Biodegradation of Emerging Contaminants Controlled by Biological and Chemical Factors
Abstract
1. Introduction
2. Chemical Characteristics of Common Emerging Contaminants
2.1. Pharmaceutical and Personal Care Products
2.2. Per- and Poly-Fluoroalkyl Substances
2.3. Pesticides
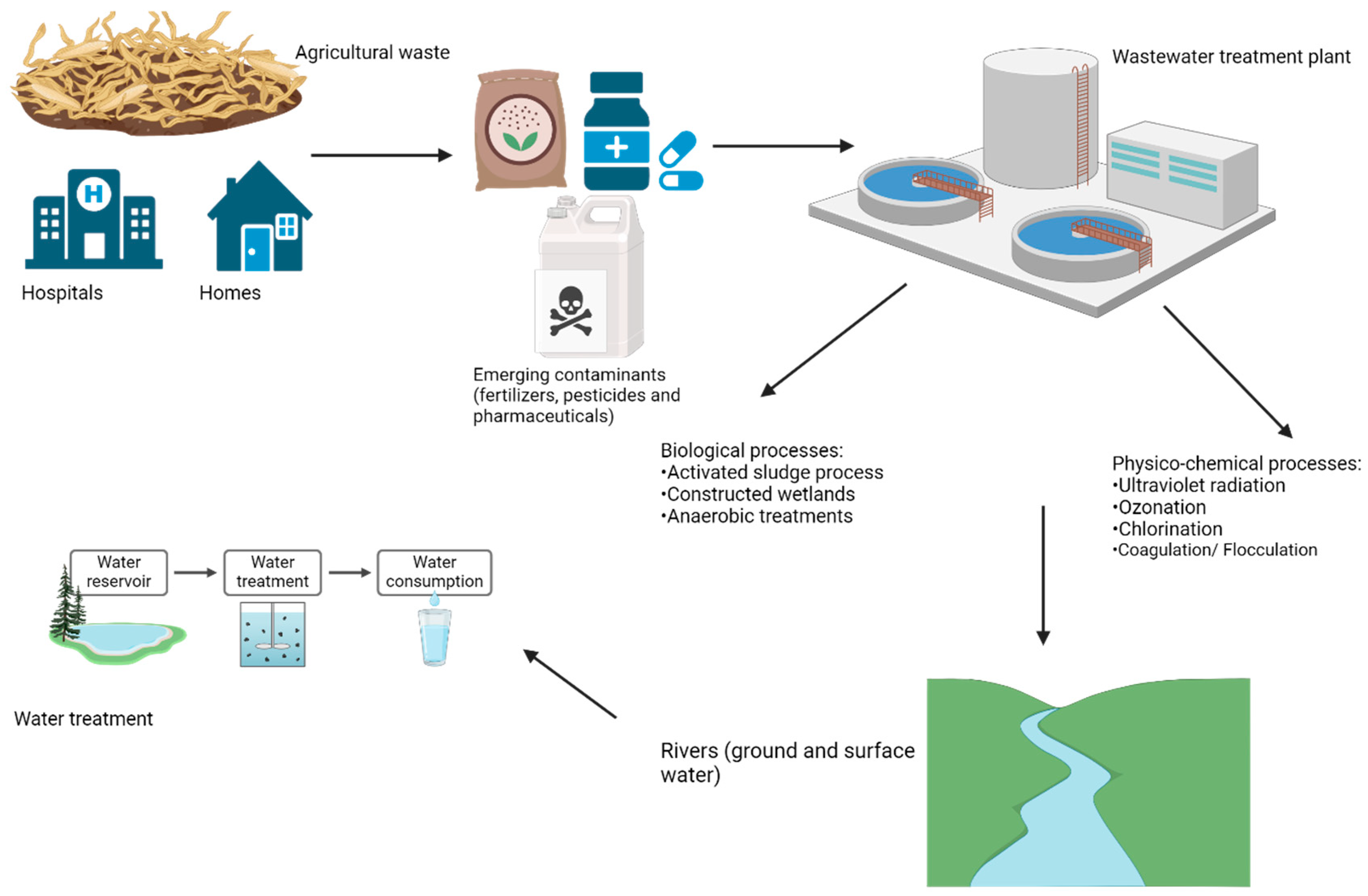
3. Sources and Fate of Emerging Contaminants
4. Microbial Biodegradation of Emerging Contaminants
4.1. Bacterial Degradation of Emerging Contaminants
| Emerging Contaminants | Genes Associated with Metabolism | Enzymes | Bacterial Species | References |
|---|---|---|---|---|
| Atrazine | trZn, atZA, atZB, atZC | Atrazine chlorohydrolase, Hydroxyl-atrazine ethylamine hydrolase, N-isopropylammelide isopropyl amino hydrolase, allophanate hydrolase | Acinetobacter spp., Bacillus sp., Microbacter spp. | [113,123] |
| Acetaminophen | amiB, ampD | Amidases Atrazine chlorohydrolase Hydroxyl-atrazine ethylamine hydrolase | Pseudomonas spp. | [124] |
| Terbuthylazine | trZn, atZA, atZB, atZC atZA, atZB, atZC | N-isopropylammelide isopropyl amino hydrolase, allophanate hydrolase | Pseudomonas putida | [117,124] |
| Sulfamethoxazole | amoA, amoB, amoC, hao, nxrAB | Ammonia monooxygenase, hydroxylamine oxidoreductase, | Nitromonas, Nitrospira, Nitrocsococcus | [98,125] |
| Erythromycin Fluoxetine Roxithromycin | NIR, NOD, pMMO, MDH, mtdB, FDH | Nitric oxide reductase, methane monooxygenase, methane dehydrogenase | (Candidatus Methylomirabilis species—Ca. M. oxyfera; Ca. M. sinica; Ca. M. lanthanidiphila) | [24] |
| Trichloroethylene | MmoBCDXYZ (cluster) | Methane monooxygenase cluster | Methylosinus trichosporium | [85] |
| Carbamazepine | bphC | Biphenyl-2,3-dioxygenases | P. xenovorans, Pseudomonas spp. | [121,122,126] |
4.2. Fungal Biodegradation of Emerging Contaminants
| Emerging Contaminants | Genes Associated with Biodegradation | Enzymes | Fungi | References |
|---|---|---|---|---|
| Fipronil | cyp51F1 | Cyp50 moassociatednoxygenase | Trametes versicolor | [18,134] |
| Bisphenol A | p2ox | Manganese peroxidase | Phanerochaete chrysosporium | [18,135] |
| Imidacloprid | cyp51F1 | Cyp50 monooxygenase | Trametes versicolor | [18,136] |
| Carbamezapine | lcca | Laccase | Trametes versicolor | [18,135] |
| Diclofenac | lipB | Lignin peroxidase | Phanerochaete chrysosporium | [18,135] |
| Acetaminophen | lcca | Laccase | Bjerkandera spp. | [18,137] |
| Atrazine | lcca | Laccase | Trametes versicolor | [18,138] |
5. Effect of Biological and Chemical Factors on EC Biodegradation
5.1. Seasonal Variation
5.2. Microbial Structure
5.3. Oxygen Requirements
5.4. Metabolisms, Co-Metabolisms, and Synergisms
6. Effects of Emerging Contaminants on the Microbiome
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gogoi, A.; Mazumder, P.; Tyagi, V.K.; Tushara Chaminda, G.G.; An, A.K.; Kumar, M. Occurrence and Fate of Emerging Contaminants in Water Environment: A Review. Groundw. Sustain. Dev. 2018, 6, 169–180. [Google Scholar] [CrossRef]
- Noguera-Oviedo, K.; Aga, D.S. Lessons Learned from More than Two Decades of Research on Emerging Contaminants in the Environment. J. Hazard. Mater. 2016, 316, 242–251. [Google Scholar] [CrossRef]
- Tong, X.; Mohapatra, S.; Zhang, J.; Tran, N.H.; You, L.; He, Y.; Gin, K.Y.H. Source, Fate, Transport and Modelling of Selected Emerging Contaminants in the Aquatic Environment: Current Status and Future Perspectives. Water Res. 2022, 217, 118418. [Google Scholar] [CrossRef]
- Araújo, C.V.M.; Sendra, M.; Moreno-garrido, I. Effect of Erythromycin and Modulating Effect of CeO2 NPs on the Toxicity Exerted by the Antibiotic on the Microalgae Chlamydomonas reinhardtii and Phaeodactylum tricornutum. Environ. Pollut. 2018, 242, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Gomes, I.B.; Maillard, J.Y.; Simões, L.C.; Simões, M. Emerging Contaminants Affect the Microbiome of Water Systems—Strategies for Their Mitigation. NPJ Clean Water 2020, 3, 39. [Google Scholar] [CrossRef]
- van Hamelsveld, S.; Jamali-Behnam, F.; Alderton, I.; Kurenbach, B.; McCabe, A.W.; Palmer, B.R.; Gutiérrez-Ginés, M.J.; Weaver, L.; Horswell, J.; Tremblay, L.A.; et al. Effects of Selected Emerging Contaminants Found in Wastewater on Antimicrobial Resistance and Horizontal Gene Transfer. Emerg. Contam. 2023, 9, 100257. [Google Scholar] [CrossRef]
- Kurenbach, B.; Hill, A.M.; Godsoe, W.; Van Hamelsveld, S.; Heinemann, J.A. Agrichemicals and Antibiotics in Combination Increase Antibiotic Resistance Evolution. PeerJ. 2018, 6, e5801. [Google Scholar] [CrossRef]
- Xing, Y.; Wu, S.; Men, Y. Exposure to Environmental Levels of Pesticides Stimulates and Diversifies Evolution in Escherichia coli toward Higher Antibiotic Resistance. Environ. Sci. Technol. 2020, 54, 8770–8778. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.X.; Sun, Y.; Xie, X.D.; Yang, B.Z.; Cao, L.Y.; Luo, S.; Wang, Y.Y.; Mai, B.X. Bioaccumulation and Human Health Risk Assessment of DDT and Its Metabolites (DDTs) in Yellowfin Tuna (Thunnus albacares) and Their Prey from the South China Sea. Mar. Pollut. Bull. 2020, 158, 111396. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Adeel, M.; Rasheed, T.; Zhao, Y.; Iqbal, H.M.N. Emerging Contaminants of High Concern and Their Enzyme-Assisted Biodegradation—A Review. Environ. Int. 2019, 124, 336–353. [Google Scholar] [CrossRef]
- Alvarino, T.; Suarez, S.; Katsou, E.; Vazquez-Padin, J.; Lema, J.M.; Omil, F. Removal of PPCPs from the Sludge Supernatant in a One Stage Nitritation/Anammox Process. Water Res. 2015, 68, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Strong, L.C.; Rosendahl, C.; Johnson, G.; Sadowsky, M.J.; Wackett, L.P. Arthrobacter aurescens TC1 Metabolizes Diverse S-Triazine Ring Compounds. Appl. Environ. Microbiol. 2002, 68, 5973–5980. [Google Scholar] [CrossRef] [PubMed]
- Stolz, A. Molecular Characteristics of Xenobiotic-Degrading Sphingomonads. Appl. Microbiol. Biotechnol. 2009, 81, 793–811. [Google Scholar] [CrossRef]
- Poddar, K.; Sarkar, D.; Chakraborty, D.; Patil, P.B.; Maity, S.; Sarkar, A. Paracetamol Biodegradation by Pseudomonas Strain PrS10 Isolated from Pharmaceutical Effluents. Int. Biodeterior. Biodegrad. 2022, 75, 105490. [Google Scholar] [CrossRef]
- Bhalerao, T.S.; Puranik, P.R. Biodegradation of Organochlorine Pesticide, Endosulfan, by a Fungal Soil Isolate, Aspergillus niger. Int. Biodeterior. Biodegrad. 2007, 59, 315–321. [Google Scholar] [CrossRef]
- Dalecka, B.; Juhna, T.; Rajarao, G.K. Constructive Use of Filamentous Fungi to Remove Pharmaceutical Substances from Wastewater. J. Water Process Eng. 2020, 33, 100992. [Google Scholar] [CrossRef]
