The Role of Nitric Oxide in HSV-1 Infection: The Use of an Inducible Nitric Synthase Inhibitor Aminoguanidine to Treat Neuroinflammation
Abstract
1. Introduction
2. Materials and Methods
2.1. Virus and Cells
2.2. Mice and Infection
2.3. Flow Cytometry Analysis
2.4. Quantitative PCR
2.5. Confocal Microscopy
2.6. Primary Cultures
2.7. ELISA
2.8. Statistics
3. Results
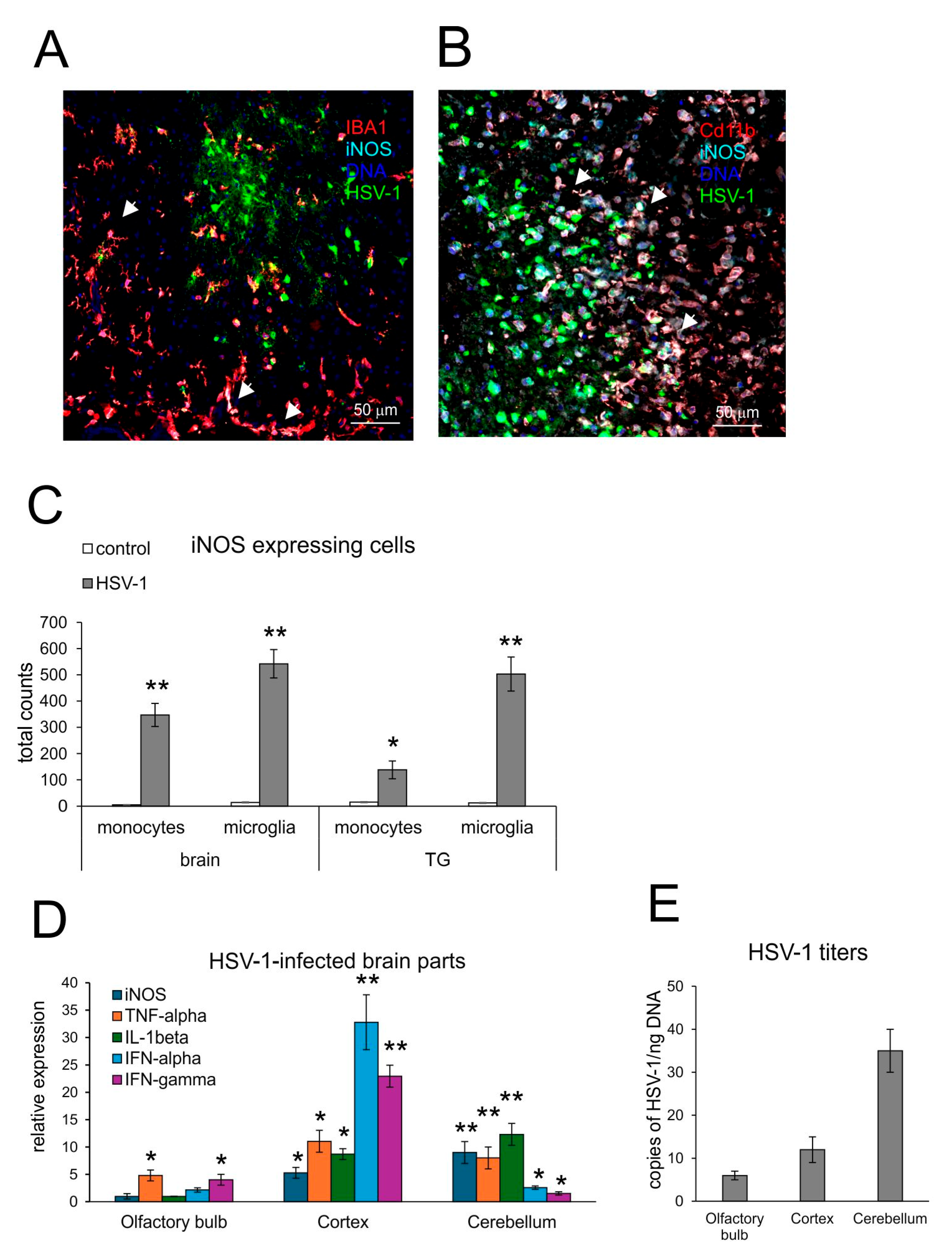
3.1. Expression of iNOS Accompanies Neuroinflammation
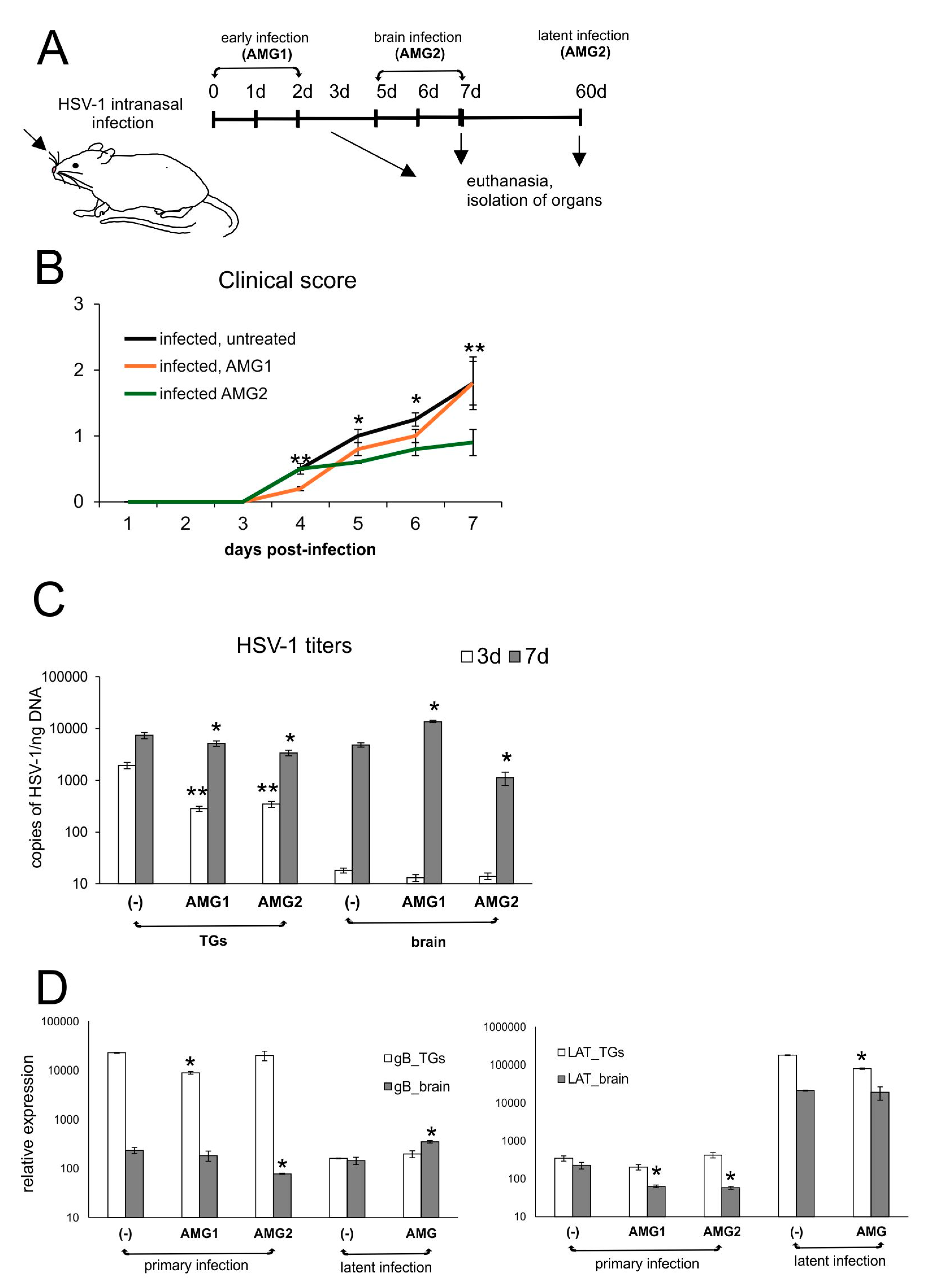
3.2. Inhibition of NO Production Induces Different Effects upon Response to HSV-1 Infection Depending on the Time of Treatment
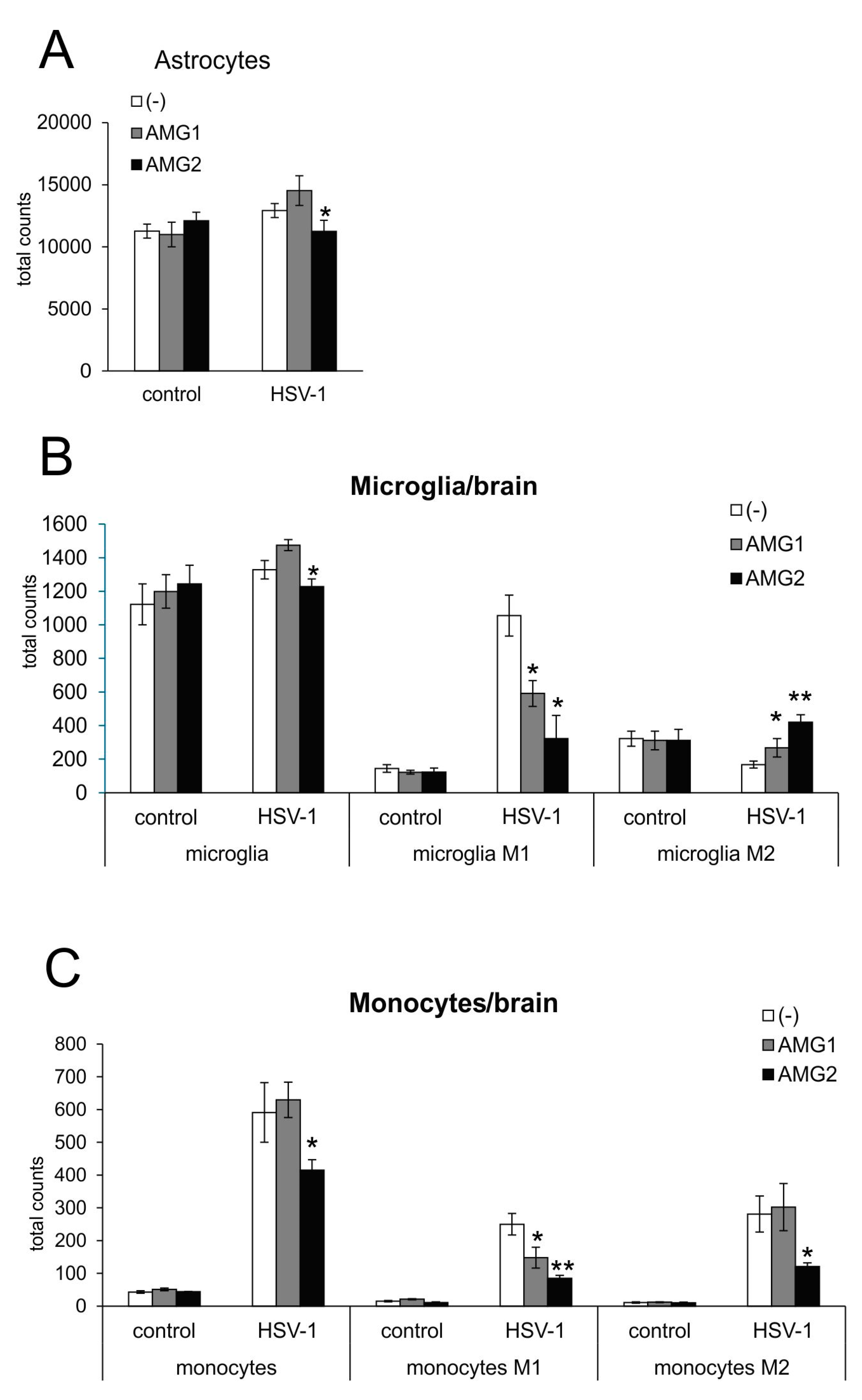
3.3. NO Exerts Cell Type-Dependent Effects for Glial Cells upon HSV-1 Infection
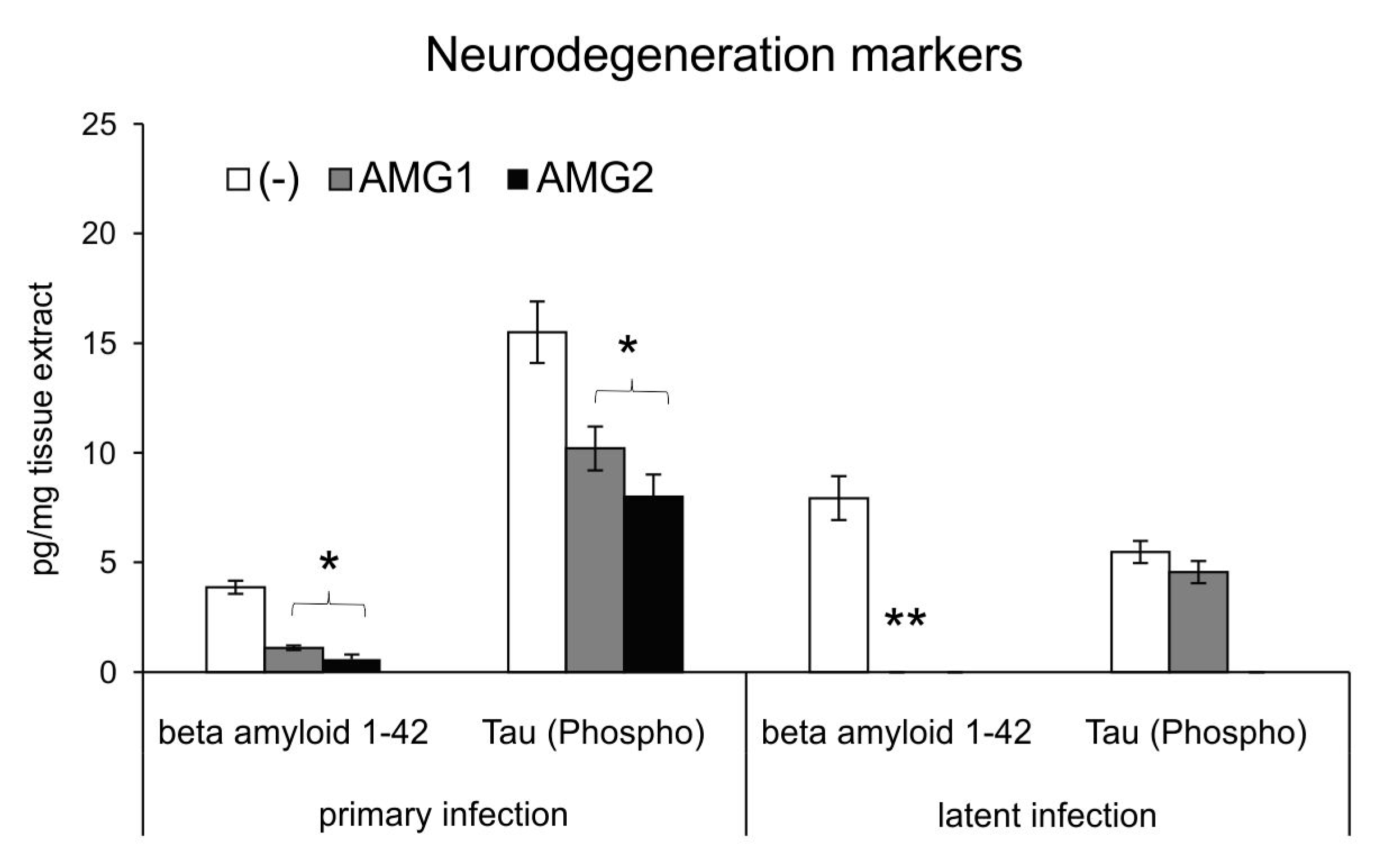
3.4. Inhibition of NO Production Early During Infection Influences HSV-1-Related Neurodegenerations Markers
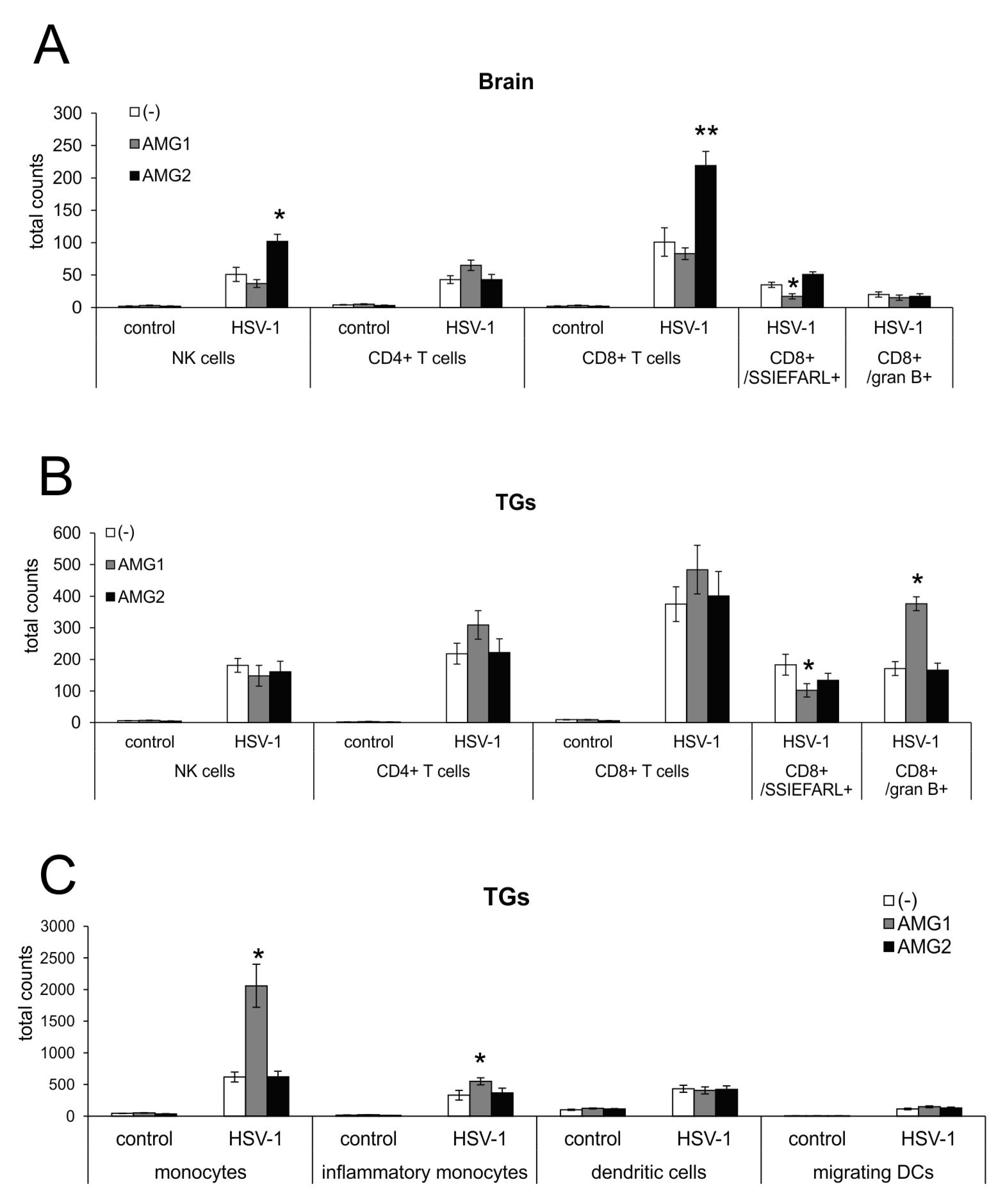
3.5. Inhibition of NO Production During HSV-1 Decreases Fas/FasL-Dependent Inflammation but May Increase Viral Titers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMG | aminoguanidine |
| SNP | sodium nitroprusside |
| TGs | trigeminal ganglia |
| NO | nitric oxide |
References
- Moncada, S.; Higgs, A. The L-arginine-nitric oxide pathway. N. Engl. J. Med. 1993, 329, 2002–2012. [Google Scholar] [PubMed]
- Moncada, S.; Palmer, R.M.; Higgs, E.A. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991, 43, 109–142. [Google Scholar] [CrossRef]
- Stuehr, D.J. Structure-function aspects in the nitric oxide synthases. Annu. Rev. Pharmacol. Toxicol. 1997, 37, 339–359. [Google Scholar] [CrossRef]
- Geller, D.A.; Billiar, T.R. Molecular biology of nitric oxide synthases. Cancer Metastasis Rev. 1998, 17, 7–23. [Google Scholar] [CrossRef]
- Goldstein, S.; Merenyi, G. The chemistry of peroxynitrite: Implications for biological activity. Methods Enzymol. 2008, 436, 49–61. [Google Scholar]
- Stamler, J.S. Redox signaling: Nitrosylation and related target interactions of nitric oxide. Cell 1994, 78, 931–936. [Google Scholar] [CrossRef]
- Stamler, J.S.; Hausladen, A. Oxidative modifications in nitrosative stress. Nat. Struct. Biol. 1998, 5, 247–249. [Google Scholar] [CrossRef] [PubMed]
