268–272. [Google Scholar] [CrossRef]
- McMahan, K.; Yu, J.; Mercado, N.B.; Loos, C.; Tostanoski, L.H.; Chandrashekar, A.; Liu, J.; Peter, L.; Atyeo, C.; Zhu, A.; et al. Correlates of Protection against SARS-CoV-2 in Rhesus Macaques. Nature 2021, 590, 630–634. [Google Scholar] [CrossRef]
- Chandrashekar, A.; Liu, J.; Martinot, A.J.; McMahan, K.; Mercado, N.B.; Peter, L.; Tostanoski, L.H.; Yu, J.; Maliga, Z.; Nekorchuk, M.; et al. SARS-CoV-2 Infection Protects against Rechallenge in Rhesus Macaques. Science 2020, 369, 812–817. [Google Scholar] [CrossRef]
- Shannon, K.L.; Osula, V.O.; Shaw-Saliba, K.; Hardick, J.; McBryde, B.; Dugas, A.; Hsieh, Y.; Hansoti, B.; Rothman, R.E. Emergency Department National Influenza Network Investigators Viral Co-infections Are Associated with Increased Rates of Hospitalization in Those with Influenza. Influenza Other Respir. Viruses 2022, 16, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Deol, P.; Miura, T.A. Respiratory Viral Coinfections: Interactions, Mechanisms and Clinical Implications. Nat. Rev. Microbiol. 2025; online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Georgakopoulou, V.E. Insights from Respiratory Virus Co-Infections. World J. Virol. 2024, 13, 98600. [Google Scholar] [CrossRef]
- Hwang, K.S.; Seo, E.U.; Choi, N.; Kim, J.; Kim, H.N. 3D Engineered Tissue Models for Studying Human-Specific Infectious Viral Diseases. Bioact. Mater. 2023, 21, 576–594. [Google Scholar] [CrossRef]
- Altamirano-Lagos, M.J.; Díaz, F.E.; Mansilla, M.A.; Rivera-Pérez, D.; Soto, D.; McGill, J.L.; Vasquez, A.E.; Kalergis, A.M. Current Animal Models for Understanding the Pathology Caused by the Respiratory Syncytial Virus. Front. Microbiol. 2019, 10, 873. [Google Scholar] [CrossRef]
- Qiu, Y.; Hu, G. Lung-on-a-Chip: From Design Principles to Disease Applications. Biomicrofluidics 2025, 19, 021501. [Google Scholar] [CrossRef]
- Fredericks, M.N.; Kolodner, Z.; Waalkes, A.; Sawatzki, K.; Hao, L.; Long, D.R.; Penewit, K.; Midkiff, C.C.; McCormick, C.J.; Beraki, S.; et al. SIV/SARS-CoV-2 Coinfection in Rhesus Macaques Impacts Viral Shedding, Host Immunity, the Microbiome, and Viral Evolution. Front. Immunol. 2025, 16, 1587688. [Google Scholar] [CrossRef] [PubMed]
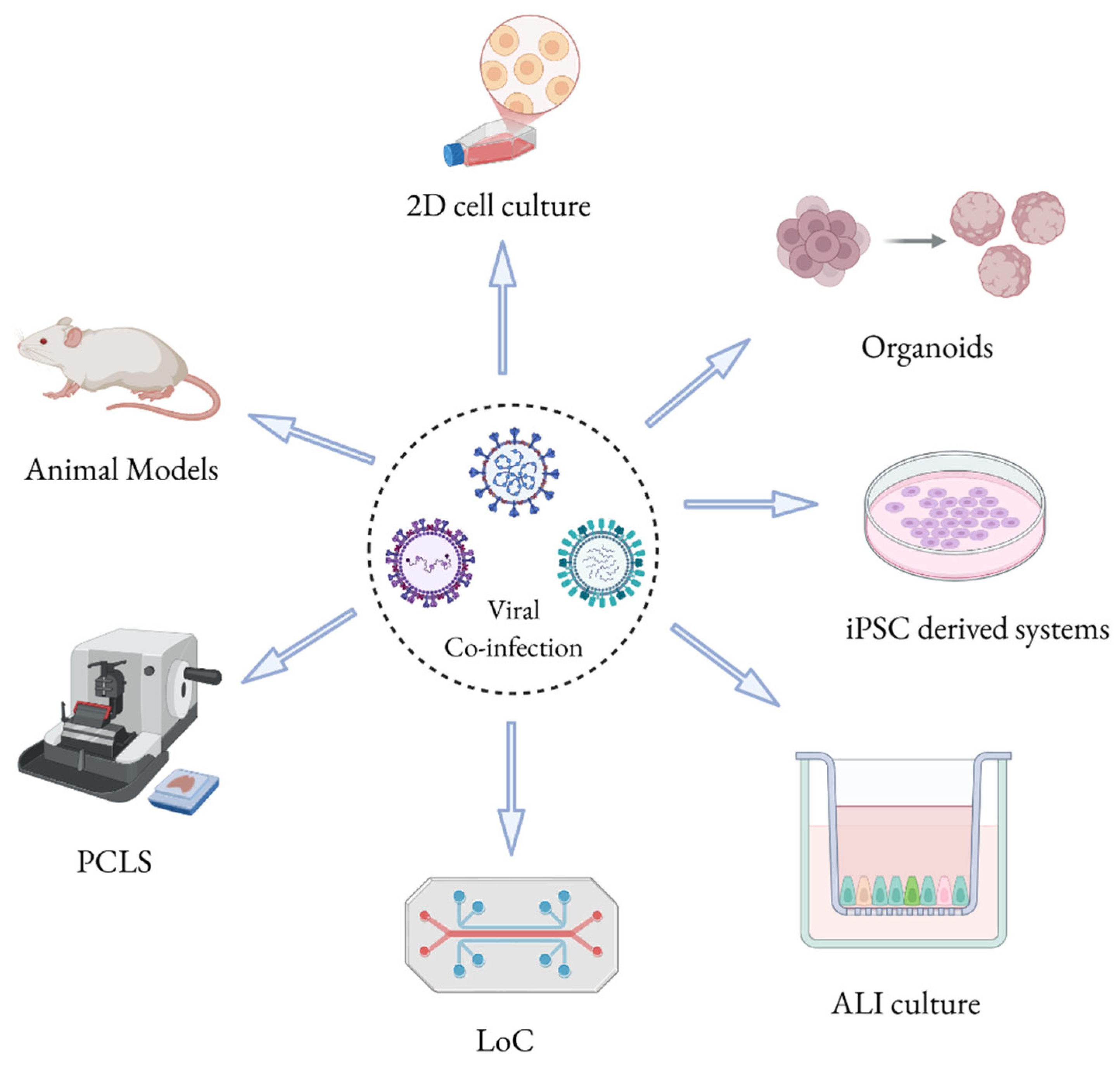
| Experimental Model | Advantages | Disadvantages |
|---|---|---|
| 2D cell culture models | Simple to handle and maintain; readily available (for immortalized cell lines); high data reproducibility (for immortalized cell lines); suitable for high-throughput testing | Do not recapitulate in vivo system; lack the immune component; no polarization |