- Morgana, S. Gonçalves Isolation of Filamentous Fungi Present in Swine Wastewater That Are Resistant and with the Ability to Remove Atrazine. Afr. J. Biotechnol. 2012, 11, 11074–11077. [Google Scholar] [CrossRef]
- Vaksmaa, A.; Guerrero-Cruz, S.; Ghosh, P.; Zeghal, E.; Hernando-Morales, V.; Niemann, H. Role of Fungi in Bioremediation of Emerging Pollutants. Front. Mar. Sci. 2023, 10, 1–21. [Google Scholar] [CrossRef]
- Angeles-de Paz, G.; Ledezma-Villanueva, A.; Robledo-Mahón, T.; Pozo, C.; Calvo, C.; Aranda, E.; Purswani, J. Assembled Mixed Co-Cultures for Emerging Pollutant Removal Using Native Microorganisms from Sewage Sludge. Chemosphere 2023, 313, 137472. [Google Scholar] [CrossRef]
- Xu, N.; Bao, M.; Sun, P.; Li, Y. Study on Bioadsorption and Biodegradation of Petroleum Hydrocarbons by a Microbial Consortium. Bioresour. Technol. 2013, 149, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; He, Y.; Li, X.; Nagarajan, D.; Chang, J.S. Enhanced Biodegradation of Chlortetracycline via a Microalgae-Bacteria Consortium. Bioresour. Technol. 2022, 343, 126149. [Google Scholar] [CrossRef]
- Kennes-Veiga, D.M.; Gónzalez-Gil, L.; Carballa, M.; Lema, J.M. Enzymatic Cometabolic Biotransformation of Organic Micropollutants in Wastewater Treatment Plants: A Review. Bioresour. Technol. 2022, 344 (Pt B), 126291. [Google Scholar] [CrossRef]
- Fischer, K.; Majewsky, M. Cometabolic Degradation of Organic Wastewater Micropollutants by Activated Sludge and Sludge-Inherent Microorganisms. Appl. Microbiol. Biotechnol. 2014, 98, 6583–6597. [Google Scholar] [CrossRef]
- Martínez-Quintela, M.; Arias, A.; Alvarino, T.; Suarez, S.; Garrido, J.M.; Omil, F. Cometabolic Removal of Organic Micropollutants by Enriched Nitrite-Dependent Anaerobic Methane Oxidizing Cultures. J. Hazard. Mater. 2021, 402, 123450. [Google Scholar] [CrossRef]
- Ohoro, C.R.; Adeniji, A.O.; Elsheikh, E.A.E.; Al-Marzouqi, A.; Otim, M.; Okoh, O.O.; Okoh, A.I. Influence of Physicochemical Parameters on PPCP Occurrences in the Wetlands. Environ. Monit. Assess. 2022, 194, 339. [Google Scholar] [CrossRef]
- Nödler, K.; Tsakiri, M.; Licha, T. The Impact of Different Proportions of a Treated Effluent on the Biotransformation of Selected Micro-Contaminants in River Water Microcosms. Int. J. Environ. Res. Public Health 2014, 11, 10390–10405. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Gil, L.; Mauricio-Iglesias, M.; Carballa, M.; Lema, J.M. Why Are Organic Micropollutants Not Fully Biotransformed? A Mechanistic Modelling Approach to Anaerobic Systems. Water Res. 2018, 142, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, N.; Porter, T.M.; Zimmerman, A.R.; Fulthorpe, R.R.; Kasozi, G.N.; Silliman, B.R.; Slater, G.F. Rapid Degradation of Deepwater Horizon Spilled Oil by Indigenous Microbial Communities in Louisiana Saltmarsh Sediments. Environ. Sci. Technol. 2013, 47, 13303–13312. [Google Scholar] [CrossRef] [PubMed]
- Rempel, A.; Gutkoski, J.P.; Nazari, M.T.; Biolchi, G.N.; Cavanhi, V.A.F.; Treichel, H.; Colla, L.M. Current Advances in Microalgae-Based Bioremediation and Other Technologies for Emerging Contaminants Treatment. Sci. Total Environ. 2021, 772, 144918. [Google Scholar] [CrossRef]
- Leahy, J.; Colwell, R. Microbial Degradation of Hydrocarbons in the Ecosystem. Microb. Action Hydrocarb. 1990, 54, 343–351. [Google Scholar] [CrossRef]
- Atlas, R.M. Microbial Degradation of Petroleum Hydrocarbons: An Environmental Perspective. Microbiol. Rev. 1981, 45, 180–209. [Google Scholar] [CrossRef]
- Liang, Y.; Van Nostrand, J.D.; Deng, Y.; He, Z.; Wu, L.; Zhang, X.; Li, G.; Zhou, J. Functional Gene Diversity of Soil Microbial Communities from Five Oil-Contaminated Fields in China. ISME J. 2011, 5, 403–413. [Google Scholar] [CrossRef]
- Cirja, M.; Ivashechkin, P.; Schäffer, A.; Corvini, P.F.X. Factors Affecting the Removal of Organic Micropollutants from Wastewater in Conventional Treatment Plants (CTP) and Membrane Bioreactors (MBR). Rev. Environ. Sci. Biotechnol. 2008, 7, 61–78. [Google Scholar] [CrossRef]
- Jafari Ozumchelouei, E.; Hamidian, A.H.; Zhang, Y.; Yang, M. Physicochemical Properties of Antibiotics: A Review with an Emphasis on Detection in the Aquatic Environment. Water Environ. Res. 2020, 92, 177–188. [Google Scholar] [CrossRef]
- Gavrilescu, M.; Demnerová, K.; Aamand, J.; Agathos, S.; Fava, F. Emerging Pollutants in the Environment: Present and Future Challenges in Biomonitoring, Ecological Risks and Bioremediation. N. Biotechnol. 2015, 30, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Manzetti, S.; Van Der Spoel, E.R.; Van Der Spoel, D. Chemical Properties, Environmental Fate, and Degradation of Seven Classes of Pollutants. Chem. Res. Toxicol. 2014, 27, 713–737. [Google Scholar] [CrossRef] [PubMed]
- Dubey, M.; Mohapatra, S.; Kumar, V.; Suthar, S.; Ahmad, A. Occurrence, Fate, and Persistence of Emerging Micropollutants in Sewage Sludge Treatment. Environ. Pollut. 2021, 273, 116515. [Google Scholar] [CrossRef]
- Delgadillo-Mirquez, L.; Lardon, L.; Steyer, J.P.; Patureau, D. A New Dynamic Model for Bioavailability and Cometabolism of Micropollutants during Anaerobic Digestion. Water Res. 2011, 45, 4511–4521. [Google Scholar] [CrossRef] [PubMed]
- El-Shahawi, M.S.; Hamza, A.; Bashammakh, A.S.; Al-Saggaf, W.T. An Overview on the Accumulation, Distribution, Transformations, Toxicity and Analytical Methods for the Monitoring of Persistent Organic Pollutants. Talanta 2010, 80, 1587–1597. [Google Scholar] [CrossRef]
- Ohoro, C.R.; Adeniji, A.O.; Okoh, A.I.; Okoh, O.O. Distribution and Chemical Analysis of Pharmaceuticals and Personal Care Products (PPCPs) in the Environmental Systems: A Review. Int. J. Environ. Res. Public Health 2019, 16, 3026. [Google Scholar] [CrossRef]
- Patel, N.; Khan, Z.A.; Shahane, S.; Rai, D.; Chauhan, D.; Kant, C.; Chaudhary, V.K. Emerging Pollutants in Aquatic Environment: Source, Effect, and Challenges in Biomonitoring and Bioremediation- A Review. Pollution 2020, 6, 99–113. [Google Scholar] [CrossRef]
- Rivera-Utrilla, J.; Sánchez-Polo, M.; Ferro-García, M.Á.; Prados-Joya, G.; Ocampo-Pérez, R. Pharmaceuticals as Emerging Contaminants and Their Removal from Water. A Review. Chemosphere 2013, 93, 1268–1287. [Google Scholar] [CrossRef]
- Ivshina, I.; Tyumina, E.; Vikhareva, E. Biodegradation of Emerging Pollutants: Focus on Pharmaceuticals. Microbiol. Aust. 2018, 39, 117–122. [Google Scholar] [CrossRef]