- Garren, M.R.; Ashcraft, M.; Qian, Y.; Douglass, M.; Brisbois, E.J.; Handa, H. Nitric oxide and viral infection: Recent developments in antiviral therapies and platforms. Appl. Mater. Today 2021, 22, 100887. [Google Scholar] [CrossRef]
- Sanders, S.P.; Proud, D.; Permutt, S.; Siekierski, E.S.; Yachechko, R.; Liu, M.C. Role of nasal nitric oxide in the resolution of experimental rhinovirus infection. J. Allergy Clin. Immunol. 2004, 113, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Dulin, H.; Hendricks, N.; Xu, D.; Gao, L.; Wuang, K.; Ai, H.; Hai, R. Impact of Protein Nitration on Influenza Virus Infectivity and Immunogenicity. Microbiol. Spectr. 2022, 10, e0190222. [Google Scholar] [CrossRef]
- Akaberi, D.; Krambrich, J.; Ling, J.; Luni, C.G.; Jarhult, J.D.; Lennerstrand, J.; Lundkvist, A. Mitigation of the replication of SARS-CoV-2 by nitric oxide in vitro. Redox Biol. 2020, 37, 101734. [Google Scholar] [CrossRef]
- Pandian, K.; Postma, R.; van Zonneveld, A.J.; Harms, A.; Hankemeier, T. Microvessels-on-chip: Exploring endothelial cells and COVID-19 plasma interaction with nitric oxide metabolites. Nitric Oxide 2025, 155, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Kandemir, O.; Polat, A.; Kaya, A. Inducible nitric oxide synthase expression in chronic viral hepatitis and its relation with histological severity of disease. J. Viral Hepat. 2002, 9, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Andrews, D.M.; Matthews, V.B.; Sammels, L.M.; Carrello, A.C.; McMinn, P.C. The severity of murray valley encephalitis in mice is linked to neutrophil infiltration and inducible nitric oxide synthase activity in the central nervous system. J. Virol. 1999, 73, 8781–8790. [Google Scholar] [CrossRef]
- Zhu, S.; Viejo-Borbolla, A. Pathogenesis and virulence of herpes simplex virus. Virulence 2021, 12, 2670–2702. [Google Scholar] [CrossRef]
- World Health Organization. Herpes Simplex Virus. 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/herpes-simplex-virus (accessed on 23 May 2025).
- Eimer, W.A.; Vijaya Kumar, D.K.; Navalpur Shanmugam, N.K.; Rodriguez, A.S.; Mitchell, T.; Washicosky, K.J.; György, B.; Breakefield, X.O.; Tanzi, R.E.; Moir, R.D. Alzheimer’s disease-associated beta-amyloid is rapidly seeded by herpesviridae to protect against brain infection. Neuron 2018, 100, 1527–1532. [Google Scholar] [CrossRef]