| Organoids | Reproduce the 3D structure, resembling in vivo tissues and host-like environment; replicate the cellular components and functionality of the native lung | Difficult to handle due to sophisticated culture conditions; possibility of reversal of epithelial polarity |
| Air–liquid interface (ALI) cultures | Differentiation in pseudostratified epithelium; able to replicate the mucociliary structures and barrier functions of the human airway; exposure to air allowing gas exchange | Requires extended culture time (minimum 21 days) for epithelial differentiation; differentiated cultures lose functionality and integrity after extended culture periods |
| iPSC-derived systems | Possibility to differentiate into patient-specific lung epithelial, endothelial, and immune cell types, capturing the individual genetic backgrounds | The process of guiding iPSCs to a specific cell type is often inefficient; time-consuming |
| Lung-on-a-chip (LoC) | Tissue-like system combined with electronic sensors; coupled with microfluidic devices, recapitulate key aspects of the alveolar-capillary interface | Limited throughput due to low sample numbers and labor-intensive setup; high technical complexity |
| Precision-cut lung slices (PCLS) | Preserve complex lung architecture, retaining all native cell types and extracellular matrix | Progressive loss of tissue architecture and function occurs during culture |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yazici, O.; Vanetti, C.; Clerici, M.; Biasin, M. Experimental Models to Investigate Viral and Cellular Dynamics in Respiratory Viral Co-Infections. Microorganisms 2025, 13, 2444. https://doi.org/10.3390/microorganisms13112444
Yazici O, Vanetti C, Clerici M, Biasin M. Experimental Models to Investigate Viral and Cellular Dynamics in Respiratory Viral Co-Infections. Microorganisms. 2025; 13(11):2444. https://doi.org/10.3390/microorganisms13112444
Chicago/Turabian StyleYazici, Ozge, Claudia Vanetti, Mario Clerici, and Mara Biasin. 2025. "Experimental Models to Investigate Viral and Cellular Dynamics in Respiratory Viral Co-Infections" Microorganisms 13, no. 11: 2444. https://doi.org/10.3390/microorganisms13112444
APA StyleYazici, O., Vanetti, C., Clerici, M., & Biasin, M. (2025). Experimental Models to Investigate Viral and Cellular Dynamics in Respiratory Viral Co-Infections. Microorganisms, 13(11), 2444. https://doi.org/10.3390/microorganisms13112444