- Behera, S.K.; Kim, H.W.; Oh, J.E.; Park, H.S. Occurrence and Removal of Antibiotics, Hormones and Several Other Pharmaceuticals in Wastewater Treatment Plants of the Largest Industrial City of Korea. Sci. Total Environ. 2011, 409, 4351–4360. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Sridharan, S.; Sawarkar, A.D.; Shakeel, A.; Anerao, P.; Mannina, G.; Sharma, P.; Pandey, A. Current Research Trends on Emerging Contaminants Pharmaceutical and Personal Care Products (PPCPs): A Comprehensive Review. Sci. Total Environ. 2023, 859 Pt S1, 160031. [Google Scholar] [CrossRef]
- Zicarelli, G.; Multisanti, C.R.; Falco, F.; Faggio, C. Evaluation of Toxicity of Personal Care Products (PCPs) in Freshwaters: Zebrafish as a Model. Environ. Toxicol. Pharmacol. 2022, 94, 103923. [Google Scholar] [CrossRef]
- Yao, L.; Zhao, J.; Liu, Y.; Zhang, Q.; Jiang, Y.; Liu, S.; Liu, W. Science of the Total Environment Personal Care Products in Wild Fi Sh in Two Main Chinese Rivers: Bioaccumulation Potential and Human Health Risks. Sci. Total Environ. 2018, 621, 1093–1102. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, K.; Tanoue, R.; Kunisue, T.; Tue, N.M.; Fujii, S.; Sudo, N.; Isobe, T.; Nakayama, K.; Sudaryanto, A.; Subramanian, A.; et al. Pharmaceuticals and Personal Care Products (PPCPs) in Surface Water and Fish from Three Asian Countries: Species-Specific Bioaccumulation and Potential Ecological Risks. Sci. Total Environ. 2023, 866, 161258. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, T.; Kelly, B.C.; Gin, K.Y. Chemosphere Bioaccumulation Behaviour of Pharmaceuticals and Personal Care Products in a Constructed Wetland. Chemosphere 2019, 222, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.; Abdollahi, M. Environmental Distribution of Personal Care Products and Their Effects on Human Health. Iran. J. Pharm. Res. 2021, 20, 216–253. [Google Scholar] [CrossRef]
- Verlicchi, P.; Zambello, E. Pharmaceuticals and Personal Care Products in Untreated and Treated Sewage Sludge: Occurrence and Environmental Risk in the Case of Application on Soil—A Critical Review. Sci. Total Environ. 2015, 538, 750–767. [Google Scholar] [CrossRef] [PubMed]
- Cizmas, L.; Sharma, V.K.; Gray, C.M.; McDonald, T.J. Pharmaceuticals and Personal Care Products in Waters: Occurrence, Toxicity, and Risk. Environ. Chem. Lett. 2015, 13, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Batayi, B.; Okonkwo, J.O.; Daso, P.A.; Rimayi, C.C. Poly- and Perfluoroalkyl Substances (PFASs) in Sediment Samples from Roodeplaat and Hartbeespoort Dams, South Africa. Emerg. Contam. 2020, 6, 367–375. [Google Scholar] [CrossRef]
- Androulakakis, A.; Alygizakis, N.; Bizani, E.; Thomaidis, N.S. Current Progress in the Environmental Analysis of Poly- and Perfluoroalkyl Substances (PFAS). Environ. Sci. Adv. 2022, 1, 705–724. [Google Scholar] [CrossRef]
- Nakayama, S.F.; Yoshikane, M.; Onoda, Y.; Nishihama, Y.; Iwai-Shimada, M.; Takagi, M.; Kobayashi, Y.; Isobe, T. Worldwide Trends in Tracing Poly- and Perfluoroalkyl Substances (PFAS) in the Environment. TrAC-Trends Anal. Chem. 2019, 121, 115410. [Google Scholar] [CrossRef]
- Khan, S.; Naushad, M.; Govarthanan, M.; Iqbal, J.; Alfadul, S.M. Emerging Contaminants of High Concern for the Environment: Current Trends and Future Research. Environ. Res. 2022, 207, 112609. [Google Scholar] [CrossRef]
- Yadav, I.C.; Devi, N.L. Pesticides Classification and Its Impact on Human and Environment. In Environmental Science and Engineering; Studium Press LLC: Houston, TX, USA, 2017; Volume 6, pp. 140–158. [Google Scholar]
- Tadeo, J.L. Analysis of Pesticides in Food and Environmental Samples; CRC Press: Boca Raton, FL, USA, 2008; ISBN 9781420007756. [Google Scholar]
- Zacharia; Tano, J. Identity, Physical and Chemical Properties of Pesticides. In Pesticides in the Modern World—Trends in Pesticides Analysis; IntechOpen: London, UK, 2011. [Google Scholar]
- Jjemba, P.K. Excretion and Ecotoxicity of Pharmaceutical and Personal Care Products in the Environment. Ecotoxicol. Environ. Saf. 2006, 63, 113–130. [Google Scholar] [CrossRef] [PubMed]
- Campos-Mañas, M.C.; Plaza-Bolaños, P.; Martínez-Piernas, A.B.; Sánchez-Pérez, J.A.; Agüera, A. Determination of Pesticide Levels in Wastewater from an Agro-Food Industry: Target, Suspect and Transformation Product Analysis. Chemosphere 2019, 232, 152–163. [Google Scholar] [CrossRef]
- Tang, Y.; Yin, M.; Yang, W.; Li, H.; Zhong, Y.; Mo, L.; Liang, Y.; Ma, X.; Sun, X. Emerging Pollutants in Water Environment: Occurrence, Monitoring, Fate, and Risk Assessment. Water Environ. Res. 2019, 91, 984–991. [Google Scholar] [CrossRef]
- Pal, A.; Gin, K.Y.H.; Lin, A.Y.C.; Reinhard, M. Impacts of Emerging Organic Contaminants on Freshwater Resources: Review of Recent Occurrences, Sources, Fate and Effects. Sci. Total Environ. 2010, 408, 6062–6069. [Google Scholar] [CrossRef]
- Gani, K.M.; Hlongwa, N.; Abunama, T.; Kumari, S.; Bux, F. Emerging Contaminants in South African Water Environment- a Critical Review of Their Occurrence, Sources and Ecotoxicological Risks. Chemosphere 2021, 269, 128737. [Google Scholar] [CrossRef]
- Wilkinson, J.; Hooda, P.S.; Barker, J.; Barton, S.; Swinden, J. Occurrence, Fate and Transformation of Emerging Contaminants in Water: An Overarching Review of the Field. Environ. Pollut. 2017, 231 Pt S1, 954–970. [Google Scholar] [CrossRef] [PubMed]
- Rimayi, C.; Odusanya, D.; Weiss, J.M.; de Boer, J.; Chimuka, L. Contaminants of Emerging Concern in the Hartbeespoort Dam Catchment and the uMngeni River Estuary 2016 Pollution Incident, South Africa. Sci. Total Environ. 2018, 627, 1008–1017. [Google Scholar] [CrossRef]
- Bischel, H.N.; Özel Duygan, B.D.; Strande, L.; McArdell, C.S.; Udert, K.M.; Kohn, T. Pathogens and Pharmaceuticals in Source-Separated Urine in eThekwini, South Africa. Water Res. 2015, 85, 57–65. [Google Scholar] [CrossRef]
- Madhi, S.A.; Cutland, C.; Ismail, K.; O’Reilly, C.; Mancha, A.; Klugman, K.P. Ineffectiveness of Trimethoprim-Sulfamethoxazole Prophylaxis and the Importance of Bacterial and Viral Coinfections in African Children with Pneumocystis Carinii Pneumonia. Clin. Infect. Dis. 2002, 35, 1120–1126. [Google Scholar] [CrossRef]
- Wood, T.P.; Duvenage, C.S.J.; Rohwer, E. The Occurrence of Anti-Retroviral Compounds Used for HIV Treatment in South African Surface Water. Environ. Pollut. 2015, 199, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yu, X.; Yu, F.; Huang, X. Occurrence, Sources and Fate of Pharmaceuticals and Personal Care Products and Artificial Sweeteners in Groundwater. Environ. Sci. Pollut. Res. Int. 2021, 28, 20903–20920. [Google Scholar] [CrossRef]