- Bourgade, K.; Frost, E.H.; Dupuis, G.; Witkowski, J.M.; Laurent, B.; Calmettes, C.; Ramassamy, C.; Desroches, M.; Rodrigues, S.; Fülöp, T. Interaction Mechanism Between the HSV-1 Glycoprotein B and the Antimicrobial Peptide Amyloid-β. J. Alzheimers Dis. Rep. 2022, 6, 599–606. [Google Scholar] [CrossRef] [PubMed]
- D’Aiuto, L.; Caldwell, J.K.; Edwards, T.G.; Zhou, C.; McDonald, M.L.; Di Maio, R.; Joel, W.A.; Hyde, V.R.; Wallace, C.T.; Watkins, S.C.; et al. Phosphorylated-tau associates with HSV-1 chromatin and correlates with nuclear speckles decondensation in low-density host chromatin regions. Neurobiol. Dis. 2025, 206, 106804. [Google Scholar] [CrossRef]
- Carbone, I.; Lazzarotto, T.; Ianni, M.; Porcellini, E.; Forti, P.; Masliah, E.; Gabrielli, L.; Licastro, F. Herpes virus in Alzheimer’s disease: Relation to progression of the disease. Neurobiol. Aging 2014, 35, 122–129. [Google Scholar] [CrossRef]
- Sait, A.; Angeli, C.; Doig, A.J.; Day, P.J.R. Viral involvement in Alzheimer’s disease. ACS Chem. Neurosci. 2021, 12, 1049–1060. [Google Scholar] [CrossRef]
- Prosswimmer, T.; Heng, A.; Daggett, V. Mechanistic insights into the role of amyloid-β in innate immunity. Sci. Rep. 2024, 14, 5376. [Google Scholar] [CrossRef]
- Reinert, L.S.; Lopušná, K.; Winther, H.; Sun, C.; Thomsen, M.K.; Nandakumar, R.; Mogensen, T.H.; Meyer, M.; Vægter, C.; Nyengaard, J.R.; et al. Sensing of HSV-1 by the cGAS-STING pathway in microglia orchestrates antiviral defence in the CNS. Nat. Commun. 2016, 7, 13348. [Google Scholar] [CrossRef]
- Chhatbar, C.; Detje, C.N.; Grabski, E.; Borst, K.; Spanier, J.; Ghita, L.; Elliott, D.A.; Jordao, M.J.C.; Mueller, N.; Sutton, J.; et al. Type I interferon receptor signaling of neurons and astrocytes regulates microglia activation during viral encephalitis. Cell Rep. 2018, 25, 118–129. [Google Scholar] [CrossRef]
- Marques, C.P.; Cheeran, M.C.; Palmquist, J.M.; Hu, S.; Urban, S.L.; Lokensgard, J.R. Prolonged microglial cell activation and lymphocyte infiltration following experimental herpes encephalitis. J. Immunol. 2008, 181, 6417–6426. [Google Scholar] [CrossRef]
- Marques, C.P.; Cheeran, M.C.; Palmquist, J.M.; Hu, S.; Lokensgard, J.R. Microglia are the major cellular source of inducible nitric oxide synthase during experimental herpes encephalitis. J. NeuroVirology 2008, 14, 229–238. [Google Scholar] [CrossRef]