- Bai, X.; Lutz, A.; Carroll, R.; Keteles, K.; Dahlin, K.; Murphy, M.; Nguyen, D. Occurrence, Distribution, and Seasonality of Emerging Contaminants in Urban Watersheds. Chemosphere 2018, 200, 133–142. [Google Scholar] [CrossRef]
- Semple, K.T.; Morriss, A.W.J.; Paton, G.I. Bioavailability of Hydrophobic Organic Contaminants in Soils: Fundamental Concepts and Techniques for Analysis. Eur. J. Soil Sci. 2003, 54, 809–818. [Google Scholar] [CrossRef]
- Ren, X.; Zeng, G.; Tang, L.; Wang, J.; Wan, J.; Liu, Y.; Yu, J.; Yi, H.; Ye, S.; Deng, R. Sorption, Transport and Biodegradation—An Insight into Bioavailability of Persistent Organic Pollutants in Soil. Sci. Total Environ. 2018, 610–611, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Jelić, A.; Gros, M.; Petrović, M.; Ginebreda, A.; Barceló, D. Emerging and Priority Pollutants in Rivers: Bringing Science into River Management Plans; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; Volume 19, ISBN 9783642257216. [Google Scholar]
- Wilkinson, J.L.; Hooda, P.S.; Barker, J.; Barton, S.; Swinden, J. Ecotoxic Pharmaceuticals, Personal Care Products, and Other Emerging Contaminants: A Review of Environmental, Receptor-Mediated, Developmental, and Epigenetic Toxicity with Discussion of Proposed Toxicity to Humans. Crit. Rev. Environ. Sci. Technol. 2016, 46, 336–381. [Google Scholar] [CrossRef]
- Tran, N.H.; Reinhard, M.; Gin, K.Y.H. Occurrence and Fate of Emerging Contaminants in Municipal Wastewater Treatment Plants from Different Geographical Regions-a Review. Water Res. 2018, 133, 182–207. [Google Scholar] [CrossRef]
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U.; Mohan, D. Pharmaceuticals of Emerging Concern in Aquatic Systems: Chemistry, Occurrence, Effects, and Removal Methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, J.; Zhou, S.; Lian, M. Microbial Degradation of Carbamazepine by a Newly Isolated of Gordonia polyophrenivorans. Environ. Technol. Innov. 2023, 32, 103322. [Google Scholar] [CrossRef]
- Richard, C.; Canonica, S. Aquatic Phototransformation of Organic Contaminants Induced by Coloured Dissolved Natural Organic Matter. In Environmental Photochemistry Part II.; Springer: Berlin/Heidelberg, Germany, 2005; Volume 2, pp. 299–323. [Google Scholar]
- Zhou, W.; Li, M.; Achal, V. A comprehensive review on environmental and human health impacts of chemical pesticide usage. Emerg Contam. 2025, 11, 100410. [Google Scholar] [CrossRef]
- Remucal, C.K. The Role of Indirect Photochemical Degradation in the Environmental Fate of Pesticides: A Review. Environ. Sci. Process. Impacts 2014, 16, 628. [Google Scholar] [CrossRef] [PubMed]
- Da̧browska, D.; Kot-Wasik, A.; Namieśnik, J. The Importance of Degradation in the Fate of Selected Organic Compounds in the Environment. Part II. Pho-todegradation and Biodegradation. Polish J. Environ. Stud. 2004, 13, 607–616. [Google Scholar]
- Latch, D.E.; Stender, B.L.; Packer, J.L.; Arnold, W.A.; McNeill, K. Photochemical Fate of Pharmaceuticals in the Environment: Cimetidine and Ranitidine. Environ. Sci. Technol. 2003, 37, 3342–3350. [Google Scholar] [CrossRef]
- Ruggeri, G.; Ghigo, G.; Maurino, V.; Minero, C.; Vione, D. Photochemical Transformation of Ibuprofen into Harmful 4-Isobutylacetophenone: Pathways, Kinetics, and Significance for Surface Waters. Water Res. 2013, 47, 6109–6121. [Google Scholar] [CrossRef]
- Nzila, A. Update on the Cometabolism of Organic Pollutants by Bacteria. Environ. Pollut. 2013, 178, 474–482. [Google Scholar] [CrossRef]
- Ahmed, I.; Iqbal, H.M.N.; Dhama, K. Enzyme-Based Biodegradation of Hazardous Pollutants—An Overview. J. Exp. Biol. Agric. Sci. 2017, 5, 402–411. [Google Scholar] [CrossRef]
- Murdoch, R.W.; Hay, A.G. Formation of Catechols via Removal of Acid Side Chains from Ibuprofen and Related Aromatic Acids. Appl. Environ. Microbiol. 2005, 71, 6121–6125. [Google Scholar] [CrossRef] [PubMed]
- Aulestia, M.; Flores, A.; Acosta-Jurado, S.; Santero, E.; Camacho, E.M. Genetic Characterization of the Ibuprofen-Degradative Pathway of Rhizorhabdus wittichii MPO218. Appl. Environ. Microbiol. 2022, 88, e0038822. [Google Scholar] [CrossRef]
- De Gusseme, B.; Vanhaecke, L.; Verstraete, W.; Boon, N. Degradation of Acetaminophen by Delftia tsuruhatensis and Pseudomonas aeruginosa in a Membrane Bioreactor. Water Res. 2011, 45, 1829–1837. [Google Scholar] [CrossRef]
- Mohamed, M.; Ismail, W.; Heider, J.; Fuchs, G. Aerobic Metabolism of Phenylacetic Acids in Azoarcus evansii. Arch. Microbiol. 2002, 178, 180–192. [Google Scholar] [CrossRef] [PubMed]
- da Cruz, G.F.; de Vasconcellos, S.P.; Angolini, C.F.F.; Dellagnezze, B.M.; Garcia, I.N.S.; de Oliveira, V.M.; dos Santos Neto, E.V.; Marsaioli, A.J. Could Petroleum Biodegradation Be a Joint Achievement of Aerobic and Anaerobic Microrganisms in Deep Sea Reservoirs? AMB Express 2011, 1, 1–10. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Guan, F.; Yang, Z.; Zhai, X.; Zhang, Y.; Tang, X.; Duan, J.; Xiao, H. The Isolation of Anaerobic and Facultative Anaerobic Sulfate-Reducing Bacteria (SRB) and a Comparison of Related Enzymes in Their Sulfate Reduction Pathways. Microorganisms 2023, 11, 2019. [Google Scholar] [CrossRef]
- Yang, X.; Li, E.; Liu, F.; Xu, M. Interactions of PAH-Degradation and Nitrate-/Sulfate-Reducing Assemblages in Anaerobic Sediment Microbial Community. J. Hazard. Mater. 2020, 388, 122068. [Google Scholar] [CrossRef]
- Gilmour, C.C.; Elias, D.A.; Kucken, A.M.; Brown, S.D.; Palumbo, A.V.; Schadt, C.W.; Wall, J.D. Sulfate-Reducing Bacterium Desulfovibrio desulfuricans ND132 as a Model for Understanding Bacterial Mercury Methylation. Appl. Environ. Microbiol. 2011, 77, 3938–3951. [Google Scholar] [CrossRef]
- Burland, S.M.; Edwards, E.A. Anaerobic Benzene Biodegradation Linked to Nitrate Reduction. Appl. Environ. Microbiol. 1999, 65, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.D.; Kraus, P.F.; Lawson, P.A.; Drake, G.R.; Balkwill, D.L.; Tanner, R.S. Desulfovibrio carbinoliphilus sp. Nov., a Benzyl Alcohol-Oxidizing, Sulfate-Reducing Bacterium Isolated from a Gas Condensate-Contaminated Aquifer. Int. J. Syst. Evol. Microbiol. 2008, 58, 1313–1317. [Google Scholar] [CrossRef]
- Keen, P.L.; Patrick, D.M. Tracking Change: A Look at the Ecological Footprint of Antibiotics and Antimicrobial Resistance. Antibiotics 2013, 2, 191–205. [Google Scholar] [CrossRef]
- Tran, N.H.; Urase, T.; Ngo, H.H.; Hu, J.; Ong, S.L. Insight into Metabolic and Cometabolic Activities of Autotrophic and Heterotrophic Microorganisms in the Biodegradation of Emerging Trace Organic Contaminants. Bioresour. Technol. 2013, 146, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Dai, Y.; Huan, Y.; Liu, Z.; Sun, L.; Zhou, Q.; Zhang, W.; Sang, Q.; Wei, H.; Yuan, S. Different Utilizable Substrates Have Different Effects on Cometabolic Fate of Imidacloprid in Stenotrophomonas Maltophilia. Appl. Microbiol. Biotechnol. 2013, 742, 140530. [Google Scholar] [CrossRef]
- Granatto, C.F.; Grosseli, G.M.; Sakamoto, I.K.; Fadini, P.S.; Varesche, M.B.A. Methanogenic Potential of Diclofenac and Ibuprofen in Sanitary Sewage Using Metabolic Cosubstrates. Sci. Total Environ. 2020, 235, 140530. [Google Scholar] [CrossRef]
- Liu, J.; Amemiya, T.; Chang, Q. Toluene Dioxygenase Expression Correlates with Trichloroethylene Degradation Capacity in Pseudomonas putida F1 Cultures. Biodegradation 2012, 23, 683–691. [Google Scholar] [CrossRef]
- Morono, Y.; Unno, H.; Tanji, Y.; Hori, K. Addition of Aromatic Substrates Restores Trichloroethylene Degradation Activity in Pseudomonas putida F1. Appl. Environ. Microbiol. 2004, 70, 2830–2835. [Google Scholar] [CrossRef]
- Bordel, S.; Muñoz, R.; Díaz, L.F.; Villaverde, S. New Insights on Toluene Biodegradation by Pseudomonas putida F1: Influence of Pollutant Concentration and Excreted Metabolites. Appl. Microbiol. Biotechnol. 2007, 74, 857–866. [Google Scholar] [CrossRef]
- Leahy, J.G.; Byrne, A.M.; Olsen, R.H. Comparison of Factors Influencing Trichloroethylene Degradation by Toluene-Oxidizing Bacteria. Appl. Environ. Microbiol. 1996, 62, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Hori, K.; Morono, Y.; Tanji, Y.; Unno, H. Kinetic Analyses of Trichloroethylene Cometabolism by Toluene-Degrading Bacteria Harboring a Tod Homologous Gene. Biochem. Eng. J. 2005, 26, 59–64. [Google Scholar] [CrossRef]
- Omotayo, A.E.; Ilori, M.O.; Obayori, O.S.; Oladipo, O. Influence of pH, Temperature and Nutrient Addition on The Degradation of Atrazine by Nocardioides spp. Isolated from Agricultural Soil in Nigeria. MJM 2016, 12, 270–278. [Google Scholar] [CrossRef]
- Boopathy, R. Anaerobic Degradation of Atrazine. Int. Biodeterior. Biodegrad. 2017, 119, 626–630. [Google Scholar] [CrossRef]
- Nsenga Kumwimba, M.; Meng, F. Roles of Ammonia-Oxidizing Bacteria in Improving Metabolism and Cometabolism of Trace Organic Chemicals in Biological Wastewater Treatment Processes: A Review. Sci. Total Environ. 2019, 659, 419–441. [Google Scholar] [CrossRef]
- Kunkel, U.; Radke, M. Biodegradation of Acidic Pharmaceuticals in Bed Sediments: Insight from a Laboratory Experiment. Environ. Sci. Technol. 2008, 42, 7273–7279. [Google Scholar] [CrossRef] [PubMed]
- Kolvenbach, B.A.; Helbling, D.E.; Kohler, H.P.E.; Corvini, P.F.X. Emerging Chemicals and the Evolution of Biodegradation Capacities and Pathways in Bacteria. Curr. Opin. Biotechnol. 2014, 27, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Fani, R.; Fondi, M. Origin and Evolution of Metabolic Pathways. Phys. Life Rev. 2009, 6, 23–52. [Google Scholar] [CrossRef] [PubMed]
- Gedalanga, P.B.; Pornwongthong, P.; Mora, R.; Chiang, S.Y.D.; Baldwin, B.; Ogles, D.; Mahendraa, S. Identification of Biomarker Genes to Predict Biodegradation of 1,4-Dioxane. Appl. Environ. Microbiol. 2014, 80, 3209–3218. [Google Scholar] [CrossRef] [PubMed]
- Sene, L.; Converti, A.; Secchi, G.A.R.; Simão, R. de C.G. New Aspects on Atrazine Biodegradation. Braz. Arch. Biol. Technol. 2010, 53, 487–496. [Google Scholar] [CrossRef]
- Jan-Roblero, J.; Cruz-Maya, J.A. Ibuprofen: Toxicology and Biodegradation of an Emerging Contaminant. Molecules 2023, 28, 1–15. [Google Scholar] [CrossRef]
- Reis, A.C.; Kolvenbach, B.A.; Nunes, O.C.; Corvini, P.F.X. Biodegradation of Antibiotics: The New Resistance Determinants—Part I. N. Biotechnol. 2020, 54, 34–51. [Google Scholar] [CrossRef]
- Arp, D.J.; Yeager, C.M.; Hyman, M.R. Molecular and Cellular Fundamentals of Aerobic Cometabolism of Trichloroethylene. Biodegradation 2001, 12, 81–103. [Google Scholar] [CrossRef]
- Shapir, N.; Mongodin, E.F.; Sadowsky, M.J.; Daugherty, S.C.; Nelson, K.E.; Wackett, L.P. Evolution of Catabolic Pathways: Genomic Insights into Microbial s-Triazine Metabolism. J. Bacteriol. 2007, 189, 674682. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, W.; Li, M.; Liu, S.; Peng, D.; Zhao, F.; Wu, X.; Tan, H. Biodegradation Characteristics and Mechanism of Terbuthylazine by the Newly Isolated Agrobacterium rhizogenes Strain AT13. J. Hazard. Mater. 2023, 456, 131664. [Google Scholar] [CrossRef] [PubMed]
- Ming, H.; Zhang, H.; Chen, Q.; Wang, Y.; Su, J.; Zhao, X.; Fan, J. Abundance and Community Structure of Ammonium Monooxygenase (AmoA) Genes in the Wet Season of Liaohe Estuary Sediments. Cont. Shelf Res. 2020, 209, 104253. [Google Scholar] [CrossRef]
- Wang, Q.; Xie, S.; Hu, R. Bioaugmentation with Arthrobacter sp. Strain DAT1 for Remediation of Heavily Atrazine-Contaminated Soil. Int. Biodeterior. Biodegrad. 2013, 77, 63–67. [Google Scholar] [CrossRef]
- Aryal, N.; Wood, J.; Rijal, I.; Deng, D.; Jha, M.K.; Ofori-Boadu, A.; Khan, H.K.; Rehman, M.Y.A.; Malik, R.N.; Maculewicz, J.; et al. Transformation Products of Pharmaceuticals in the Environment: Their Fate, (Eco)Toxicity and Bioaccumulation Potential. J. Environ. Manage. 2020, 271, 1587–1594. [Google Scholar] [CrossRef]
- Nešvera, J.; Rucká, L.; Pátek, M. Catabolism of Phenol and Its Derivatives in Bacteria: Genes, Their Regulation, and Use in the Biodegradation of Toxic Pollutants. Adv. Appl. Microbiol. 2015, 93, 107–160. [Google Scholar] [CrossRef]
- Sharma, A.; Kalyani, P.; Trivedi, V.D.; Kapley, A.; Phale, P.S. Nitrogen-Dependent Induction of Atrazine Degradation Pathway in Pseudomonas sp. Strain AKN5. FEMS Microbiol. Lett. 2019, 366, 1–9. [Google Scholar] [CrossRef]
- Fajardo, C.; Saccà, M.L.; Gibello, A.; Martinez-Iñigo, M.J.; Nande, M.; Lobo, C.; Martin, M. Assessment of S-Triazine Catabolic Potential in Soil Bacterial Isolates Applying Atz Genes as Functional Biomarkers. Water. Air. Soil Pollut. 2012, 223, 3385–3392. [Google Scholar] [CrossRef]
- Kassotaki, E.; Buttiglieri, G.; Ferrando-Climent, L.; Rodriguez-Roda, I.; Pijuan, M. Enhanced Sulfamethoxazole Degradation through Ammonia Oxidizing Bacteria Co-Metabolism and Fate of Transformation Products. Water Res. 2016, 94, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Rucká, L.; Nešvera, J.; Pátek, M. Biodegradation of Phenol and Its Derivatives by Engineered Bacteria: Current Knowledge and Perspectives. World J. Microbiol. Biotechnol. 2017, 33, 174. [Google Scholar] [CrossRef]