- Zolini, G.P.; Lima, G.K.; Lucinda, N.; Silva, M.A.; Dias, M.F.; Pessoa, N.L.; Coura, B.P.; Cartelle, C.T.; Arantes, R.M.; Kroon, E.G.; et al. Defense against HSV-1 in a murine model is mediated by iNOS and orchestrated by the activation of TLR2 and TLR9 in trigeminal ganglia. J. Neuroinflamm. 2014, 11, 20. [Google Scholar] [CrossRef] [PubMed]
- Gamba, G.; Cavalieri, H.; Courreges, M.C.; Massouh, E.J.; Benencia, F. Early inhibition of nitric oxide production increases HSV-1 intranasal infection. J. Med. Virol. 2004, 73, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Benencia, F.; Gamba, G.; Cavalieri, H.; Courreges, M.C.; Benedetti, R.; Villamil, S.M.; Massouh, E.J. Nitric oxide and HSV vaginal infection in BALB/c mice. Virology 2003, 309, 75–84. [Google Scholar] [CrossRef]
- Fan, D.; Gu, Y.T.; Lv, H.; Tang, T.; Xu, Z.H.; Song, Z.Q.; Tong, X.J. The protective mechanism for the blood-brain barrier induced by aminoguanidine in surgical brain injury in rats. Cell. Mol. Neurobiol. 2011, 31, 1213–1219. [Google Scholar] [CrossRef]
- Cymerys, J.; Kowalczyk, A.; Mikołajewicz, K.; Słońska, A.; Krzyżowska, M. Nitric Oxide Influences HSV-1-Induced Neuroinflammation. Oxid. Med. Cell. Longev. 2019, 11, 2302835. [Google Scholar] [CrossRef] [PubMed]
- Krzyzowska, M.; Kowalczyk, A.; Skulska, K.; Thörn, K.; Eriksson, K. Fas/FasL Contributes to HSV-1 Brain Infection and Neuroinflammation. Front. Immunol. 2021, 12, 714821. [Google Scholar] [CrossRef]
- Krzyzowska, M.; Janicka, M.; Chodkowski, M.; Patrycy, M.; Obuch-Woszczatyńska, O.; Tomaszewska, E.; Ranoszek-Soliwoda, K.; Celichowski, G.; Grobelny, J. Epigallocatechin Gallate-Modified Silver Nanoparticles Show Antiviral Activity against Herpes Simplex Type 1 and 2. Viruses 2023, 15, 2024. [Google Scholar] [CrossRef]
- Menendez, C.M.; Jinkins, J.K.; Carr, D.J.J. Resident T Cells are unable to control herpes simplex virus-1 activity in the brain ependymal region during latency. J. Immunol. 2016, 197, 1262–1275. [Google Scholar] [CrossRef] [PubMed]
- Åkerstrom, S.; Gunalan, V.; Keng, C.T.; Tan, Y.-J.; Mirazimi, A. Dual effect of nitric oxide on SARS-CoV replication: Viral RNA production and palmitoylation of the S protein are affected. Virology 2009, 395, 1–9. [Google Scholar] [CrossRef]