- Elyamine, A.M.; Kan, J.; Meng, S.; Tao, P.; Wang, H.; Hu, Z. Aerobic and Anaerobic Bacterial and Fungal Degradation of Pyrene: Mechanism Pathway Including Biochemical Reaction and Catabolic Genes. Int. J. Mol. Sci. 2021, 22, 8202. [Google Scholar] [CrossRef]
- Ganesh Kumar, A.; Manisha, D.; Sujitha, K.; Magesh Peter, D.; Kirubagaran, R.; Dharani, G. Genome Sequence Analysis of Deep Sea Aspergillus sydowii BOBA1 and Effect of High Pressure on Biodegradation of Spent Engine Oil. Sci. Rep. 2021, 11, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Novianty, R.; Saryono; Awaluddin, A.; Pratiwi, N.W.; Hidayah, A.; Juliantari, E. The Diversity of Fungi Consortium Isolated from Polluted Soil for Degrading Petroleum Hydrocarbon. Biodiversitas 2021, 22, 5077–5084. [Google Scholar] [CrossRef]
- Kameshwar, A.K.S.; Qin, W. Metadata Analysis of Phanerochaete Chrysosporium Gene Expression Data Identified Common CAZymes Encoding Gene Expression Profiles Involved in Cellulose and Hemicellulose Degradation. Int. J. Biol. Sci. 2017, 13, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Syed, K.; Yadav, J.S. P450 Monooxygenases (P450ome) of the Model White Rot Fungus Phanerochaete chrysosporium. Crit. Rev. Microbiol. 2012, 38, 339–363. [Google Scholar] [CrossRef]
- Doddapaneni, H.; Chakraborty, R.; Yadav, J.S. Genome-Wide Structural and Evolutionary Analysis of the P450 Monooxygenase Genes (P450ome) in the White Rot Fungus Phanerochaete chrysosporium: Evidence for Gene Duplications and Extensive Gene Clustering. BMC Genom. 2005, 6, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Ohno, H.; Ichinose, H.; Kawagishi, H.; Hirai, H. White-Rot Fungus Phanerochaete chrysosporium Metabolizes Chloropyridinyl-Type Neonicotinoid Insecticides by an N-Dealkylation Reaction Catalyzed by Two Cytochrome P450s. J. Hazard. Mater. 2021, 402, 123831. [Google Scholar] [CrossRef]
- Wolfand, J.M.; Lefevre, G.H.; Luthy, R.G. Metabolization and Degradation Kinetics of the Urban-Use Pesticide Fipronil by White Rot Fungus: Trametes Versicolor. Environ. Sci. Process. Impacts 2016, 18, 1256–1265. [Google Scholar] [CrossRef]
- Zhuo, R.; Fan, F. A Comprehensive Insight into the Application of White Rot Fungi and Their Lignocellulolytic Enzymes in the Removal of Organic Pollutants. Sci. Total Environ. 2021, 778, 146132. [Google Scholar] [CrossRef]
- Hu, K.; Barbieri, M.V.; López-García, E.; Postigo, C.; López de Alda, M.; Caminal, G.; Sarrà, M. Fungal Degradation of Selected Medium to Highly Polar Pesticides by Trametes Versicolor: Kinetics, Biodegradation Pathways, and Ecotoxicity of Treated Waters. Anal. Bioanal. Chem. 2022, 414, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.R.; Kim, S.Y.; Kang, M.; Lee, T.K. Removal of Pharmaceuticals and Personal Care Products Using Native Fungal Enzymes Extracted during the Ligninolytic Process. Environ. Res. 2021, 195, 110878. [Google Scholar] [CrossRef] [PubMed]
- Bastos, A.C.; Magan, N. Trametes Versicolor: Potential for Atrazine Bioremediation in Calcareous Clay Soil, under Low Water Availability Conditions. Int. Biodeterior. Biodegrad. 2009, 63, 389–394. [Google Scholar] [CrossRef]
- Liu, Y.; He, M.; Wang, B.; Wu, L. Dry—Wet Seasonal Variations of Microbially Mediated Carbon Metabolism in Soils of a Floodplain Lake. Ecohydrology 2023, 16, e2510. [Google Scholar] [CrossRef]
- Manzoni, S.; Schimel, J.P.; Porporato, A. Responses of Soil Microbial Communities to Water Stress: Results from a Meta-Analysis. Ecology 2012, 93, 930–938. [Google Scholar] [CrossRef]
- Gliksman, D.; Haenel, S.; Grünzweig, J.M. Biotic and Abiotic Modifications of Leaf Litter during Dry Periods Affect Litter Mass Loss and Nitrogen Loss during Wet Periods. Funct. Ecology. 2018, 32, 831–839. [Google Scholar] [CrossRef]
- Letnik, I.; Avrahami, R.; Rokem, J.S.; Greiner, A.; Zussman, E.; Greenblatt, C. Living Composites of Electrospun Yeast Cells for Bioremediation and Ethanol Production. Biomacromolecules. 2015, 16, 3322–3328. [Google Scholar] [CrossRef]
- Sekhar, A.; Horemans, B.; Aamand, J.; Sørensen, S.R.; Vanhaecke, L.; Bussche, J.V.; Hofkens, J.; Springael, D. Surface Colonization and Activity of the 2,6-Dichlorobenzamide (BAM) Degrading Aminobacter sp. Strain MSH1 at Macro- and Micropollutant BAM Concentrations. Environ Sci Technol. 2016, 50, 10123–10133. [Google Scholar] [CrossRef]
- Gomes, I.B.; Simões, L.C.; Simões, M. The Effects of Emerging Environmental Contaminants on Stenotrophomonas maltophilia Isolated from Drinking Water in Planktonic and Sessile States. Sci. Total Environ. 2018, 643, 1348–1356. [Google Scholar] [CrossRef]
- Zhao, Y.; Qu, D.; Zhou, R.; Yang, S.; Ren, H. Efficacy of Forming Biofilms by Pseudomonas migulae AN-1 toward in Situ Bioremediation of Aniline-Contaminated Aquifer by Groundwater Circulation Wells. Environ. Sci. Pollut. Res. 2016, 23, 11568–11573. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Huang, Y.; Li, J.; Wu, X.; Zhou, Z.; Lei, Q. Chemosphere Biofilm-Mediated Bioremediation Is a Powerful Tool for the Removal of Environmental Pollutants. Chemosphere 2022, 294, 133609. [Google Scholar] [CrossRef] [PubMed]
- Wilson, L.P.; Bouwer, E.J. Biodegradation of Aromatic Compounds under Mixed Oxygen/Denitrifying Conditions: A Review. J. Ind. Microbiol. Biotechnol. 1997, 18, 116–130. [Google Scholar] [CrossRef]
- Ghattas, A.K.; Fischer, F.; Wick, A.; Ternes, T.A. Anaerobic Biodegradation of (Emerging) Organic Contaminants in the Aquatic Environment. Water Res. 2017, 116, 268–295. [Google Scholar] [CrossRef]
- Conkle, J.L.; Gan, J.; Anderson, M.A. Degradation and Sorption of Commonly Detected PPCPs in Wetland Sediments under Aerobic and Anaerobic Conditions. J. Soils Sediments 2012, 12, 1164–1173. [Google Scholar] [CrossRef]
- Motteran, F.; Varesche, M.B.A.; Lara-Martin, P.A. Assessment of the Aerobic and Anaerobic Biodegradation of Contaminants of Emerging Concern in Sludge Using Batch Reactors. Environ. Sci. Pollut. Res. 2022, 29, 84946–84961. [Google Scholar] [CrossRef]
- Gangadharan Puthiya Veetil, P.; Vijaya Nadaraja, A.; Bhasi, A.; Khan, S.; Bhaskaran, K. Degradation of Triclosan under Aerobic, Anoxic, and Anaerobic Conditions. Appl. Biochem. Biotechnol. 2012, 167, 1603–1612. [Google Scholar] [CrossRef]
- Bergmann, F.D.; Selesi, D.; Meckenstock, R.U. Identification of New Enzymes Potentially Involved in Anaerobic Naphthalene Degradation by the Sulfate-Reducing Enrichment Culture N47. Arch. Microbiol. 2011, 193, 241–250. [Google Scholar] [CrossRef]
- Mohatt, J.L.; Hu, L.; Finneran, K.T.; Strathmann, T.J. Microbially Mediated Abiotic Transformation of the Antimicrobial Agent Sulfamethoxazole under Iron-Reducing Soil Conditions. Environ. Sci. Technol. 2011, 45, 4793–4801. [Google Scholar] [CrossRef]
- König, A.; Weidauer, C.; Seiwert, B.; Reemtsma, T.; Unger, T.; Jekel, M. Reductive Transformation of Carbamazepine by Abiotic and Biotic Processes. Water Res. 2016, 101, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Morrica, P.; Giordano, A.; Seccia, S.; Ungaro, F.; Ventriglia, M. Degradation of Imazosulfuron in Soil. Pest Manag. Sci. 2001, 57, 360–365. [Google Scholar] [CrossRef]
- Chiu, T.Y.; Paterakis, N.; Cartmell, E.; Scrimshaw, M.D.; Lester, J.N. A Critical Review of the Formation of Mono-and Dicarboxylated Metabolic Intermediates of Alkylphenol Polyethoxylates during Wastewater Treatment and Their Environmental Significance. Crit. Rev. Environ. Sci. Technol. 2010, 40, 199–238. [Google Scholar] [CrossRef]
- Caldwell, M.E.; Suflita, J.M. Detection of Phenol and Benzoate as Intermediates of Anaerobic Benzene Biodegradation under Different Terminal Electron-Accepting Conditions. Environ. Sci. Technol. 2000, 34, 1216–1220. [Google Scholar] [CrossRef]
- Zhang, M.C.; Bennett, G.N. Biodegradation of Xenobiotics by Anaerobic Bacteria. Appl Microbiol Biotechnol. 2005, 600–618. [Google Scholar] [CrossRef]
- Foght, J. Anaerobic Biodegradation of Aromatic Hydrocarbons: Pathways and Prospects. J. Mol. Microbiol. Biotechnol. 2008, 15, 93–120. [Google Scholar] [CrossRef] [PubMed]
- Bremer, J.R.A. Aerobic and Anaerobic Biodegradation. J. Ecosyst. Ecography 2022, 12, 1000325. [Google Scholar] [CrossRef]
- Zamarro, M.T.; Martín-Moldes, Z.; Díaz, E. The ICEXTD of Azoarcus sp. CIB, an Integrative and Conjugative Element with Aerobic and Anaerobic Catabolic Properties. Environ. Microbiol. 2016, 18, 5018–5031. [Google Scholar] [CrossRef]
- Li, Y.; Li, B.; Wang, C.P.; Fan, J.Z.; Sun, H.W. Aerobic Degradation of Trichloroethylene by Co-Metabolism Using Phenol and Gasoline as Growth Substrates. Int. J. Mol. Sci. 2014, 15, 9134–9148. [Google Scholar] [CrossRef]
- Behki, R.; Topp, E.; Dick, W.; Germon, P. Metabolism of the Herbicide Atrazine by Rhodococcus Strains. Appl. Environ. Microbiol. 1993, 59, 1955–1959. [Google Scholar] [CrossRef]
- Rios-Miguel, A.B.; Smith, G.J.; Cremers, G.; van Alen, T.; Jetten, M.S.M.; Op den Camp, H.J.M.; Welte, C.U. Microbial Paracetamol Degradation Involves a High Diversity of Novel Amidase Enzyme Candidates. Water Res. X 2022, 16, 100152. [Google Scholar] [CrossRef]
- Emily, V.; Rui, W.R.; Hara, Y.; Adnan, A.M.; Hock, O.G.; Kee, W.K. Fungal and Bacterial Species in Degrading Carbamazepine: A Metabolite Perspective: Mini-Review. J. Exp. Biol. Agric. Sci. 2022, 10(5), 922–931. [Google Scholar] [CrossRef]
- Chen, S.F.; Chen, W.J.; Huang, Y.; Wei, M.; Chang, C. Insights into the Metabolic Pathways and Biodegradation Mechanisms of Chloroacetamide Herbicides. Environ. Res. 2023, 229, 115918. [Google Scholar] [CrossRef]
- Van Hylckama Vlieg, J.E.T.; Janssen, D.B. Formation and Detoxification of Reactive Intermediates in the Metabolism of Chlorinated Ethenes. J. Biotechnol. 2001, 85, 81–102. [Google Scholar] [CrossRef] [PubMed]
- Nyakundi, W.O.; Magoma, G.; Ochora, J.; Nyende, A.B. Biodegradation of Diazinon and Methomyl Pesticides By White Rot Fungi From Selected Horticultural Farms in Rift Valley and Central Kenya. Access 2011, 1, 107–124. [Google Scholar]
- Ellegaard-Jensen, L.; Knudsen, B.E.; Johansen, A.; Albers, C.N.; Aamand, J.; Rosendahl, S. Fungal-Bacterial Consortia Increase Diuron Degradation in Water-Unsaturated Systems. Sci. Total Environ. 2014, 466–467, 699–705. [Google Scholar] [CrossRef]
- Knudsen, B.E.; Ellegaard-Jensen, L.; Albers, C.N.; Rosendahl, S.; Aamand, J. Fungal Hyphae Stimulate Bacterial Degradation of 2,6-Dichlorobenzamide (BAM). Environ. Pollut. 2013, 181, 122–127. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Zhao, Z.; Chen, J.; Lu, H.; Liu, G.; Zhou, J.; Guan, X. PAHs Accelerate the Propagation of Antibiotic Resistance Genes in Coastal Water Microbial Community. Environ. Pollut. 2017, 231, 1145–1152. [Google Scholar] [CrossRef]
- Kim, S.W.; Park, S.B.; Im, S.P.; Lee, J.S.; Jung, J.W.; Gong, T.W.; Lazarte, J.M.S.; Kim, J.; Seo, J.S.; Kim, J.H.; et al. Outer Membrane Vesicles from β-Lactam-Resistant Escherichia coli Enable the Survival of β-Lactam-Susceptible E. coli in the Presence of β-Lactam Antibiotics. Sci. Rep. 2018, 8, 5402. [Google Scholar] [CrossRef] [PubMed]
- Proia, L.; Lupini, G.; Osorio, V.; Pérez, S.; Barceló, D.; Schwartz, T.; Amalfitano, S.; Fazi, S.; Romaní, A.M.; Sabater, S. Response of Biofilm Bacterial Communities to Antibiotic Pollutants in a Mediterranean River. Chemosphere 2013, 92, 1126–1135. [Google Scholar] [CrossRef]
- Isidori, M.; Lavorgna, M.; Nardelli, A.; Pascarella, L.; Parrella, A. Toxic and Genotoxic Evaluation of Six Antibiotics on Non-Target Organisms. Sci. Total Environ. 2005, 346, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hu, C.; Shen, Y.; Shi, B.; Zhao, D.; Xing, X. Response of Microorganisms in Biofilm to Sulfadiazine and Ciprofloxacin in Drinking Water Distribution Systems. Chemosphere 2019, 218, 197–204. [Google Scholar] [CrossRef]
- Song, T.; Zhang, X.; Li, J.; Xie, W.; Dong, W.; Wang, H. Sulfamethoxazole Impact on Pollutant Removal and Microbial Community of Aerobic Granular Sludge with Filamentous Bacteria. Bioresour. Technol. 2023, 379, 128823. [Google Scholar] [CrossRef]
- Chen, W.; Liu, W.; Pan, N.; Jiao, W.; Wang, M. Oxytetracycline on Functions and Structure of Soil Microbial Community. J. Soil Sci. Plant Nutr. 2013, 13, 967–975. [Google Scholar] [CrossRef]
- Gao, M.; Song, W.; Zhou, Q.; Ma, X.; Chen, X. Interactive Effect of Oxytetracycline and Lead on Soil Enzymatic Activity and Microbial Biomass. Environ. Toxicol. Pharmacol. 2013, 36, 667–674. [Google Scholar] [CrossRef]
- Xiang, Y.; Jia, M.; Xu, R.; Xu, J.; He, L.; Peng, H.; Sun, W.; Wang, D.; Xiong, W.; Yang, Z. Carbamazepine Facilitated Horizontal Transfer of Antibiotic Resistance Genes by Enhancing Microbial Communication and Aggregation. Bioresour. Technol. 2024, 391, 129983. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lu, J.; Mao, L.; Li, J.; Yuan, Z.; Bond, P.L.; Guo, J. Antiepileptic Drug Carbamazepine Promotes Horizontal Transfer of Plasmid-Borne Multi-Antibiotic Resistance Genes within and across Bacterial Genera. ISME J. 2019, 13, 509–522. [Google Scholar] [CrossRef]