- Freidkin, L.; Garsiel Katz, T.; Peles, I.; Ben Shitrit, I.; Abayev, M.; Almog, Y.; Galante, O.; Fuchs, L. Medium-Term Effect of Inhaled Nitric Oxide in Mechanically Ventilated COVID-19 Patients. J. Clin. Med. 2025, 14, 806. [Google Scholar] [CrossRef] [PubMed]
- Drake, M.G.; Bivins-Smith, E.R.; Proskocil, B.J.; Nie, Z.; Scott, G.D.; Lee, J.J.; Lee, N.A.; Fryer, A.D.; Jacoby, D.B. Human and mouse eosinophils have antiviral activity against parainfluenza virus. Am. J. Respir. Cell Mol. Biol. 2016, 55, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Bochkov, Y.A.; Voelker, D.R.; Foster, M.W.; Que, L.G. Identification of a Novel Inhibitor of Human Rhinovirus Replication and Inflammation in Airway Epithelial Cells. Am. J. Respir. Cell Mol. Biol. 2019, 60, 58–67. [Google Scholar] [CrossRef]
- Pinheiro, M.B.M.; Rozini, S.V.; Quirino-Teixeira, A.C.; Barbosa-Lima, G.; Lopes, J.F.; Sacramento, C.Q.; Bozza, F.A.; Bozza, P.T.; Hottz, E.D. Dengue induces iNOS expression and nitric oxide synthesis in platelets through IL-1R. Front. Immunol. 2022, 13, 1029213. [Google Scholar] [CrossRef]
- Thein, T.-L.; Wong, J.; Leo, Y.-S.; Ooi, E.-E.; Lye, D.; Yeo, T.W. Association between increased vascular nitric oxide bioavailability and progression to dengue hemorrhagic fever in adults. J. Infect. Dis. 2015, 212, 711–714. [Google Scholar] [CrossRef]
- Madhu, B.P.; Singh, K.P.; Saminathan, M.; Singh, R.; Shivasharanappa, N.; Sharma, A.K.; Malik, Y.S.; Dhama, K.; Manjunatha, V. Role of nitric oxide in the regulation of immune responses during rabies virus infection in mice. Virusdisease 2016, 27, 387–399. [Google Scholar] [CrossRef]
- Fujii, S.; Akaike, T.; Maeda, H. Role of nitric oxide in pathogenesis of herpes simplex virus encephalitis in rats. Virology 1999, 256, 203–212. [Google Scholar] [CrossRef]
- Benencia, F.; Courreges, M.C.; Gamba, G.; Cavalieri, H.; Massouh, E.J. Effect of aminoguanidine, a nitric oxide synthase inhibitor, on ocular infection with herpes simplex virus in Balb/c mice. Invest. Ophthalmol. Vis. Sci. 2001, 42, 1277–1284. [Google Scholar]
- Meyding-Lamadé, U.; Seyfer, S.; Haas, J.; Dvorak, F.; Kehm, R.; Lamadé, W.; Hacke, W.; Wildemann, B. Experimental herpes simplex virus encephalitis: Inhibition of the expression of inducible nitric oxide synthase in mouse brain tissue. Neurosci. Lett. 2002, 318, 21–24. [Google Scholar] [CrossRef]
- Hasson, J.; Weidenfeld, J.; Mizrachi-Kol, R.; Ben-Hur, T.; Ovadia, H. The effect of herpes simplex virus-1 on nitric oxide synthase in the rat brain: The role of glucocorticoids. Neuroimmunomodulation 2011, 18, 111–116. [Google Scholar]
- Lucinda, N.; Figueiredo, M.M.; Pessoa, N.L.; Santos, B.S.; Lima, G.K.; Freitas, A.M.; Machado, A.M.; Kroon, E.G.; Antonelli, L.R.; Campos, M.A. Dendritic cells, macrophages, NK and CD8+ T lymphocytes play pivotal roles in controlling HSV-1 in the trigeminal ganglia by producing IL1-beta, iNOS and granzyme B. Virol. J. 2017, 14, 37. [Google Scholar]
- Bingisser, R.M.; Tilbrook, P.A.; Holt, P.G.; Kees, U.R. Macrophage-Derived Nitric Oxide Regulates T cell Activation via Reversible Disruption of the Jak3/STAT5 Signaling Pathway. J. Immunol. 1998, 160, 5729–5734. [Google Scholar] [PubMed]
- Niedbala, W.; Wei, X.-Q.; Campbell, C.; Thomson, D.; Komai-Koma, M.; Liew, F.Y. Nitric oxide preferentially induces type 1 T cell differentiation by selectively up-regulating IL-12 receptor β2 expression via cGMP. Proc. Natl. Acad. Sci. USA 2002, 99, 16186–16191. [Google Scholar] [PubMed]
- Niedbala, W.; Wei, X.-Q.; Piedrafita, D.; Xu, D.; Liew, F.Y. Effects of nitric oxide on the induction and differentiation of Th1 cells. Eur. J. Immunol. 1999, 29, 2498–2505. [Google Scholar] [CrossRef]
- Lee, D.H.; Ghiasi, H. Roles of M1 and M2 macrophages in herpes simplex virus 1 infectivity. J. Virol. 2017, 91, e00578-17. [Google Scholar] [CrossRef]
- Jaggi, U.; Yang, M.; Matundan, H.H.; Hirose, S.; Shah, P.K.; Sharifi, B.G.; Ghiasi, H. Increased phagocytosis in the presence of enhanced M2-like macrophage responses correlates with increased primary and latent HSV-1 infection. PLoS Pathog. 2020, 16, e1008971. [Google Scholar] [CrossRef]
- Seitz, S.; Clarke, P.; Tyler, K.L. Pharmacologic depletion of microglia increases viral load in the brain and enhances mortality in murine models of flavivirus-induced encephalitis. J. Virol. 2018, 92, e00525-18. [Google Scholar] [CrossRef]
- Wheeler, D.L.; Sariol, A.; Meyerholz, D.K.; Perlman, S. Microglia are required for protection against lethal coronavirus encephalitis in mice. J. Clin. Investig. 2018, 128, 931–943. [Google Scholar] [CrossRef]