- Tian, L.; Fang, H.; Mao, Q.; Bai, Y.; Ye, Z.; Hu, D.; Wang, X.; Hou, Y.; Ye, N.; Zhang, S.; et al. Low Concentrations of Antibiotics Alter Microbial Communities and Induce High Abundances of Antibiotic-Resistant Genes in Ornamental Water. Water 2023, 15, 3047. [Google Scholar] [CrossRef]
- Lee, S.S.; Paspalof, A.M.; Snow, D.D.; Richmond, E.K.; Rosi-Marshall, E.J.; Kelly, J.J. Occurrence and Potential Biological Effects of Amphetamine on Stream Communities. Environ. Sci. Technol. 2016, 50, 9727–9735. [Google Scholar] [CrossRef] [PubMed]
- Wolfson, S.J.; Porter, A.W.; Villani, T.S.; Simon, J.E.; Young, L.Y. The Antihistamine Diphenhydramine Is Demethylated by Anaerobic Wastewater Microorganisms. Chemosphere 2018, 202, 460–466. [Google Scholar] [CrossRef]
- Güzelant, A.; Apiliogullari, S.; Kara, I.; Turhan, V.; Apiliogullari, B.; Yilmaz, H.; Balasar, M.; Duman, A. The Preventive Effect of Diphenhydramine on Bacterial Growth in Propofol: A Laboratory Study. Eur. J. Anaesthesiol. 2008, 25, 737–740. [Google Scholar] [CrossRef]
| Compound | Application | Chemical Formula | Molecular Weight (g/mol) | pKa | LogKow | Solubility (mg/L) | References |
|---|---|---|---|---|---|---|---|
| Acetaminophen | Analgesic | C8H9NO2 | 151.16 | 9.38 | 0.46 | 14 × 103 | [29,44] |
| Atenolol | β-Blocker | C14H22N2O3 | 266.34 | 9.6 | 0.16 | 13 × 103 | [29,44] |
| Carbamazepine | Anti-epileptic | C15H12N2O | 236.27 | 13.9 | 2.45 | 18 | [29,44] |
| Diclofenac | Anti-inflammatory | C14H11Cl12NO2 | 296.14 | 4.15 | 4.51 | 4.47 | [29,44] |
| Ibuprofen | Anti-inflammatory | C13H18O2 | 206.28 | 4.91 | 3.97 | 21 | [29,44] |
| Ranitidine | Antihistamine | C13H22N4O3S | 314.40 | 8.4 | 0.99 | 1000 | [29,42] |
| Sulfamethoxazole | Antibiotic | C12H14N4O2S | 278.33 | 7.59 | 0.14 | 610 | [29,44] |
| Salicylic acid | Analgesic | C7H6O3 | 138.12 | 2.97; 13.6 | 2.26 | 2240 | [29] |
| Compound | Application | Chemical Formula | Molecular Weight (g/mol) | pKa | LogKow | Solubility (mg/L) | References |
|---|---|---|---|---|---|---|---|
| Climbazole | Antifungal | C15H17ClN2O2 | 292.76 | - | - | - | [46] |
| Ethinylestradiol | Hormone | C20H24O2 | 296.40 | 1.70 | - | 1.70 | [45,46] |
| Galaxolide | Fragrances | C18H26O | 258.40 | - | - | 1.75 | [29,46] |
| Tonalide | Fragrances | C18H26O | 258.4 | - | - | 1.25 | [29,46] |
| Triclosan | Anti-microbial | C12H7Cl3O2 | 289.53 | 7.9 | 4.76 | 10 | [29,46] |
| Compound | Application | Chemical Formula | Molecular Weight (g/mol) | pKa | LogKow | Solubility (mg/L) | References |
|---|---|---|---|---|---|---|---|
| Trifluoroacetic acid | Adhesive and sealant | C2HF3O2 | 111.02 | 0.52 | - | - | [55] |
| Perfluorobutanoic acid | Firefighting foams | C4HF9O3S | 300.10 | - | - | - | [54,55] |
| Perfluorohexane phosphonic acid | Food packaging | C6HF13O3S | 400.11 | −3.45 | - | - | [54,55] |
| Hexafluoropropylene oxide trimer acid | Industrial organofluorine chemistry | C3F6O | 166.02 | - | - | - | [54,55] |
| Compound | Application | Chemical Formula | Molecular Weight (g/mol) | pKa | LogKow | Solubility (mg/L) | References |
|---|---|---|---|---|---|---|---|
| Organochlorides (DDT) | Insecticide | C14H9Cl15 | 702.00 | - | 6.91 | 0.006 | [57,58] |
| Organophosphates (Azinphos-methyl) | Insecticide | C10H12N3O2PS2 | 300.00 | - | 2.96 | 28 | [57,58] |
| Carbamates (Aldicarb) | Insecticide | C7H14N2O2S | 190.00 | - | 1.15 | 4930 | [57,58] |
| Pyrethroids (Acrinathrin) | Insecticide | C26H21F6NO5 | 541.00 | - | 5.6 | <0.02 | [57,58] |
| Emerging Contaminant | Source | Concentration (ng/L) | References |
|---|---|---|---|
| Atrazine | Surface water | 1237 | [64] |
| Terbutylazine | Surface water | 1969 | [64] |
| Atenolol | Groundwater | 25,900 | [64] |
| Sulfamethoxazole | Wastewater | 34,500 | [64] |
| Nevirapine | Surface water | 1480 | [66] |
| Zidovudine | Surface water | 973 | [66] |
| Lamivudine | Surface water | 242 | [66] |
| Stavudine | Surface water | 778 | [66] |
| Emerging Contaminant | Impact | Microbial Response | Reference |
|---|---|---|---|
| Sulfamethoxazole | Decrease the bacterial biomass in biofilms by inhibiting the production of dihydrofolic acid. | The microbial cells produce more EPSs to act as a protective barrier against the compound. | [173,174,176] |
| Oxytetracycline | Increase in microbial biomass carbon, which led to an increase in nitrification potential and dehydrogenase activity. | The microbial community adapts to the metabolic shift, resulting in a microbial shift to a community in favor of members that are more tolerant to the ECs and capable of EC biodegradation. | [177,178] |
| Carbamazepine | Disruption in the microbial diversity within the community leads to the selection of antibiotic-resistant genes. | The microbial community acquires antibiotic-resistant genes as a result of horizontal gene transfer. | [179,180,181] |
| Amphetamine | Amphetamine disrupts the photosynthetic process, which can cause a decrease in the oxygen levels, shifting to a more anaerobic community and eventually decreases the bacterial composition. | The microbial community may shift towards anaerobic biodegraders with enhanced expression of detoxification enzymes. | [182] |
| Diphenhydramine | Inhibit the growth and proliferation of bacteria, which can lead to a decline in aerobic fast-oxidizing microbes. | Bacterial species adapt to diphenhydramine concentrations in wastewater and demethylate diphenhydramine into N-desmethyl diphenhydramine. | [183,184] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mqambalala, A.; Maleke, M.; Osman, J.R.; Hernandez, J.C. Biodegradation of Emerging Contaminants Controlled by Biological and Chemical Factors. Microorganisms 2025, 13, 2354. https://doi.org/10.3390/microorganisms13102354
Mqambalala A, Maleke M, Osman JR, Hernandez JC. Biodegradation of Emerging Contaminants Controlled by Biological and Chemical Factors. Microorganisms. 2025; 13(10):2354. https://doi.org/10.3390/microorganisms13102354
Chicago/Turabian StyleMqambalala, Avela, Maleke Maleke, Jorge R. Osman, and Julio Castillo Hernandez. 2025. "Biodegradation of Emerging Contaminants Controlled by Biological and Chemical Factors" Microorganisms 13, no. 10: 2354. https://doi.org/10.3390/microorganisms13102354
APA StyleMqambalala, A., Maleke, M., Osman, J. R., & Hernandez, J. C. (2025). Biodegradation of Emerging Contaminants Controlled by Biological and Chemical Factors. Microorganisms, 13(10), 2354. https://doi.org/10.3390/microorganisms13102354