- Ronaldson, P.T.; Bendayan, R. HIV-1 viral envelope glycoprotein gp120 produces oxidative stress and regulates the functional expression of multidrug resistance protein-1 (Mrp1) in glial cells. J. Neurochem. 2008, 106, 1298–1313. [Google Scholar] [CrossRef]
- de Souza, K.P.; Silva, E.G.; de Oliveira Rocha, E.S.; Figueiredo, L.B.; de Almeida-Leite, C.M.; Arantes, R.M.; de Assis Silva Gomes, J.; Ferreira, G.P.; de Oliveira, J.G.; Kroon, E.G.; et al. Nitric oxide synthase expression correlates with death in an experimental mouse model of dengue with CNS involvement. Virol. J. 2013, 10, 267. [Google Scholar] [CrossRef] [PubMed]
- Forrester, V.; McMenamin, P.G.; Dando, S.J. CNS infection and immune privilege. Nat. Rev. Neurosci. 2018, 19, 655–671. [Google Scholar] [CrossRef]
- Cai, Z.; Hussain, M.D.; Yan, L.J. Microglia, neuroinflammation, and beta-amyloid protein in Alzheimer’s disease. Int. J. Neurosci. 2014, 124, 307–321. [Google Scholar] [CrossRef]
- Xu, L.; He, D.; Bai, Y. Microglia-mediated inflammation and neurodegenerative disease. Mol. Neurobiol. 2016, 53, 6709–6715. [Google Scholar] [CrossRef]
- Griffin, W.S. Neuroinflammatory cytokine signaling and Alzheimer’s disease. N. Engl. J. Med. 2013, 368, 770–771. [Google Scholar] [CrossRef] [PubMed]
- De Chiara, G.; Piacentini, R.; Fabiani, M.; Mastrodonato, A.; Marcocci, M.E.; Limongi, D.; Napoletani, G.; Protto, V.; Coluccio, P.; Celestino, I.; et al. Recurrent herpes simplex virus-1 infection induces hall arks of neurodegeneration and cognitive deficits in mice. PLoS Pathog. 2019, 15, e1007617. [Google Scholar]
- Colzani, M.; De Maddis, D.; Casali, G.; Carini, M.; Vistoli, G.; Aldini, G. Reactivity, Selectivity, and Reaction Mechanisms of Aminoguanidine, Hydralazine, Pyridoxamine, and Carnosine as Sequestering Agents of Reactive Carbonyl Species: A Comparative Study. ChemMedChem 2016, 11, 1778–1789. [Google Scholar] [CrossRef]
- Yuste, J.E.; Tarragon, E.; Campuzano, C.M.; Ros-Bernal, F. Implications of glial nitric oxide in neurodegenerative diseases. Front. Cell Neurosci. 2015, 9, 322. [Google Scholar]
- Yildiz, G.; Demiryürek, A.T.; Sahin-Erdemli, I.; Kanzik, I. Comparison of antioxidant activities of aminoguanidine, methylguanidine and guanidine by luminol-enhanced chemiluminescence. Br. J. Pharmacol. 1998, 124, 905–910. [Google Scholar]
- Mielcarska, M.B.; Rouse, B.T. Viruses and the Brain—A Relationship Prone to Trouble. Viruses 2025, 17, 203. [Google Scholar] [CrossRef] [PubMed]
- Ethell, D.W.; Buhler, L.A. Fas ligand-mediated apoptosis in degenerative disorders of the brain. J. Clin. Immunol. 2003, 23, 363–370. [Google Scholar] [PubMed]
- Lind, L.; Svensson, A.; Thörn, K.; Krzyzowska, M.; Eriksson, K. CD8+ T cells in the central nervous system of mice with herpes simplex infection are highly activated and express high levels of CCR5 and CXCR3. J. NeuroVirology 2021, 27, 145–153. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patrycy, M.; Janicka, M.; Kauc, A.; Osińska, A.; Antos-Bielska, M.; Bylińska, K.; Obuch-Woszczatyńska, O.; Szymański, P.; Chodkowski, M.; Krzyżowska, M. The Role of Nitric Oxide in HSV-1 Infection: The Use of an Inducible Nitric Synthase Inhibitor Aminoguanidine to Treat Neuroinflammation. Microorganisms 2025, 13, 2222. https://doi.org/10.3390/microorganisms13102222
Patrycy M, Janicka M, Kauc A, Osińska A, Antos-Bielska M, Bylińska K, Obuch-Woszczatyńska O, Szymański P, Chodkowski M, Krzyżowska M. The Role of Nitric Oxide in HSV-1 Infection: The Use of an Inducible Nitric Synthase Inhibitor Aminoguanidine to Treat Neuroinflammation. Microorganisms. 2025; 13(10):2222. https://doi.org/10.3390/microorganisms13102222
Chicago/Turabian StylePatrycy, Magdalena, Martyna Janicka, Agnieszka Kauc, Aleksandra Osińska, Małgorzata Antos-Bielska, Klaudia Bylińska, Oliwia Obuch-Woszczatyńska, Paweł Szymański, Marcin Chodkowski, and Małgorzata Krzyżowska. 2025. "The Role of Nitric Oxide in HSV-1 Infection: The Use of an Inducible Nitric Synthase Inhibitor Aminoguanidine to Treat Neuroinflammation" Microorganisms 13, no. 10: 2222. https://doi.org/10.3390/microorganisms13102222
APA StylePatrycy, M., Janicka, M., Kauc, A., Osińska, A., Antos-Bielska, M., Bylińska, K., Obuch-Woszczatyńska, O., Szymański, P., Chodkowski, M., & Krzyżowska, M. (2025). The Role of Nitric Oxide in HSV-1 Infection: The Use of an Inducible Nitric Synthase Inhibitor Aminoguanidine to Treat Neuroinflammation. Microorganisms, 13(10), 2222. https://doi.org/10.3390/microorganisms13102222







